Abstract
Recent Vibrio parahaemolyticus outbreaks associated with consumption of raw shellfish in the United States focused attention on the occurrence of this organism in shellfish. From March 1999 through September 2000, paired oyster samples were collected biweekly from two shellfish-growing areas in Mobile Bay, Ala. The presence and densities of V. parahaemolyticus were determined by using DNA probes targeting the thermolabile hemolysin (tlh) and thermostable direct hemolysin (tdh) genes for confirmation of total and pathogenic V. parahaemolyticus, respectively. V. parahaemolyticus was detected in all samples with densities ranging from <10 to 12,000 g−1. Higher V. parahaemolyticus densities were associated with higher water temperatures. Pathogenic strains were detected in 34 (21.8%) of 156 samples by direct plating or enrichment. Forty-six of 6,018 and 31 of 6,992 V. parahaemolyticus isolates from enrichments and direct plates, respectively, hybridized with the tdh probe. There was an apparent inverse relationship between water temperature and the prevalence of pathogenic strains. Pathogenic strains were of diverse serotypes, and 97% produced urease and possessed a tdh-related hemolysin (trh) gene. The O3:K6 serotype associated with pandemic spread and recent outbreaks in the United States was not detected. The efficient screening of numerous isolates by colony lift and DNA probe procedures may account for the higher prevalence of samples with tdh+ V. parahaemolyticus than previously reported.
Vibrio parahaemolyticus is a gram-negative, halophilic bacterium that occurs naturally in estuarine environments worldwide (21). Its densities in the environment and seafoods vary greatly by season, location, sample type, fecal pollution, and analytical methodology (5, 8-10, 14, 21, 22, 36). Pathogenic V. parahaemolyticus generally produces a thermostable direct hemolysin (TDH), the product of the tdh gene (20). More than 90% of clinical V. parahaemolyticus isolates but fewer than 1% of food or environmental strains produce TDH or possess tdh (9, 10, 14, 22, 26, 31, 35). The frequency of TDH or tdh detection in environmental samples and seafoods ranges from 0 to 6% (5, 14, 22, 26, 31, 34). Quantitative data for tdh+ V. parahaemolyticus have been reported in only one study and are based on one or two isolates from each of four oyster samples (10).
A series of oyster-associated outbreaks of V. parahaemolyticus (3, 4, 6, 7) in 1997 (Washington State) and 1998 (Texas, New York and Connecticut, and Washington State) prompted the development of nonradioactive DNA probes (28, 29) and direct-plating methods for rapid and efficient determination of the abundance of total and pathogenic V. parahaemolyticus in oysters. The Food and Drug Administration (FDA) initiated a surveillance program using these new methods in Alabama in March 1999. The objective of this study was to correlate quantitative data on the abundance of total and pathogenic V. parahaemolyticus with each other and with environmental parameters such as water temperature and salinity. Direct-plating methods were used to collect quantitative data for total and tdh+ V. parahaemolyticus. Additionally, the prevalence of total and tdh+ V. parahaemolyticus was determined as described in the FDA Bacteriological Analytical Manual (15), especially when levels were below the detection sensitivity of direct-plating methods. Pathogenic strains were serotyped to determine the prevalence of specific strains and the potential presence of outbreak strains.
MATERIALS AND METHODS
Sample collection.
Oysters were collected biweekly with hand tongs from a small boat at two sites near Mobile Bay (Fig. 1) from March 1999 through September 2000. One site was on Cedar Point Reef (lower salinity) in the Mississippi Sound, and the other was in Dauphin Island Bay (higher salinity). Both sites are in National Shellfish Sanitation Program conditionally approved shellfish-growing areas with commercial harvesting. Oysters (25 from each site) were placed on top of bagged ice in insulated chests, transported to the FDA Gulf Coast Seafood Laboratory, and analyzed within 3 h of collection. Bottom (1 to 2 m) water temperature and salinity were determined with a model 85 salinometer (YSI, Yellow Springs, Ohio).
FIG. 1.
Harvest sites near Mobile Bay.
Enrichment and isolation of V. parahaemolyticus.
The formulation of the bacteriological media used, unless listed, was that in the FDA Bacteriological Analytical Manual (11), and the source was Difco Laboratories, Sparks, Md., unless stated otherwise. Paired samples of oysters (10 to 12 oysters/sample) from each site were scrubbed, shucked, diluted 1:1 with alkaline peptone water (APW), and blended for 90 s in a Waring blender (Waring, Hartford, Conn.). A 50-g portion of the APW-oyster homogenate was placed into a 1-liter Erlenmeyer flask containing 200 ml of APW (1:10) and incubated overnight at 35°C. Four thiosulfate-citrate-bile salts-sucrose (TCBS) agar plates were streaked from each flask of APW-oyster enrichment for V. parahaemolyticus isolation (one 1.0-μl loopful was taken from the surface pellicle, one was taken from the bottom, and two were taken after the flask was swirled). TCBS plates were incubated overnight at 35°C; up to 48 typical green (sucrose-negative) colonies were transferred with sterile toothpicks to either the first six or the last six columns of a 96-well plate preloaded with 100 μl of APW. The 96-well plates were incubated at 35°C on a rocker for 4 to 6 h. Growth was transferred to duplicate Vibrio vulnificus agar (VVA) plates (37) with a 48-prong replicator (Bokel Industries, Feasterville, Pa.). The VVA plates were incubated overnight at 35°C.
Direct plating for V. parahaemolyticus.
T1N3 agar (1% Tryptone, 3% NaCl, 2% agar) plates were spread plated in triplicate with 0.1-g (0.2 g of 1:1 APW-oyster homogenate) and 0.01-g (100 μl of 1:10 APW-oyster homogenate from the flask described above) portions of homogenized oyster tissue. T1N3 spread plates were incubated overnight at 35°C; colony lifts for DNA probes were prepared as described below.
Colony hybridization for total and pathogenic V. parahaemolyticus.
Colony lifts were prepared by placing Whatman 541 filters (8.5-cm diameter) onto appropriate VVA (enrichment) and T1N3 (direct plating) plates and applying gentle pressure with a plastic spreading rod. Colonies were tested for the presence of the thermolabile hemolysin (tlh) gene and the thermostable direct hemolysin (tdh) gene to verify the identity of the V. parahaemolyticus species and its pathogenicity, respectively (28, 29). Positive and negative controls spotted onto a separate strip of Whatman 541 filter paper were placed into each bag of five filters and included V. parahaemolyticus strains TX-2103, SPRC-10290, and GCSL-DI-B9 (tlh+ tdh+); Dal-1094 (tlh+ tdh); and V. vulnificus MO6-24 and V. hollisae 8393 (tlh tdh). Alkaline phosphatase-labeled DNA probes for the tlh and tdh genes were obtained from DNA Technology (Aarhus, Denmark); the probe sequences and hybridization conditions were as previously described (28, 29).
Strain characterization.
Isolates hybridizing with both the tlh and tdh DNA probes were recovered and purified on T1N3 agar. Isolates were identified as V. parahaemolyticus with API 20E diagnostic strips (bioMérieux, Hazelwood, N.J.). In addition to API 20E testing, urease production was also determined with Christensen's urea agar (17). Both DNA probes (28, 29) and a PCR method (2) were used to test for the presence of tdh, while the presence of trh and the thermolabile direct hemolysin gene (tlh), which is species specific, was determined by multiplex PCR (2). Test strains were grown, and their O:K serovars were determined by using specific antisera (Denka, Seiken Corp., Tokyo, Japan) as previously described (15).
Statistical analysis.
Total V. parahaemolyticus counts were analyzed by regression of the mean log10 density of replicate samples against environmental factors (water temperature, salinity, and harvest site). The effect of temperature was further summarized by rank correlation and the use of a smoothing technique (moving average) in which V. parahaemolyticus counts corresponding to temperatures within a range of 5°C were pooled to estimate geometric mean counts at the midpoint of successive intervals. Nondetectable counts (<10 CFU/g) of total V. parahaemolyticus were replaced by half of the limit of detection (5 CFU/g). Environmental effects on tdh+ V. parahaemolyticus counts were investigated by generalized linear regression. Counts of tdh+ V. parahaemolyticus were not log10 transformed because of the high prevalence of nondetectable samples; they were modeled as either Poisson or binomially distributed, with P values adjusted for overdispersion by the method of scaling predicted variance by the deviance (30). Differences between the frequencies of detection of tdh+ V. parahaemolyticus by direct plating versus enrichment were evaluated by McNemar's test, and differences in the frequency of detection of pathogenic V. parahaemolyticus at different sites were evaluated by Fisher's exact test. All statistical analyses were performed with the Statistical Analysis System (SAS Institute, Cary, N.C.).
RESULTS
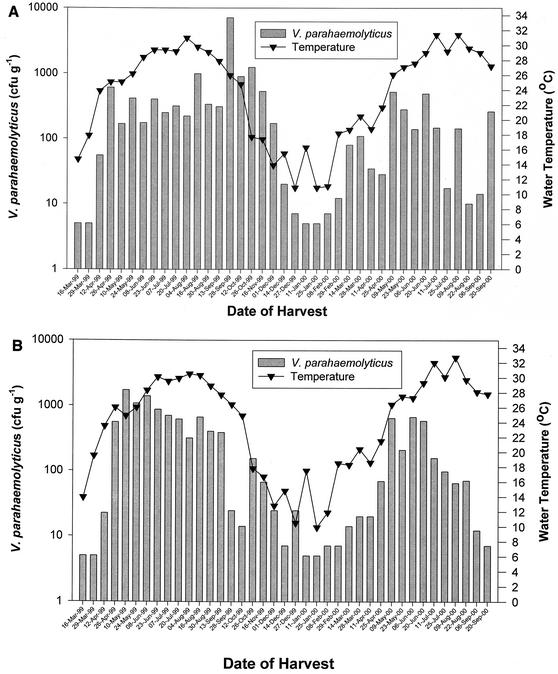
Similar seasonal trends in total V. parahaemolyticus abundance at the two harvest sites are demonstrated in Fig. 2A (Cedar Point) and B (Dauphin Island Bay). In 1999, V. parahaemolyticus densities in oysters usually ranged from 100 to 1,000 CFU g−1 from April through November at Cedar Point and April to mid-September at Dauphin Island Bay. From December through March, V. parahaemolyticus densities were generally <100 CFU g−1. In 2000, only samples collected in May and June consistently exceeded 100 CFU g−1 at either location. Samples collected in March 1999 and January 2000 were below the level of detection (10 CFU g−1) by the direct-plating procedure, but V. parahaemolyticus was detected in all samples with the 25-g enrichments. Only one sample, collected from Cedar Point on 28 September 1999, exceeded 10,000 CFU g−1. Except for three occasions (12 April 1999, 14 December 1999, and 20 September 2000), duplicate samples from the same site did not vary by more than 1 log.
FIG. 2.
Density of V. parahaemolyticus in oysters from Cedar Point Reef (A) and Dauphin Island Bay Reef (B), Ala. Each bar represents the geometric mean of two samples. Bars at 5 CFU g−1 indicate that samples were taken but V. parahaemolyticus was not detected in either sample (count, <10 CFU g−1).
Water temperatures from May through September in 1999 and 2000 were similar and exceeded 25°C. Mean salinities at Cedar Point and Dauphin Island Bay from May through September were approximately 6 ppt higher in 2000 (23.6 and 27.5, respectively) than in 1999 (17.6 and 20.7, respectively). In March 2000, temperatures were 1 to 5°C higher than in 1999 and V. parahaemolyticus levels increased earlier in 2000 than in 1999.
Water temperature and salinity were significantly associated (P < 0.05) with log10 total V. parahaemolyticus counts. The abundance of total V. parahaemolyticus in Alabama oysters was seasonal and correlated well (r = 0.51, P < 0.0001) with the water temperature. The effect of the harvest site on total V. parahaemolyticus densities was not significant. The best estimate of the temperature effect was a 1.2-fold increase in the number of V. parahaemolyticus CFU per gram per degree Celsius. The effect of salinity was quadratic, with a predicted optimum of 17 ppt. The linear regression model that best fits the data is log10(V. parahaemolyticus/g) = −1.53 + 0.082 × temperature + 0.20 × salinity − 0.006 × salinity2.
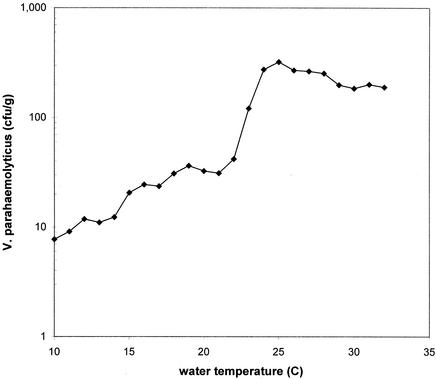
The model has an R2 of 0.42, indicating that 42% of the observed variation in log10 total V. parahaemolyticus density was attributable to differences in water temperature and salinity. The relationship of V. parahaemolyticus densities in oysters versus water temperature from Cedar Point Reef and Dauphin Island Bay Reef, Ala., is shown in Fig. 3. The mean density rose rapidly as the temperature increased above 20°C, reaching an apparent plateau at temperatures above 25°C.
FIG. 3.
Relationship between water temperature and V. parahaemolyticus density in oysters from Cedar Point Reef and Dauphin Island Bay Reef, Ala.
Pathogenic strains (i.e., tdh+) were detected at both sites by the direct-plating and enrichment methods. The frequencies of isolation of pathogenic V. parahaemolyticus by direct plating and enrichment are shown in Table 1. Paired samples from the same collection were both positive for tdh+ on 2 (2.6%) of 78 occasions by direct plating and on only 1 (1.3%) of 78 occasions by enrichment. The rates of detection from individual samples were higher, with 20 (12.8%) of 156 positive by direct plating and 15 (9.6%) of 156 positive after enrichment. Overall, the observed difference in the frequency of detection of the tdh gene by direct plating versus the enrichment method was not significant. Combining results from replicate samples and detection methods, pathogenic strains were detected on 20 (51.3%) of 39 sampling occasions at Dauphin Island Bay compared to 10 (25.6%) of 39 sampling occasions at Cedar Point (P < 0.05) and in 34 (21.8%) of 156 samples overall.
TABLE 1.
Number of pathogenic (tdh+) V. parahaemolyticus isolates versus temperature and the total number of V. parahaemolyticus isolates
| Temp (°C) or V. parahaemolyticus count (log10 g−1) | No. of samples | No. of tdh+ samples (direct plates) | No. of tdh+ isolates/ no. of tlh+ isolates from direct plates (%) | No. of tdh+ samples (enrichments) | No. of tdh+ isolates/ no. of tlh+ isolates from enrichments (%) |
|---|---|---|---|---|---|
| Temp | |||||
| <15 | 22 | 2 | 3/63 (4.8) | 4 | 19/858 (2.2) |
| 15-20 | 30 | 1 | 1/459 (0.22) | 3 | 17/1,134 (1.5) |
| 20-25 | 18 | 1 | 1/660 (0.15) | 2 | 2/641 (0.31) |
| 25-30 | 68 | 14 | 24/5,206 (0.46) | 6 | 8/2,699 (0.30) |
| >30 | 18 | 2 | 2/604 (0.33) | 0 | 0/686 (0.0) |
| Vibrio counts | |||||
| <1 | 28 | 5 | 8/0a | 4 | 30/1,077 (2.8) |
| 1-2 | 46 | 2 | 2/170 (1.2) | 4 | 7/1,555 (0.45) |
| 2-3 | 65 | 11 | 19/2,504 (0.76) | 4 | 5/2,646 (0.19) |
| >3 | 17 | 2 | 2/4,318 (0.05) | 3 | 4/740 (0.54) |
| Overall | 156 | 20 | 31/6,992 (0.44) | 15 | 46/6,018 (0.76) |
Ratio of tdh to tlh is indeterminate.
The number of tdh+ V. parahaemolyticus colonies was low and variable across samples collected under similar conditions; however, a higher isolation frequency was observed when water temperatures and total V. parahaemolyticus densities were low (Table 1). The results of regression analyses to estimate the magnitude of this effect were found to be sensitive to the specification of link function or model structure. The most consistent effect identified by regression analysis was sampling site. The ratio of pathogenic to total V. parahaemolyticus isolates after APW enrichment was significantly higher at Dauphin Island Bay (1.35%) than at Cedar Point (0.17%). The average tdh+ V. parahaemolyticus density determined by direct plating was 2.7 CFU g−1 at Dauphin Island Bay compared to 1.3 CFU g−1 at Cedar Point (P < 0.05).
Table 2 lists the sources and serotypes of the pathogenic V. parahaemolyticus isolates tested. All of the strains that hybridized with both the tlh and tdh DNA probes were confirmed as V. parahaemolyticus by the API 20E system. Except for a single O4:K8 isolate from an oyster sample collected at Cedar Point reef on 16 August 1999, all 39 pathogenic isolates were trh+ and urease+ by both the API 20E system and Christensen's urea agar (data not shown). Pathogenic V. parahaemolyticus isolates were serologically heterogeneous. In fact, five different serotypes of tdh+ V. parahaemolyticus were isolated from a single sample from Dauphin Island Bay on 16 March 1999. The O3:K6 serotype was not detected.
TABLE 2.
Sources and serotypes of tdh+ V. parahaemolyticus isolates
| Harvest date | Site/samplea | No. of tdh+ isolatesb | Serotype(s) |
|---|---|---|---|
| 16-Mar-99 | DI/A | 6 | O4:K9 |
| 16-Mar-99 | DI/B | 7 | O1:K22, O1:K56, O4:K9, O1:Kuk,c O11:K40 |
| 24-May-99 | DI/B | 2 | O1:Kuk |
| 08-Jun-99 | DI/B | 1 | O1:K56 |
| 07-Jul-99 | DI/B | 1 | O1:K56 |
| 16-Aug-99 | CP/B | 1 | O4:K8 |
| 27-Dec-99 | DI/A | 4 | O11:Kuk |
| 11-Jan-00 | DI/A | 1 | O11:Kuk |
| 11-Jan-00 | DI/B | 11 | O11:Kuk |
| 25-Jan-00 | DI/B | 1 | O1:K30 |
| 14-Mar-00 | DI/A | 1 | O1:K55 |
| 23-May-00 | DI/B | 1 | O8:K70 |
| 06-Jun-00 | DI/B | 1 | O4:K9 |
| 20-Jun-00 | DI/B | 1 | O1:K54 |
DI: Dauphin Island Bay; CP: Cedar Point. A and B are subsamples.
A total of 39 isolates were tested.
uk, capsular antigen untypeable with available antisera.
DISCUSSION
The abundance of total V. parahaemolyticus in Alabama oysters at both sites was seasonal and was in agreement with previous studies (9, 21, 24). The abundance of V. parahaemolyticus was affected more by temperature than by salinity; however, V. parahaemolyticus levels were lower in the summer of 2000 than in that of 1999 but the temperatures were similar. Salinities were closer to the optimum of 17 ppt during the summer of 1999 than in that of 2000, and this may account for the difference. On some occasions, V. parahaemolyticus levels appeared to fluctuate independently of temperature and salinity. V. parahaemolyticus levels were more than 2 logs higher in a sample collected at Cedar Point on 20 September 2000 than in one collected on the same day at Dauphin Island Bay or in samples collected 2 weeks earlier at both locations, when the temperatures and salinities were similar. The oyster, as a living host, may have contributed to the variation in V. parahaemolyticus numbers independently of temperature and salinity because of fluctuations in physiology resulting from reproductive status, diet, and health.
V. parahaemolyticus levels in Alabama oysters during the summer of 1999 varied little (102 to 103 g−1) and were consistent with observations in Galveston Bay after the 1998 outbreak (10). The erratic trend of total V. parahaemolyticus levels in Alabama oysters during the summer of 2000 was similar to that observed in Washington State oysters after an outbreak in 1997 (10). V. parahaemolyticus levels greater than 104 g−1 were observed on one occasion in this study; this was consistent with a growing body of evidence that this level seldom occurs in oysters prior to harvest (1, 9, 10, 22, 33). These levels stand in contrast to those reported in a recent study of U.S. market shellstock oysters (5) that frequently found V. parahaemolyticus levels of greater than 104 g−1 in Gulf Coast oysters and that suggested that these high levels are probably the result of postharvest growth.
The use of a direct-plating method for total V. parahaemolyticus probably accounts for the close agreement in V. parahaemolyticus levels between replicate samples. A strong correlation (r = 0.78) between the direct-plating and Bacteriological Analytical Manual most-probable number methods for determining total V. parahaemolyticus densities in market level oysters has been reported (16).
Pathogenic V. parahaemolyticus was detected in 20 (12.8%) of 156 samples by the quantitative direct-plating procedure. The rate of pathogenic V. parahaemolyticus detection by an equivalent direct-plating procedure in an earlier study in Galveston Bay was considerably lower, at 3 (2.8%) of 106 samples (10). Other researchers employed direct-plating methods (9, 23-25) and found much lower frequencies of samples yielding either TDH+ (1%) or tdh+ (4%) V. parahaemolyticus isolates. This may be because they tested relatively few selected typical colonies for pathogenicity instead of using the colony lift format as in the present study. The qualitative procedure (enrichment of 25-g portions of oyster homogenate, followed by isolation on TCBS) yielded a lower rate of pathogenic V. parahaemolyticus detection (9.6%) than the quantitative (0.1 g of oyster homogenate) spread plating procedure. Both methods detected a prevalence of pathogenic V. parahaemolyticus higher than the 0 to 6% reported in previous studies (5, 14, 22, 26, 31). The low frequency at which both paired samples were found to have the tdh gene by either method (1.3% by enrichment and 2.6% by direct plating) suggests that collection of multiple samples at each site would substantially increase the detection of pathogenic V. parahaemolyticus.
The average tdh+ V. parahaemolyticus density determined by direct plating was 2.7 CFU g−1 at Dauphin Island Bay and 1.3 CFU g−1 at Cedar Point. Similar densities of tdh+ V. parahaemolyticus were reported in a previous study of Pacific Coast oysters (10). Alabama oysters were not linked to any V. parahaemolyticus illnesses during the study.
Similar numbers of colonies were examined for the tdh gene after enrichment and direct plating. The tdh gene was detected in a small proportion of V. parahaemolyticus isolates after enrichment (46 of 6,018) and in directly plated oyster homogenates (31 of 6,992). The tdh+ colonies recovered from enrichments and by direct plating were low in number and unevenly distributed. Few total or tdh+ V. parahaemolyticus colonies were found on direct plates during the winter, whereas a constant number (47 isolates per sample) was found in enrichments throughout the year. In two samples collected at Dauphin Island Bay (16 March 1999 and 11 January 2000), 13 and 12 of 47 isolates, respectively, obtained after enrichment were tdh+; other samples yielded much fewer tdh+ isolates. The ratio of pathogenic to total V. parahaemolyticus was seasonal; pathogenic strains were more prevalent in the winter. The greater prevalence of pathogenic V. parahaemolyticus in the winter was not observed in previous surveys, and this may explain why V. parahaemolyticus illnesses are less seasonal on the Gulf Coast than in other regions (19). The greater rate of detection of pathogenic V. parahaemolyticus in the winter may be due to a decreased prevalence of total V. parahaemolyticus and not to a greater abundance of pathogenic strains. During warm weather, V. parahaemolyticus multiplies in oysters (18); it probably multiplies in many other marine environments that contain adequate nutrients. Growth slows or stops as winter approaches, and we are not aware of data suggesting that tdh+ strains are more cold tolerant than other V. parahaemolyticus strains.
Vibrios are associated with mammals and birds (11, 27), and animal passage can select for virulent strains (13). Feces from warm-blooded animals could be a more significant source of vibrios under cold conditions that are unfavorable for environmental growth. Pathogenic strains of V. cholerae were most prevalent during the winter in Apalachicola Bay, Fla. (12). A wildlife refuge borders Dauphin Island Bay, and diverse species of birds are abundant year round. During the winter of 2000, raccoons were routinely sighted feeding on oysters along the shoreline of Dauphin Island Bay. Raccoons have been shown to harbor high densities of V. parahaemolyticus in their intestines (11).
Nearly all (97.4%) of the tdh+ V. parahaemolyticus strains examined in this study produced urease and possessed the trh gene. These traits are normally associated with V. parahaemolyticus from the Pacific Northwest and have not been reported in seafood or environmental samples from the Gulf Coast (22). Some have hypothesized that these traits may increase virulence, but the recently emerged O3:K6 clone associated with pandemic spread and high attack rates did not possess these traits (32). Additionally, approximately 40% of recent (1997 to 2001) human V. parahaemolyticus isolates did not produce urease or possess trh (Cheryl Bopp, Centers for Disease Control and Prevention, personal communication, June 2002). While diverse serotypes were found in this study, no O3:K6 strains were observed.
This study provides novel information on the abundance, ecology, and characteristics of pathogenic V. parahaemolyticus in U.S. Gulf Coast oysters. The higher prevalence of pathogenic V. parahaemolyticus observed in the present study is probably due to the testing of many more colonies than in most previous investigations. Extensive quantification of pathogenic V. parahaemolyticus in any environmental matrix has not previously been reported. Our quantitative data suggest a higher ratio of pathogenic to total V. parahaemolyticus strains in oysters during cooler weather and may explain why illness along the Gulf Coast is more prevalent in winter months than expected on the basis of levels of total V. parahaemolyticus. U.S. isolates of tdh+ V. parahaemolyticus possessing the trh gene and producing urease were previously reported only along the Pacific coast; our results indicate that the overwhelming majority of pathogenic strains from the Gulf Coast also possess these potential virulence traits. These data should be valuable for assessment of the human health risk due to consumption of raw oysters.
Acknowledgments
We appreciate the assistance of Nancy Puhr of the Centers for Disease Control and Prevention with V. parahaemolyticus serotyping and thank Susan McCarthy for manuscript review.
REFERENCES
- 1.Baross, J. A., J. Liston, and R. Y. Morita. 1978. Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl. Environ. Microbiol. 36:492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bej, A. K., D. P. Patterson, C. W. Brasher, M. C. L. Vickery, D. D. Jones, and C. Kaysner. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods 36:215-225. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific northwest, 1997. Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 5.Cook, D. W., P. O'Leary, J. C. Hunsucker, E. M. Sloan, J. C. Bowers, R. J. Blodgett, and A. DePaola. 2002. Vibrio vulnificus and Vibrio parahaemolyticus in U.S. retail shell oysters: a national survey June 1998 to July 1999. J. Food Prot. 65:79-87. [DOI] [PubMed] [Google Scholar]
- 6.Daniels, N. A., B. Ray, A. Easton, N. Marano, E. Kahn, A. L. McShan, L. Del Rosario, T. Baldwin, M. A. Kingsley, N. D. Puhr, J. G. Wells, and F. J. Angulo. 2000. Emergence of a new Vibrio parahaemolyticus serotype in raw oysters. JAMA 284:1541-1545. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, N., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. Hammond, S. Thompson, S. Wilson, N. Bean, P. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 8.DePaola, A., L. H. Hopkins, and R. M. McPhearson. 1988. Evaluation of four methods for enumeration of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 54:617-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz, and R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Appl. Environ. Microbiol. 56:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePaola, A., C. A. Kaysner, J. C. Bowers, and D. W. Cook. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters following outbreaks in Washington, Texas, and New York (1997, 1998). Appl. Environ. Microbiol. 66:4649-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola, A., and M. L. Motes. 1984. Isolation of Vibrio parahaemolyticus from wild raccoons in Florida, p. 563-566. In R. R. Colwell (ed.), Vibrios in the environment. John Wiley & Sons, Inc., New York, N.Y.
- 12.DePaola, A., M. W. Presnell, R. E. Becker, M. L. Motes, Jr., S. R. Zywno, J. F. Musselman, J. Taylor, and L. Williams. 1984. Distribution of Vibrio cholerae in the Apalachicola (Florida) bay estuary. J. Food Prot. 47:549-553. [DOI] [PubMed] [Google Scholar]
- 13.Dutta, N. K., M. V. Panse, and H. I. Jhala. 1963. Choleragenic property of certain strains of El Tor, nonagglutinable, and water vibrios confirmed experimentally. Br. Med. J. 1:1200-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle, P. M., and F. D. Crisley. 1975. Isolation and characterization of Vibrio parahaemolyticus from Cape Cod soft-shell clams (Mya arenaria). Appl. Microbiol. 29:635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliot, E. L., C. A. Kaysner, L. Jackson, and M. L. Tamplin. 1998. Vibrio cholerae, V. parahaemolyticus, V. vulnificus, and other Vibrio spp., p. 9.01-9.27. In U.S. Food and Drug Administration bacteriological analytical manual. A.O.A.C. International, Gaithersburg, Md.
- 16.Ellison, R. K., E. Malnati, A. DePaola, J. C. Bowers, and G. E. Rodrick. 2001. Populations of Vibrio parahaemolyticus in retail oysters from Florida using two methods. J. Food Prot. 64:682-686. [DOI] [PubMed] [Google Scholar]
- 17.Ewing, W. H. 1986. Edwards and Ewing's identification of Enterobacteriaceae. Elsevier Science Publishing Co. Inc., New York, N.Y.
- 18.Gooch, J. A., A. DePaola, C. A. Kaysner, and D. L. Marshall. 2001. Evaluation of two direct-plating methods using nonradioactive probes for enumeration of Vibrio parahaemolyticus in oysters. Appl. Environ. Microbiol. 67:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hlady, W. G. 1997. Vibrio infections associated with raw oyster consumption in Florida, 1981-1994. J. Food Prot. 60:353-357. [DOI] [PubMed] [Google Scholar]
- 20.Honda, T., and T. Iida. 1993. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med. Microbiol. 4:106-113. [Google Scholar]
- 21.Kaneko, T., and R. R. Colwell. 1975. Incidence of Vibrio parahaemolyticus in Chesapeake Bay. Appl. Microbiol. 30:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaysner, C. A., C. Abeyta, Jr., R. F. Stott, J. L. Lilja, and M. M. Wekell. 1990. Incidence of urea-hydrolyzing Vibrio parahaemolyticus in Willapa Bay, Washington. Appl. Environ. Microbiol. 56:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly, M. T., and E. M. Stroh. 1988. Occurrence of Vibrionaceae in natural and cultivated oyster populations in the Pacific Northwest. Diagn. Microbiol. Infect. Dis. 9:1-5. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, M. T., and E. M. D. Stroh. 1988. Temporal relationship of Vibrio parahaemolyticus in patients and the environment. J. Clin. Microbiol. 26:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, M. T., and E. M. D. Stroh. 1989. Urease-positive, Kanagawa-negative Vibrio parahaemolyticus from patients and the environment in the Pacific northwest. J. Clin. Microbiol. 27:2820-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiiyukia, C., K. Venkateswaran, I. M. Navarro, H. Nakano, H. Kawakami, and H. Hashimoto. 1989. Seasonal distribution of Vibrio parahaemolyticus serotypes along the oyster beds in Hiroshima coast. J. Fac. Appl. Biol. Sci. 28:49-61. [Google Scholar]
- 27.Lee, J. V., D. J. Bashford, T. J. Donovan, A. L. Furniss, and P. A. West. 1982. The incidence of Vibrio cholerae in water, animals and birds in Kent, England. J. Appl. Bacteriol. 52:281-291. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy, S. A., A. DePaola, D. W. Cook, C. A. Kaysner, and W. E. Hill. 1999. Evaluation of alkaline phosphatase- and digoxigenin-labelled probes for detection of the thermolabile hemolysin (tlh) gene of Vibrio parahaemolyticus. Lett. Appl. Microbiol. 28:66-70. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy, S. A., A. DePaola, C. A. Kaysner, W. E. Hill, and D. W. Cook. 2000. Evaluation of nonisotopic DNA hybridization methods for detection of the tdh gene of V. parahaemolyticus. J. Food Prot. 63:1660-1664. [DOI] [PubMed] [Google Scholar]
- 30.McCollaugh, P., and J. A. Nelder. 1989. Generalized linear models. Chapman & Hall, London, England.
- 31.Ogawa, H., H. Tokunou, T. Kishimoto, S. Fukuda, K. Umemura, and M. Takata. 1989. Ecology of Vibrio parahaemolyticus in Hiroshima Bay. Hiroshima J. Vet. Med. 4:47-57. [Google Scholar]
- 32.Okuda, J., M. Ishibashi, E. Hayashi, T. Nishino, Y. Takeda, A. K. Mukhopadhyary, S. Garg, S. K. Bhattacharya, B. G. Nair, and M. Nishibuchi. 1997. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 35:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tepedino, A. A. 1982. Vibrio parahaemolyticus in Long Island oysters. J. Food Prot. 45:150-151. [DOI] [PubMed] [Google Scholar]
- 34.Thompson, C. A., and C. Vanderzant. 1976. Serological and hemolytic characteristics of Vibrio parahaemolyticus from marine sources. J. Food Sci. 41:204-205. [Google Scholar]
- 35.Wagatsuma, S. 1974. Ecological studies on Kanagawa phenomenon-positive strains of Vibrio parahaemolyticus, p. 91-96. In T. Fujino, G. Sakaguchi, R. Sakazaki, and Y. Takeda (ed.), International Symposium on Vibrio parahaemolyticus. Saikon Publishing Co., Ltd., Tokyo, Japan.
- 36.Watkins, W. D., and V. J. Cabelli. 1985. Effect of fecal pollution on Vibrio parahaemolyticus densities in an estuarine environment. Appl. Environ. Microbiol. 49:1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright, A. C., G. A. Miceli, W. L. Landry, J. B. Christy, W. D. Watkins, and J. G. Morris. 1993. Rapid identification of Vibrio vulnificus on nonselective media with an alkaline phosphatase-labeled oligonucleotide probe. Appl. Environ. Microbiol. 59:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]