Abstract
Although many promising objective methods (measuring systems) are available, there are no truly validated instruments for monitoring intensive care unit (ICU) sedation. Auditory evoked potentials can be used only for research in patients with a deep level of sedation. Other measuring systems require further development and validation to be useful in the ICU. Continuing research will provide an objective system to improve the monitoring and controlling of this essential treatment for ICU patients. Subjective methods (scoring systems) that are based on clinical observation have proven their usefulness in guiding sedative therapy. The Glasgow Coma Score modified by Cook and Palma (GCSC) achieves good face validity and reliability, which assures its clinical utility for routine practice and research. Other scales, in particular the Ramsay Scale, can be recommended preferably for clinical use. An accurate use of available instruments can improve the sedative treatment that we deliver to our patients.
Keywords: ICU sedation monitoring, quality of sedation, sedation measuring systems, sedation scoring systems, sedative agents
Introduction
Anxiety and agitation are experienced by more than 70% of ICU patients. Their prevalence is even greater in critically ill patients undergoing mechanical ventilation. Consequently, the routine assessment of sedation should be a part of total care for critically ill patients, in a manner similar to that in which cardiorespiratory parameters are monitored. The difficulty in monitoring sedation, however, is that there is no gold standard tool for this purpose [1].
Current techniques have been proposed, but they are often not supported by conclusive clinical evidence. A critical review of the available studies reveals three methodological problems. The first is the fact that these instruments have commonly been tested in postoperative patients, who do not suffer the multiple organ disorders experienced by critically ill patients [2]. Second, much of the available medical literature is based on the opinions of experts, rather than on results of controlled clinical trials. Finally, the third problem is the weak design of most of the published studies, which are often poorly controlled and nonrandomized. So far there have only been two reported trials that have employed a double-blind protocol [3]. It is therefore not surprising that routine sedation is often an empirical clinical exercise, without adequate assessment according to scientific evidence.
The clinical utility of each available tool must be evaluated rigorously before deciding on whether they have applications in routine practice, because assessment of sedation is a primary objective in intensive care management.
From both practical and evidence-based points of view, the present review assesses the available instruments for monitoring and controlling sedation in ICU patients who are undergoing mechanical ventilation. It is structured in two parts. The first part introduces some methodological concepts to facilitate a critical review of each instrument. The second part provides practical recommendations, on the basis of evidence and experience, for a clinical approach to the application of each tool in ICU settings.
Methodological concepts regarding the assessment of sedation
Considerations concerning validity and reliability of instruments
The clinical utility of each new instrument should be assessed according to a rational evaluation of its validity, reliability and applicability.
`Validity' is the ability of a tool to actually measure the parameter that it is designed for. In monitoring of sedation this concept implies the ability to document agitation and distress symptoms (anxiety, delirium and pain), as well as to identify the end-points of each level of sedation that each sedative agent can achieve. It is indispensable also that it measures the reality from an interpretation scheme with validity on both content and construct in order to ensure that the same clinical situations will always be interpreted in the same way.
If a standard criterion exits, then the new test should compare favourably with it. The `gold standard' is easy to define for some conditions, but is more much elusive when testing diagnosis of subjective experiences such as anxiety or agitation. For this reason, the validation of these instruments is more much difficult and complex. Accordingly, validity tends to be confirmed on the basis of opinion of recognized practitioners (face validity) searching for signs and symptoms as logical consequences (or constructs) of the target disorders.
`Reliability' is the capacity of a new test to obtain similar measures with different observers (inter-rater) or with the same observer at different times (intrarater). In clinical research, interobserver agreement (calculated as weighted κ index) is the best indicator of the reliability of these instruments.
`Applicability' in this context implies that an instrument is easy to learn to operate, and that it is suitable for routine use by physicians and nurses.
In order to assist the reader in determining the relative methodological authority of the evidence presented herein, a rating system is employed that facilitates objective analysis (Table 1) [1].
Table 1.
Rating system to determine the clinical utility of instruments for monitoring ICU sedation
| Condition | Description | Points |
| Validity | Capacity to document agitation- and distress-related symptoms (pain, anxiety, delirium) | 0-5 |
| Content and construct validity | 0-10 | |
| Exact definition of level of sedation end-points | 0-10 | |
| Reliability | Inter-rater and intra-rater agreement | 0-20 |
| Applicability | Easy learning and routine recording by physicians and nurses | 0-5 |
| Total points | 0-50 |
Considerations regarding clinical and experimental evidence
The technical assessment of each instrument should not only be subjected to a critical analysis of its methodology, but also to a review of the quality of the studies on which it has been based. This is essential in order to make a critique of the advantages and limitations of such instruments. The quality of these studies can be classified according to three categories:
(1) class I evidence – the instrument has been validated by one or more double-blinded, or randomized controlled clinical trials carried out in an adequate sample of critically ill patients;
(2) class II evidence – the instrument has been validated according to data provided by one or more adequately designed and controlled clinical studies, but not in critically ill patients; and
(3) class III evidence – the instrument has not been validated or has only been validated on the basis of experts' opinions, studies with historical controls, or case reports.
The references have been qualified according to the classification described above.
Rational scheme for adjusting doses of sedatives
Sedation level should be assessed on a continuous basis for each instrument. Three levels of sedation can be considered: adequate – sedation is measured accurately within the desired range; insufficient – the measured level of sedation is lower than the actual level of sedation, within the desired range; and excessive – the measured level of sedation is higher than the actual level of sedation, within the desired range. It is critical that the level of sedation is measured accurately, because if the measured level is insufficient or excessive, then the intravenous infusion will be increased or decreased (Fig. 1).
Figure 1.

Rational scheme for adjusting doses of sedatives
Adequacy of sedation in ICU settings: individualized level of sedation
The vast majority of experts agree that the adequate level of sedation level is different for each patient, according to their different clinical circumstances (severity of the respiratory insufficiency, haemodynamic state, etc) [4,5]. In addition, the different definitions of the ideal levels of sedation are only the opinions of investigators, and are not necessarily based on experimental evidence. For this reason, these subjective concepts are not applicable to the general population of critical care patients. Nevertheless, the concept that must be emphasized is the need for sedation to be applied on an individualized basis, under the clinical criteria of intensivists, because any definition is valid for all the sedation needs that our patients require in daily practise.
There are recent contributions to this topic. The most remarkable is the study of Kress et al [6]. Those investigators suggested that the interruption of sedation can in some patients reduce the necessary time of mechanical ventilation. Also, in the methodology of this work is that it is the intensivist who must decide which patients can be awakened. Each physician also decides the adequate level of sedation for each patient included in the study. I agree with the author in considering that sedation must be adjusted in an individualized way. This approach is currently the best way to administer sedation.
Quality of sedation
Undersedation and oversedation cause several problems. Undersedation usually produces sudden changes in the level of consciousness as a consequence of stress. These changes result in inadequate ventilation, hypertension, tachycardia and discomfort, all of which have adverse consequences for the outcome of ICU patients [7]. Oversedation often occurs as a result of accumulation of sedative and analgesic agents, and it can be associated with prolongation of mechanical ventilation and weaning [8].
To avoid these problems a reliable definition of quality of sedation is necessary. This concept should be defined as the percentage of hours in which a patient maintains an adequate or desired level of sedation according to the assessment method used [9]:
Quality of sedation = adequate sedation hours/ total hours of sedation × 100
Thus, quality of sedation is expressed as a percentage. A reasonable goal is to achieve a quality of sedation greater than 85% [9]. To have a method to measure the quality of sedation is as important as choosing the best instrument.
Practical recommendations
Assessments systems for sedation
The methods that are suitable for assessing the depth of sedation can be considered under two headings – objective or subjective assessment – depending on whether the techniques require the application of an index that is derived from a quantifiable physiological variable (measurement system) or of a scoring system, respectively [5].
Objective methods: sedation measuring systems
The development of methods for objective measurement of sedation has paralleled the development of methods for assessment of the depth of surgical anaesthesia. In addition, the available equipment has been developed from technology used in anaesthesia. Unfortunately, none of these measures has been validated for clinical use in the ICU [11]. Most of them are in the process of development and validation. However, they may be used as alternatives in sedated patients for whom sedation scores cannot be applied. Table 2 illustrates the characteristics and rating classifications of these methods.
Table 2.
Characteristics of measurement systems
| Clinical utility | |||
| Measuring system | Advantage and limitations | (rating points 0-50) | Reference |
| Plasma drug concentration | Lack of agreement with level of sedation | 5 | [10] |
| Frontalis electromyography | Interindividual variation | 10 | [11] |
| Lower oesophageal contractility | Low sensitivity | 10 | [12] |
| Continuous electroencephalography | Difficult interpretation | 15 | [13] |
| Interagent variation | |||
| Cerebral function monitor | Complex and difficult to interpret | 20 | [14] |
| Cerebral function analyzing monitor | Complex and difficult to interpret | 25 | [15] |
| Power spectral analysis | Not available for clinical use | 5 | [16] |
| AEPs | Limited reliability in light sedation | 35 | [17] |
| Adequate for research |
Plasma drug concentration
Although monitoring of therapeutic drug concentrations is useful for antibiotics, anticonvulsants and antiarrythmics, there is no correlation between plasma concentration and the effect of a drug at its site of action. In contrast to postoperative patients, critically ill patients often have continuously varying degrees of organic dysfunction that lead to alterations in their responses to drugs (pharmacodynamics) and in their ability to eliminate drugs by biotransformation and excretion (pharmacokinetics). Sample processing delays before results are available result in an inadequate reference on which to base adjustment of sedation. A further limitation pertains to interindividual variation in the sedative effects of some agents, such as benzodiazepines. High plasma concentrations of midazolam have been associated with an increase in agitation [12]. Consequently, this method cannot be recommended for monitoring sedation in daily practice.
Frontalis electromyogram
The frontalis muscle contracts in response to stress, and this contraction can be recorded even in the presence of neuromuscular blocking agents. This method was used by Edmonds et al [13] to monitor anaesthesia. Unfortunately, this technique is not sufficiently sensitive for the purpose of monitoring the level of sedation in ICU patients.
Lower oesophageal contractility
Evans et al [14] observed a correlation of peristaltic contractility with depth of anaesthesia. Spontaneous lower oesophageal contractility represents a response to stress. However, this response is subject to considerable interpatient variation, and it may also be influenced by atropine.
Continuous electroencephalography
This method was proposed by Peter [15] 50 years ago for monitoring of opiate intravenous anaesthesia. It provides a record of cortical activity, measured from a series of scalp electrodes, against time. Decreases in the level of consciousness result in changes in electroencephalography readings. However, different sedatives alter the readings in different ways, making interpretation very difficult. In addition, electroencephalography does not correlate with sedation score or plasma drug concentration [11].
In centres that have experience in collecting electroencephalography records, this information can be useful in interpreting neurological function in patients after head injury or in evaluating coma versus brain death. However, continuous electroencephalography cannot be recommended for routine monitoring of ICU sedation.
Cerebral function monitor
The cerebral function monitor was described by Dubois et al [16] for the purpose of monitoring the level of general anaesthesia. It is a device that processes electroencephalography readings, but remains in time domain. The electroencephalography is filtered to minimize the impact of low frequency activity and is rectified to eliminate its biphasic value. The electrical activity is recorded from one channel, and is displayed as a low-speed paper trace.
The adequacy of the cerebral function monitor has not been confirmed in critically ill patients because it is drug dependent [13]. For this reason, the cerebral function monitor cannot be recommended for clinical use.
Cerebral function analyzing monitor
This system uses two electroencephalography channels. It was used by Sebel et al [17] to monitor the depth of anaesthesia. It produces information that is easy to interpret and that varies with depth of sedation. It is not as yet available for daily practice. Further studies are necessary to validate the use of this device in ICU settings.
Power spectral analysis
This technique employs an alternative analysis of the electroencephalography signal. The signal is digitized at fixed time, analyzed and subjected to power-spectrum calculation. Vesalis et al [18] described a correlation between spectral edge frequency and sedation with midazolam in ICU patients. Recently, the median frequency has been used to regulate a closed loop of total intravenous anaesthesia. This finding has not been confirmed in patients with more severe organic dysfunction [18]. The utility of this technique in critically ill patients remain unknown.
Sensory evoked potentials
Evoked potentials (EPs) are electrophysiologic responses of the nervous system to sensory stimulation. A computer is used to average individual time-locked responses to repeated stimuli. EPs are divided into three classes on the basis of latency. Long-latency EPs (hundreds of milliseconds) are suppressed under surgical anaesthesia, and are not useful for monitoring of sedation. Medium-latency EPs (tens of milliseconds) are often recordable under anaesthesia, and can be affected by anaesthetic state. Short-latency EPs (milliseconds), which are predominantly generated at subcortical levels, are sufficiently strong to be recorded under sedation.
The auditory evoked potentials (AEPs) are the simplest EPs to produce. Auditory stimulation is accomplished with filtered clicks delivered by headphones. EPs are recorded from scalp electrodes in response to a standard noise.
There is clinical evidence regarding the clinical utility of AEPs for monitoring of sedation. A recent study [19] correlated this method with the results from five different scoring systems. Further studies are required to validate the use of AEPs in monitoring of sedation. AEPs can only be recommended for research in patients with deep levels of sedation, because light levels of sedation resulted in AEPs that are poorly correlated with scoring system results (Fig. 2).
Figure 2.

Correlation between AEPs (low latency Nb) and scoring system (more than 10 points corresponds to deep sedation).
The clinical utilities of other types of evoked response (motor or visual) have not been demonstrated.
Subjective methods: scoring systems
The only instruments that have demonstrated usefulness in the critical care setting are the scoring systems that are widely known as scales of sedation [18]. These instruments are based on clinical observation. The scores are recorded according to direct evaluation by an observer. Each scale should be validated according to the training and characteristics of the professionals that use it.
Interobserver agreement (calculated as weighted κ index) is the most important feature in assessing scales of sedation. It is also desirable that other characteristics are included in the assessment, such as simplicity, reliability, accuracy and minimal additional discomfort to the patient [20].
There is no ideal scoring system. More than 30 clinical scales have been described in the medical literature. However, only two of these instruments have been adequately validated for use in the ICU: the Ramsay scale and the GCSC. Because there is no goal standard against which to validate the sedation scales, researchers have targetted their investigations at determining the `clinimetric' properties of these scales. De Jonghe et al [21], in a recent systematic review, observed the high reliability and satisfactory correlation with other scales achieved by the Ramsay scale and Comfort scale. The only limitation observed was the lack of studies that validated these scale in detecting changes in the sedation status over time.
Ramsay scale
This scoring system was described by Ramsay et al [22] in 1974 for the purpose of monitoring sedation with alphaxolone/alphadolone. It continues to be the most widely used scale for monitoring sedation in daily practice, as well as in clinical research.
This instrument identifies situations of agitation or sleep visually (Table 3). Some experts consider that it is more a scale of consciousness than a tool for measurement of sedation [23]. Those authors have also suggested that the sedation levels identified using this scale are not clearly defined or fully conclusive. Consequently, they consider that this scale is excessively subjective and that it has poor validity.
Table 3.
Ramsay scale
| Level | Characteristics |
| 1 | Patient awake, anxious, agitated, or restless |
| 2 | Patient awake, cooperative, orientated and tranquil |
| 3 | Patient drowsy, with response to commands |
| 4 | Patient asleep, brisk response to glabella tap or loud auditory |
| stimulus | |
| 5 | Patient asleep, sluggish response to stimulus |
| 6 | Patient has no response to firm nail-bed pressure or other |
| noxious stimuli |
Contrary to these opinions, in our experience this method has good reliability, with good interobserver agreement (Cohen κ index 0.79; P <0.0001) [24]. This qualifies this scale as sufficiently reproducible for clinical practice.
Glasgow Coma Scale modified by Cook and Palma
Described by Cook in 1987 [25], this instrument provides a score of the reactivity of the patient under mechanical ventilation according to response to external stimuli (Table 4). Opening of the eyes is considered to be indicative of higher functioning, and motor response is evaluated on the basis of somatic stimulation. It causes minimal additional discomfort to the patient.
Table 4.
Glasgow Coma Scale modified by Cook and Palma
| Characteristic | Score |
| Eyes open | |
| Spontaneously | 4 |
| In response to speech | 3 |
| In response to pain | 2 |
| None | 1 |
| Response to nursing procedures | |
| Obeys commands | 5 |
| Purposeful movements | 4 |
| Nonpurposeful flexion | 3 |
| Nonpurposeful extension | 2 |
| None | 1 |
| Cough | |
| Spontaneous strong | 4 |
| Spontaneous weak | 3 |
| On suction only | 2 |
| None | 1 |
| Respiration | |
| Obeys commands | 5 |
| Spontaneous intubated | 4 |
| Spontaneous intermittent mandatory ventilator triggering | 3 |
| Respiration against ventilator | 2 |
| No respiratory efforts | 1 |
The above cited study [24] confirmed the reproducibility of this scale, with good agreement between observers (Cohen κ index 0.94; P <0.0001) which validated it for practice as well as for clinical research [13]. Fig. 3 illustrates these findings.
Figure 3.
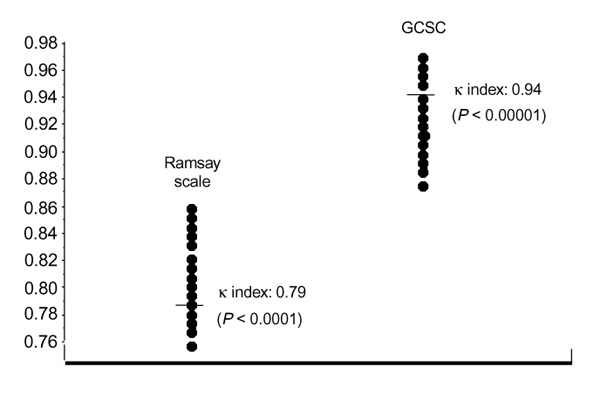
Interobserver agreement in two sedation scales with two observers.
Bion scale
In 1988, Bion [26] designed a three-dimensional linear analogue scale that combines evaluation of depth of sedation and degree of distress (a level of comprehension of consciousness). Although this system provides useful information, it is too complex for use in daily practice.
Comfort scale
1994, Max et al [27] reported experience with a new designated scale in 85 mechanically ventilated children. Those authors consider this scale to be sufficiently reliable, despite the fact that discrepancies between observers were observed in 38% of the measurements. The characteristics of this method mean that it has little application in adults.
Sedation-agitation scale
This scoring system was described by Riker et al [28] for the purpose of controlling treatment with haloperidol in agitated ICU patients. It achieved good inter-rater correlation (Cohen κ index 0.92), but it does not evaluate the relation between patient and ventilator. However, it is sufficiently reproducible for use in clinical practice.
Motor activity assessment scale
This scale was described by Clemmer, and has recently been evaluated in surgical ICU patients by Devlin et al [29]. The reliability observed in this study was good (Cohen κ index 0.83), but this agreement should be confirmed in critically ill patients.
Rational selection of sedation scales
All validated scales can be used in clinical practice. However, for research purposes the GCSC [13] is preferable. Table 5 summarizes the characteristics and rating classification of sedation scales.
Table 5.
Characteristics of scoring systems
Practical recommendations to guide sedative therapy according to ventilatory mode
The following recommendations are guidelines for specialists. There is, to my knowledge, only one published article regarding this topic [30]. For this reason, the following recommendations are based on my experience. Obviously, the selection of treatment must be made according to clinical judgement, adapting it to the clinical circumstances of each patient. Table 6 summarize these recommendations.
Table 6.
Recommendations to guide sedative therapy according to ventilatory mode
| Ventilatory mode | Therapeutic goal | Therapeutic goal |
| (Ramsay scale level) | (GCSC points) | |
| Assisted CMV (<24 h) | 3 | 8-12 |
| Assisted CMV (24-72 h) | 3 | 8-12 |
| Assisted CMV (>72 h) | 3 | 8-12 |
| Pressure controlled ventilation | 4-5 | 15-18 |
| Synchronous intermittent | 2 | 13-15 |
| mandatory ventilation | ||
| Pressure support ventilation | 2 | 13-15 |
| Continuous positive airway | 2 | 13-15 |
| pressure |
Assisted-controlled mechanical ventilation
The traditional controlled mechanical ventilation (CMV) mode does not permit synchronization between spontaneous breaths of the patient and the cycle of the respirator. For this reason, modern ventilators have substituted CMV with assisted CMV. The assisted CMV mode is more flexible and permits spontaneous patient breathing at some points in the cycle. This means that it is not necessary for sedation to suppress totally the spontaneous breathing of patient. Assisted CMV is the recommended mode for initiating mechanical ventilation, because it assures a specific minute volume even in the absence or weakness of respiratory stimuli. Generally, this mode achieves good synchronization between the ventilator cycle and the effort of the patient.
In order to formulate the sedation regimen, two fundamental factors must be considered. First, the degree of patient resistance must be evaluated. At present, we think that the best approach is to maintain a light and comfortable level of sedation, if it is reasonably possible, in order to decrease the incidence of adverse effects of high doses of sedatives. The ideal sedation depth for most of these patients is mean sedation (level 3 of the Ramsay scale or between 8 and 12 points of the GCSC).
The second factor is the anticipated duration of mechanical ventilation. If it is anticipated that sedation of less than 24 h (short-term sedation) will be required, then both propofol or midazolam should be selected. The initial dose of propofol should be 1 mg/kg, preceded by 1 mg/kg as a bolus. Adjustments to the dose should be made in 10-20% increments or decrements. Midazolam should be administered at an initial dose of 0.1 mg/kg, preceded by 0.05 mg/kg as a bolus. Adjustments to the dose should also be made in 10-20% increments or decrements. If the anticipated duration of sedation is between 24 and 72 h (medium-term sedation) then propofol is preferable to midazolam because of the unnecessary accumulation and extended sedation that can occur with this drug. Nevertheless, if midazolam is used, then the dose should be titrated 12-24 h before sedation is due to be discontinued. If it is expected that sedation will need to be sustained for longer than 3 days (long-term sedation), then midazolam is preferable. Two days before discontinuation of sedation, midazolam should be substituted with propofol to facilitate elimination of the benzodiazepines before the patient awakes.
Complementary analgesia will also be necessary in most cases, because none of the available sedatives have analgesic properties. In my opinion, analgesics should always be administered if communication with the patient is not possible. The initial plan should be to administer morphine as a continuous infusion (0.5-1.5 mg/kg per h), with a supplementary bolus of 3-5 mg if indicated. Alternatively, fentanyl may be used at equivalent doses.
Pressure controlled ventilation
Some authors have postulated that this ventilatory mode can reduce the incidence of barotrauma, decreasing airway pressure and alveolar overdistension. This mode has been used in refractory hypoxaemia, and it has been associated with other ventilatory techniques as the inversion of the inspiration-expiration relationship or high frequency ventilation. There are practical limitations to the use of this mode because it can cause a degree of patient-ventilator asynchrony, so most of the patients need deep sedation (level 4-5 of the Ramsay scale or 8-12 points on the GCSC). The recommended sedation regimen is the same as that for assisted CMV (see above).
Synchronized intermittent mandatory ventilation
This mode is a partial support system that requires preservation of spontaneous breathing. It requires a degree of cooperation from the patient. It is commonly used without continuous sedation in patients who are being weaned from mechanical ventilation.
Some patients ventilated in this manner require sedation because of agitation as a result of nonrespiratory cause. If it is necessary to administer sedatives, the patient should be carefully monitored; if there is confusional symptomatology, then a combined regimen of a sedative at a low dose and a neuroleptic should be administered. The therapeutic goal is light sedation (level 2 of the Ramsay scale or 13-15 points on the GCSC). For this purpose, propofol is indicated at anxiolytic doses (0.5 mg/kg per h, initially). Alternatively, midazolam can be used at a low dose (0.05 mg/kg per h), provided that the treatment is not extended beyond than 24 h. This combination achieves excellent results in patients under assisted ventilation, and it has little effect on respiratory stimuli [16]. If the patient is in a confused state, then haloperidol may be administered in a continuous infusion at a dose of 1-2 mg/h, with an initial bolus of 5 mg.
For patients who experience disturbance in nocturnal sleep, overnight sedation under controlled ventilation is recommended, according to the plan described above [17].
The preferred analgesic regimen is a combination of a nonsteroideal anti-inflammatory drug with morphine or pethidine, administered parenterally. The recommended plan is a fixed regimen combined with an `on-demand' regimen if the patient is able to cooperate. In patients with refractory pain, morphine administration at bolus doses of 1-2.5 mg, subcutaneously or intravenously, via a patient-controlled analgesia pump can achieve excellent results.
Pressure support ventilation
This form of ventilation is also considered partial support. It was designed for use during weaning from mechanical ventilation. Pressure support ventilation requires that the patient is able to initiate breathing. If the patient is adequately ventilated, with an appropriate degree of analgesia, then sedation is not generally required. In exceptional cases, in which the patient presents with agitation resulting from a nonrespiratory cause, propofol or midazolam may be administered at anxyolitic doses as was described for synchronized intermittent mandatory ventilation (light sedation; level 2 of the Ramsay scale or 13-15 points on the GCSC) under close respiratory monitoring.
Continuous positive airway pressure
This mode of partial respiratory support is widely used because of technical considerations, and in order to avoid total respiratory substitution with controlled ventilation. It also requires that the patient is able to initiate breathing. Therefore, the recommendations are similar to those for pressure support ventilation, described above.
Future strategies for selected patients
New evidence suggests that daily interruption of sedation in selected patients may improve their situation by allowing clinicians to optimize the administration of sedatives while ensuring optimal comfort for the patient [6]. A word of caution is necessary regarding these findings, because this approach is appropriate only for certain, stable patients. Further research is necessary to confirm these findings in the general population of ICU patients. However, these promising strategies might be an exciting prospect for the future.
Instruments to monitor neuromuscular blockade
The above-described scoring systems are not useful for monitoring sedation in patients treated with muscular blocking agents. Because these agents have a very low margin of safety, they should be reserved for extreme situations in which the potential benefit to the patient justifies their use. In such circumstances, we recommend discontinuing their administration every 24 or 48 h in order to perform an accurate assessment of neuromuscular function. If this is not possible, an alternative can be to monitor neuromuscular function using a nerve stimulator [31]. The purpose of this device is to provide sufficient stimuli to produce action potentials in all fibres of a nerve. The simplest way to stimulate nerves is to apply single stimuli at intervals no shorter than 10 s. Neuromuscular monitoring offers the advantage of rapid response and objective data.
Conclusion
The present review describes and compares the available instruments for monitoring ICU sedation, from a practical and evidence-based point of view. Although many promising objective methods (measuring systems) are available, there are no truly validated instruments for use in clinical practice. AEPs can be used only for research in patients with deep levels of sedation. Other measuring systems require further development and validation before they can be considered useful in ICU. Continuing research will provide an objective system for improving the monitoring and controlling of this essential treatment for ICU patients.
Subjective methods (scoring systems) that are based on clinical observations have proven utility in guiding sedative therapy. The GCSC achieves good face validity and reliability, which assures its clinical utility for routine practice and research. Other scales, in particular the Ramsay scale, can be recommended for clinical use.
A conclusion that arises from the present review is that the appropriate use of instruments to monitor sedation can improve the sedative treatment that we deliver to our patients. It is anticipated that future contributions to this forgotten field will result in improvements in the quality of care that our patients need and deserve.
References
- Carrasco G, Cabré Ll. Sedation in intensive medicine [in Spanish]. Farmacia Hospitalaria. 1994;19:59–64. [Google Scholar]
- Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23:1596–1600. doi: 10.1097/00003246-199509000-00021. [DOI] [PubMed] [Google Scholar]
- Carrasco G, Cabré L, Sobrepere G, et al. Synergistic sedation with propofol and midazolam in intensive care patients after coronary artery bypass grafting. Crit Care Med. 1998;26:844–851. doi: 10.1097/00003246-199805000-00015. [DOI] [PubMed] [Google Scholar]
- O'Sullivan GF, Park GR. The assessment of sedation in critically ill patients. Clin Intensive Care. 1991;1:116–122. [Google Scholar]
- Hole A. Monitoring sedation in the ICU patient. Clin Intensive Care. 1993;(Suppl 2):27–29. [Google Scholar]
- Kress JP, Pohlman RN, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- Fowler SB, Hertzog J, Wagner BK. Pharmacological interventions for agitation in head injured patients in the acute care setting. J Neurosci Nursing. 1995;27:119–123. doi: 10.1097/01376517-199504000-00011. [DOI] [PubMed] [Google Scholar]
- Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- Costa J, Cabré L, Molina R, Carrasco G. Cost of ICU sedation: comparison of empirical and controlled sedation methods. Clin Intensive Care. 1994;5:17–21. [PubMed] [Google Scholar]
- Cruspinera A, Gimeno G, Figueras MJ, et al. Nurses control of patients in critically ill patients [abstract; in Spanish]. . Med Intensiva. 1993;17:N7. [Google Scholar]
- Bion JF, Ledingham IMcA. Sedation in intensive care–a postal survey. Intensive Care Med. 1987;13:215–216. [PubMed] [Google Scholar]
- Shelly MP, Mendel L, Park GR. Failure of critically ill patients to metabolise midazolam. Anaesthesia. 1987;42:619–626. doi: 10.1111/j.1365-2044.1987.tb03086.x. [DOI] [PubMed] [Google Scholar]
- Edmonds HL, Paloheimo M. Computerised monitoring of the EMG and EEG during anaesthesia: an evaluation of the anaesthesia and brain function motor. Intensive Clin Monit Comput. 1985;1:201–210. doi: 10.1007/BF01720184. [DOI] [PubMed] [Google Scholar]
- Evans JM, Bithell JF, Vlachonikolic IG. Relationship between lower oesophageal contractility, clinical signs and surgery in man. Br J Anesth. 1987;59:1346–1355. doi: 10.1093/bja/59.11.1346. [DOI] [PubMed] [Google Scholar]
- Peter S. Effects of high dose fentanyl anaesthesia on the electroencephalogram. Anesthesiology. 1959;20:359–376. [Google Scholar]
- Dubois M, Savege TM, O'Carroll TM, Frank M. General anaesthesia and changes on the cerebral function monitor. Anaesthesia. 1978;33:157–164. doi: 10.1111/j.1365-2044.1978.tb08343.x. [DOI] [PubMed] [Google Scholar]
- Sebel PS, Maynard DE, Major E, Frank M. The cerebral function analysing monitor (CFAM). Br J Anaesth. 1983;55:1265–1270. doi: 10.1093/bja/55.12.1265. [DOI] [PubMed] [Google Scholar]
- Vesalis RA, carlon GC, Bedford SF. Spectral edge frequency correlates with sedation level in ICU patients receiving i.v. midazolam [abstract]. Anesthesiology. 1989;71:A156. [Google Scholar]
- Schulte-Tamburen AM, Scheier J, Briegel J, Scwender D, Peter K. Comparison of five sedation scoring systems by means of auditory evoked potentials. Intensive Care Med. 1999;25:377–382. doi: 10.1007/s001340050861. [DOI] [PubMed] [Google Scholar]
- Carrasco G, Molina R, Costa J, Soler JM, Paniagua J, Cobra Ll. Usefulness of sedation scales in ICU. A comparative-randomised study in patients sedated with propofol, midazolam or opiates plus benzodiazepines [abstract]. Intensive Care Med. 1992;18 (Suppl 2):57. [Google Scholar]
- De Jonghe B, Cook D, Appere-De-Vecchi C, Meade M, Outin H. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26:275–285. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxolone/alphadolone. Br Med J. 1974;ii:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Flaschen J, Cowen J, Polomano RC. Beyond the Ramsay scale: need for a validated measure of sedating drug efficacy in the intensive care unit. Crit Care Med. 1994;22:732–733. [PubMed] [Google Scholar]
- Carrasco G, Cabré L, Serra J. What can you associate with opiates for sedation and resuscitation [in French]. Rean Urg. 1995;4 (Suppl):33–38. [Google Scholar]
- Cook S, Palma O. Propofol as a sole agent for prolonged infusion in intensive care. J Drug Dev. 1989;(Suppl 2):65–67. [Google Scholar]
- Bion JF. Sedation and analgesia in the intensive care unit. . Hospital Update. 1988;14:1272–1286. [Google Scholar]
- Max CM, Smith PG, Lowie LH, et al. Optimal sedation of mechanically ventilated pediatrical patients. Crit Care Med. 1994;22:163–170. doi: 10.1097/00003246-199401000-00029. [DOI] [PubMed] [Google Scholar]
- Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- Devlin JW, Boleski G, Mlinarek M, et al. Motor activity assessment scale: a valid and reliable sedation scale for use with mechanically ventilated patients in adult surgical intensive care unit. . Crit Care Med. 1997;27:1271–1275. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Carrasco G, Cabré L. Control of sedation in patients with acute respiratory distress syndrome. Acute Respiratory Distress Syndrome [in French]. Edited by Mancebo J, Blanch L. Paris: Elsevier; 1999:68–81. [Google Scholar]
- Wheeler A. Sedation, analgesia, and paralyisis in the intensive care unit. Chest. 1993;104:566–577. doi: 10.1378/chest.104.2.566. [DOI] [PubMed] [Google Scholar]


