Abstract
Greater understanding of the pathophysiology of carbon dioxide kinetics during steady and nonsteady state should improve, we believe, clinical care during intensive care treatment. Capnography and the measurement of end-tidal partial pressure of carbon dioxide (PETCO2) will gradually be augmented by relatively new measurement methodology, including the volume of carbon dioxide exhaled per breath (VCO2,br) and average alveolar expired PCO2 (PA̅E̅CO2). Future directions include the study of oxygen kinetics.
Keywords: airway, capnography, carbon dioxide, carbon dioxide kinetics, expirogram, nonsteady state, ventilation
Introduction
Carbon dioxide is produced in the tissues by aerobic plus/minus anaerobic metabolism (Fig. 1a), transported in blood to the lung by venous return (essentially equal to cardiac output [QT]), and eliminated from the lung by minute ventilation (VE) [1]. In this model the lung is a simple mixing chamber and the alveolar fractional carbon dioxide (FACO2) is given by
Figure 1.
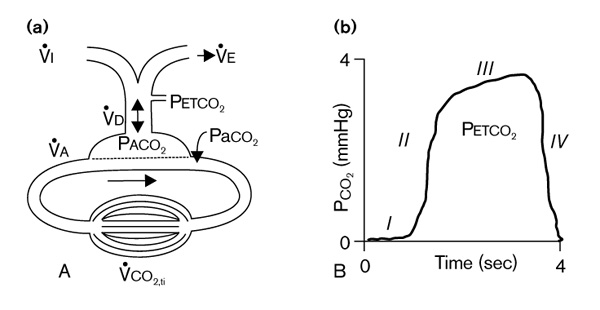
(a) Scheme of carbon dioxide stores and transport. PaCO2, arterial PCO2; PACO2, alveolar PCO2; PETCO2, end-tidal PCO2; V̇A, alveolar ventilation; V̇CO2,ti, tissue carbon dioxide production; V̇D, dead space ventilation; V̇E, expired ventilation; V̇I, inspired ventilation. (b) Normal capnogram (tidal PCO2 versus time). Phase I, inspiratory baseline; Phase II, expiratory upstroke; Phase III, alveolar plateau; and Phase IV, inspiratory downstroke. Adapted from Breen [61].
FACO2 = V̇CO2,ti/V̇A + FICO2 (1)
where V̇CO2,ti is the tissue carbon dioxide production, V̇A is alveolar ventilation, and FICO2 is the inspired FCO2. If one assumes no diffusion defect for carbon dioxide, then the partial carbon dioxide tension (PCO2) of arterial blood (PaCO2) leaving the lung is the perfusion-weighted average alveolar PCO2 (PACO2). Note that pulmonary shunt will add mixed venous blood with high PCO2 (PVCO2) to arterial blood and slightly increase PaCO2 [2]. V̇A is the product of respiratory frequency and expired tidal volume (VT). Expired VT is composed of alveolar VT and total physiologic dead space (VDphy). The fraction VDphy/VT is given by
VDphy/VT = (PaCO2 - PĒCO2)/PaCO2 (2)
where PĒCO2 is the mixed expired PCO2 [2]. In turn, VDphy is partitioned into anatomic dead space (VDana; conducting airways that do not participate in gas exchange) and alveolar dead space (VDalv; ventilated alveolar units that are devoid of perfusion; Fig. 2). VDalv/VTalv is given by
Figure 2.
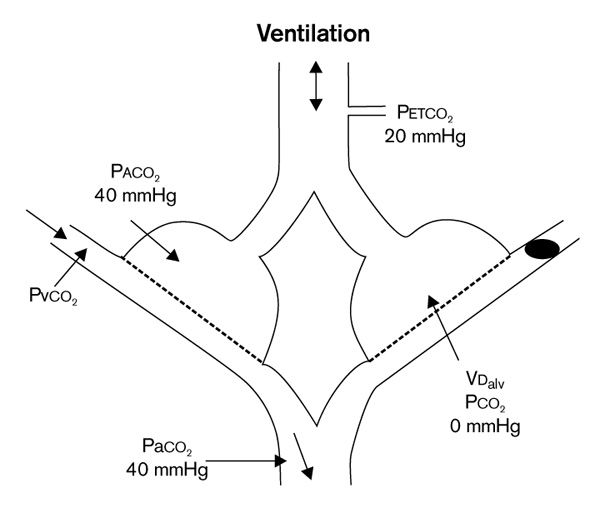
Effect of alveolar dead space (VDalv). The right lung compartment receives no perfusion and contains no carbon dioxide (ignoring interlung unit ventilation). By mass balance for carbon dioxide, VDalv/VTalv = (PaCO2 - PETCO2)/PaCO2. For the sample condition shown, VDalv/VTalv = (40-20)/40= 50%. PaCO2, arterial PCO2; PACO2, alveolar PCO2; PETCO2, end-tidal PCO2; P CO2, mixed venous PCO2;VTalv, alveolar tidal volume.Adapted from Breen [61].
CO2, mixed venous PCO2;VTalv, alveolar tidal volume.Adapted from Breen [61].
VDalv/VTalv = (PaCO2 - PACO2)/PaCO2 (3)
where PACO2 is the alveolar PCO2, estimated either from PETCO2 or PA̅E̅CO2 [2] (see below). The PaCO2-PETCO2 gradient results from the presence of VDalv or high alveolar ventilation-to-blood flow (VA/Q) lung regions (see also Capnography during weaning from mechanical ventilation, below).
The normal capnogram is the measurement of PCO2 at the airway opening during the ventilatory cycle (Fig. 1b) [1]. Phase I (inspiratory baseline) reflects inspired gas, which is normally devoid of carbon dioxide. Phase II (expiratory upstroke) is the transition between VDana, which does not participate in gas exchange, and alveolar gas from the respiratory bronchioles and alveoli. Phase III is the alveolar plateau. Traditionally, PCO2 of the last alveolar gas sampled at the airway opening is called the PETCO2. Finally, phase IV is the inspiratory downstroke, the beginning of the next inspiration.
However, the capnogram contains no volume information. Accordingly, the PA̅E̅CO2 [2,3], which is the volume-averaged alveolar PCO2, is a better index of PACO2 than is PETCO2, which is just a single measurement of PCO2 at the end of exhalation [2]. A more informative determination of pulmonary carbon dioxide elimination is VCO2,br, which is starting to garner clinical acceptance. VCO2,br is the multiplication and integration of airway flow and PCO2 over an entire respiratory cycle [4,5,6]. See the section on Future directions of carbon dioxide kinetics monitoring, below, for an interpretation and contrast of the measurements of VCO2,br and PETCO2.
The disposition of carbon dioxide can also be represented in a hydraulic model (Fig. 3) [3,7]. The large peripheral tissue compartment drains through a conduit ( T) into the small central pulmonary compartment. The central compartment can be further divided into pulmonary shunt (V̇A/
T) into the small central pulmonary compartment. The central compartment can be further divided into pulmonary shunt (V̇A/ = 0), normal lung (V̇A/
= 0), normal lung (V̇A/ near unity), and high V̇A/Q lung units, including VDalv. The tissues produce carbon dioxide (VCO2,ti), which empties into the peripheral tissue compartment. Then, carbon dioxide flows by gravity (QT) from the higher level peripheral tissue compartment to the lower level pulmonary compartment. V̇A, which equals V̇E minus VDana and the effects of high V̇A/Q units, eliminates carbon dioxide from the lung. In this model,
near unity), and high V̇A/Q lung units, including VDalv. The tissues produce carbon dioxide (VCO2,ti), which empties into the peripheral tissue compartment. Then, carbon dioxide flows by gravity (QT) from the higher level peripheral tissue compartment to the lower level pulmonary compartment. V̇A, which equals V̇E minus VDana and the effects of high V̇A/Q units, eliminates carbon dioxide from the lung. In this model,  T affects the distribution and total amount of carbon dioxide in the body. For example, at low
T affects the distribution and total amount of carbon dioxide in the body. For example, at low  T, retention of carbon dioxide occurs in the peripheral tissue compartment, and higher PVCO2 is required to restore carbon dioxide delivery to the lungs. This hydraulic model can help to understand the meaning of PETCO2 during successful cardiopulmonary resuscitation (CPR), and to compare PETCO2 with PaCO2 in the assessment of ventilator parameters. See the section, Effect of positive end-expiratory pressure on carbon dioxide kinetics, below, which highlights the utility of the hydraulic model.
T, retention of carbon dioxide occurs in the peripheral tissue compartment, and higher PVCO2 is required to restore carbon dioxide delivery to the lungs. This hydraulic model can help to understand the meaning of PETCO2 during successful cardiopulmonary resuscitation (CPR), and to compare PETCO2 with PaCO2 in the assessment of ventilator parameters. See the section, Effect of positive end-expiratory pressure on carbon dioxide kinetics, below, which highlights the utility of the hydraulic model.
Figure 3.
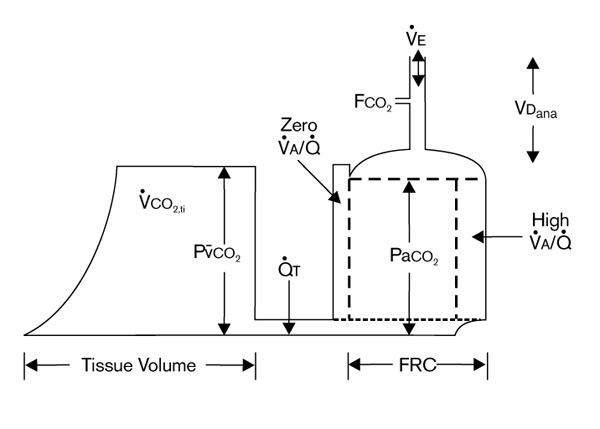
Hydraulic model of carbon dioxide kinetics in the body. Large peripheral tissue carbon dioxide compartment (left) drains through cardiac output ( T) into the smaller central pulmonary carbon dioxide compartment (right). FCO2, fractional carbon dioxide; FRC, functional residual capacity; PaCO2 arterial PCO2; P
T) into the smaller central pulmonary carbon dioxide compartment (right). FCO2, fractional carbon dioxide; FRC, functional residual capacity; PaCO2 arterial PCO2; P CO2, mixed venous PCO2; V̇A/
CO2, mixed venous PCO2; V̇A/ , ventilation : perfusion ratio; VCO2,ti, tissue carbon dioxide production; VDana, anatomical dead space; V̇E, exhaled ventilation (see text). Adapted from Breen and Mazumdar [3].
, ventilation : perfusion ratio; VCO2,ti, tissue carbon dioxide production; VDana, anatomical dead space; V̇E, exhaled ventilation (see text). Adapted from Breen and Mazumdar [3].
Capnometry: current technologies
Capnometry is the measurement of FCO2 in tidal gas at the airway opening [1,8]. Capnography is the graphic display of measured FCO2 versus time. Capnometry most commonly utilizes infrared light absorption or mass spectrometry [9]. Both methods are reliable and relatively accurate. Capnometers that are used in clinical practice use two different sampling techniques: sidestream or mainstream sampling. A mainstream capnometer has an airway adaptor cuvette attached in-line and close to the endotracheal tube (ETT). The cuvette incorporates an infrared light source and sensor that senses carbon dioxide absorption to measure PCO2. A sidestream capnometer uses a sampling line that attaches to a T-piece adapter at the airway opening, through which the instrument continually aspirates tidal airway gas for analysis of carbon dioxide.
Mainstream capnometry
The main advantage of the mainstream analyzer is its rapid response, because the measurement chamber is part of the breathing circuit. The sample cuvette lumen, through which inspired and expired gases pass, is large in order to minimize the work of breathing, and pulmonary secretions generally do not interfere with carbon dioxide analysis. Compared with sidestream sampling, the airway cuvette is relatively bulky and can add dead space. However, within the past few years lighter and smaller airway cuvettes have been developed to allow its use in neonates [10,11]. The analyzer is warmed to prevent condensation on the sample chamber window, and caution must be taken to prevent burns. The monitoring of PETCO2 in nonintubated patients is more difficult with mainstream sampling.
Sidestream capnometry
The sidestream PCO2 analyzer adds only a light T-adapter to the breathing circuit, and can be easily adapted to nonintubation forms of airway control. Because the sampling tubing is small-bore, it can be blocked by secretions. During sidestream capnography, the dynamic response, the steepness of the expiratory upstroke and inspiratory downslope, tends to be blunted because of the dispersive mixing of gases through the sampling line [4,12,13], where gas of high PCO2 mixes with gas of low PCO2. In addition, a washout time is required for the incoming sampled gas to flush out the volume of the measuring chamber. The overall effect is an averaging of the capnogram, resulting in a lowering of the alveolar plateau and an elevation of the inspiratory baseline. Thus, PETCO2 may be underestimated and rebreathing can be simulated [12,14]. These problems are exacerbated by high ventilatory rates and by the use of long sampling catheters. In addition, the capnogram is delayed in time by transport delay, the time required to aspirate gas from the airway opening adapter through the sampling tubing to the sampling chamber [4,12]. In conditions of low fresh gas flow (eg closed circle circuit anesthesia), the amount of gas sampled and removed from the breathing circuit needs to be considered.
Portable capnometers
Although portable capnometers exist, their use in the field can be hindered by cost and requirement for calibration [15]. The portable infrared analyzer will not operate in temperatures that are subzero or greater than 40°C. Another device that is used for measurement of PCO2 is the chemical colorimetric airway detector [16], which uses a pH-sensitive indicator to detect breath-by-breath exhaled carbon dioxide [15]. The colorimetric airway detector is interposed between the ETT and the ventilation device. They have an unopened shelf-life of 15 months. Both adult and pediatric adaptors exist, but they cannot be used in infants who weigh less than 1 kg. Because of excessive flow resistance, they are not suited for patients who are able to breath spontaneously, and excessive humidity will render them inoperative in 15-20 min. The devices can be damaged by mucous, edematous or gastric contents, and by administration of intratracheal epinephrine. Despite these drawbacks, colorimetric sensors have been found to be useful in guiding prehospital CPR both in intubated patients and those with a laryngeal mask airway [15,17].
Traditional use of capnography: airway patency and assessment of ventilation
Because the lung is the only body compartment in which carbon dioxide normally and continuously accumulates, the presence of cyclic exhaled carbon dioxide can be used to confirm airway patency and pulmonary ventilation. Although initially adopted for anesthesia monitoring in the operating room, the use of capnography to confirm airway patency and lung ventilation has expanded over the past 8 years to include critical care, emergency medicine, field resuscitation, and conscious sedation settings [1,8,15,18,19,20,21,22].
However, there are pitfalls in the use of capnography to confirm endotracheal intubation. Potential problems with technology are described above. In addition, several scenarios have been described that impact on the ability of capnography to assess the airway and ventilation.
First, during circulatory arrest, pulmonary ventilation will result in low and decreasing values of exhaled carbon dioxide because QT and carbon dioxide transport from the tissues to the lung are decreased or absent in the presence of continuing VA [23,24,25]. In the clinical setting, however, Vukmir et al [19] demonstrated that infrared capnography was 100% specific and sensitive in the detection of endotracheal versus esophageal intubation in 100 critical care cases of airway management, 17 of which were cardiac arrests.
Second, positive-pressure ventilation by face mask can force pharyngeal gas, containing exhaled carbon dioxide from the previous breath, into the esophagus and stomach [1]. Likewise, ingestion of carbonated beverages can also generate carbon dioxide in the stomach [26]. Subsequent esophageal intubation and gastric ventilation can result in initial cyclic `exhaled' carbon dioxide. However, esophageal intubation usually causes an initial `PETCO2' that is less than 10 mmHg and that decreases with each `exhaled breath' as inspiration dilutes carbon dioxide in the stomach [27]. In the case of suspected esophageal intubation, consider interpreting the value of exhaled carbon dioxide after the sixth breath [15].
Third, in a case report in a neonate weighing under 700 g [28], although the ETT tube was correctly positioned in the trachea, displacement of the ETT against the lateral wall of the trachea resulted in a flat capnogram and an erroneous diagnosis of esophageal intubation.
Fourth, pathology that causes absence of ventilation, including severe bronchospasm, patient apnea, or plugged ETT will result in absence of expired carbon dioxide and a falsely negative diagnosis that the ETT is not in the trachea.
Finally, it is prudent to remember that a normal capnogram confirms ventilation of the lungs through a patent airway, but not necessarily a secure airway. In a case report [29], a normal capnogram resulted during ventilation through an ETT positioned at the glottic opening, but not securely placed in the trachea.
Despite these potential drawbacks, capnography remains the most reliable monitor of airway patency in a variety of experimental and clinical settings. Mickelson et al [30] demonstrated that exhaled carbon dioxide was the most reliable indicator of esophageal intubation in canine model. Likewise, Knapp et al [31] studied current methods of verifying tracheal tube placement in the critical care setting, and found that capnography was superior to auscultation or other devices such as the lighted stylet. Capnography can also recognize esophageal intubation in neonates [32]. In the field, compared with other devices carbon dioxide monitoring best detects esophageal intubation by limiting the number of false negatives and false positives [15].
In addition to confirmation of ETT placement in the trachea, capnography may aid in cases of difficult intubation. During awake, blind, nasotracheal intubation, the end of a sidestream capnometer sampling probe can be placed through and positioned at the distal end of the ETT [33]. Then, increasing values of cyclic exhaled PCO2 can help guide the ETT to the glottic opening. During a difficult intubation, effective ventilation can be maintained through a tube at the tip of the pharynx (guided by the expiratory carbon dioxide waveform), until other adjuncts to intubation are available [34].
Sidestream capnography adapts well to the nonintubated, sedated patient. Croswell et al [35] compared monitoring by capnography, pulse oximetry and clinical observation in sedated, pediatric, dental patients. Capnography provided a minimum 15 s warning of potential arterial desaturation, and was the most sensitive method for detecting airway compromise, especially during deeper levels of sedation. With oral/nasal capnometry in pediatric patients after active seizures, Abramo et al [36] demonstrated that PETCO2 is a useful predictor of hypercapnia and is more sensitive than pulse oximetry in predicting impending respiratory failure. Other studies [8,37] have supported the assertion that capnography provides the earliest warning of airway obstruction and respiratory compromise.
Finally, capnography is a useful monitor during transport of intubated, critically ill patients [38,39]. Beside the obvious advantage of early warning against ETT dislodgment and/or compromise of ventilation, monitoring of PETCO2 (as an estimate of PaCO2) may aid the management of patients in whom hypercapnia is detrimental, such as patients with head injury with raised intracranial pressure and pediatric patients with pulmonary hypertension [38].
Capnography during weaning from mechanical ventilation
Capnography has been considered a potentially useful noninvasive monitor to assess the weaning of patients from mechanical ventilation in critical care settings [40]. However, studies have shown variable results in the ability of PETCO2 to predict PaCO2. Whether the use of PETCO2 can limit the need for invasive arterial blood gas monitoring has yet to be established.
In a 1985-1991 literature review of the efficacy of noninvasive blood gas monitoring in the adult critical care unit [41], the Technology Subcommittee of the Working Group on Critical Care (Ontario Ministry of Health) concluded that changes in PETCO2 need to be interpreted with extreme caution. Healey et al [42] compared the correlation of PETCO2 with PaCO2 before and after withdrawal of assist control mechanical ventilation. PETCO2 paralleled changes in PaCO2 (r = 0.82). Saura et al [43], in a prospective study to evaluate the relationship between PaCO2 and PETCO2 before and during weaning with continuous positive airway pressure ventilation, also found that PETCO2 could detect clinically relevant hypercapnic episodes. However, there was a high incidence of false positives that led to arterial blood gas sampling. Withington et al [44] found that, after a gradient between PaCO2 and PETCO2 was established, PETCO2 was a useful parameter in the weaning of postcardiac surgery patients.
The assessment of PETCO2 may be misleading if not considered in the context of changing hemodynamics and ventilatory pattern. Although there can be significant correlation of PETCO2 with PaCO2, clinically acceptable sensitivity and specificity may only occur in the absence of significant changes in  T or V̇A/Q relationships. In evaluating the use of capnography as a noninvasive monitor of PaCO2 in critical care patients, Morley et al [45] observed that PETCO2 was useful as a predictor only in patients without significant parenchymal lung disease. Prause [46] found that PETCO2 was useful for the adjustment of ventilatory parameters in prehospital emergency care patients only if they had no major cardiopulmonary damage. As depicted in Fig. 2, the gradient between PETCO2 and PaCO2 depends on VDalv (ie the amount of lung regions with high or infinite V̇A/
T or V̇A/Q relationships. In evaluating the use of capnography as a noninvasive monitor of PaCO2 in critical care patients, Morley et al [45] observed that PETCO2 was useful as a predictor only in patients without significant parenchymal lung disease. Prause [46] found that PETCO2 was useful for the adjustment of ventilatory parameters in prehospital emergency care patients only if they had no major cardiopulmonary damage. As depicted in Fig. 2, the gradient between PETCO2 and PaCO2 depends on VDalv (ie the amount of lung regions with high or infinite V̇A/ ratios) [2,25]. Lung regions with high V̇A/
ratios) [2,25]. Lung regions with high V̇A/ ratios can result from high alveolar pressures (eg large VT, positive end-expiratory pressure [PEEP]), low pulmonary perfusion pressures (eg low
ratios can result from high alveolar pressures (eg large VT, positive end-expiratory pressure [PEEP]), low pulmonary perfusion pressures (eg low  T, upright position), and obstruction of pulmonary blood flow (eg thrombus, gas, or fat embolism). Thus, in the critically ill patient, VDalv often changes and affects the ability of PETCO2 to predict PaCO2 and be a substitute for arterial blood gas sampling.
T, upright position), and obstruction of pulmonary blood flow (eg thrombus, gas, or fat embolism). Thus, in the critically ill patient, VDalv often changes and affects the ability of PETCO2 to predict PaCO2 and be a substitute for arterial blood gas sampling.
Capnography during nonsteady-state conditions
Capnography during cardiopulmonary resuscitation
An important and relatively successful application of capnography in the nonsteady-state clinical setting has been during CPR [1,3,25]. During cardiac arrest, the abrupt decrease in  T results in reduction in carbon dioxide transport from the tissues to lung and, hence, decreased carbon dioxide elimination from the lung. With subsequent successful CPR, the increase in
T results in reduction in carbon dioxide transport from the tissues to lung and, hence, decreased carbon dioxide elimination from the lung. With subsequent successful CPR, the increase in  T restores pulmonary blood flow and carbon dioxide transport, and increases pulmonary elimination of carbon dioxide. Contrast this nonsteady-state effect of
T restores pulmonary blood flow and carbon dioxide transport, and increases pulmonary elimination of carbon dioxide. Contrast this nonsteady-state effect of  T upon carbon dioxide kinetics with the steady-state equation (Eqn 1) for carbon dioxide kinetics.
T upon carbon dioxide kinetics with the steady-state equation (Eqn 1) for carbon dioxide kinetics.  T does not even appear in Equation 1, although it is the conduit for V̇CO2,ti.
T does not even appear in Equation 1, although it is the conduit for V̇CO2,ti.
The measurement of exhaled carbon dioxide is the best signal of return of spontaneous circulation during CPR [23,24]. Capnography is also a useful noninvasive index of the adequacy of pulmonary perfusion during closed-chest cardiac compression [47,48]. In fact, capnography may be used to compare the efficacy of different modes of chest compression [49].
Moreover, the quantitative measurement of PETCO2 may have predictive value during CPR. This was recognized as early as 1939, when Eisenmenger wrote "If during a resuscitation attempt the analysis of the expired air, performed about twice per hour, still shows plenty of carbon dioxide, then continuation of artificial respiration (and circulation) would be indicated" [50]. Asplin and White [20] measured the 1-min value, the 2-min value, and the maximum value of PETCO2 during CPR in 27 patients. The initial PETCO2 values were prognostic for return of spontaneous circulation. Finally, the predictive value of PETCO2 has been studied in hospital settings. Domsky et al [51], in a retrospective chart review of 100 critically ill surgery patients, found that a persistent PETCO2 of 28 mmHg or less was associated with a mortality rate of 55%, versus a mortality of 17% in patients with higher PETCO2. Mortality rate was also increased in patients with a persistent PaCO2-PETCO2 difference of 8 mmHg or more. Quantitative capnography during resuscitation will continue to evolve.
Future directions of carbon dioxide kinetics monitoring
The following three sections examine how clinically relevant perturbations (application of PEEP, onset of pulmonary embolism, and recovery from pulmonary embolism) affect nonsteady-state carbon dioxide kinetics. The use of relatively new measurements (VCO2,br, PA̅E̅CO2) will help define pathophysiology and will improve, we believe, clinical diagnosis and treatment.
Effect of positive end-expiratory pressure on carbon dioxide kinetics
The addition of PEEP to mechanical ventilation should acutely decrease VCO2,br, due to decreased V̇A (increased VDphy) and decreased carbon dioxide transfer to the lung (decreased  T and venous return) [1,3]. Then, gradual recovery of VCO2,br would occur if peripheral tissue carbon dioxide retention caused sufficient increase in PVCO2 (especially at sustained low
T and venous return) [1,3]. Then, gradual recovery of VCO2,br would occur if peripheral tissue carbon dioxide retention caused sufficient increase in PVCO2 (especially at sustained low  T) to restore carbon dioxide delivery to the lung (Fig. 3).
T) to restore carbon dioxide delivery to the lung (Fig. 3).
The initial effects during the first 25 breaths after adding 11 cmH2O PEEP to mechanical ventilation of anesthetized dogs are shown in Fig. 4 [3]. The summation of the decreases in VT, compared with the baseline value, permitted calculation of increased functional residual capacity (FRC) at 1152 ml. PETCO2 paralleled the decrease in VT, but recovered to baseline by breath 10. VCO2,br decreased from baseline (7.6 ml) to zero in the first couple of exhalations. However, VCO2,br had only increased to 4.9 ml by breath 25. From a baseline value (3.3 l/min), QT (ascending aorta flow probe) decreased to 1.6 l/min by breath 10, which was sustained through breath 25. During measurements extended to 25 min, depressed QT was sustained and VCO2,br was still 17% less than baseline. PEEP caused an immediate and sustained increase in VDphy from 312 to 366 ml, resulting entirely from the increase in VDana. PETCO2 continued to increase to the 25 min value (43 ± 6 mmHg), which was significantly greater than baseline. There were parallel changes in PaCO2 and PVCO2.
Figure 4.
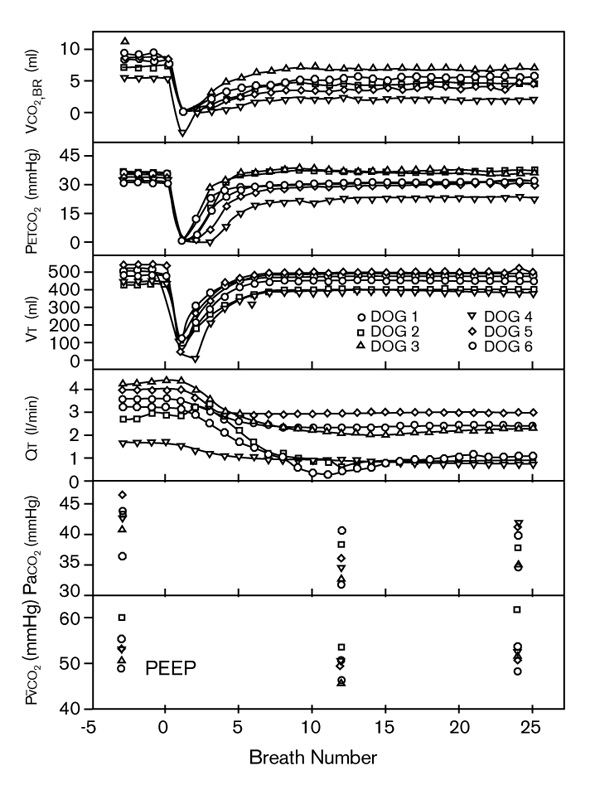
Initial breath-by-breath effects of adding 11 cmH2O PEEP in mechanically ventilated anesthetized dogs on carbon dioxide volume exhaled per breath (VCO2,br), end-tidal PCO2 (PETCO2), exhaled tidal volume (VT), and cardiac output (QT, aorta flow probe). PaCO2, arterial PCO2; P CO2, mixed venous PCO2. Adapted from Breen and Mazumdar [3].
CO2, mixed venous PCO2. Adapted from Breen and Mazumdar [3].
A study of the hydraulic model of carbon dioxide kinetics (Fig. 3) will help to summarize [1,3]. PEEP immediately decreased VCO2,br by the following mechanisms:decreased V̇A, itself caused by the increase in VDana and by appearance of new high V̇A/ lung units; and decreased PACO2 caused by decreased
lung units; and decreased PACO2 caused by decreased  T and, hence, reduced carbon dioxide transfer from the tissues to the lung. Dilution of PACO2 with fresh gas as FRC increased at onset of PEEP was offset by decreased VCO2,br (including the effect of initial decreased exhaled VT). Sustained decrease in VCO2,br at 25 min occurred because V̇A remained depressed from continued increased VDphy. Then, VCO2,br could only recover to the baseline value if PACO2 significantly increased. However, at persistently decreased
T and, hence, reduced carbon dioxide transfer from the tissues to the lung. Dilution of PACO2 with fresh gas as FRC increased at onset of PEEP was offset by decreased VCO2,br (including the effect of initial decreased exhaled VT). Sustained decrease in VCO2,br at 25 min occurred because V̇A remained depressed from continued increased VDphy. Then, VCO2,br could only recover to the baseline value if PACO2 significantly increased. However, at persistently decreased  T, the increase in tissue carbon dioxide retention and PVCO2 were not enough to restore carbon dioxide delivery to the lung and sufficiently increase PACO2. PETCO2, because it does not measure exhaled volume, failed to correctly estimate VCO2,br. Steady state was not reached by 25 min of PEEP. A parallel study of the effects of PEEP on carbon dioxide kinetics in anesthetized patients [52] demonstrated less marked changes, presumably because the intact thorax in patients blunted the increase in FRC and VDana and the intact pleural pressure gradient limited the generation of high V̇A/
T, the increase in tissue carbon dioxide retention and PVCO2 were not enough to restore carbon dioxide delivery to the lung and sufficiently increase PACO2. PETCO2, because it does not measure exhaled volume, failed to correctly estimate VCO2,br. Steady state was not reached by 25 min of PEEP. A parallel study of the effects of PEEP on carbon dioxide kinetics in anesthetized patients [52] demonstrated less marked changes, presumably because the intact thorax in patients blunted the increase in FRC and VDana and the intact pleural pressure gradient limited the generation of high V̇A/ lung units. Other studies, in patients with acute respiratory failure [53,54], have demonstrated the limitations of interpreting changes in the PaCO2-PETCO2 gradient during PEEP.
lung units. Other studies, in patients with acute respiratory failure [53,54], have demonstrated the limitations of interpreting changes in the PaCO2-PETCO2 gradient during PEEP.
Effect of pulmonary embolism on carbon dioxide kinetics
Pulmonary embolism should cause a different VA/Q abnormality, the generation of pure VDalv. The embolus will block perfusion to lung units, converting them into VDalv [1,25]. The increase in VDalv will increase VDphy and result in decreased VA and, hence, VCO2,br. Eventually, tissue carbon dioxide retention and increased PVCO2 would restore carbon dioxide delivery from the tissue to the lung and VCO2,br. Presumably, persistent VDalv during pulmonary embolus would preclude the accuracy of PETCO2 as an estimate of either PaCO2 or VCO2,br.
To examine these hypotheses, an animal model similar to the PEEP study (above) was invoked, except that the perturbation was abrupt tightening of a snare around the right pulmonary artery (RPA) [1,55]. Compared with baseline (9.3 ml), average VCO2,br decreased to 7.0 ml by 1 min after RPA occlusion (Fig. 5). At the same time, PETCO2 decreased from 29 to 22 mmHg. During the following 70 min of RPA occlusion, VCO2,br steadily increased to approach the baseline value. In contrast, at 70 min of RPA occlusion, PETCO2 was still 13% less than baseline. PaCO2 and PVCO2 progressively converged on their maxima (high values) by 70 min.  T, despite an initial tendency to decrease, did not change significantly.
T, despite an initial tendency to decrease, did not change significantly.
Figure 5.
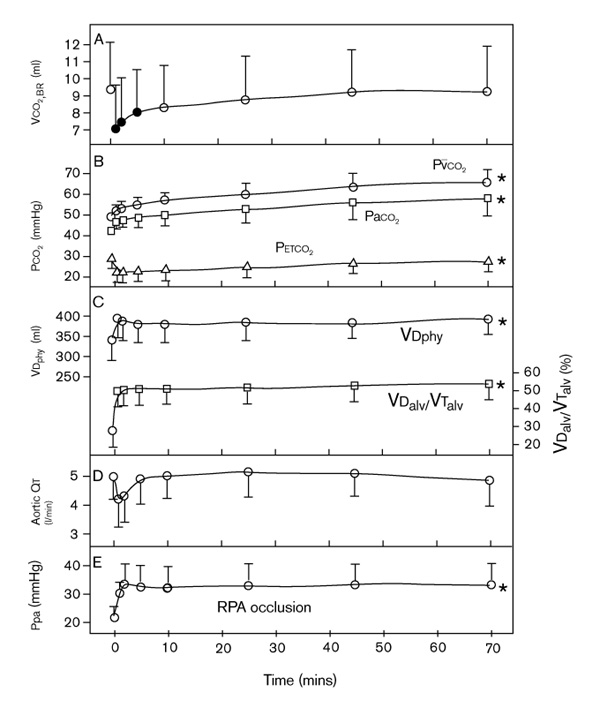
In five mechanically ventilated dogs, effect of 70 min of RPA occlusion on the following: (a) carbon dioxide volume exhaled per breath (VCO2,br); (b) PCO2; (c) dead space (VD); (d) ascending aortic cardiac output ( T); and (e) mean pulmonary artery pressure (Ppa). RPA occlusion began after time 0 (baseline). Solid symbol denotes significant difference (P < 0.05) from baseline measurement. *All stages during RPA occlusion were significantly different from baseline. PaCO2, arterial PCO2; PETCO2, end-tidal PCO2; P
T); and (e) mean pulmonary artery pressure (Ppa). RPA occlusion began after time 0 (baseline). Solid symbol denotes significant difference (P < 0.05) from baseline measurement. *All stages during RPA occlusion were significantly different from baseline. PaCO2, arterial PCO2; PETCO2, end-tidal PCO2; P CO2, mixed venous PCO2; VDalv/VTalv, alveolar dead space : tidal volume fraction; VDphy, physiologic dead space. From Breen et al [55].
CO2, mixed venous PCO2; VDalv/VTalv, alveolar dead space : tidal volume fraction; VDphy, physiologic dead space. From Breen et al [55].
In summary, large experimental pulmonary embolus immediately decreased VCO2,br by 25%, almost entirely due to an increase in VDalv (Fig. 2). VCO2,br increased and recovered to baseline as carbon dioxide was retained in the body, signaled by the progressive increases in PaCO2 and PVCO2. PETCO2 remained significantly less than baseline due to persistent increased VDalv, and detected neither the increase and recovery of VCO2,br nor the increase in PaCO2. Because  T did not significantly decrease, PVCO2 could increase sufficiently to restore carbon dioxide delivery to the lung.
T did not significantly decrease, PVCO2 could increase sufficiently to restore carbon dioxide delivery to the lung.
Resolution of pulmonary embolism
Patients with large pulmonary embolism can suffer progressive hypercapnia and may require emergent embolectomy, either by the transvenous or open thoracic approach. Conceivably, the functional recovery of carbon dioxide exchange could signal reperfusion of the affected pulmonary circulation and help guide the course of surgical therapy.
Accordingly, using the experimental model of pulmonary embolism (above), the RPA was occluded for 70 min to approach steady state. Then, the RPA snare was abruptly released and measurements were repeated during 70 min of RPA reperfusion [1,56]. At onset of RPA reperfusion, VCO2,br abruptly increased from 9 to 12 ml. By 70 min of RPA reperfusion, VCO2,br returned to the baseline value. Immediately after RPA reperfusion, PETCO2 increased from 25 to 33 mmHg because VDalv/VTalv decreased by 41%.At 70 min, PETCO2 was still greater than baseline. PaCO2 and PVCO2 steadily decreased during 70 min of RPA reperfusion, modeling the release of carbon dioxide retention in the central pulmonary and peripheral tissue carbon dioxide compartments. QT did not change significantly.
In summary, VCO2,br detects and follows the resolution of carbon dioxide retention in lung and tissues during reperfusion after experimental pulmonary embolus. In contrast, PETCO2 did not detect the secondary slow decrease in VCO2,br back to baseline because PETCO2 measures neither exhaled volume nor the shape of the PCO2 waveform.
Accordingly, during onset and resolution of pulmonary embolism, this analysis of nonsteady-state carbon dioxide kinetics may aid the clinical assessment of pulmonary embolism [57].
Although beyond the scope of the present review, volumetric capnography (ie the carbon dioxide expirogram, the plot of exhaled PCO2 versus exhaled volume) can also yield information about lung volume [58], dead space [59], and pulmonary blood flow (carbon dioxide rebreathing technique) [60].
Conclusion
In our opinion, better understanding of pathophysiology of carbon dioxide kinetics during steady and nonsteady state should improve clinical care during intensive care treatment. Capnography and the measurement of PETCO2 will gradually be augmented by relatively new measurement methodology (including VCO2,br and PA̅E̅CO2). Future directions include the study of oxygen kinetics [1].
Acknowledgments
Acknowledgement
Supported by National Heart, Lung, and Blood Institute grant HL-42637.
References
- Breen PH. Carbon dioxide kinetics during anesthesia: pathophysiology and monitoring. Respiration in Anesthesia: Pathophysiology and Clinical Update. Edited by Breen PH. Philadelphia: WB Saunders, Anesthesiology Clinics of North America. 1998;16:259–293. [Google Scholar]
- Breen PH, Mazumdar B, Skinner SC. Comparison of end-tidal PCO2 and average alveolar expired PCO2 during positive end-expiratory pressure. Anesth Analg. 1996;82:368–373. doi: 10.1097/00000539-199602000-00027. [DOI] [PubMed] [Google Scholar]
- Breen PH, Mazumdar B. How does positive end-expiratory pressure decrease CO2 elimination from the lung? Respir Physiol. 1996;103:233–242. doi: 10.1016/0034-5687(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Breen PH, Isserles SA, Harrison BA, Roizen MF. Simple, computer measurement of pulmonary VCO2 per breath. J Appl Physiol. 1992;72:2029–2035. doi: 10.1152/jappl.1992.72.5.2029. [DOI] [PubMed] [Google Scholar]
- Breen PH, Serina ER. Bymixer provides on-line calibration of measurement of CO2 volume exhaled per breath. Ann Biomed Eng. 1997;25:164–171. doi: 10.1007/BF02738547. [DOI] [PubMed] [Google Scholar]
- Breen PH, Serina ER, Barker SJ. Measurement of pulmonary CO2 elimination must exclude inspired CO2 measured at the capnometer sampling site. J Clin Monit. 1996;12:231–236. doi: 10.1007/BF00857644. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Longobardo GS. Oxygen and carbon dioxide gas stores of the body. Physiol Rev. 1970;50:196–243. doi: 10.1152/physrev.1970.50.2.196. [DOI] [PubMed] [Google Scholar]
- Dang K, Breen PH. Ambulatory capnography. J Respir Care Pract. 1998;Jun/Jul:25–30. [Google Scholar]
- Stock MC. Capnography for adults. Crit Care Clin. 1995;11:219–232. [PubMed] [Google Scholar]
- Rozycki HJ, Sysyn GD, Marshall MK, et al. Mainstream end tidal carbon dioxide monitoring in the neonatal intensive care unit. . Pediatrics. 1998;101:648–653. doi: 10.1542/peds.101.4.648. [DOI] [PubMed] [Google Scholar]
- McEvedy BA, McLeod ME, Kirpalani H, Volgyesi GA, Lerman J. End tidal carbon dioxide measurements in critically ill neonates: a comparison of side-stream and mainstream capnometers. Can J Anaesth. 1990;37:322–326. doi: 10.1007/BF03005583. [DOI] [PubMed] [Google Scholar]
- Breen PH, Mazumdar B, Skinner SC. Capnometer transport delay: measurement and clinical implications. Anesth Analg. 1994;78:584–586. doi: 10.1213/00000539-199403000-00027. [DOI] [PubMed] [Google Scholar]
- Pascucci RC, Schena JA, Thompson JE. Comparison of a side-stream and mainstream capnometer in infants. Crit Care Med. 1989;17:560–562. doi: 10.1097/00003246-198906000-00016. [DOI] [PubMed] [Google Scholar]
- Doyle DJ. Time constant-related capnograph distortion: a theoretical analysis. J Biomed Eng. 1991;13:500–502. doi: 10.1016/0141-5425(91)90098-r. [DOI] [PubMed] [Google Scholar]
- Cardoso MM, Banner MJ, Melker RJ, Bjoraker DG. Portable devices used to detect endotracheal intubation during emergency situations: a review. Crit Care Med. 1998;26:957–964. doi: 10.1097/00003246-199805000-00036. [DOI] [PubMed] [Google Scholar]
- Kelly JS, Wilhoit RD, Brown RE, James R. Efficacy of the FEF colorimetric end-tidal carbon dioxide detector in children. Anesth Analg. 1992;75:45–50. [PubMed] [Google Scholar]
- Nakatani K, Yukioka H, Fujimori M, et al. Utility of colorimetric end-tidal carbon dioxide detector for monitoring during prehospital cardiopulmonary resuscitation. Am J Emerg Med. 1999;17:203–206. doi: 10.1016/s0735-6757(99)90062-1. [DOI] [PubMed] [Google Scholar]
- Birmingham PK, Cheney FW, Ward RJ. Esophageal intubation: a review of detection techniques. Anesth Analg. 1986;65:886–891. [PubMed] [Google Scholar]
- Vukmir RB, Heller MB, Stein KL. Confirmation of endotracheal tube placement: a miniaturized infrared qualitative CO2 detector. Ann Emerg Med. 1991;20:726–729. doi: 10.1016/s0196-0644(05)80831-5. [DOI] [PubMed] [Google Scholar]
- Asplin BR, White RD. Prognostic value of end-tidal carbon dioxide pressures during out-of-hospital cardiac arrest. Ann Emerg Med. 1995;25:756–761. doi: 10.1016/s0196-0644(95)70203-2. [DOI] [PubMed] [Google Scholar]
- Liu S-Y, Lee T-S, Bongard F. Accuracy of capnography in nonintubated surgical patients. Chest. 1992;102:1512–1515. doi: 10.1378/chest.102.5.1512. [DOI] [PubMed] [Google Scholar]
- Sanders AB. Capnometry in emergency medicine. Ann Emerg Med. 1989;18:1287–1290. doi: 10.1016/s0196-0644(89)80260-4. [DOI] [PubMed] [Google Scholar]
- Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Eng J Med . 1988;318:607–611. doi: 10.1056/NEJM198803103181005. [DOI] [PubMed] [Google Scholar]
- Garnett AR, Ornato JP, Gonzalez ER, Johnson EB. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512–515. [PubMed] [Google Scholar]
- Isserles SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg . 1991;73:808–814. doi: 10.1213/00000539-199112000-00023. [DOI] [PubMed] [Google Scholar]
- Garnett AR, Gervin CA, Gervin AS. Capnographic waveforms in esophageal intubation: effect of carbonated beverages. Ann Emerg Med. 1989;18:387–390. doi: 10.1016/s0196-0644(89)80576-1. [DOI] [PubMed] [Google Scholar]
- Ping STS, Mehta MP, Symreng T. Reliability of capnography in identifying esophageal intubation with carbonated beverage or antacid in the stomach. Anesth Analg. 1991;73:333–337. doi: 10.1213/00000539-199109000-00018. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Maniscalco WM. A novel cause of error in capographic confirmation of intubation in the neonatal intensive care unit. . Pediatrics. 1995;95:140–142. [PubMed] [Google Scholar]
- Werman HA, Talcone RE. Glottic positioning of the endotracheal tube tip: a diagnostic dilemna. Ann Emerg Med. 1998;31:643–646. doi: 10.1016/s0196-0644(98)70213-6. [DOI] [PubMed] [Google Scholar]
- Mickelson KS, Sterner SP, Ruiz E. Exhaled PCO2 as a predictor of endotracheal tube placement [abstract]. Ann Emerg Med. 1986;15:208–657. [Google Scholar]
- Knapp S, Kofler J, Stoiser B, et al. The assessment of four different methods to verify tracheal tube placement in the critical care setting. Anesth Analg. 1999;88:766–770. doi: 10.1097/00000539-199904000-00016. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Maniscalco WM, Cohen AR, Litman RS, Chhibber A. The use of capnography for recognition of esophageal intubation in the neonatal intensive care unit. Pediatr Pulmonol. 1995;19:262–268. doi: 10.1002/ppul.1950190504. [DOI] [PubMed] [Google Scholar]
- Linko K, Paloheimo M. Capnography facilitates blind nasotracheal intubation. Acta Anesthesiol Belg. 1983;34:117–122. [PubMed] [Google Scholar]
- Bund M, Walz R, Lobbes W, Seitz W. A tube in the pharynx for emergency ventilation. Acta Anaesthesiol Scand. 1997;41:529–530. doi: 10.1111/j.1399-6576.1997.tb04736.x. [DOI] [PubMed] [Google Scholar]
- Croswell RJ, Dilley DC, Lucas WJ, Van WF. A comparison of conventional versus electronic monitoring of sedated dental patients. . Pediatr Dent. 1995;17:332–339. [PubMed] [Google Scholar]
- Abramo TJ, Wiebe RA, Scott S, Goto CS, McIntire DD. Noninvasive capnometry monitoring for respiratory status during pediatric seizures. . Crit Care Med. 1997;25:1242–1246. doi: 10.1097/00003246-199707000-00029. [DOI] [PubMed] [Google Scholar]
- Wright SW. Conscious sedation in the emergency department: the value of capnography and pulse oximetry. Ann Emerg Med. 1992;21:551–555. doi: 10.1016/s0196-0644(05)82523-5. [DOI] [PubMed] [Google Scholar]
- Palmon SC, Liu M, Moore LE, Kirsch JR. Capnography facilitates tight control of ventilation during transport. Crit Care Med. 1996;24:608–611. doi: 10.1097/00003246-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Link J, Krause H, Wagner W, Papadopoulos G. Intrahospital transport of critically ill patients. Crit Care Med. 1990;18:1427–1429. doi: 10.1097/00003246-199012000-00024. [DOI] [PubMed] [Google Scholar]
- Carlon GC, Cole R, Miodownik S, Kopec I, Groeger JS. Capnography in mechanically ventilated patients. Crit Care Med. 1988;16:550–556. doi: 10.1097/00003246-198805000-00016. [DOI] [PubMed] [Google Scholar]
- Technology Subcommittee of the Working Group on Critical Care, Ontario Ministry of Health Noninvasive blood gas monitoring: a review for use in the adult critical care unit. Can Med Assoc J. 1992;146:703–712. [PMC free article] [PubMed] [Google Scholar]
- Healey CJ, Fedullo AJ, Swinburne AJ, Wahl GW. Comparison of noninvasive measurements of carbon dioxide tension during withdrawal from mechanical ventilation. Crit Care Med. 1987;15:764–768. doi: 10.1097/00003246-198708000-00011. [DOI] [PubMed] [Google Scholar]
- Saura P, Blanch L, Lucangelo U, Fernandez R, Mestre J, Artigas A. Use of capnography to detect hypercapnic episodes during weaning from mechanical ventilation. Intensive Care Med. 1996;22:374–381. doi: 10.1007/BF01712151. [DOI] [PubMed] [Google Scholar]
- Withington DE, Ramsay JG, Saoud AT, Bilodeau J. Weaning from ventilation after cardiopulmonary bypass: evaluation of a non-invasive technique. Can J Anaesth. 1991;38:15–19. doi: 10.1007/BF03009157. [DOI] [PubMed] [Google Scholar]
- Morley TF, Giaimo J, Maroszan E, et al. Use of capnography for assessment of the adequacy of alveolar ventilation during weaning from mechanical ventilation. Am Rev Respir Dis. 1993;148:339–344. doi: 10.1164/ajrccm/148.2.339. [DOI] [PubMed] [Google Scholar]
- Prause G, Hetz H, Lauda P, Pojer H, Smolle-Juettner F, Smolle J. A comparison of the end-tidal CO2 documented by capnometry and the arterial pCO2 in emergency patients. . Resuscitation. 1997;35:145–148. doi: 10.1016/S0300-9572(97)00043-9. [DOI] [PubMed] [Google Scholar]
- White RD, Asplin BR. Out-of-Hospital quantitative monitoring of end-tidal carbon dioxide pressure during CPR. Ann Emerg Med. 1994;23:25–30. doi: 10.1016/s0196-0644(94)70003-6. [DOI] [PubMed] [Google Scholar]
- Szaflarski NL, Cohen NH. Use of capnography in critically ill adults. Heart Lung. 1991;20:363–372. [PubMed] [Google Scholar]
- Ward KR, Menegazzi JJ, Zelenak RR, Sullivan RJ, McSwain NE. A comparison of chest compressions between mechanical and manual CPR by monitoring end-tidal PCO2 during human cardiac arrest. Ann Emerg Med. 1993;22:669–674. doi: 10.1016/s0196-0644(05)81845-1. [DOI] [PubMed] [Google Scholar]
- Koetter K, Maleck WH. Effectiveness of mechanical versus manual chest compressions in out-of-hospital cardiac resuscitation [letter]. . Am J Emerg Med. 1999;17:210. doi: 10.1016/s0735-6757(99)90065-7. [DOI] [PubMed] [Google Scholar]
- Domsky M, Wilson RF, Heins J. Intraoperative end-tidal carbon dioxide values and derived calculations correlated with outcome: prognosis and capnography. Crit Care Med. 1995;23:1497–1503. doi: 10.1097/00003246-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Breen PH. How does positive end-expiratory pressure decrease pulmonary CO2 elimination in anesthetized patients? . Respir Physiol. 1999;118:227–236. doi: 10.1016/s0034-5687(99)00087-0. [DOI] [PubMed] [Google Scholar]
- Jardin F, Genevray B, Pazin M, Margairaz A. Inability to titrate PEEP in patient with acute respiratory failure using end-tidal carbon dioxide measurements. Anesthesiology. 1985;62:530–533. doi: 10.1097/00000542-198504000-00031. [DOI] [PubMed] [Google Scholar]
- Blanch L, Fernandez R, Benito S, Mancebo J, Net A. Effect of PEEP on the arterial minus end-tidal carbon dioxide gradient. . Chest. 1987;92:451–454. doi: 10.1378/chest.92.3.451. [DOI] [PubMed] [Google Scholar]
- Breen PH, Mazumdar B, Skinner SC. How does experimental pulmonary embolism decrease CO2 elimination? Respir Physiol. 1996;105:217–224. doi: 10.1016/0034-5687(96)00036-9. [DOI] [PubMed] [Google Scholar]
- Breen PH, Mazumdar B, Skinner SC. Carbon dioxide elimination measures resolution of experimental pulmonary embolus in dogs. . Anesth Analg. 1996;83:247–253. doi: 10.1097/00000539-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Chopin C, Fesard P, Mangalaboyi J, et al. Use of capnography in diagnosis of pulmonary embolism during acute respiratory failure of chronic obstructive pulmonary disease. Crit Care Med . 1990;18:353–357. doi: 10.1097/00003246-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Stenz RI, Grenier B, Thompson JE, Arnold JH. Single-breath CO2 analysis as a predictor of lung volume in a healthy animal model during controlled ventilation. Crit Care Med. 1998;26:1409–1413. doi: 10.1097/00003246-199808000-00028. [DOI] [PubMed] [Google Scholar]
- Arnold JH, Thompson JE, Arnold LW. Single breath CO2 analysis: description and validation of a method. Crit Care Med . 1996;24:96–102. doi: 10.1097/00003246-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Blanch L, Fernandez R, Benito S, Mancebo J, Calaf N, Net A. Accuracy of an indirect carbon dioxide Fick method in determination of the cardiac output in critically ill mechanically ventilated patients. . Intensive Care Med. 1988;14:131–135. doi: 10.1007/BF00257466. [DOI] [PubMed] [Google Scholar]
- Breen PH. Capnography: the science behind the lines. . A.S.A. Annual Refresher Course Lectures. Park Ridge, IL: American Society of Anesthesiologists. 1994;126:1–7. [Google Scholar]


