Abstract
Hyperactivation of Cdc2 in fission yeast causes cells to undergo a lethal premature mitosis called mitotic catastrophe. This phenotype is observed in cdc2-3w wee1-50 cells at high temperature. Eleven of 17 mutants that suppress this phenotype define a single complementation group, mcs1. The mcs1-77 mutant also suppresses lethal inactivation of the Wee1 and Mik1 tyrosine kinases and thus delays mitosis independently of Cdc2 tyrosine phosphorylation. We have cloned mcs1 by isolating suppressors of the cell cycle arrest phenotype of mcs1-77 cdc25-22 cells and found that it encodes Res2, a component of the START gene–specific transcription factor complex MBF (also known as DSC-1). The mcs1-77 mutant bears a single point mutation in the DNA-binding domain of Res2 that causes glycine 68 to be replaced by a serine residue. Importantly, two substrates of the anaphase-promoting complex (APC), the major B-type cyclin, Cdc13, and the anaphase inhibitor, Cut2, are unstable in G2-phase mcs1-77 cells. Consistent with this, we observe abnormal sister chromatid separation in mcs1-77 cdc25-22 cells at the restrictive temperature. Mutation of either Cdc10 or Res1 also deregulates MBF-dependent transcription and causes a G2 delay. We find that this cell cycle delay is abolished in the absence of the APC regulator Ste9/Srw1 and that the periodic expression of Ste9/Srw1 is controlled by the MBF complex. These data suggest that in fission yeast the MBF complex plays a key role in the inactivation of cyclin B and Cut2 destruction by controlling the periodic production of APC regulators.
INTRODUCTION
In eukaryotic cells, both the onset of DNA replication (S phase) and the initiation of mitosis (M phase) are triggered by members of the Cdk family in association with a regulatory cyclin subunit. Considerable efforts have been made in the past 10 years to understand exactly how Cdk/cyclin complexes are regulated. In the fission yeast, both major transitions are catalyzed by a single gene product, Cdc2 (Nurse and Bissett, 1981). Although Cdc2 associates with four distinct cyclins encoded by the cdc13, cig1, cig2, and puc1 genes, only one of these, Cdc13, is indispensable for cell cycle progression and is sufficient to trigger both S phase and the initiation of mitosis in the absence of the other cyclins (reviewed by Fisher and Nurse, 1995; Stern and Nurse, 1996). Productive complex formation with the Cdc2 kinase requires phosphorylation of the catalytic subunit on a conserved threonine residue (Gould et al., 1991). The activity of the Cdc2/Cdc13 complex periodically oscillates through the cell cycle, peaking in M phase, and is maintained in an inactive state in G1 by both direct binding of a Cdk inhibitor, Rum1, and proteolytic degradation of the cyclin subunit (Moreno et al., 1989; Correa-Bordes and Nurse, 1995; Kominani et al., 1998). Ubiquitination and subsequent degradation of cyclin B is initiated by a specialized multisubunit ubiquitin ligase complex called the anaphase-promoting complex (APC) (reviewed by Page and Hieter, 1999; Zachariae and Nasmyth, 1999). The APC responsible for cyclin B degradation is found in association with an adaptor protein called Ste9/Srw1 (a homologue of CDH1/Fizzy-related) (Yamaguchi et al., 1997; Fang et al., 1998; Kitamura et al., 1998; Kominani et al., 1998). The APC is also responsible for the degradation of Cut2, an inhibitor of the metaphase-to-anaphase transition, but in this case APC associates with a distinct adaptor protein called Slp1 (a homologue of CDC20/Fizzy) (Funabiki et al., 1996; Matsumoto, 1997). The activity of the APC itself is cell cycle regulated in that ubiquitination and proteolysis of cyclin B occur only in mitosis and G1 (Amon et al., 1994; Brandeis and Hunt, 1996; Iringer and Nasmyth, 1997). On passage through START, a key regulatory event in late G1, APC-mediated degradation is inhibited and the Cdc2/Cdc13 cyclin complex accumulates, increasing to a peak in late G2. The mechanisms that trigger cessation of APC-mediated degradation, however, are unknown.
Although Cdc2 is required for the initiation of S phase, the activity of the complex is restrained in S phase and G2 by two functionally overlapping kinases, Wee1 and Mik1, which maintain an inhibitory phosphorylation of Cdc2 on a conserved tyrosine residue (Russell and Nurse, 1987; Lundgren et al., 1991). At the initiation of mitosis, the Cdc2/Cdc13 complex is fully activated by tyrosine dephosphorylation of this residue by the Cdc25 phosphatase (Millar et al., 1991). In addition to being required to induce S phase and mitosis, the presence or activity of the Cdc2/Cdc13 complex is believed to play an additional role in preventing the reinitiation of S phase during G2, because either deletion of cdc13 or overexpression of the rum1 Cdk inhibitor induces cells to undergo rereplication in the absence of an intervening mitosis (Hayles et al., 1994; Correa-Bordes and Nurse, 1995). Conversely, hyperactivation of Cdc2/Cdc13 kinase induces premature entry into mitosis (or mitotic catastrophe), which results in cell death (Russell and Nurse, 1987). Together, these results have led researchers to propose a quantitative model to explain how the Cdc2/Cdc13 complex can trigger both S phase and mitosis in the correct sequential cell cycle order (Stern and Nurse, 1996).
Although this model may be relevant to the rapid early divisions of metazoan embryonic cells, it fails to explain how cell cycle–regulated transcription is coordinated with periodic alteration in Cdk/cyclin activity. In particular, passage through START requires the function of an essential transcription factor complex called MBF, also known as DSC-1, which controls the expression of a number of genes critical for S-phase initiation (Lowndes et al., 1991, 1992). In fission yeast, the MBF complex, which contains the Cdc10 (Lowndes et al., 1992), Res1 (Tanaka et al.,1992; Caligiuri and Beach, 1993), Res2 (Miyamoto et al., 1994; Zhu et al., 1994; Aytéet al., 1997; Zhu et al., 1997), and Rep2 (Nakashima et al., 1995) proteins, binds to MluI cell cycle box elements in the promoters of a small number of periodically expressed genes, which so far include cdc18, cdc22, cdt1, cdt2, and cig2 (Caligiuri and Beach, 1993; Kelly et al., 1993; Hofmann and Beach, 1994; Obara-Ishihara and Okayama, 1994; Zhu et al., 1994). Of these, the cdc18 gene appears to be a critical target because temperature-sensitive mutations of cdc10 can be rescued by ectopic expression of cdc18 (Kelly et al., 1993). Not only is Cdc18 critical for S-phase initiation, but high-level expression can induce continuous DNA synthesis in the absence of an intervening mitosis (Nishitani and Nurse, 1995; Greenwood et al., 1998). Thus, accurate periodic expression of cdc18 is thought to be important for ensuring that cells initiate S phase only once per cell cycle (Nishitani and Nurse, 1995; Baum et al., 1998). Although MBF was initially thought to be the transcriptionally active complex, recent analysis has indicated that it can be isolated by electrophoretic mobility shift assay only in G2, when periodic transcription of target genes is repressed (Reymond et al., 1993; McInerny et al. 1995; Baum et al. 1997). The MBF complex disappears at the onset of mitosis and reappears during S phase of the next cell cycle, suggesting that it is directly or indirectly under the control of Cdk/cyclin activity (Reymond et al., 1993; Baum et al. 1997). Paradoxically, however, the induction, maintenance, and repression of target genes appear not to require the Cdc2 kinase (Baum et al., 1997). Thus, how cell cycle regulation of the MBF complex is coordinated with periodic activity of Cdk/cyclin complexes and the APC remains unclear.
To determine the mechanisms governing Cdc2/Cdc13 activity, we have focused on a number of mutants that display potent genetic interactions with cdc2 and thus may encode novel regulators of the Cdc2/Cdc13 complex. In particular, cdc2-3w wee1-50 cells, which express a dominantly active Cdc2 kinase and a temperature-sensitive Wee1 kinase, undergo premature and lethal mitotic initiation (Russell and Nurse, 1987). This phenotype is suppressed by mutations in one of six mitotic catastrophe suppressors (mcs1–mcs6) (Molz et al., 1989). We and others have recently shown that the mcs2 and mcs6 genes code for a cyclin H–like molecule and its Cdk, respectively, which interact to form fission yeast Cdc2-activating kinase (Buck et al., 1995; Damagnez et al., 1995). In this paper, we demonstrate that mcs1-77 bears a point mutation in the Res2 transcription factor, a component of the MBF complex. Mutations in Res2 and other components of the MBF complex cause a G2 delay that is exacerbated in cells bearing a partially defective Cdc25 phosphatase. We show that the Cdc13 cyclin B and the anaphase regulator Cut2 are unstable in mcs1-77 cells. These results suggest that inactivation of the START gene–specific transcription factor complex is required for the timely inactivation of APC-mediated destruction of mitotic targets. We show that, at least in the case of Cdc13 cyclin B, this instability is due to ectopic expression of the APC regulator Ste9/Srw1, a previously unrecognized transcriptional target of the MBF complex.
MATERIALS AND METHODS
Media and General Techniques
Media and genetic methods for studying fission yeast have been reviewed (Moreno et al., 1991). General DNA methods used standard techniques (Sambrook et al., 1989). Cell length measurements were made with the use of log-phase cells with a Nikon (Garden City, NY) filar eyepiece drum micrometer at 1200× magnification. Transformations were regularly performed by means of the lithium acetate method (Moreno et al., 1991) or by electroporation (Prentice, 1991) with the use of a Bio-Rad (Richmond, CA) Gene Pulser.
Analysis of DNA Content by Flow Cytometry
Samples containing ∼106 cells were fixed with 70% ethanol, treated successively with RNase and pepsin, and stained with 50 mg/ml propidium iodide essentially as described previously (Corliss and White, 1981). DNA content was then analyzed with a Becton Dickinson (Franklin Lakes, NJ) FACScan and CELL Quest software (Becton Dickenson, Oxford, United Kingdom).
Isolation and Transposon Mutagenesis of the res2 Genomic Clone
A genomic library, pURB1 (Barbet et al., 1992), was introduced into a mcs1-77 cdc25-22 ura4-D18 h− strain by lithium acetate transformation and plated on medium lacking uracil. A total of 60,000 transformants were screened. Transformants were replica plated twice to 30°C, and 89 complementing colonies were identified. DNA from these colonies was isolated by transformation into Escherichia coli. Plasmids encoding cdc25 and nim1 were identified by a combination of restriction mapping, PCR, and Southern blot analysis. The 42 remaining plasmids were found to be related by restriction mapping. The gene responsible for suppression was identified by transposon mutagenesis of the smallest genomic clone, pURB1-mcs1, with the use of TnHIS3, as described previously (Sedgwick and Morgan, 1994). Complementing activity was tested by transforming transposed DNA into a mcs1-77 cdc25-22 ura4-D18 strain that was assayed for growth at 30°C. Automated sequencing was performed with the Applied Biosystems (Foster City, CA) cycle sequencing kit with the use of previously described primers to the 5′ and 3′ ends of the transposon.
Sequencing of the mcs1-77 Mutation
The res2 ORF was amplified from mcs1-77 cells by PCR with the use of an Expand high-fidelity polymerase (Boehringer Mannheim, Indianapolis, IN) and cloned into pCRII (Invitrogen, Carlsbad, CA). Two independent PCR products were sequenced in both directions with the Applied Biosystems cycle sequencing kit with the use of T3 and T7 primers.
RNA Isolation and Hybridization
To isolate RNA, Schizosaccharomyces pombe cells were cultured in YEPD to exponential phase. Approximately 10 μg of total RNA was isolated and resolved by agarose gel electrophoresis before transfer to nitrocellulose for hybridization. Probing with [32P]dCTP-labeled DNA was as described previously (Zhu et al., 1997). A fragment of the ste9/srw1 ORF was amplified by PCR with the 5′ oligonucleotide CTTAGTAGCCCTTTTATCAAAT and the 3′ oligonucleotide GATTCGCGACATCGCAAAA for use as probes. Similarly, a probe for cdc18 was generated by PCR with the use of the 5′ oligonucleotide ATGGATGAATTTGATGGTTT and the 3′ oligonucleotide TTACCGTATTTTCATTGTACG. Probes for cdc2 and his3 were as described previously (Zhu et al., 1997)
Electrophoretic Mobility Shift Assay
Cells were grown to midlog phase, harvested, washed with 1 ml of H2O, resuspended in 40 μl of lysis buffer (25 mM HEPES, pH 7.6, 0.1 mM EDTA, 150 mM KCl, 0.1% Triton X-100, 25% glycerol, 1 M urea, 1 mM DTT, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM EGTA, 1 mM tetrasodium pyrophosphate, 100 μM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate), and lysed as described above. Lysate (30 μg) was incubated in 20 μl of binding buffer containing 25 mM HEPES, pH 7.6, 34 mM KCl, 5 mM MgCl2, and 2 μg of poly dIdC for 10 min at room temperature, and then for 20 min with 1 ng of [32P]dNTP-labeled probe. Reactions were run on a 4% acrylamide gel in 0.5 M TBE (100 mM tris-borate, 2 mM EDTA), dried, and exposed for autoradiography.
Western Blot Analysis
Western blot analysis of cell extracts was performed as described previously (Buck et al., 1995). Affinity-purified polyclonal antisera to Res2 were used at a 1:500 dilution and visualized with the use of anti-rabbit conjugated HRP. Autoradiographs were scanned with the use of a Molecular Dynamics Personal Densitometer (Amersham, Arlington Heights, IL). Anti-cyclin B polyclonal antibody (Alfa et al., 1990) was used at a 1:1000 dilution and visualized with the use of anti-rabbit conjugated HRP. Anti-hemagglutinin 12CA5 antibody (BAbCO, Richmond, CA) was used at a 1:1000 dilution and visualized with the use of anti-mouse conjugated HRP.
RESULTS
Mcs1-77 Mutant Bypasses the Requirement for Cdc2 Tyrosine Phosphorylation
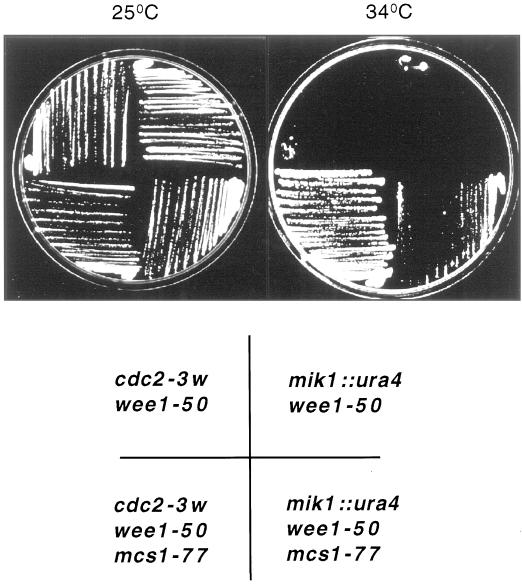
The fission yeast strain cdc2-3w wee1-50 is viable at low temperature but undergoes a lethal premature entry into mitosis at the restrictive temperature for wee1-50 (Russell and Nurse, 1987) (Figure 1). Seventeen mitotic catastrophe suppressors that were able to grow at the restrictive temperature of 37°C were identified (Molz et al., 1989). Eleven of these were found to reside in a single complementation group, mcs1. We have characterized a mutant from this group, mcs1-77 (Table 1). In addition to the Wee1 kinase, Cdc2 is phosphorylated on tyrosine 15 by the action of the Mik1 tyrosine kinase. Cells lacking both wee1 and mik1 also undergo a premature mitotic catastrophe (Lundgren et al., 1991). To determine whether mcs1-77 cells could proliferate in the complete absence of the Wee1 and Mik1 kinases, a wee1-50 Δmik1 mcs1-77 strain was constructed and incubated at both the permissive and restrictive temperatures for wee1-50. Whereas a wee1-50 Δmik1 strain underwent mitotic catastrophe at 34°C, a wee1-50 Δmik1 mcs1-77 strain was still able to form colonies, although growth was poor (Figure 1). These results indicate that mcs1-77 exerts a cell cycle delay in G2 that is independent of the tyrosine phosphorylation state of Cdc2.
Figure 1.
mcs1-77 cells bypass the requirement for the Wee1 and Mik1 tyrosine kinases. cdc2-3w wee1-50, cdc2-3w wee1-50 mcs1-77, mik1::ura4 wee1-50, and mik1::ura4 wee1-50 mcs1-77 cells were grown on YEPD at 25°C and then streaked on the same medium at either 25°C (left) or 34°C (right), and growth of the colonies was examined after 4 d at these temperatures.
Table 1.
Strains used in this study
| Strain | Genotype | Reference/source |
|---|---|---|
| PR 109 | h− | P. Russell |
| SP 820 | mcs1-77 ura4+ h+ | Molz et al. (1989) |
| JM 1344 | mcs1-77 his7-366 h− | This study |
| JM 1346 | mcs1-77 ade6-M216 h+ | This study |
| NT 9 | cdc10-129 h− | N. Jones |
| Y 337 | cdc10-c4 h− | L. Johnston |
| PN 1359 | res1∷ura4 ade6-M210 h− | P. Nurse |
| NT 38 | res2∷ura4 ade6-M210 h− | N. Jones |
| PN 1404 | rep2∷ura4 h− | P. Nurse |
| JM 1272 | rad3∷ura4 ade6-704 h− | A. Carr |
| JM 2059 | ste9∷ura4 h | T. Toda |
| PR 12 | cdc2-3w wee1-50 h− | P. Russell |
| SP 763 | cdc2-3w wee1-50 mcs1-77 h− | Molz et al. (1989) |
| PR 337 | mik1∷ura4 wee1-50 h− | P. Russell |
| JM 2038 | mik1∷ura4 wee1-50 mcs1-77 | This study |
| JM 1351 | cdc25-22 ade6-M210 his7-366 h+ | Shieh et al. (1997) |
| JM 1518 | mcs1-77 cdc25-22 | This study |
| JM 1604 | mcs1-77 cdc25-22 rad3∷ura4 | This study |
| JM 2060 | mcs1-77 cdc25-22 ste9∷ura4 | This study |
| HF 178 | cut2-364∷cut2+-HA-LEU2 ura4+ h− | M. Yanagida |
| JM 1603 | mcs1-77 cdc25-22 cut2-364∷cut2+-HA-LEU2 | This study |
All strains were leu1-32 ura4-D18 unless indicated otherwise.
Mcs1 Is Required for the G1-to-S Transition and Meiotic Progression
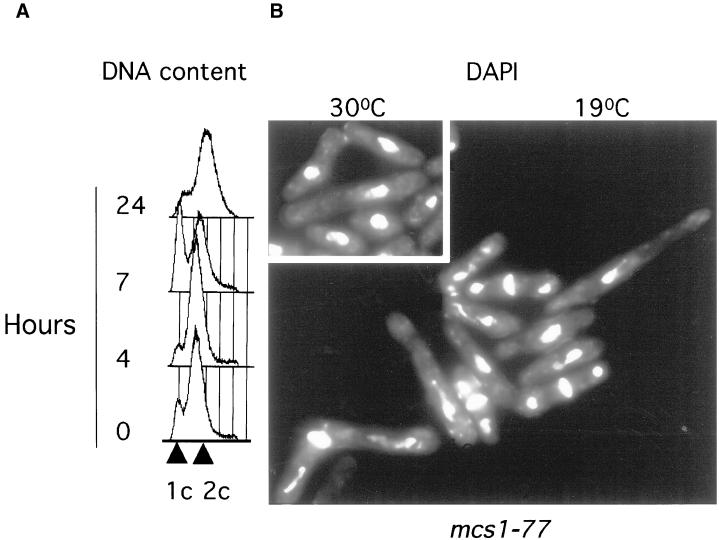
The mcs1-77 allele was characterized further. After prolonged incubation at low temperature (19°C), mcs1-77 cells elongate and undergo cell cycle arrest (Molz et al., 1989). FACS analysis revealed that after 7 h of incubation at 19°C, a large proportion of mcs1-77 cells delay in G1 before eventually arresting in G2 (Figure 2A). This suggests that Mcs1, like Cdc2, may be required for both the transition through START and the initiation of mitosis. However, cells did not arrest uniformly with a single nucleus but rather with abnormal chromatin structures, suggesting a chromosome segregation defect (Figure 2B). This phenotype is strikingly similar to that observed in cells that bypass the cdc10 START gene (Marks et al., 1992). We also found that mcs1-77 cells displayed a profound meiotic defect that cosegregated with the cold-sensitive cdc− phenotype. In self-crosses, wild-type cells give rise to >94% four-spored asci, whereas mcs1-77 cells produced asci with aberrant numbers of spores (Table 2). These result indicate that mcs1 not only controls the mitotic cell cycle but also is required for meiotic progression.
Figure 2.
mcs1-77 cells arrest at low temperature in G1 with abnormal chromatin structures. (A) mcs1-77 cells transiently arrest in G1. Log-phase cultures of mcs1–77 cells growing in Edinburgh minimal medium (EMM) medium at 30°C were analyzed for DNA content after shift to 19°C for the times indicated. (B) mcs1-77 cells cell cycle arrest at low temperature with abnormal chromatin structures. Log-phase cultures of mcs1-77 cells growing in EMM medium at 30°C were transferred to the same medium at either 30°C (inset) or 19°C for 24 h. After this time, cells were stained with DAPI to visualize nuclei.
Table 2.
Effect of mcs1-77 mutation on ascospore formation
| Strain | Spores per ascus (%)
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Wild type | 0 | 1.5 | 2.5 | 1.5 | 94.5 |
| mcs1-77 | 38 | 17 | 31 | 4 | 13 |
mcs1-77 Contains a Point Mutation in the DNA-binding Domain of Res2
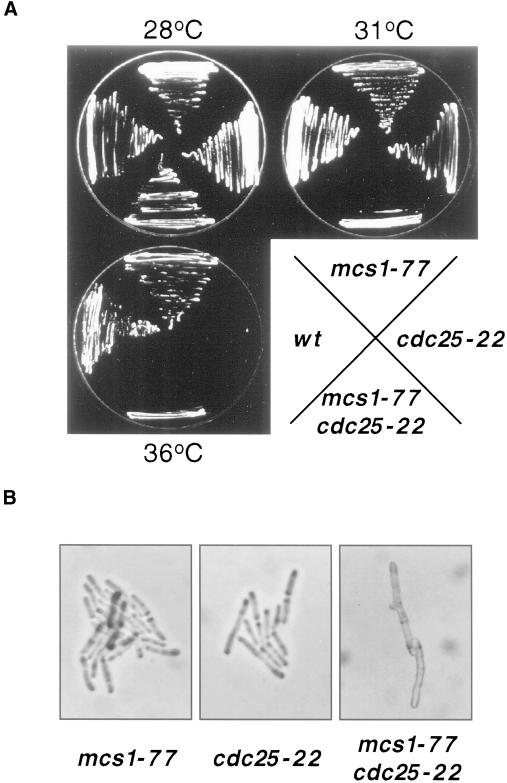
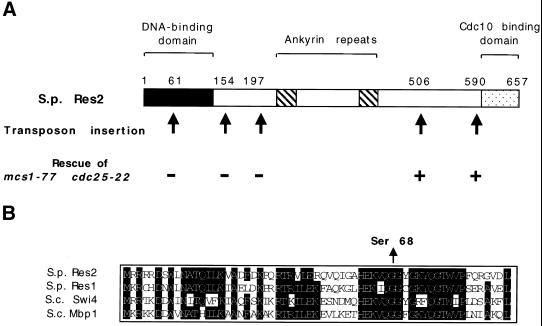
Consistent with a role for mcs1 in controlling the G2/M transition, the mcs1-77 mutant forms a conditional lethal genetic interaction with cdc25-22 that encodes a temperature-sensitive version of the Cdc25 phosphatase (Molz et al., 1989). Although mcs1-77 cdc25-22 cells can proliferate at 28°C (Figure 3A), they undergo cell cycle arrest at the intermediate temperature of 31°C, whereas mcs1-77 and cdc25-22 single mutants are still able to form colonies. At this temperature, the mcs1-77 cdc25-22 double mutants arrest as highly elongated cells, indicating G2 arrest (Figure 3). We made use of the observation that mcs1-77 cdc25-22 double mutant cells arrest at 31°C to clone the mcs1 gene. A genomic library was introduced into mcs1-77 cdc25-22 ura4-D18 cells, and from a total of 60,000 transformants growing at 28°C, 89 plasmid-dependent colonies were isolated at 31°C. Forty-seven of these suppressors represented two known genes, cdc25 (36 clones) and nim1/cdr1 (11 clones). The restriction maps of the remaining 42 suppressors were found to be related. To localize the region responsible for suppression of the mcs1-77 cdc25-22 cell cycle arrest, one of these clones (pURB1-mcs1) was subjected to transposon mutagenesis (Sedgwick and Morgan, 1994). Surprisingly, noncomplementing insertions were found to reside in a known gene, res2, that encodes a component of the START gene–specific transcription factor complex MBF (Figure 4A). Notably, two transpositional insertions in res2 that remove the C-terminal 67 and 151 amino acids were still able to rescue mcs1-77 cdc25-22 cells at the restrictive temperature. Because the C-terminal domain of Res2 is required for interaction with Cdc10 and to confer periodic transcriptional regulation to the MBF complex, this suppression is due to heteroallelic complementation, suggesting that the MBF complex contains more than one molecule of Res2 (Zhu et al., 1997).
Figure 3.
mcs1 delays the initiation of mitosis. (A) mcs1-77 is synthetically lethal with cdc25-22. Wild-type, mcs1-77, cdc25-22, and mcs1-77 cdc25-22 cells were grown on YEPD at 28°C and then streaked onto YEPD plates at 28, 31, or 36°C, and the growth of the strains was monitored after 3 d at these temperatures. (B) mcs1-77 cdc25-22 arrested cells are highly elongated. mcs1-77 cells were crossed to cdc25-22 cells, and the resulting asci were subjected to tetrad dissection on YEPD plates at 31°C. Colonies from a tetratype that were genotypically mcs1-77 (left), cdc25-22 (middle), or mcs1-77 cdc25-22 (right) were visualized after 2 d of growth at 31°C.
Figure 4.
mcs1-77 encodes a point mutant in the DNA-binding domain of Res2. (A) Localization of inactivating transposon insertions in a genomic clone containing Res2. Simultaneous localization and sequencing of complementing regions was performed by transposon mutagenesis of the pURB1-Res2 genomic clone. The structure of the Res2 protein is shown with the N-terminal DNA-binding domain (black bar), central ankyrin repeats (gray bar), and the C-terminal Cdc10 interaction domain (hatched bar). Arrows indicate the location of transposon insertions, and the ability of the transposed plasmid to rescue mcs1-77 cdc25-22 cells at 31°C is shown as either rescue (+) or no rescue (−) after 3 d of growth on Edinburgh minimal medium (EMM) plates lacking uracil. (B) Sequence of the DNA-binding domain of Res2 from wild-type and mcs1-77 cells. Alignment of amino acids 28–89 of Schizosaccharomyces pombe (S.p.) Res2 protein with homologous regions of S.p. Res1 and Saccharomyces cerevisiae (S.c.) Mbp1 and S.c. Swi4 transcription factors. The arrow indicates the change of glycine 68 to a serine residue in mcs1-77 cells.
Cells deleted for res2 undergo a cold-sensitive cell cycle arrest in G1 and are defective in premeiotic DNA synthesis, which results in aberrant spore formation (Miyamoto et al., 1994; Zhu et al., 1994; Aytéet al., 1997; Zhu et al., 1997). This persuaded us to determine the relationship between the mcs1-77 mutation and the res2 genomic clone. To achieve this, mcs1-77 cells were crossed to res2::ura4 mutants, and the progeny were scored for cold-sensitive cell cycle arrest. Because this cross also gave rise to inviable asci, it was performed with cells expressing res2 from an episomal plasmid. The progeny from >2000 spores plated were all found to undergo a cold-sensitive cell cycle arrest, indicating that mcs1-77 and res2 are allelic. To localize the mutation in res2, genomic DNA from mcs1-77 cells was amplified by PCR, and the product was sequenced. mcs1-77 cells were found to contain a single point mutation in the DNA-binding domain of res2, resulting in the mutation of glycine 68 to a serine residue (Figure 4B) (Xu et al., 1994). These results indicate that Res2, which has been implicated as a repressor of Cdc10-dependent gene transcription in G2, plays an unexpected additional role in controlling the timing of mitotic initiation.
Mcs1-77 Cells Are Partially Defective in Mitotic Gene Transcription
Our previous analysis suggested that mcs1-77 cells are phenotypically indistinguishable from Δres2 cells. To discover how a component of the START gene–specific transcription factor complex can be implicated in the G2/M transition, we characterized the mcs1-77 mutation in more detail. Consistent with the nature of the mcs1-77 mutation, we observed that antibodies to Res2 were able to detect a full-length protein in Western blot analysis of cell extracts from mcs1-77 but not Δres2 cells (Figure 5A). However, using a fragment of the cdc22 promoter in electrophoretic mobility shift assays with extracts of either mcs1-77 or Δres2 cells, we were unable to detect the MBF complex (Figure 5B). To examine expression of the MBF target gene cdc18, mRNA was extracted from cells that were synchronized in S phase by incubating in the presence of hydroxyurea and then released. Under these conditions, expression of cdc18 in Δres2 cells was found to be invariant through the cell cycle compared with wild type, whereas in Δrep2 cells expression was substantially decreased and periodic, as observed previously (Figure 5C) (Baum et al., 1997). Under the same conditions, expression of cdc18 in mcs1-77 cells was seen to fluctuate, although the maximal levels and rates of accumulation were decreased significantly with respect to wild type (Figure 5C). Thus, mcs1-77 is not a null allele of the res2 gene.
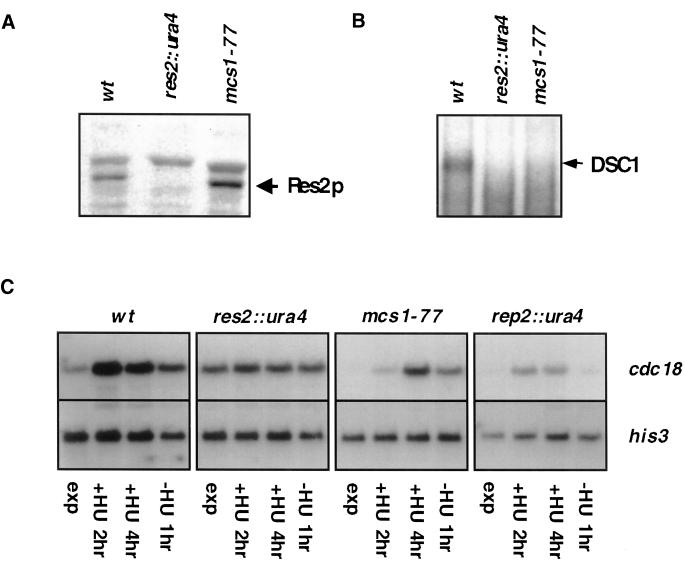
Figure 5.
mcs1-77 cells are partially defective in periodic transcription of cdc18. (A) Expression of Res2 in mcs1-77 cells. Ten micrograms of cell extract prepared from log-phase cultures of wild-type (wt), res2::ura4, or mcs1-77 cells were probed for the presence of Res2 polypeptide with the use of anti-Res2 antiserum after SDS-PAGE and Western blotting. (B) The DSC1 (MBF) complex cannot be isolated from mcs1-77 cells. Electrophoretic mobility shift assays were performed with the labeled cdc22 probe with the use of extracts derived from log-phase cultures of wild-type (wt), res2::ura4, or mcs1-77 cells. (C) Expression of cdc18 mRNA in mcs1-77 cells. Log-phase cultures growing in YEPD at 30°C of wild-type (wt), res2::ura4, rep2::ura4, or mcs1-77 cells were incubated in the same medium containing 2 mM hydroxyurea for the times indicated and then incubated in YEPD for an additional 1 h after extensive washing. Total RNA was extracted, and equal quantities were separated by electrophoresis and probed with the use of DNA specific to the cdc18 gene. Represented blots were probed with the same probe and for the same length of time. Reprobing was performed with his3-specific probes to verify approximately equal loading of RNA.
Cells Lacking the MBF Complex Are Delayed in G2
The MBF transcription factor complex, which contains the Res2, Cdc10, Res1, and Rep2 proteins, can be isolated from fission yeast cell lysates only when S phase transcription is repressed in G2 (Baum et al. 1997; Zhu et al., 1997). Mutations in genes that prevent the appearance of this complex, however, have different effects on cell cycle–regulated transcription of target genes. For example, deletion of res2 or removal of the C-terminal 61 residues in the Cdc10 protein (cdc10-c4) causes high constitutive expression of target genes, whereas deletion of res1 causes low constitutive expression of target genes (McInerny et al., 1995; Baum et al., 1997). Regardless, Δres2, cdc10-c4, and Δres1 cells undergo a nonconditional cell cycle arrest in combination with cdc25-22, a partially defective form of the Cdc25 phosphatase (Table 3). Conversely, the MBF complex can be isolated from Δrep2 cells in which transcription is also periodic but maximal accumulation is greatly diminished (Figure 5C) (Baum et al., 1997). Deletion of rep2 has no effect on the timing of mitotic initiation alone or in combination with cdc25-22 (Table 3). These results suggest a strong correlation between deregulation of MBF-dependent transcription and a delay in the timing of mitotic initiation. The reason why inactivating mutations in res1 were not found in the screen for mitotic catastrophe suppressors in the cdc2-3w wee1-50 strain is probably that this screen was performed at 37°C, at which temperature res1− cells are inviable (Molz et al., 1989).
Table 3.
Mutation of MBF components causes a G2 delay
| Strain | Wild type | Cdc25-22 |
|---|---|---|
| Wild type | +++ | +++ |
| mcs1-77 | +++ | cdc− |
| res2∷ura4 | +++ | cdc− |
| res1∷ura4 | +++ | cdc− |
| cdc10-c4 | +++ | cdc− |
| cdc10-129 | +++ | cdc− |
| rep2∷ura4 | +++ | +++ |
Mitotic Delay Is Not Due to Activation of the DNA Damage or Replication Checkpoint
All of the known targets of the MBF complex, including cdc22, cdc18, cdt1, and cig2, are important for the initiation of S phase. Aberrant initiation of DNA replication forks causes activation of a DNA replication checkpoint that delays the onset of mitosis. This signal is dependent on the activity of the Rad3 kinase, which is also required to inhibit mitosis when DNA is damaged. To determine whether deregulation of the MBF complex delays mitosis by activating either the DNA damage or DNA replication checkpoint, we examined the effect of deleting rad3 in mcs1-77 cdc25-22 cells. The resulting rad3::ura4 mcs1-77 cdc25-22 triple mutant underwent cell division at the same length as mcs1-77 cdc25-22 cells at all temperatures, indicating that the mitotic delay observed in mcs1-77 cells is not due to activation of the DNA damage or replication checkpoint (Table 4). Similar results were obtained when other components of these pathways were deleted, including chk1 (our unpublished results).
Table 4.
Mitotic delay is not dependent on Rad3 kinase
| Strain | 28°C | 31°C |
|---|---|---|
| mcs1-77 cdc25-22 | +++ | cdc− |
| mcs1-77 cdc25-22 rad3∷ura4 | +++ | cdc− |
The mcs1-77 Mutant Causes Instability of Cyclin B and Cut2 in G2
Activation of the Cdc2/Cdc13 complex is the rate-limiting factor responsible for M-phase initiation in fission yeast. Because transcription of cdc13 or cdc2 was unaffected in mcs1-77 cells (our unpublished results), we focused on the Cdc2/Cdc13 protein complex itself. The level of the Cdc13 protein oscillates through the cell cycle, reaching a peak in late G2 (Moreno et al., 1989). This is due in part to the fact that Cdc13 is unstable in M and G1 phase and stabilizes after cells pass through START. To determine whether mcs1-77 affects either the steady-state level and/or the stability of Cdc13, cdc25-22 and mcs1-77 cdc25-22 cells were synchronized in late G2 and cell extracts were probed for the presence of Cdc13. As the results in Figure 6 show, the steady-state level of Cdc13 was decreased significantly in mcs1-77 cells. To determine whether this is due to a decreased protein synthesis rate or to an increased rate of degradation, cells were incubated in the presence of the protein synthesis inhibitor cycloheximide for various times and the level of Cdc13 was monitored. No change in the level of Cdc13 was observed in cdc25-22 cells at the restrictive temperature, even after protein synthesis was inhibited for prolonged periods (Figure 6). This confirms that instability of cyclin B is normally confined to the M and G1 phases of the cell cycle. In contrast, we observed a more rapid decrease in the level of Cdc13 in mcs1-77 cdc25-22 cells under the same conditions (Figure 6). This indicates that cyclin B destruction is not suppressed in G2-phase mcs1-77 cells.
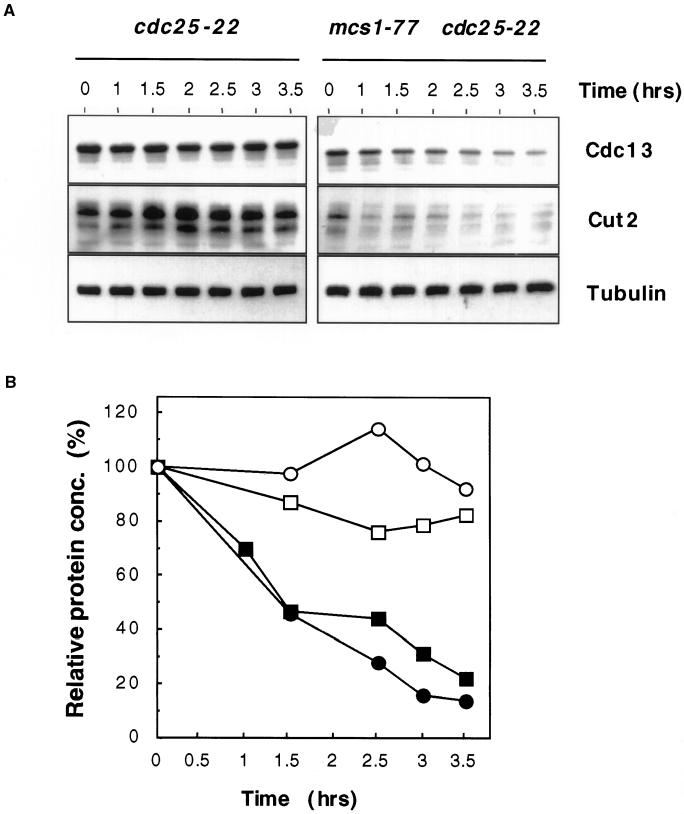
Figure 6.
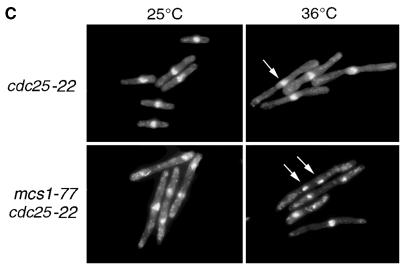
Cdc13 and Cut2 are unstable in mcs1-77 cells in G2 phase. (A) Stability of cyclin B and Cut2 proteins in mcs1-77 cdc25-22 cells. Log-phase cultures of either cdc25-22 cut2-364::cut2+-HA-LEU2 or mcs1-77 cdc25-22 cut2-364::cut2+-HA-LEU2 cells growing in YEPD at 28°C were shifted to 35.5°C for 3 h and then incubated in the same medium containing 100 μg/ml cycloheximide at 35.5°C for the times indicated. Cell extracts were prepared and probed for the presence of either Cdc13, with the use of affinity-purified anti-Cdc13 polyclonal antibodies, or Cut2, with the use of anti-hemagglutinin mAbs. (B) Quantification of cyclin B and Cut2 stability from A. The abundance of Cdc13 (squares) and Cut2 (circles) was expressed as a percentage of the initial level after the addition of cycloheximide to either cdc25-22 cut2-364::cut2+-HA-LEU2 (○, □) or mcs1-77 cdc25-22 cut2-364::cut2+-HA-LEU2 cells (●, ▪). (C) Aberrant chromosome segregation in mcs1-77 cdc25-22 cells. Log-phase cultures of either cdc25-22 or mcs1-77 cdc25-22 cells growing in YEPD at 25°C were shifted to 36°C for 3 h. Cells were then fixed and stained with DAPI to visualize chromatin.
Because Cdc13 degradation is triggered by the action of the APC, we asked whether other known substrates of the APC were also unstable in mcs1-77 cells. In particular, the anaphase inhibitor Cut2, like cyclin B, is normally degraded only in the M and G1 phases of the cell cycle. To monitor Cut2 levels, a chromosomal C-terminally hemagglutinin-tagged cut2 gene was introduced into cdc25-22 and mcs1-77 cdc25-22 cells. We found that the steady-state level of Cut2 was higher in cdc25-22 cells at the restrictive temperature than in mcs1-77 cdc25-22 cells (Figure 6A). This can also be accounted for by a dramatically increased rate of protein degradation observed when protein synthesis was blocked in the presence of cycloheximide (Figure 6). These results suggest that APC-mediated degradation is deregulated when the MBF complex is compromised. Destruction of Cut2 during mitosis is required for the separation of sister chromatids into two daughter cells (Funabiki et al., 1996). To determine the effect of Cut2 degradation in vivo, nuclei from either cdc25-22 or mcs1-77 cdc25-22 cells were stained with DAPI after shift to the restrictive temperature for 4 h and the number of binucleate cells or cells with abnormal chromatin structures were determined. At the permissive temperature of 25°C, 19% of cdc25-22 cells were binucleate compared with 38% of mcs1-77 cdc25-22 cells. At the restrictive temperature, cdc25-22 cells arrested with a single elongated nucleus, with only 1.5% displaying binucleate or segregated DNA (Figure 6C). In contrast, 47% of mcs1-77 cdc25-22 cells arrested, with a large number of either binucleate cells or cells showing aberrantly segregated chromosomes, under the same conditions (Figure 6C). These results are consistent with the inability of mcs1-77 cells to maintain sister chromatid cohesion in the G2 phase of the cell cycle. Together, these data suggest that APC-mediated degradation is ectopically activated in cells lacking the MBF complex.
Deletion of Ste9/Srw1 Bypasses the G2 Delay in mcs1-77 Cells
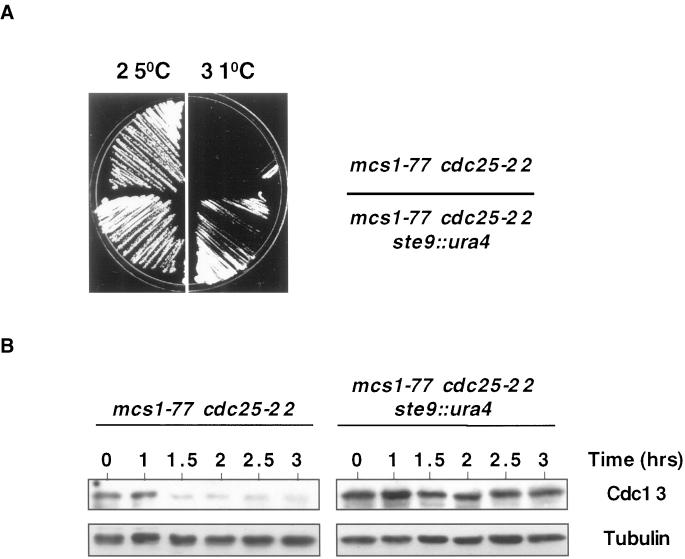
Degradation of both cyclin B and Cut2 by the APC requires association with WD40-domain containing adaptor proteins, which in fission yeast are encoded by the slp1 (fizzy) and ste9/srw1 (fizzy-related) genes. We noted that ste9/srw1 was initially identified as a multicopy suppressor of the mitotic catastrophe phenotype of Δmik1 wee1-50 cells at high temperature (Yamaguchi et al., 1997; H. Okayama, personal communication). This prompted us to determine whether the G2 delay in mcs1-77 cells is due to deregulated activity of APCSte9, resulting in ectopic degradation of cyclin B. To do this, we examined the effect of deleting ste9/srw1 in mcs1-77 cdc25-22 cells. At the permissive temperature, the resulting ste9::ura4 mcs1-77 cdc25-22 triple mutant underwent cell division at 20.5 ± 0.2 μm compared with 28 ± 0.3 μm for mcs1-77 cdc25-22 cells and failed to arrest in G2 when incubated at 31°C (Figure 7A). These results indicate that perturbation of the MBF complex causes a G2 delay that is due in part to deregulation of APCSte9, resulting in ectopic degradation of cyclin B. To analyze this directly, mcs1-77 cdc25-22 or ste9::ura4 mcs1-77 cdc25-22 cells were arrested in G2 at high temperature and the stability of Cdc13 was assessed after addition of cycloheximide. Whereas the steady-state level of Cdc13 decreased in mcs1-77 cells, it was unchanged in mcs1-77 ste9::ura4 cells during the same period (Figure 7B). It should be noted that 35% of ste9::ura4 mcs1-77 cdc25-22 cells were either binucleate or displayed some aberrant chromosome structures (our unpublished results), indicating that Ste9/Srw1 is not the sole target by which the MBF complex controls APC activity.
Figure 7.
Instability of Cdc13 in mcs1-77 cells is due to the activity of APCSte9 (A) Deletion of Ste9/Srw1 rescues the cell cycle delay in mcs1-77 cells. mcs1-77 cdc25-22 or ste9::ura4 mcs1-77 cdc25-22 cells were grown on YEPD at 25°C and then streaked onto YEPD plates at either 25 or 31°C, and the growth of the strains was monitored after 3 d at these temperatures. (B) Deletion of Ste9 stabilizes Cdc13 cyclin B in mcs1-77 cells. Log-phase cultures of either mcs1-77 cdc25-22 or ste9::ura4 mcs1-77 cdc25-22 cells growing in YEPD at 28°C were shifted to 35.5°C for 3 h and then incubated in the same medium containing 100 μg/ml cycloheximide at 35.5°C for the times indicated. Cell extracts were prepared and probed for the presence of Cdc13 with the use of affinity-purified anti-Cdc13 polyclonal antibodies.
Cell Cycle–regulated Expression of Ste9/Srw1 Is Controlled by the MBF Complex
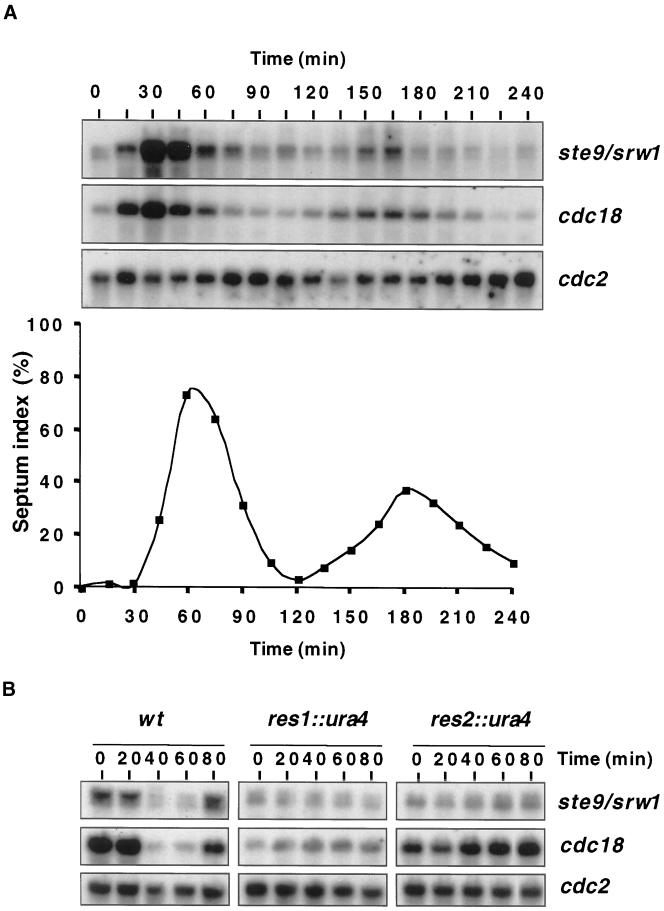
We postulated that the MBF complex could control APCSte9 activity by directly regulating the transcription of the ste9/srw1 gene. We noted that the promoter region of ste9/srw1 contains two elements that correspond to the consensus core sequence (A/TCGCGA/T) recognized by the MBF complex. To determine whether ste9/srw1 is cell cycle regulated, a cdc25-22 strain was synchronized by blocking in G2 at the restrictive temperature of 35.5°C and then releasing to 25°C. Northern blot analysis of total RNA isolated from this culture at various times after release indicated that the mRNA for ste9/srw1 was strongly cell cycle regulated and peaked just before S phase, which occurs as the division septa are laid down (Figure 8A). Notably, the peak of ste9/srw1 expression was exactly coincident with that of cdc18, a known MBF target (Figure 8A). To determine whether the expression of ste9/srw1 is regulated by the MBF complex, wild-type cells or cells lacking either Res1 or Res2 were synchronized by blocking in S phase with hydroxyurea and then releasing to G2. Northern blot analysis showed that in wild-type cells the expression of both ste9/srw1 and cdc18 was high in S phase but declined as cells entered G2 (Figure 8B). Importantly, no periodicity of either cdc18 or ste9/srw1 expression was observed in either Δres1 or Δres2 cells under the same conditions, strongly indicating that the MBF complex regulates expression of ste9/srw1 (Figure 8B). Under the same conditions, periodic transcription of ste9/srw1 was also deregulated in mcs1-77 cells, but to a lesser extent than Δres2 cells (our unpublished results).
Figure 8.
MBF complex controls periodic transcription of ste9/srw1. (A) Transcription of ste9/srw1 is cell cycle regulated. A log-phase culture of cdc25-22 cells growing in YES was arrested in G2 by incubation at 36.5°C for 4 h. Cells were shifted to 25°C and collected for Northern blot analysis and examined microscopically for the appearance of septa at the times indicated. Total RNA was extracted, and equal quantities were separated by electrophoresis and probed with the use of DNA specific to the ste9/srw1 and cdc18 genes. Reprobing with cdc2-specific probes verified approximately equal loading of RNA. (B) Res1 and Res2 control transcription of ste9/srw1. Log-phase cultures of wild-type, res1::ura4, or res2::ura4 cells growing in YES were incubated in the presence of 11 mM hydroxyurea for 4 h and then washed and resuspended in fresh YES lacking hydroxyurea for the times indicated. Cells were harvested, and total RNA was extracted. Equal quantities of RNA were separated by electrophoresis and probed with the use of DNA specific to the ste9/srw1 and cdc18 genes. Reprobing with cdc2-specific probes verified approximately equal loading of RNA.
DISCUSSION
We have characterized mutants that bypass the lethal hyperactivation of Cdc2 in fission yeast to identify novel mechanisms by which Cdc2/cyclin B activity is controlled. In a previous genetic screen, 11 of 17 mutants that suppress this phenotype were found to reside in a single complementation group, mcs1 (Molz et al., 1989). We have characterized the mcs1-77 mutant and found it to contain a point mutation in the DNA-binding domain of Res2, a component of the S phase–specific transcription factor complex MBF. The MBF complex, which contains the Cdc10, Res1, Res2, and Rep2 proteins, is responsible for the periodic transcription of a number of genes essential for the onset of S phase. Mutations in other MBF components that prevent the periodic appearance of the complex in G2 also display a synthetic genetic interaction with cdc25-22, suggesting that the MBF complex plays a role in determining the timing of mitotic initiation.
We have shown previously that mcs2 and mcs6, mutants of which bypass the mitotic catastrophe phenotype of cdc2-3w wee1-50, code for components of a Cdk-activating kinase that controls Cdc2 phosphorylation on threonine 167 (Buck et al., 1995; Damagnez et al., 1995). Our observation that the mcs1-77 mutation can bypass the loss of Wee1 and Mik1 tyrosine kinases indicates that Res2 also controls the activity of the Cdc2/Cdc13 complex, but by a mechanism that is independent of Cdc2 tyrosine phosphorylation. This is consistent with our finding that the G2 delay observed in mcs1-77 cells is not dependent on activation of either the DNA damage or DNA replication checkpoint pathway, because these act through Cdc2 tyrosine phosphorylation. Instead, we have found that the rate of degradation of the major cyclin B, Cdc13, is increased in mcs1-77 cells blocked at the G2/M transition. Cdc13 is targeted for degradation by the APC, a specialized E3 ubiquitin ligase (Page and Hieter, 1999; Zachariae and Nasmyth, 1999). Because APC-mediated degradation ceases after cells pass START, we suggest that repression of the MBF complex may play a key role in the inactivation of APC at this time. We considered that this may be due to either cell cycle–regulated production or modification of an APC subunit(s) or adaptor proteins such as Ste9/Srw1 (Yamaguchi et al., 1997; Kitamura et al., 1998; Kominani et al., 1998). In particular, we focused our attention on ste9/srw1, because it had been isolated previously as a multicopy suppressor of the mitotic catastrophe phenotype of Δmik1 wee1-50 cells at high temperature (Yamaguchi et al., 1997; H. Okayama, personal communication). This persuaded us to examine whether the G2 delay observed in mcs1-77 cells was due to ectopic activity of APCSte9, and we found that this was indeed the case. Furthermore, we found that the expression of Ste9/Srw1 was profoundly cell cycle regulated and that this periodicity was regulated by the MBF complex. This leads to a simple explanation of how the mcs1-77 mutation rescues the mitotic catastrophe of a cdc2-3w wee1-50 strain: ectopic expression of Ste9/Srw1 leads to enhanced degradation of Cdc13 in G2, thereby reducing the effective concentration of active Cdc2/Cdc13 kinase.
Intriguingly, a recent report has suggested that the accumulation of cyclin B1 in mammalian cells is triggered by the E2F-mediated expression of cyclin A, which in association with Cdk2 phosphorylates and inactivates APCCdh1 (Lukas et al., 1999). Although we cannot formally rule out the possibility that MBF-mediated accumulation of the Cig2 cyclin in fission yeast may contribute to the accumulation of Cdc13 through phosphorylation and inactivation of APCSte9, deletion of Cig2 did not rescue the lethality observed in cdc2-3w wee1-50 cells at high temperature. Therefore, we would argue that repression of START-dependent transcription of Ste9/Srw1 may be more important for the inactivation of APCSte9 and thus the accumulation of cyclin B. It should be noted that levels of CDH1 also fluctuate in mammalian cells, being present primarily in G1, although it is not known whether this is due to regulation by E2F (Kramer et al., 2000). Regardless, our results highlight a role for the S phase–specific gene transcription factor complex in the timely inactivation of cyclin B destruction as cells pass through S phase.
In addition to Cdc13, we found that another APC substrate, the anaphase inhibitor Cut2, was also unstable in mcs1-77 cells. Consistent with this, we observed a high frequency of chromosomal abnormalities in mcs1-77 cells in G2, as assessed by DAPI staining of nuclei. However, we found that deletion of ste9/srw1 had little effect on the frequency of chromosomal abnormalities observed in mcs1-77 cells and by inference Cut2 stability. Because ubiquitination of Cut2 is not regulated by APCSte9 but rather by a related complex, APCSlp1, our results suggest that the MBF complex has an additional role in controlling APC activity (Funabiki et al., 1996; Matsumoto, 1997; Yamaguchi et al., 1997; Kitamura et al., 1998; Kominani et al., 1998). One obvious possibility is that the MBF complex may control the periodic transcription of one or more components or activators of the APCSlp1 complex.
It is now well recognized that the APC plays a central role in controlling the destruction of key cell cycle regulators and, as a consequence, the order and timing of cell cycle progression in all eukaryotes. Deregulation of this activity is very likely to contribute to the chromosome instability and missegregation associated with the uncontrolled proliferation of many tumor cells. This highlights the need to understand how various forms of the APC are cell cycle regulated and how APC activity is restrained in response to cellular damage. Importantly, the composition of the fission yeast APC and the mechanism by which it is inactivated upon spindle damage closely resemble those observed in higher eukaryotes (reviewed by Peters, 1999). Thus, it is highly likely that cell cycle cues governing APC activity, such as those described in this paper, may also be operative in higher eukaryotes.
ACKNOWLEDGMENTS
The authors thank Dr. Lee Johnston, Dr. Jerome Wuarin, Dr. Vicky Buck, and members of the Division of Yeast Genetics for helpful advice and discussions and critical reading of the manuscript. We are particularly grateful to Dr. Simon Whitehall (University of Newcastle) for assistance with the electrophoretic mobility shift assay. The authors also thank Dr. David Beach (Cold Spring Harbor Laboratories), Dr. Nic Jones (Imperial Cancer Research Fund), Dr. Paul Nurse (ICRF), Dr. Haruo Okayama (University of Tokyo), Dr. Paul Russell (The Scripps Research Institute), and Dr. Mitsuhiro Yanagida (University of Kyoto) for strains and reagents. We also thank Dr. Haruo Okayama for communicating results before publication. This research was supported by the Medical Research Council.
REFERENCES
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Amon A, Iringer S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Ayté J, Leis JF, DeCaprio JA. The fission yeast protein p73res2 is an essential component of the mitotic MBF complex and a master regulator of meiosis. Mol Cell Biol. 1997;17:6246–6254. doi: 10.1128/mcb.17.11.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N, Muriel WJ, Carr AM. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992;114:59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- Baum B, Nishitani H, Yanow S, Nurse P. Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J. 1998;17:5689–5698. doi: 10.1093/emboj/17.19.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Wuarin J, Nurse P. Control of S-phase periodic transcription in the fission yeast mitotic cycle. EMBO J. 1997;16:4676–4688. doi: 10.1093/emboj/16.15.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Buck V, Russell P, Millar JBA. Identification of a cdk-activating kinase in fission yeast. EMBO J. 1995;14:6173–6183. doi: 10.1002/j.1460-2075.1995.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M, Beach D. Sct1 functions in partnership with Cdc10 in a transcription complex that activates cell cycle START and inhibits differentiation. Cell. 1993;72:607–619. doi: 10.1016/0092-8674(93)90079-6. [DOI] [PubMed] [Google Scholar]
- Corliss DA, White WEJ. Fluorescence of yeast vitally stained with ethidium bromide and propidium iodide. J Histochem Cytochem. 1981;29:45–48. doi: 10.1177/29.1.6162881. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Damagnez V, Makela TP, Cottarel G. Schizosaccharomyces pombe Mop1-Mcs2 is related to mammalian CAK. EMBO J. 1995;14:6164–6172. doi: 10.1002/j.1460-2075.1995.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschener MW. Direct binding of CDC20 family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- Fisher D, Nurse P. Cyclins of the fission yeast Schizosaccharomyces pombe. Semin Cell Biol. 1995;6:73–78. doi: 10.1016/1043-4682(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood E, Nishitani H, Nurse P. Cdc18p can block mitosis by two independent mechanisms. J Cell Sci. 1998;111:3101–3108. doi: 10.1242/jcs.111.20.3101. [DOI] [PubMed] [Google Scholar]
- Hayles J, Fisher D, Woolard A, Nurse P. Temporal order of S-phase and mitosis in fission yeast is determined by the state of the p34cdc2/mitotic cyclin B complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Hofmann JF, Beach D. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J. 1994;13:425–434. doi: 10.1002/j.1460-2075.1994.tb06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iringer S, Nasmyth K. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Martin GS, Forsburg SL, Stephen RJ, Russo A, Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Maekawa H, Shimoda C. Fission yeast Ste9, a homologue of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1 phase. Mol Biol Cell. 1998;9:1065–1080. doi: 10.1091/mbc.9.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominani K, Seth-Smith H, Toda T. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell cycle arrest in fission yeast. EMBO J. 1998;17:5388–5399. doi: 10.1093/emboj/17.18.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes NF, Johnson AL, Johnston LH. Coordination of expression of DNA synthesis genes in budding yeast by a cell-cycle regulated trans factor. Nature. 1991;350:247–250. doi: 10.1038/350247a0. [DOI] [PubMed] [Google Scholar]
- Lowndes NF, McInerny CJ, Johnson AL, Fantes PA, Johnston LH. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+ Nature. 1992;355:449–453. doi: 10.1038/355449a0. [DOI] [PubMed] [Google Scholar]
- Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Walworth RM, Booher M, Demski M, Kirschner M, Beach D. mik1 and wee1 co-operate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Marks J, Fankhauser C, Reymond A, Simanis V. Cytoskeletal and DNA structure abnormalities result from bypass of requirement for the cdc10 start gene in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1992;101:517–528. doi: 10.1242/jcs.101.3.517. [DOI] [PubMed] [Google Scholar]
- Matsumoto T. A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol Cell Biol. 1997;17:742–750. doi: 10.1128/mcb.17.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerny CJ, Kersey PJ, Creanor J, Fantes PA. Positive and negative roles for cdc10 in cell cycle gene expression. Nucleic Acids Res. 1995;23:4761–4768. doi: 10.1093/nar/23.23.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JB, McGowan CH, Lenaers G, Jones R, Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Tanaka K, Okayama H. res2+, a new member of the cdc10+/SWI4 family, controls the ‘start’ of mitotic and meiotic cycles in fission yeast. EMBO J. 1994;13:1873–1880. doi: 10.1002/j.1460-2075.1994.tb06456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molz L, Booher R, Young P, Beach D. cdc2 and the regulation of mitosis: six interacting mcs genes. Genetics. 1989;122:773–782. doi: 10.1093/genetics/122.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakashima N, Tanaka K, Sturm S, Okayama H. Fission yeast Rep2 is a putative transcriptional activator subunit for the cell cycle ‘start’ function of Res2-Cdc10. EMBO J. 1995;14:4794–4802. doi: 10.1002/j.1460-2075.1995.tb00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Nurse P. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell. 1995;83:397–405. doi: 10.1016/0092-8674(95)90117-5. [DOI] [PubMed] [Google Scholar]
- Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara T, Okayama H. A B-type cyclin negatively regulates conjugation via interacting with cell cycle ‘start’ genes in fission yeast. EMBO J. 1994;13:1863–1872. doi: 10.1002/j.1460-2075.1994.tb06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AM, Hieter P. The anaphase-promoting complex: new subunits and regulators. Annu Rev Biochem. 1999;68:583–609. doi: 10.1146/annurev.biochem.68.1.583. [DOI] [PubMed] [Google Scholar]
- Peters JM. Subunits and substrates of the anaphase-promoting complex. Exp Cell Res. 1999;248:339–349. doi: 10.1006/excr.1999.4443. [DOI] [PubMed] [Google Scholar]
- Prentice HL. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1991;20:621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, Marks J, Simanis V. The activity of S. pombe MBF-like factor is cell cycle regulated and dependent on the activity of p34cdc2. EMBO J. 1993;12:4325–4334. doi: 10.1002/j.1460-2075.1993.tb06117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sedgwick SG, Morgan BA. Locating, DNA sequencing, and disrupting yeast genes using tagged Tn1000. Methods Mol Genet. 1994;3:131–140. [Google Scholar]
- Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- Tanaka K, Okazaki K, Okazaki N, Ueda T, Sugiyama A, Nojima H, Okayama H. A new cdc gene required for S phase entry of Schizosaccharomyces pombe encodes a protein similar to the cdc10+ and SWI4 gene products. EMBO J. 1992;11:4923–4932. doi: 10.1002/j.1460-2075.1992.tb05599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Sheppard KA, Peng CY, Yee AS, Pwinica-Worms H. Cyclin A/Cdk2 binds directly to E2F-1 and inhibits the DNA binding activity of E2F/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Murakami H, Okayama H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Takeda T, Nasmyth K, Jones N. pct1+, which encodes a new DNA-binding partner of p85cdc10, is required for meiosis in the fission yeast Schizosaccharomyces pombe. Genes Dev. 1994;8:885–898. doi: 10.1101/gad.8.8.885. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Takeda T, Whitehall S, Peat N, Jones N. Functional characterization of the fission yeast Start-specific transcription factor Res2. EMBO J. 1997;16:1023–1034. doi: 10.1093/emboj/16.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]