Abstract
The distribution of the concentration of potential indicators of fecal viral pollution in shellfish was analyzed under diverse conditions over 18 months in diverse geographical areas. These microorganisms have been evaluated in relation to contamination by human viral pathogens detected in parallel in the analyzed shellfish samples. Thus, significant shellfish-growing areas from diverse countries in the north and south of Europe (Greece, Spain, Sweden, and the United Kingdom) were defined and studied by analyzing different physicochemical parameters in the water and the levels of Escherichia coli, F-specific RNA bacteriophages, and phages infecting Bacteroides fragilis strain RYC2056 in the shellfish produced, before and after depuration treatments. A total of 475 shellfish samples were studied, and the results were statistically analyzed. According to statistical analysis, the presence of human viruses seems to be related to the presence of all potential indicators in the heavily contaminated areas, where E. coli would probably be suitable as a fecal indicator. The F-RNA phages, which are present in higher numbers in Northern Europe, seem to be significantly related to the presence of viral contamination in shellfish, with a very weak predictive value for hepatitis A virus, human adenovirus, and enterovirus and a stronger one for Norwalk-like virus. However, it is important to note that shellfish produced in A or clean B areas can sporadically contain human viruses even in the absence of E. coli or F-RNA phages. The data presented here will be useful in defining microbiological parameters for improving the sanitary control of shellfish consumed raw or barely cooked.
Shellfish are filter-feeding organisms that accumulate and concentrate pathogenic microorganisms present in the water, which remain infectious for a certain period (5). The role of shellfish in the epidemiology of fecally-orally transmitted infectious diseases is well known (5, 11). Traditionally, coliform bacteria and Escherichia coli have been used as indicators of the sanitary quality of shellfish, and this has led to success in the prevention of shellfish-borne infections by fecal bacteria. However, it has been clearly established that bacterial standards do not always reveal the presence of viruses or the presence of members of the genus Vibrio (9, 24). Hence, there is a need for indicators of viral fecal pollution in order to improve the microbiological control of shellfish. Somatic coliphages (24), bacteriophages infecting Bacteroides fragilis (16, 17, 20), and F-specific RNA (F-RNA) bacteriophages (12, 13, 16) have been proposed as potential indicators of infectious viruses. Additionally, the detection of human adenovirus by PCR has been proposed as a molecular index of viral contamination of human origin (23).
The main objective of this study was to analyze the distribution of the proposed indicators of viral contamination in shellfish produced in highly diverse geographical areas. For this purpose, shellfish samples collected in shellfish-growing areas in the Atlantic Ocean, the Skagerrak Sea, and the eastern and western Mediterranean Sea were analyzed for E. coli, somatic coliphages, F-RNA bacteriophages, and phages infecting B. fragilis RYC2056. In addition, physicochemical parameters of the shellfish-growing areas were measured. The values obtained for potential indicators through 18 months of sampling in the four areas were also compared with the presence of human adenovirus (ADV), enterovirus (EV), hepatitis A virus (HAV), and Norwalk-like viruses, including genogroup I (NLVI) and genogroup II (NLVII), in shellfish samples, described in more detail in a previous article (8). The information obtained in the study is highly valuable for improving microbiological control of shellfish and increasing the level of safety for the population.
MATERIALS AND METHODS
Sampling.
Bivalve molluscan shellfish were collected in shellfish-growing areas with different levels of fecal pollution in Greece, Spain, Sweden, and the United Kingdom on a monthly basis over 18 months. These areas are classified in accordance with European Union legislation as A areas (<230 E. coli organisms/100 g of shellfish flesh and liquor), B areas (<4,600 E. coli organisms/100 g in 90% of samples), and C areas, which exceed the mentioned limits. In Greece, Mytilus galloprovincialis from two B areas and four A areas and Crassostrea gigas from two B areas were harvested. In Spain, M. galloprovincialis and C. gigas from an A area and a B area located in the western Mediterranean Sea and Ostrea edulis from a nonclassified area in the Atlantic Ocean was harvested. In Sweden, M. galloprovincialis from an A area, a B area, and a nonclassified area were collected. Finally, C. gigas from an A area and a B area, Mytilus edulis from a B area, and C. gigas and M. edulis from a B, a C, and a prohibited area in the United Kingdom were harvested.
Once collected, the shellfish were shipped directly to each laboratory via cold storage within 24 h, and E. coli and bacteriophages were measured immediately. Processed samples were stored at −70 ± 10°C and later used for human enteric virus detection by PCR as described by Formiga-Cruz et al. (8). The sampling regimen also included paired samples directly from B harvesting areas (1), and after the depuration treatment the end product was tested to provide information on the effectiveness of commercial depuration processes for virus removal.
Shellfish processing.
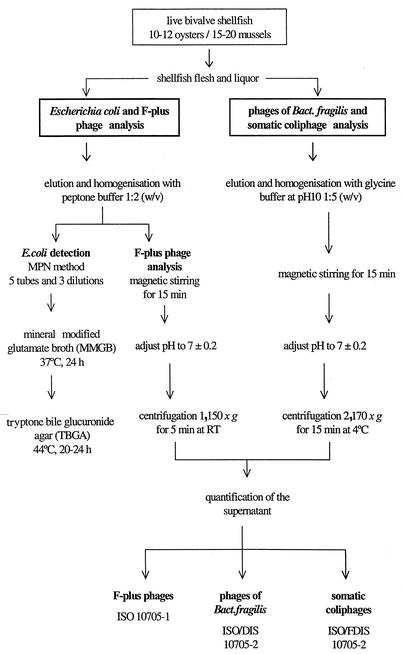
Shellfish were washed, scrubbed under clean running water, and opened with a sterile shucking knife. For the analysis of phages infecting B. fragilis, somatic coliphages and F-RNA phages, 10 to 12 oysters and 15 to 20 mussels were selected. Shellfish flesh and liquor were collected into a sterile beaker and diluted with glycine buffer, pH 10 (1:5, wt/vol), for the detection of somatic coliphages and bacteriophages infecting B. fragilis (21, 23). For F-plus bacteriophages, shellfish meat was eluted with peptone water (1:2, wt/vol), since these phages have been shown to be more stable at neutral pH (7). Once eluted, they were homogenized in a blender and magnetically stirred for 15 min. After the pH was adjusted to 7.2 ± 0.2, the homogenate was centrifuged at 2,170 × g for 15 min at 4°C, and the supernatant was used for phage enumeration by the procedures outlined below (Fig. 1).
FIG. 1.
Procedure for quantification of bacteriophages and E. coli.
Performance assessment program.
Concentrated suspensions of bacteriophages φX174, MS2, and B56-3 were distributed to all the laboratories to be used as reference suspensions in internal controls. Each bacteriophage was cultured by the appropriate method described in the corresponding ISO protocol (2, 3, 4). The bacteriophage suspension obtained was treated with chloroform at a 2.5:1 (vol/vol) culture ratio and centrifuged at 3,000 × g for 20 min. This high-titer phage suspension was divided into 2.2-ml volumes and stored at −70 ± 10°C. Prior to reference material preparation, each frozen vial was defrosted at room temperature to prepare 10-fold dilution series in peptone saline. Before distribution into vials in 2.2-ml volumes and storage at −70 ± 10°C, a first titration was carried out to assess homogeneity between samples. Further titrations were carried out during a long enough period to correctly assess homogeneity.
Commercially preprepared lenticules (National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom) for E. coli were analyzed in order to evaluate the level of performance of the selected procedure by each laboratory. Lenticules are plano-convex discs containing biologically active material in a solid water-soluble matrix. They are easily transported and robust and can be used to provide a reproducibly countable number of CFU. Upon receipt, the lenticule contents were rehydrated in 100 ml of peptone (producing a 1:10 dilution, from which a further serial dilution was prepared) and analyzed within 1 h. Once prepared, the samples were analyzed by all partners using a two-stage, five-tube, three-dilution most-probable-number (MPN) method.
Control charts for standard suspensions of the above-mentioned phages and E. coli were prepared by all laboratories during the study, and sporadic discrepancies were detected and immediately corrected.
E. coli analysis.
The procedure for detection of E. coli was, with little modification, that described by Donovan et al. (6), which consists of a two-stage, five-tube, three-dilution MPN method. In brief, it requires inoculation into mineral-modified glutamate broth and further confirmation by subculturing the contents of positive tubes onto a chromogenic agar to detect β-glucuronidase activity.
Analysis of bacteriophages.
All phages were quantified by the double-agar-layer method. E. coli WG5 grown on modified Scholten's broth was used as the host strain for the quantification of somatic coliphages. Salmonella enterica serovar Typhimurium WG49 (14) grown on tryptone-yeast extract-glucose broth was used as the host strain for F-specific bacteriophages. B. fragilis RYC2056 grown on Bacteroides phage recovery medium broth was used as the host strain for the quantification of B. fragilis bacteriophages. All procedures are described in the corresponding standardized protocol (2, 3, 4).
Human enteric virus detection.
Prior to detection of enteric viruses (ADV, EV, HAV, and NLV) by nested PCR, shellfish samples collected in Greece, Spain, and Sweden were processed by the method based on elution with 0.25 N glycine buffer at pH 10 (1:5, wt/vol) described by Pina et al. (23) and Muniain-Mujika et al. (21), with some modifications (8). The laboratory in the United Kingdom analyzed bivalve mollusks by a procedure based on direct nucleic acid extraction (8, 19). The methods exhibited equivalent sensitivities in viral standard suspensions at the PCR level, and the method used in the United Kingdom laboratory also produced a high number of positive results for human viruses in shellfish.
Physicochemical parameters.
The parameters of the shellfish-growing waters that were studied were temperature, salinity, pH, and dissolved oxygen content. The temperature was measured at the depth at which shellfish were collected. The pH was determined upon arrival at the laboratory with an Orion SA250 pH meter with a temperature-compensatory system and calibrated with pH 7 and 4 buffers. Salinity was measured with a conductivity meter (Wissenschaflich Tecnische Werkstätten; LF 196) at the depth at which shellfish were collected. Dissolved oxygen was measured with a mobile potency meter that gives measurements as saturation percentage. Calibration in air (100% saturation) was done before every measurement.
Statistical analysis.
In order to perform the statistical analysis, the variables for E. coli, bacteriophages infecting B. fragilis, somatic coliphages, and F-RNA phages were transformed by the log10(x + 1) function. All statistical tests were done with the statistical package SPSS 10.0.7 in a Pentium III machine running MS Windows 2000 Professional. Values that fell below the level of detection (31 PFU/100 g for phages and 20 [MPN]/100 g for E. coli) were considered zeroes in the statistical analysis.
The first block of the analysis included two logistic regression models. It was intended to measure the predictive capacity of a set of classificatory variables and covariates on the binary variables ADV, EV, NLVI, and NLVII. The first logistic model had the mollusk type and the country as classificatory variables and phages infecting B. fragilis, somatic coliphages, F-RNA phages, E. coli, and temperature as covariates. The second logistic model did not include the temperature as a covariate, because some samples were collected under conditions that did not allow measurement of the water temperature, i.e., low tides and other technical difficulties. It is important to note that the two models must be carefully compared because of the differences between the sample sizes. P values were obtained at the last step of each stepwise regression procedure. Stepwise regression is an iterative procedure that explores the statistical significance of the relations between a set of prediction variables and a response variable. In our case, the method was backward stepwise: it started with a logistic model that included all the classificatory variables (the temperature and all the phages above) and in several steps discarded the nonsignificant variables. The procedure stopped when the model was reduced to significant variables, showing a final equation that related the response and the prediction variables. This method requires a probability level, the POUT value, in order to discard nonsignificant relations along the iterative process (POUT = 0.10). The second block of the analysis was a standard test of nonparametric regression for every pair of parameters: somatic coliphages, phages infecting B. fragilis, F-RNA phages, E. coli, and temperature.
RESULTS
Level of fecal contamination in the studied areas according to E. coli standards.
Bacteriological results have been described elsewhere (8). Briefly, areas in northern Europe yielded in general higher values than those in the south. Thus, 83% of the tested samples from the B area in Spain yielded E. coli values lower than 230 per 100 g. In Greece, 76 to 94% of the samples from the B areas analyzed yielded similar values. In the United Kingdom, 56 to 76% of samples presented the same levels of fecal contamination. In Sweden, 94% of samples from a B area had values below 230 E. coli organisms/100 g, whereas in the other B area examined these levels were detected in 46% of the samples. Regarding areas in the Skagerrak Sea, an increase of E. coli numbers occurred in the three Swedish areas due to flooding and ice (thawing of the water in the ground) to more than 4,600 E. coli organisms per 100 g of shellfish flesh in the samples collected in March to April 2001.
Physicochemical parameters.
Temperatures in the Mediterranean Sea shellfish-growing areas were higher than those in the areas located in the Atlantic Ocean and the Skagerrak Sea (Table 1). Regarding salinity, the values were also higher in the areas of the Mediterranean Sea, and the Swedish shellfish-growing areas presented the lowest salinities. The differences may be explained by the diverse source of water influents affecting the shellfish-growing area. In contrast, pH and dissolved oxygen content remained similar in all areas (Table 1).
TABLE 1.
Physicochemical parameters measured
| Country | Site | Classificationa | Temperature (°C) | pH | Salinity (‰) | Dissolved oxygen content (% saturation) |
|---|---|---|---|---|---|---|
| Spain | 1 | A | 13.2-25.3 | 8.1-8.32 | 34.4-37.8 | 8.1-102 |
| 2 | B | 8.8-27.2 | 8.01-8.58b | 17.3-37.6 | 90-105 | |
| Greece | 1 | B | 13-27 | 7.1-8.2 | 35.6-38.13 | 72-103 |
| 2 | B | 12-26.8 | 7-8.35 | 35.7-37.6 | 91-105 | |
| 3 | A | 12-26.7 | 7.2-8.2 | 35-38.2 | 88-103 | |
| 4 | A | 13-27 | 7.1-8.3 | 35.6-37.52 | 89-102 | |
| 5 | A | 12-26.8 | 7-8.35 | 35.7-38.2 | 76-105 | |
| 6 | A | 11-23 | 6.87-8.3 | 34.7-39.15 | 6-107 | |
| 7 | A | 11.5-25.6 | 6.9-8.47 | 34.5-37.69 | 52-104 | |
| 8 | A | 10.7-26 | 6.1-8.58 | 33.67-37.76 | 54-107 | |
| United Kingdom | 1 | A | 5-21.5 | 6.9-8.1 | 14.8-34 | 66-102 |
| 2 | A/B | 3.9-12.3 | 6.6-7.5 | 30-34 | 6-100 | |
| 3 | B1 | NTc | 7.2-7.8 | 20-26 | 83-105 | |
| Sweden | 1 | B | 1-18.1 | 8 | 19.9-26.8 | NT |
| 2 | NCd | 0.8-18.2 | 8 | 19.4-26.9 | NT | |
| 3 | A | 1.2-19.9 | 8 | 13.8-26.4 | NT |
According to European Union guidelines.
Measured at 12 noon.
NT, not tested.
NC, nonclassified.
Distribution of the bacteriophage levels in shellfish.
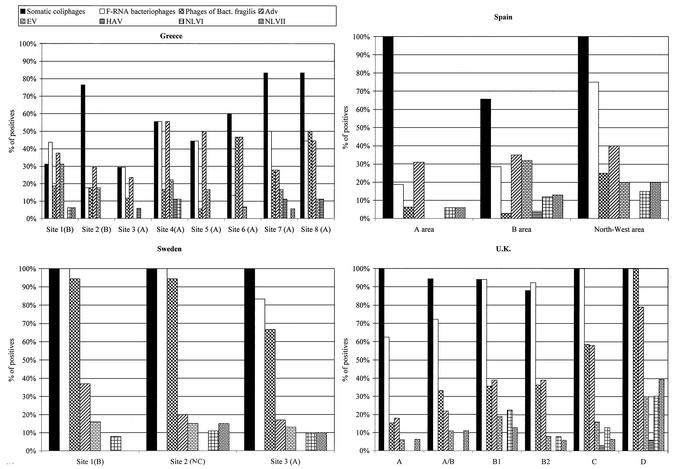
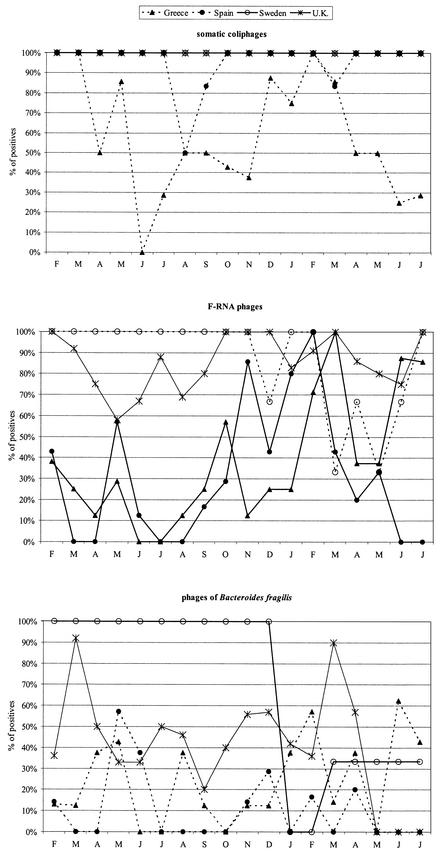
In general, somatic coliphages were detected at higher levels than the other groups of phages tested (Table 2) as well as in a higher percentage of samples in all areas (Fig. 2). Additionally, the Mediterranean sampling areas presented generally low levels of E. coli and both F-plus phages and phages infecting B. fragilis, whereas in the analyzed Atlantic Ocean and Skagerrak Sea shellfish-growing areas, higher values of E. coli and a higher number of samples presenting F-plus phages were observed. The numbers of phages infecting B. fragilis were more similar across all examined zones (Table 2 and Fig. 2). F-RNA bacteriophages were shown to be the unique group of bacteriophages with a seasonal distribution and presented higher numbers in all areas and regions during the winter months. Figure 3 presents percentages of positive results, which were very high in Sweden almost every month and may be affected by water flow occurring in March to April 2001. Depuration effectively reduced E. coli counts but was less effective at decreasing the bacteriophage levels. In fact, in some cases the numbers of phages detected went up substantially after depuration (Table 3).
TABLE 2.
Levels of E. coli and phages at each sampling site
| Country | Site | Classifi- cationa | No. of samples tested | Level (geometric mean ± SD) ofb:
|
|||
|---|---|---|---|---|---|---|---|
| E. coli (MPN/100 g) | Somatic coliphages | F-RNA phages | Phages infecting B. fragilis | ||||
| Spain | 1 | A | 16 | 23 ± 18 | 1,239 ± 2,567 | 33 ± 10 | 33 ± 2 |
| 2 | B | 68 | 34 ± 355 | 1,132 ± 10,366 | 35 ± 10,554 | 36 ± 34 | |
| 3 | NC | 20 | 105 ± 238 | 6,943.74 ± 8,299.55 | 31 ± 0 | 39 ± 67 | |
| Greece | 1 | B | 17 | 56 ± 174 | 72 ± 1,072 | 123 ± 716 | 58 ± 499 |
| 2 | B | 17 | 26 ± 310 | 277 ± 1,668 | 55 ± 340 | 61 ± 803 | |
| 3 | A | 17 | 71 ± 924 | 70 ± 1,105 | 80 ± 1,201 | 48 ± 478 | |
| 4 | A | 18 | 100 ± 1,506 | 241 ± 37,714 | 158 ± 726 | 54 ± 757 | |
| 5 | A | 18 | 37 ± 559 | 206 ± 3,116 | 127 ± 873 | 34 ± 39 | |
| 6 | A | 15 | 94 ± 849 | 244 ± 1,635 | 60 ± 4,383 | 196 ± 1,678 | |
| 7 | A | 18 | 85 ± 763 | 576 ± 1,608 | 136 ± 571 | 87 ± 990 | |
| 8 | A | 18 | 127 ± 2,302 | 1,416 ± 89,163 | 165 ± 971 | 158 ± 585 | |
| Sweden | 1 | A | 18 | 227 ± 37,558 | 2,018 ± 2,089 | 479 ± 592 | 168 ± 1,108 |
| 2 | A/B | 18 | 228 ± 37,618 | 1,157 ± 718 | 238 ± 413 | 1,108 ± 884 | |
| 3 | B1 | 18 | 61 ± 37,700 | 1,872 ± 6,810 | 138 ± 434 | 57 ± 1,219 | |
| United Kingdom | 1 | B | 68 | 93 ± 207 | 1,202 ± 5,907 | 167 ± 6,411 | 32 ± 9 |
| 2 | NC | 17 | 185 ± 552 | 1,256 ± 4,233 | 70 ± 516 | 45 ± 50 | |
| 3 | A | 18 | 133 ± 2,910 | 3,410 ± 29,175 | 979 ± 4,857 | 42 ± 465 | |
| 4 | B | 35 | 67 ± 615 | 1,302 ± 5,711 | 237 ± 4,857 | 41 ± 98 | |
According to European Union guidelines. NC, nonclassified.
Values for phages are in PFU per 100 g of shellfish flesh.
FIG. 2.
Percentage of shellfish samples positive for phages and viruses in shellfish harvested in different shellfish-growing areas of four countries.
FIG. 3.
Distribution of the percentage of positive shellfish samples for three groups of bacteriophages over 18 months of sampling.
TABLE 3.
Mean levels of E. coli and the three groups of bacteriophages in bivalve shellfish from a B area before and after a depuration treatment
| Shellfish (no. of samples) | Mean levela of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
E. coli
|
Somatic coliphages
|
F-RNA bacteriophages
|
Phages of B. fragilis
|
|||||
| ND | D | ND | D | ND | D | ND | D | |
| C. gigas (34) | 83 (61) | 1 (6) | 5,061 (94) | 7,312 (100) | 134 (33) | 43 (25) | 2 (6) | 30 (13) |
| M. galloprovincialis (34) | 269 (61) | 0 (6) | 3,814 (94) | 5,768 (88) | 99 (28) | 53 (19) | 0 (0) | 17 (25) |
| Combined data | 176 (61) | 1 (6) | 4,438 (94) | 6,540 (94) | 116 (31) | 48 (22) | 1 (3) | 24 (19) |
ND, nondepurated samples; D, depurated samples. E. coli values are MPN per 100 g; phage values are PFU per 100 g. Values in parentheses are percent positive samples.
Comparative analysis of the distribution of indicators and human viruses in shellfish.
The results on the presence of human viruses in the shellfish samples analyzed for bacteriophages in this study are described in detail in a previous article (8), and the comparison with the values obtained for the three groups of bacteriophages is described in Fig. 2. From all the studied viruses, only F-RNA bacteriophages and NLV (8) presented a seasonal distribution, with higher numbers during the cool months. In Fig. 2, the percentage of positive samples for all groups of phages and the human viruses analyzed in the diverse shellfish-growing areas studied is presented.
Statistical analysis.
For the first block of the analysis, two different logistic regression models were made in order to examine the predictive capacity of different classificatory variables and several covariates. In one model, temperature was excluded as a classificatory variable. According to Table 4, F -RNA phages were significantly related to the four viruses. The country classification had significant differences in the variables EV, NLVI, and NLVII. Finally, somatic coliphages had a significant relation to EV, and the mollusk classification showed significant differences for NLVI. Regarding the smaller set of data, when the temperature was included in the model (Table 5) the temperature appeared to be a significantly related variable for EV, NLVI, and NLVII but not for ADV, while F-RNA did not appear to be related to EV, NLVI, or NLVII but was related to ADV.
TABLE 4.
Logistic regression model without temperature as a classificatory variable (468 cases)a
| Virus |
P values for classificatory variable
|
% of samples
|
||||||
|---|---|---|---|---|---|---|---|---|
| Somatic coliphages | F-RNA phages | Phages of B. fragilis | Mollusk type | Country | With correct classification | Positive, correctly classified | Negative, correctly classified | |
| ADV | <0.001* | 60.5 | 46.2 | 69.6 | ||||
| EV | 0.027* | 0.022* | 0.092 | 0.005* | 64.6 | 54.0 | 67.0 | |
| NLVI | <0.001* | 0.004* | <0.001* | 75.5 | 74.3 | 75.6 | ||
| NLVII | 0.069 | 0.038* | <0.001* | 75.1 | 67.4 | 75.8 | ||
Asterisks indicate significant values showing dependence between classificatory variables and human viruses. No values were obtained for E. coli.
TABLE 5.
Logistic regression model with temperature as a classificatory variable (306 cases)a
| Virus |
P values for classificatory variable
|
% of samples
|
||||||
|---|---|---|---|---|---|---|---|---|
| Somatic coliphages | F-RNA phages | Mollusk type | Country | Temp | With correct classification | Positive, correctly classified | Negative, correctly classified | |
| ADV | <0.25* | 61.8 | 27.8 | 80.3 | ||||
| EV | 0.021* | <0.001* | 0.021* | 61.7 | 58.6 | 61.1 | ||
| NLVI | 0.030* | 0.091 | <0.001* | 84.0 | 60.9 | 85.9 | ||
| NLVII | 0.041* | <0.008* | <0.027* | 81.7 | 63.0 | 83.5 | ||
Asterisks indicate significant values showing dependence between classificatory variables and human viruses. No values were obtained for E. coli and phages of B. fragilis.
The differences between Tables 4 and 5 could be interpreted by taking into account the smaller sample size used for Table 5 and also by acknowledging multicollinearity effects when temperature was added in the second model. Multicollinearity appears in regression models when some (or all) prediction variables are related. This undesired situation produces a general instability in the estimation of regression because of the numerical impossibility of accurate estimates of the regression coefficients. A common consequence of multicollinearity is that a few changes in the data set (e.g., dropping several cases) may dramatically change the significance of several variables in a stepwise procedure. It was then reasonable to assume that here multicollinearity played a role in the differences between the two tables because of the significant relations between phages infecting B. fragilis, somatic coliphages, F-RNA phages, and temperature (see the interpretation of Table 6 below).
TABLE 6.
Spearman correlation coefficients between potential indicators
| Indicator | Correlation coefficient (significancea)
|
|||
|---|---|---|---|---|
| F-RNA phages | Phages of B. fragilis | E. coli | Temp | |
| Somatic coliphages | 0.527 (<0.001*) | 0.091 (<0.001*) | 0.401 (<0.001*) | −0.485 (<0.001*) |
| F-RNA phages | 0.340 (<0.001*) | 0.447 (<0.001*) | −0.316 (<0.001*) | |
| Phages of B. fragilis | 0.249 (<0.001*) | −0.190 (0.001) | ||
| E. coli | −0.252 (<0.001*) | |||
Asterisks indicate significant values showing dependence between potential indicators.
It is important to note the differences observed when the data from each country were examined separately. Thus, whereas F-RNA phages correlate with NLVI and NLVII in the United Kingdom and with NLVI and NLVII in Sweden and Greece, respectively, they correlate only with EV in Spain. Additionally, somatic coliphages correlate well with ADV and EV in Sweden, EV in Greece, and NLVII in Spain, while in the United Kingdom there is no relation of these phages to any of the analyzed viruses.
The second block of the analysis (Table 6) was a standard test of nonparametric regression between pairs formed by the phages infecting B. fragilis, somatic coliphages, F-RNA phages, E. coli, and the temperature variables. Table 6 shows significant relations between all the pairs, but again, as in the logistic regression analysis, all were weak relations. Notice finally the negative correlation between temperature and the phages and E. coli.
DISCUSSION
This is the first report on the predictive efficacy of indicator organisms across a wide range of geographical regions, with the concomitant variations in physicochemical characteristics and social trends.
Indicator microorganisms were analyzed by harvesting whole soft tissues and shell liquor to provide a procedure which is easily applied in routine food control laboratories. On the other hand, human viruses were studied by dissecting out the digestive glands to design a test with the highest sensitivity. This difference in procedures may have some implication for comparison of the results. However, 3 g of the whole animal was tested for indicator microorganisms, whereas the equivalent of 1 to 2 g of the whole animal was analyzed for human viruses.
Depuration as currently commercially practiced did not appreciably reduce the levels of F-RNA bacteriophages, phages of B. fragilis, somatic coliphages, or the occurrence of human pathogenic viruses in either of the countries (United Kingdom and Spain) where this was examined, though its effectiveness in reducing E. coli levels was confirmed. On the basis of these findings, it is suggested that the current legislative standards for E. coli in postpurification bivalve mollusks do not effectively protect the consumer from the risk of exposure to pathogenic viruses associated with fecal contamination. This finding is supported by numerous reports of viral illness outbreaks following consumption of depurated shellfish (15, 18). It further follows that it is important to reexamine the protection afforded to shellfish consumers by the requirements for depuration in current legislation. A particular issue for examination is the reliance on removal of E. coli organisms and fecal coliforms to determine the duration of depuration. A recently published study suggests 5-day depuration treatment under technically well-controlled conditions in order to ensure elimination of viruses in mussels (22).
The quantification of F-RNA bacteriophages, somatic coliphages, and bacteriophages of B. fragilis as indicators of viral pollution in shellfish through 18 months of sampling was carried out in four geographical areas, and the relationship between these and both E. coli and human enteric viruses was evaluated. Initial analysis revealed a degree of correlation between all indicator organisms, but a high variability was observed in the data, particularly at sites classified as A, A/B, and B. Closer association was demonstrated with less overall variability at more heavily polluted sites (B areas in Northern Europe). However, it should also be noted that in cleaner shellfish areas (with very low levels of E. coli, such as A and B areas in Spain), the correlation between all fecal pollution indicators and the presence of human enteric viruses was less robust. Therefore, titers of somatic bacteriophages were highly variable and did not correlate well with occurrence of enteric viral pathogens. In addition, the method for this indicator isolates diverse families of phages, rather than a single family, which markedly complicates the interpretation of the data and the potential setting of standards.
Among the bacteriophages studied, the F-RNA bacteriophages demonstrated the most significant relationship to the presence of human viruses in shellfish, although with very weak predictive capability for ADV, EV, and HAV and a stronger predictive capability for NLV. Phages infecting B. fragilis were less frequently detected than the F-RNA phages in all areas studied. Distribution of F-RNA bacteriophages was also shown to be seasonal, with higher numbers recorded in all areas and regions during the winter months; this trend was also observed in the identification of typified NLV but not with detection of ADV, EV, or HAV. However, levels of F-RNA bacteriophages often fell below the limit of sensitivity of the assay (31 PFU/100 g), particularly in shellfish collected from southern European waters, where shellfish samples negative for F-RNA phages contained human viruses. It has been reported previously that the viability and the stability of viral particles in seawater are highly influenced by temperature (10) and by prolonged exposure to higher-intensity UV radiation during the summer months. It has been suggested that somatic coliphages may multiply under specific circumstances in the environment, though F-RNA phages apparently do not (25). This may influence the concentration of the detected phages to a limited extent. The impact of fluctuations in environmental parameters would be particularly notable in shallow waters and may offer a partial explanation for the low phage recovery in some studied areas. It may then be necessary to consider testing specific viral pathogens by RT-PCR or to evaluate by PCR ADVs (DNA viruses), which are exclusively human viruses and the most prevalent human viral parameter in shellfish. In addition, ADVs exhibit a significant relation to the presence of other viruses, as shown by M. Formiga-Cruz et al. (8).
The results of the statistical analysis of the data produced in this study showed that the only group of phages with a significant relationship to all studied viruses was F-RNA phages, but only when all 475 results were analyzed together. However, the utility of this parameter is not clear in the evaluation of the viral contamination in shellfish samples collected in A or clean B areas, especially in countries of southern Europe. In these areas, most of the samples yielding viruses were negative for F-RNA phages, and so this parameter would not provide any additional indication of the presence of human viruses in shellfish compared with E. coli tests. It is important, however, to note that the method used for the quantification of the studied phages was well standardized and was successfully implemented, without significant quality problems or cost, in all laboratories involved in the study, according to a performance assessment program. Quantification of F-RNA phages may be useful as a parameter complementary to E. coli and as an indicator for specific parameters in specific locations, for instance, NLV in the United Kingdom. Furthermore, although shellfish ready for human consumption in Europe yield <230 E. coli organisms/100 g of shellfish, the addition of a viral indicator ensuring, if possible, the absence of F-RNA phages could improve the microbiological control of the shellfish distributed to be consumed raw or lightly cooked with a reasonable cost for monitoring the final product in specific geographical areas.
Acknowledgments
This research was carried out with financial support from the Commission of the European Communities, Agriculture and Fisheries (FAIR) Project CT98-4039. M. Formiga-Cruz is a fellow of the Spanish Government.
We thank the National Center of Aquaculture of St. Carles de la Rapita (Spain) for technical support and Rosa Bufías Obón for her technical assistance. We also thank Miquel Calvo for his assistance with statistical analysis.
REFERENCES
- 1.Anonymous. 1991. Council directive of 15th July laying down the health conditions for the production and placing on the market of live bivalve molluscs (91/492/EEC). Off. J. Eur. Communities L268:1-13.
- 2.Anonymous. 1996. ISO 10705-1 (amended version): water quality. Detection and enumeration of bacteriophages—part 1: enumeration of F-specific RNA bacteriophages. International Organization for Standardization, Geneva, Switzerland.
- 3.Anonymous. 1999. ISO/FDIS 10705-2: water quality. Detection and enumeration of bacteriophages—part 2: enumeration of somatic coliphages. International Organization for Standardization, Geneva, Switzerland.
- 4.Anonymous. 1999. ISO/DIS 10705-4: water quality. Detection and enumeration of bacteriophages—part 4: enumeration of bacteriophages infecting Bacteroides fragilis. International Organization for Standardization, Geneva, Switzerland.
- 5.Desenclos, J.-C. A., K. C. Klontz, M. H. Wilder, O. V. Nainan, H. S. Margolis, and R. A. Gunn. 1991. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am. J. Public Health 81:1268-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan, T. J., S. Gallacher, N. J. Andrews, M. H. Greenwood, J. Graham, J. E. Russell, D. Roberts, and R. Lee. 1998. Modification of the standard procedure used in the United Kingdom for counting Escherichia coli in live bivalve molluscs. Commun. Dis. Public Health 1:188-196. [PubMed] [Google Scholar]
- 7.Doré, W., K. Henshilwood, and D. N. Lees. 2000. Evaluation of F-specific RNA bacteriophages as a candidate of human enteric virus for bivalve molluscan shellfish. Appl. Environ. Microbiol. 66:1280-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formiga-Cruz, M., G. Tofiño-Quesada, S. Bofill-Mas, D. N. Lees, K. Henshilwood, A. K. Allard, A.-C. Condin-Hansson, B. E. Hernroth, A. Vantarakis, A. Tsibouxi, M. Papapetropoulou, D. Furones, and R. Girones. 2002. Distribution of human viral contamination in shellfish from different growing areas in Greece, Spain, Sweden and the United Kingdom. Appl. Environ. Microbiol. 68:5990-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerba, C. P. 1979. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health 69:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girones, R., J. Jofre, and A. Bosch. 1989. Natural inactivation of enteric viruses in seawater. J. Environ. Qual. 18:34-39. [Google Scholar]
- 11.Halliday. M. L., L.-Y. Kang, T.-K. Zhou, M. D. Hu, Q. C. Pan, T. Y. Fu, Y. S. Huang, and S. L. Hu. 1991. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J. Infect. Dis. 164:852-859. [DOI] [PubMed] [Google Scholar]
- 12.Havelaar, A. H., and W. H. Hogeboom. 1984. A method for the enumeration of male-specific bacteriophages in sewage. J. Appl. Bacteriol. 56:439-447. [DOI] [PubMed] [Google Scholar]
- 13.Havelaar, A. H., K. Furuse, and W. M. Hogeboom. 1986. Bacteriophages and indicator bacteria in human and animal faeces. J. Appl. Bacteriol. 60:255-262. [DOI] [PubMed] [Google Scholar]
- 14.Havelaar, A. H., M. van Olphen, and Y. C. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller, D., O. N. Gill, E. Raynham, T. Kirkland, P. M. Zadick, and R. Stanwell-Smith. 1986. An outbreak of gastrointestinal illness associated with consumption of raw depurated oysters. Br. Med. J. 292:1726-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IAWPRC Study Group on Health Related Water Microbiology. 1991. Bacteriophages as model viruses in water quality control. Water Res. 25:529-545. [Google Scholar]
- 17.Jofre, J., A. Bosch, F. Lucena, R. Girones, and C. Tartera. 1986. Evaluation of Bacteroides fragilis bacteriophages as indicators of the virological quality of water. Water Sci. Technol. 18:167-173. [Google Scholar]
- 18.Lees, D. 2000. Viruses and bivalve shellfish. Int. J. Food Microbiol. 59:81-116. [DOI] [PubMed] [Google Scholar]
- 19.Lees, D. N., K. Henshilwood, and W. J. Dore. 1994. Development of a method for detection of enteroviruses in shellfish by PCR with poliovirus as a model. Appl. Environ. Microbiol. 60:2999-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucena, F., J. Lasobras, D. McIntosh, M. Forcadell, and J. Cofre. 1994. Effect of distance from the polluting focus on relative concentrations of Bacteroides fragilis phages and coliphages in mussels. Appl. Environ. Microbiol. 60:2272-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muniain-Mujika, I., R. Girones, and F. Lucena. 2000. Viral contamination of shellfish: evaluation of methods and analysis of bacteriophages and human viruses. J. Virol. Methods 89:109-118. [DOI] [PubMed] [Google Scholar]
- 22.Muniain-Mujika, I., R. Girones, G. Tofiño-Quesada, M. Calvo, and F. Lucena. 2002. Depuration dynamics of viruses in shellfish. Int. J. Food Microbiol. 77:125-133. [DOI] [PubMed] [Google Scholar]
- 23.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughn, J. M., and T. G. Metcalf. 1974. Coliphages as indicators of enteric viruses in shellfish and shellfish raising estuarine waters. Water Res. 8:613-616. [Google Scholar]
- 25.Woody, M. A., and D. O. Cliver. 1995. Effects on temperature and host cell growth phase on replication of F-specific RNA coliphages Qβ. Appl. Environ. Microbiol. 61:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]