Abstract
Macropinocytosis results from the closure of lamellipodia generated by membrane ruffling, thereby reflecting cortical actin dynamics. Both transformation of Rat-1 fibroblasts by v-Src or K-Ras and stable transfection for expression of dominant-positive, wild-type phosphoinositide 3-kinase (PI3K) regulatory subunit p85α constitutively led to stress fiber disruption, cortical actin recruitment, extensive ruffling, and macropinosome formation, as measured by a selective acceleration of fluid-phase endocytosis. These alterations closely correlated with activation of PI3K and phosphatidylinositol-specific phospholipase C (PI-PLC), as assayed by 3-phosphoinositide synthesis in situ and in vitro and inositol 1,4,5 trisphosphate steady-state levels, respectively; they were abolished by stable transfection of v-Src–transformed cells for dominant-negative truncated p85α expression and by pharmacological inhibitors of PI3K and PI-PLC, indicating a requirement for both enzymes. Whereas PI3K activation resisted PI-PLC inhibition, PI-PLC activation was abolished by a PI3K inhibitor and dominant-negative transfection, thus placing PI-PLC downstream of PI3K. Together, these data suggest that permanent sequential activation of both PI3K and PI-PLC is necessary for the dramatic reorganization of the actin cytoskeleton in oncogene-transformed fibroblasts, resulting in constitutive ruffling and macropinocytosis.
INTRODUCTION
Macropinocytosis refers to the formation of large, irregular primary endocytic vesicles by the closure of lamellipodia generated at ruffling membrane domains (for review, see Swanson and Watts, 1995). This phenomenon, therefore, is basically different from the endocytic recapture of the limiting membrane from large secretory granules after regulated secretion (Thilo, 1985). Although macropinocytosis was the first discovered form of nonparticulate endocytosis, i.e., pinocytosis (Lewis, 1931), research has focused for decades on receptor-mediated endocytosis in view of its specificity, physiopathological implications, and regulation by a multiprotein machinery of increasing complexity (for a recent review, see Marsh and McMahon, 1999). In addition to supporting receptor-mediated endocytosis, clathrin-coated micropinocytic pits certainly contribute a substantial fraction of fluid-phase endocytosis, if not its totality (Cupers et al., 1994).
Recently, however, macropinocytosis has received increasing attention. From a fundamental point of view, macropinocytosis depends directly on membrane–cytoskeleton interactions, thereby reflecting cortical actin dynamics and possibly cell motility. From a functional perspective, macropinocytosis may account for total nutrient supply in axenic strains of the amoeba Dictyostelium discoideum (Hacker et al., 1997), for sampling of foreign antigens by immature dendritic cells (Sallusto et al., 1995) to be presented by class II or even class I major histocompatibility complexes (Norbury et al., 1995), and for the burst of uptake of the thyroid hormone precursor thyroglobulin in response to acute thyroid-stimulating hormone stimulation (Ketelbant-Balasse et al., 1973; Ericson et al., 1983). Presumably, macropinocytosis has also been subverted by pathogens such as Salmonella and Shigella that trigger their uptake to float in spacious phagosomes (Alpuche-Aranda et al., 1994), and it may be used by Chlamydia for entry into dendritic cells (Ojcius et al., 1998). Interestingly, the regulation of macropinocytosis and phagocytosis in D. discoideum are different (Seastone et al., 1999).
In most cell types, macropinocytosis is a transient response to phorbol esters (Swanson, 1989) or growth factors such as insulin, platelet-derived growth factor (PDGF), or macrophage colony-stimulating factor (M-CSF) (Racoosin and Swanson, 1992), i.e., it vanishes rapidly despite continuous stimulation. In contrast, this activity is constitutive in axenic strains of Dictyostelium (Hacker et al., 1997; Seastone et al., 1999), in macrophages and dendritic cells (Swanson and Watts, 1995), and in v-Src–transformed fibroblasts (Veithen et al., 1996, 1998). In the latter cells, topological origin, kinetics of intracellular movement, and sensitivity to a variety of pharmacological agents clearly distinguish macropinosomes from endosomes labeled by receptor-mediated endocytosis of transferrin (Veithen et al., 1998). Bar-Sagi and Feramisco (1986) have reported that microinjection of Ras into fibroblasts also produces ruffling and the appearance of large pinocytic vacuoles, presumably macropinosomes. Conversely, microinjection in Ras-transformed cells of anti-Ras antibodies abrogated ruffling and large pinocytic vacuole formation (Bar-Sagi et al., 1987). It is uncertain whether signaling machineries are identical for transient and constitutive macropinocytosis.
Constitutive macropinocytosis is abolished by pharmacological inhibitors of phosphoinositide 3-kinase (PI3K) and phosphatidylinositol-specific phospholipase C (PI-PLC) in v-Src–transformed fibroblasts (Veithen et al., 1998) and by targeted PI3K gene disruption in Dictyostelium (Zhou et al., 1998). Because transient signaling downstream of PDGF involves both PI3K and PI-PLC (Falasca et al., 1998; Rameh et al., 1998), it was tempting to suggest that the pathway from Src and Ras to constitutive macropinocytosis also depends on PI3K and PI-PLC and requires their permanent activation. This, in turn, would activate Rac, a key regulator of cytocortical actin dynamics.
Despite Src being a key regulator of the actin cytoskeleton, its downstream signaling is still unclear (Thomas and Brugge, 1997; Penuel and Martin, 1999). Reactivation of thermosensitive mutants of v-Src rapidly induces extensive reorganization of the actin cytoskeleton, including induction of membrane ruffling and stress fiber breakdown (Boschek et al., 1981), which precede the loss of focal adhesion (Meijne et al., 1997). Src phosphorylates several cytocortical targets, including cortactin (Dehio et al., 1995) and focal adhesion kinase (Meijne et al., 1997). Alternatively, v-Src could indirectly control the cytoskeleton by activation of PI3K upon interaction of its SH3 domain with the regulatory subunit of PI3K (Liu et al., 1993). Whether these two pathways cross-talk, and/or which predominantly controls the actin cytoskeleton, are unknown at present.
Among its multiple downstream signaling pathways, Ras, like Src, signals to Rac via PI3K (Rodriguez-Viciana et al., 1997). Ras coimmunoprecipitates with and directly activates PI3K (Sjölander et al., 1991; Rodriguez-Viciana et al., 1997). Microinjection of the constitutively activated V12 Ras mutant produces profound alterations of the cytoskeleton, including membrane ruffling, that are abolished by wortmannin or dominant-negative Rac. In contrast, ruffling produced by constitutively active Rac is not sensitive to wortmannin (Kotani et al., 1995; Nobes et al., 1995).
The heterodimeric PI3K enzymes are divided in three classes (Fruman et al., 1998). Class IA mammalian enzymes are made of a catalytic subunit, p110, that can be activated by Ras and a regulatory or adaptor subunit, p85, that includes two SH2 domains and one SH3 domain and that can be translocated to the plasma membrane by Src. In turn, the regulatory subunit recruits the PI3K catalytic subunit at the plasma membrane, where it catalyzes the phosphorylation of the hydroxyl group on the third carbon of the inositol ring of inositol lipids, producing the D3 phosphoinositides subgroup, mainly phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] and phosphatidyl-inositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] (Martin, 1998). As shown with the insulin signaling cascade leading to GLUT1 translocation, overexpression of wild-type p85α leads to a dominant-positive phenotype, whereas overexpression of a truncated form unable to interact with the catalytic subunit results in a dominant-negative phenotype (Hara et al., 1994).
PI-PLC can also be activated by plasma membrane recruitment to generate the second messengers inositol 1,4,5-trisphosphate (1P3), which increases the cytosolic calcium concentration, and diacylglycerol, which activates PKC isoenzymes. Among various PI-PLC subclasses, PLCγ isoforms include a large internal region containing two SH2 domains and one SH3 domain that mediate its interaction with tyrosine kinase–linked receptors and possibly Src kinases as well as their products. In addition, PLCγ1, but not PLCγ2, preferentially associates with membrane ruffles and can be activated directly by PI3K lipid products (Barker et al., 1998). PtdIns(3,4,5)P3 activates PLCγ isoforms in vitro upon interaction with their SH2 domains (Bae et al., 1998), and overexpression of a constitutively active PI3K increases intracellular IP3 levels, whereas, conversely, PLCγ1 activation is blocked by PI3K inhibitors in mast cells (Barker et al., 1998). However, although these observations suggest a sequential activation of PI3K and PLCγ1, the existence of independent PI3K and PLCγ1 pathways in response to PDGF activation has been suggested in endothelial cells (Rönnstrand et al., 1999).
Thus, multiple, possibly interacting, pathways documented for transient responses in other systems could be permanently activated to account for constitutive ruffling and macropinocytosis of oncogene-transformed cells. In this paper, we have explored signaling pathways that should be activated by v-Src and K-Ras to reorganize the actin cytoskeleton and result in constitutive macropinocytosis. Macropinocytosis was characterized by morphological approaches and quantified by biochemical assays. We used two unrelated pharmacological inhibitors of both PI3K and PI-PLC, measured these enzyme activities, and established two stable PI3K transfectants. The first transfectant, to express in control cells wild-type p85α and generate a dominant-positive phenotype, served to test whether PI3K activation would be sufficient for constitutive macropinocytosis. The other transfectant, to express in v-Src–transformed cells a truncated p85α and generate a dominant-negative phenotype, was used to test whether PI3K activation by the oncogene is indeed necessary for constitutive macropinocytosis. We found that transduction signaling leading to constitutive macropinocytosis in oncogene-transformed fibroblasts depends on permanent sequential activation of PI3K and PI-PLC.
MATERIALS AND METHODS
Cell Culture
Parental Rat-1 cells (Rat-1/control) and Rat-1 cells transformed by the B77 subclone of Rous sarcoma virus (Rat-1/v-Src) were obtained from Dr. Guy Rousseau (Christian de Duve Institute of Cellular Pathology; these cell lines were originally transferred to this institute by Dr. John Wyke, The Beatson Institute for Cancer Research, Glasgow, UK). The endocytic properties of these cell lines have been characterized (Veithen et al., 1996, 1998). Rat-1 cells infected by the Kirsten sarcoma virus (Rat-1/K-Ras) were kindly provided by Dr. John Wyke and subcloned in our laboratory based on the transformed phenotype. Two such clones provided identical results. The various cell lines were grown at 37°C in DMEM (Life Technologies, Grand Island, NY) supplemented with 20 mM glucose, 4 mM glutamine, 10 mM NaHCO3, 10 mM HEPES, 10 μg/ml streptomycin, 66 μg/ml penicillin, and 10% (vol/vol) FCS (Life Technologies) under 8% CO2. They were usually seeded for biochemical experiments at ∼106 cells on 8-cm2 Petri dishes and at 30,000 to 40,000 cells/cm2 on either glass chambers (Lab-Tek, Life Technologies, Nunc, Roskilde, Denmark) or glass coverslips for confocal microscopy. We have shown previously that the fluid-phase endocytic uptake of peroxidase in Rat-1/control and Rat-1/v-Src cells is independent of the cell density in the range used for our studies (Veithen et al., 1998). Before each experiment, cells were rinsed twice with FCS-free DMEM and preincubated therein for 30 min before the addition on endocytic tracers to avoid interference by short-lived growth factor effects.
Transfection
To obtain cell lines stably transfected with the wild-type (Wp85α) or mutant (Δp85α) regulatory subunit of bovine PI3K, cells were cotransfected with 10 μg of Wp85α or Δp85α expression plasmids (under the control of the SRα promotor; kindly provided by Dr. Wataru Ogawa, Kobe University, Kobe, Japan) (Hara et al., 1994; Kotani et al., 1994) and 1 μg of pSVTKNEO β selection plasmid with the use of the Lipofectamine method as recommended by the manufacturer (Life Technologies). Stable lines were selected by exposure to 0.5 mg/ml geneticin (G418; Life Technologies) and subcloned by limiting dilution.
Scanning Electron Microscopy
Cells cultured overnight on glass (50,000 cells/coverslip) were washed briefly with PBS followed by 0.14 M cacodylate and then fixed for 20 min at room temperature by the slow addition of 4% glutaraldehyde in 0.1 M cacodylate until a final 2% glutaraldehyde concentration was reached. After two washes in 0.14 M cacodylate, cells were postfixed in 2% osmium tetroxide in 0.1 M phosphate buffer for 1 h at 4°C, dehydrated in a graded ethanol series, and critical point-dried (CPD 020 unit, Balzers Union, Liechtenstein). A 10-nm gold film was sputter-coated, and specimens were observed at 80 kV in a CM12 electron microscope (Philips, Eindhoven, the Netherlands) with the use of the secondary electron detector. Images were stored in digital form with the use of the SIS analysis software, version 2.1 (Soft-Imaging Software, Münster, Germany).
Confocal Microscopy
After filling by a brief incubation in 1 mg/ml Texas Red–dextran, macropinosomes in living cells were studied with an Axiovert confocal microscope (Zeiss, Oberkochen, Germany) coupled to MRC 1024 confocal scanning equipment (Bio-Rad, Richmond, CA) with the use of both bright-field contrast and fluorescence images. General cell structure images were captured by the transmission bright-field detectors with the use of the blue light channel, instead of phase contrast, to prevent weakening of the emission light from the fluorochromes by the contrast ring of the objective. Observation was made with a Zeiss plan-apochromat 63X/1.40 oil differential interference contrast objective, without the use of a polarizer, analyzer, or the corresponding Wollaston prism. This mode corresponds to a bright-field image without phase or differential interference contrast. To achieve satisfactory contrast, classic Koehler illumination was complemented by a supplementary diaphragm (differential interference contrast, 0.3–0.4/0.9) in the condenser turret of the Axiovert confocal microscope. The formation of macropinosomes at ruffling zones was followed by time-lapse recording at 10-s interval.
For actin cytoskeleton staining, cells were plated on glass coverslips, fixed in formaldehyde, permeabilized with Triton X-100, and incubated with 1 μg/ml rhodamine–phalloidin (Molecular Probes, Eugene, OR) for 15 min at 4°C with 0.1% BSA in PBS-Ca2+ (137 mM NaCl, 5.4 mM KCl, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 3.6 mM CaCl2, 3 mM MgCl2, pH 7.3) in the dark. After extensive washing (five times) with PBS, coverslips were mounted in Mowiol (Calbiochem, San Diego, CA) with 2.5% DABCO (Janssen, Beerse, Belgium) and examined with the confocal microscope.
Fluid-Phase Endocytosis of Peroxidase
After the indicated intervals in DMEM supplemented with 4 mg/ml HRP (type 2; Boehringer Mannheim, Mannheim, Germany), cells were rapidly transferred to 4°C by immersion in PBS-Ca2+ and washed extensively (three rapid washes with 3 ml of PBS-Ca2+, one wash for 5 min in 1 ml of DMEM containing 10% outdated FCS, and three rapid washes with 3 ml of PBS-Ca2+). Cells were surface-digested at 4°C for 1 h in DMEM supplemented with 0.15% (wt/vol) pronase (Sigma Chemical, St. Louis, MO) and pelleted in a benchtop microfuge (Beckman, Fullerton, CA) for 10 min at 4°C. The pellet was washed twice by resuspension/sedimentation in PBS-Ca2+ and finally lysed in 0.01% (vol/vol) Triton X-100 (Serva Biochemicals, Heidelberg, Germany). HRP activity in the lysate was measured by a stopped colorimetric assay with the use of ortho-dianisidine as a substrate (Cupers et al., 1994), normalized to a 1 mg/ml extracellular concentration (to provide data equivalent to clearance values, expressed in nl/mg cell protein), and divided by the cell protein content measured by the bicinchoninic acid procedure with BSA as a standard (Smith et al., 1985). Endogenous peroxidase activity, equivalent to ∼15 ng HRP/mg cell protein, was subtracted from all values as blank.
For experiments with cytochalasin E, wortmannin, 2-nitro-4-carboxyphenyl N,N-diphenylcarbamate (NCDC) (all Sigma), LY294002, and U73122 (both Calbiochem), all stock solutions were made in DMSO. The final concentration in this solvent never exceeded 0.2% (vol/vol), at which concentration DMSO affected neither the quantitative intracellular uptake of HRP nor the qualitative labeling with Texas Red–dextran.
Receptor-mediated Endocytosis of Transferrin
Iron-saturated human transferrin (Sigma) was radiolabeled with [125I]iodine (100 mCi/ml; Amersham, Buckinghamshire, UK) as described (McFarlane, 1958) to a specific radioactivity of 500-1100 cpm/ng protein. Cells were incubated at 37°C in DMEM containing 2 μg/ml 125I-transferrin, washed extensively at 4°C, surface-digested with pronase, pelleted, rinsed, and lysed in Triton X-100 as described above. The radioactivities associated with the pellet and the supernatant were taken as measures of intracellular and surface-bound transferrin, respectively. Intracellular 125I-transferrin content was divided by pronase-releasable counts to yield endocytosis efficacy (Wiley and Cunningham, 1982).
Pulse-Chase Experiments
For pulse-chase experiments, cells were incubated at 37°C in DMEM supplemented with HRP for 30 min or with transferrin for 7 min, washed briefly at 4°C, and reincubated at 37°C for the indicated intervals. Thereafter, cells were transferred rapidly to 4°C, washed extensively as described above, digested with pronase, pelleted, lysed, and assayed for HRP activity or radioactivity and protein content. Results were expressed as the residual fraction of the intracellular amount of tracer at the initiation of the chase and adjusted by least-squares fitting to an exponential decay equation as reported previously (Cupers et al., 1994).
Western Blotting
Cells were solubilized in 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, a mixture of aprotinin, leupeptin, and pepstatin (5 mg/ml each), 1 mM Na3VO4, and 1 mM NaF, boiled for 3 min in Laemmli buffer with 0.1 M DTT, and separated on 7.5% polyacrylamide gels. Proteins were transferred from the gel to a polyvinylidene difluoride membrane (Boehringer Mannheim) during 16 h at 250 mA in 100 mM Tris, 16 mM glycine, and 20% methanol. After blocking for ∼2 h in 50 mM Tris, 90 mM NaCl, and 2 mM CaCl2, pH 8, containing 5% (wt/vol) BSA, the membrane was incubated overnight at 4°C under gentle shaking with 1 μg/ml in the same buffer containing 1% BSA and a 1:1000 dilution of rabbit anti-rat p85 antiserum (Upstate Biotechnology, Lake Placid, NY). Bound antibodies were detected with 125I-protein A (30 mCi/mg, 1 μCi/blot; Amersham) and then washed. Dried polyvinylidene difluoride membranes were exposed for 1–2 d to a phosphor screen, and the signal was detected with a PhosphorImager and quantified with ImageQuant (both from Molecular Dynamics, Sunnyvale, CA).
In Situ Assay of PI3K
This assay was based on metabolic labeling of lipids in living cells, followed by HPLC analyses of 3-phosphoinositides. Cells (∼20 million per 150-cm2 Petri dish) were incubated in phosphate-free DMEM containing 0.5% FCS and 250 μCi/ml [32P]orthophosphate (9000 Ci/mmol; Amersham) for 6 h at 37°C in the presence or absence of inhibitors. Cells were washed briefly in PBS at 4°C and harvested with 3.75 ml of 2.4 N HCl, and the dish was further rinsed with 3 ml of methanol. Lipids of these combined fluids were extracted with 4.5 ml of chloroform, dried under N2, and then separated by thin-layer chromatography on silica gel–coated plates (Merck, Darmstadt, Germany) with the use of chloroform:acetone:methanol:acetic acid:water (80:30:26:24:14, vol/vol). The spots corresponding to PtdIns(3,4)P2 and PtdIns(3,4,5)P3 were identified by staining with iodine vapor, scraped off, deacylated, and analyzed with HPLC on a SAX column (Whatman, Maidstone, United Kingdom), as described (Gratacap et al., 1998).
In Vitro Assay of PI3K
Cells were solubilized on ice with 500 μl of lysis buffer (50 mM HEPES, pH 7.2, 50 mM β-glycerophosphate, 50 mM KF, 5 mM NaPPi, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2% Triton X-100, 1 mM DTT, 1% Nonidet P-40, 0.5 μM microcystin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM benzamidine, 0.2 mM PMSF, 4 μg/ml trypsin inhibitor, 1 μg/ml pepstatin, 2 mM Na3VO4). To test for insulin sensitivity, control cells were incubated with 100 nM insulin for exactly 1 min. After 10 s of agitation, the lysate was frozen as aliquots in liquid N2. For the analysis, thawed samples were centrifuged at 4°C (20 min at 10,000 × g). An aliquot of the supernatant (corresponding to 1 mg of protein) was immunoprecipitated in the presence of lysis buffer for 2 h with 3 μg of rabbit anti-IRS-1 (Upstate Biotechnology) preabsorbed to 20 μl of protein A–Sepharose (Pharmacia Biotech, Uppsala, Sweden). The immunoprecipitate was washed three times with PBS containing 1 mM Na3VO4, including 1% Nonidet P-40 in the first washing. PI3K activity was finally measured as described (Krause et al. 1996).
In Situ Assay of PI-PLC
This activity was assayed by quantification of intracellular IP3 levels, as recommended by the manufacturer (New England Nuclear Life Science, Boston, MA). Briefly, cells were preincubated at 37°C in DMEM supplemented with 10% FCS overnight, washed rapidly with PBS-Ca2+, and transferred for 1 h to DMEM without serum in the presence or absence of the indicated inhibitors; the last 10 min occurred with 10 mM added LiCl. The reaction was terminated by aspirating the medium off the cells and adding 20% (wt/vol) ice-cold trichloroacetic acid. Cells were scraped off on ice, vortexed, and centrifuged at 1000 × g for 10 min. The supernatant was further incubated for 15 min at room temperature. After trichloroacetic acid was removed by extraction with trichlorotrifluoroethane:trioctylamine (3:1), IP3 in cell extracts was determined by a competitive radioligand-binding assay with the use of a standard membrane receptor preparation and 0.4 μCi/ml [3H]IP3. After 1 h of binding on ice, membranes were recovered by centrifugation at 2000 × g for 20 min and dissolved with 0.15 M NaOH, and radioactivity was determined in a scintillation counter. IP3 concentration was determined in triplicate based on a standard curve and expressed as pmol/mg protein.
RESULTS
v-Src and K-Ras Constitutively and Selectively Accelerate Fluid-Phase Endocytosis in Fibroblasts
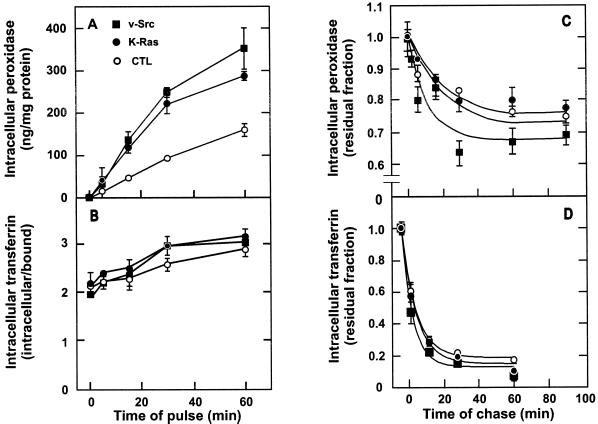
We have previously reported, in experiments with both a thermosensitive and a thermostable variant of pp60src, that v-Src causes constitutive macropinocytosis in Rat-1 fibroblasts (Veithen et al., 1996, 1998). The analysis was first extended to subcloned K-Ras–transformed Rat-1 cells. Fluid-phase endocytosis, followed by the intracellular accumulation of HRP in serum-deprived cells, was similarly accelerated approximately twofold by either v-Src or K-Ras (Figure 1A). The difference was already evident after 5 min of uptake, arguing against an effect by inhibition of fluid-phase efflux (i.e., regurgitation). This was confirmed by pulse-chase experiments, in which efflux was comparable in K-Ras–transformed fibroblasts and even moderately accelerated in v-Src–transformed fibroblasts (Figure 1C). Thus, both oncogene products accelerate fluid-phase entry. This effect was selective for fluid-phase endocytosis, because pathways of receptor-mediated endocytosis of transferrin were only marginally affected by v-Src and K-Ras, both in entry (Figure 1B) and efflux (Figure 1D). Although Src kinase was recently reported to accelerate receptor-mediated endocytosis of EGF, its effect was also marginal in fibroblasts after intervals of uptake of >1 min, as used in the present study (Wilde et al., 1999). A selective stimulation of fluid-phase endocytosis is suggestive of macropinocytosis.
Figure 1.
Constitutive stimulation of fluid-phase, but not receptor-mediated, endocytosis by v-Src and K-Ras. Control (○), v-Src–transformed (▪), or K-Ras–transformed (●) Rat-1 fibroblasts were transferred for 30 min in DMEM without serum and then incubated in DMEM supplemented with 4 mg/ml HRP for the indicated intervals of pulse (A) or 30 min (C) or with 50 nM 125I-transferrin and 1% BSA for the indicated intervals of pulse (B) or 7 min (D). Cells in C and D were briefly washed at 4°C and reincubated in DMEM at 37°C for the indicated intervals of chase. Finally, cells were washed extensively at 4°C and surface-digested with pronase. Values of intracellular accumulation of HRP were normalized as indicated in MATERIALS AND METHODS. Intracellular 125I-transferrin accumulation was divided by pronase-releasable counts (considered as surface-bound) to yield receptor-mediated endocytosis efficacy. In C and D, residual intracellular contents nor-malized as described above were expressed as percentages of corresponding values at the end of the chase, and curves were fitted to monoexponential decays. Values shown are means ± SD of three dishes from one experiment. Kinetics of uptake and chase was reproduced at least three times and twice, respectively.
Production of Macropinosomes by Constitutive Ruffling and Their Fate in Transformed Fibroblasts
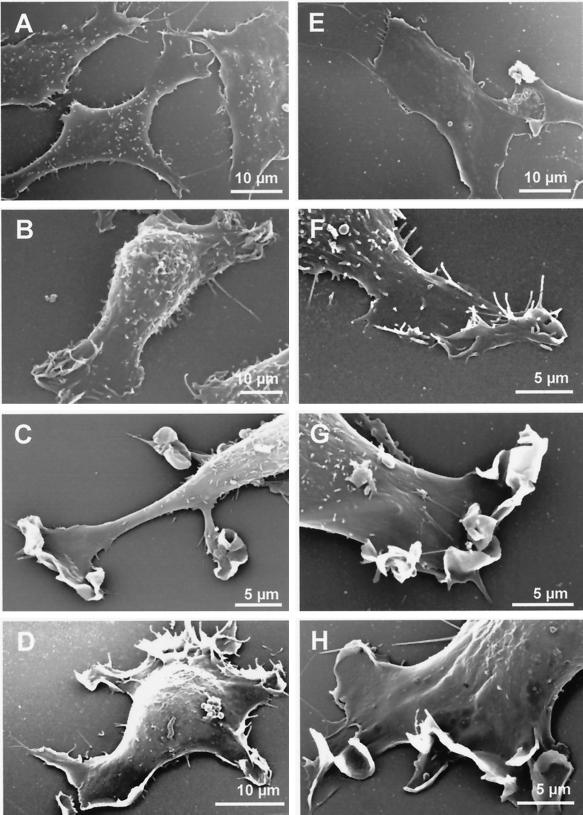
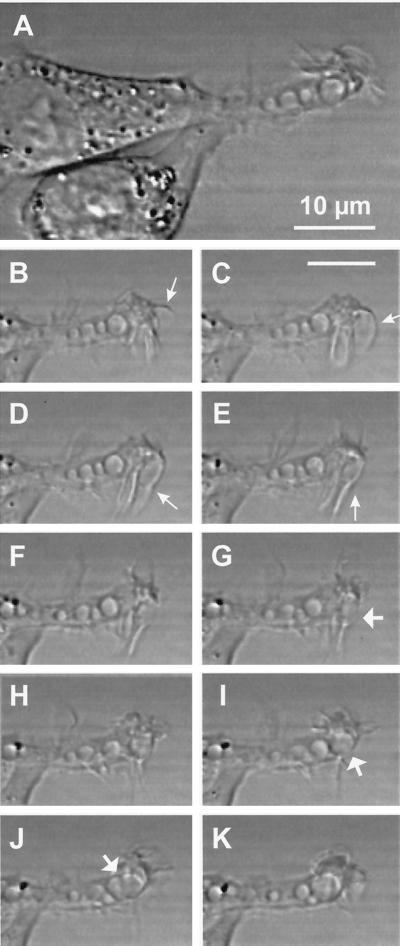
In contrast to the spindle-shaped parental Rat-1 fibroblasts, v-Src– and K-Ras–transformed Rat-1 fibroblasts generally exhibited one major or a few elongated cellular extensions that showed active ruffling at their tips. By scanning electron microscopy, these active zones generated curling lamellipodia (Figure 2). With bright-field contrast and time-lapse recording, individual lamellipodia were most conveniently studied at the tips of these cellular extensions, where they were shown to fold back against one another and isolate large fluid droplets that rounded up within 10 to 20 s, i.e., macropinosomes (Figure 3). At the peripheral ruffling zones, macropinosomes were generated one after the other, about every 1 min, producing linear alignments along the slender cellular extensions. Macropinosomes then showed a slow centripetal movement along the cellular extension, at a rate of ∼1 μm/min at ∼25°C. This kinetics is comparable to that reported for the lucent pinocytic vesicles generated at the growth cone of isolated dorsal root ganglion neurons (Nakai, 1956) or for photobleached actin filaments at lamellipodia (Condeelis, 1993). Infrequently, macropinosomes could fuse with one another; again, the fusion product rapidly rounded up, indicating external tension or internal pressure.
Figure 2.
Scanning electron microscopy. (A) Control Rat-1 fibroblasts; (B and F) v-Src fibroblasts; (C and G) K-Ras fibroblasts; (D and H) Wp85α fibroblasts; (E) v-Src/Δp85α fibroblasts.
Figure 3.
Formation of a macropinosome by membrane ruffling in a v-Src–transformed fibroblast. (A) Bright-field contrast image of overlapping cellular extensions from two adjacent v-Src fibroblasts. The extension in the focal plane contains four round electron-lucent vacuoles of irregular size, and its tip shows active ruffling. The 10 smaller images below are taken from the same field at 10-s intervals. At the ruffling membrane, a large lamellipodium (arrows in B–E) appears to fold back and to generate an ovoid macropinosome (arrow in G), which rounds up within ∼10 s (H), then docks to (I) and fuses with (J) a preexisting macropinosome. The fused organelle similarly rounds up within 10 s (K). Bars, 10 μm.
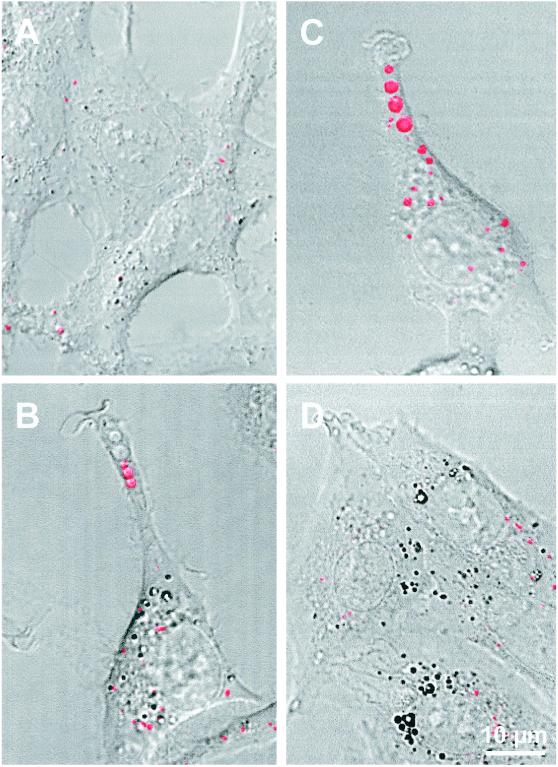
Filling of light-lucent vesicles by Texas Red–dextran could readily be seen by confocal microscopy in v-Src–transformed (Figure 4B) and K-Ras–transformed Rat-1 fibroblasts (comparable image; our unpublished results), directly demonstrating their endocytic nature. The combination of a short pulse with the appropriate setting of fluorescence detection allowed us to limit detection to macropinosomes and to leave endosomes essentially undetected (Veithen et al., 1998). Texas Red–dextran-labeled macropinosomes were exceptional in the parental Rat-1 cells (Figure 4A).
Figure 4.
Filling of macropinosomes by a fluorescent fluid-phase tracer. The indicated cells were incubated for 7 min in DMEM supplemented with 1 mg/ml Texas Red–dextran, washed, and examined immediately in the confocal microscope by fluorescence and bright-field imaging. Fused images are presented. Extensions with ruffled ends that contain several aligned macropinosomes are evident in v-Src (B) and especially Wp85α (C) fibroblasts. They are not seen in control (A) and v-Src/Δp85α (D) fibroblasts. The two nonfluorescent distal macropinosomes in B were presumably formed after washing (see also Veithen et al., 1998).
Constitutive Macropinocytosis in Transformed Fibroblasts Depends on Actin Cytoskeleton Reorganization
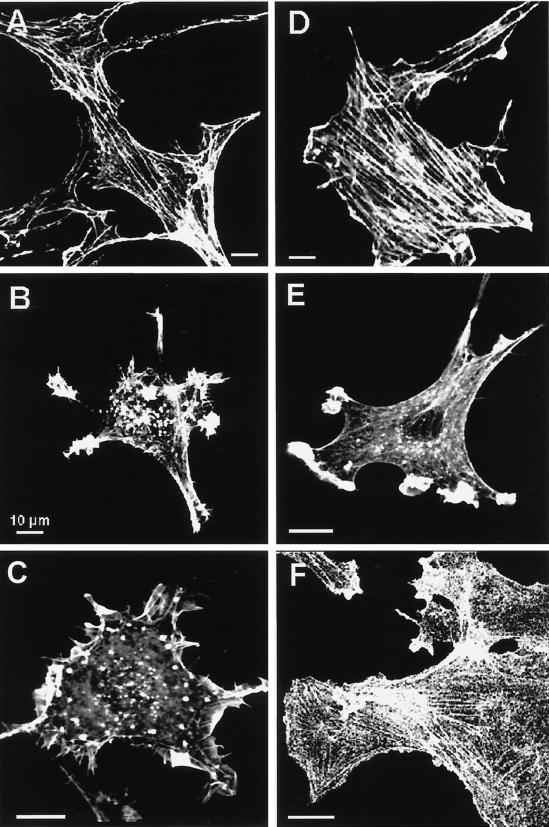
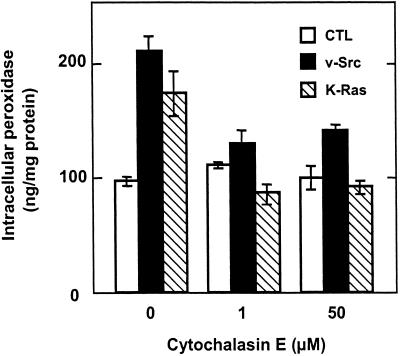
In control Rat-1 fibroblasts, F-actin was mostly associated with longitudinal stress fibers that were colinear when cells were aligned (Figure 5A). In v-Src–transformed (Figure 5B) and K-Ras–transformed Rat-1 fibroblasts (Figure 5E), stress fibers had disappeared and F-actin was mostly recruited to ruffling membrane domains. Upon incubation for 30 min in 1 μM cytochalasin E, one of the most potent members of this family of inhibitors, both stress fibers and F-actin at the ruffling zones became fragmented (our unpublished results). Whereas intracellular accumulation of peroxidase in parental Rat-1 fibroblasts was not affected by cytochalasin E, it was severely inhibited in v-Src– and K-Ras–transformed cells, almost to the level of their nontransformed counterpart (Figure 6).
Figure 5.
Actin cytoskeleton reorganization. F-actin was decorated with rhodamine–phalloidin in untreated control (A), v-Src (B), K-Ras (E), Wp85α (C), v-Src/Δp85α (D), or wortmannin-treated (100 nM for 30 min) Wp85α (F) fibroblasts. Stress fibers are almost absent in v-Src (B), K-Ras (E), and Wp85α (C) fibroblasts but reappear in these cells within 30 min after PI3K inhibition by wortmannin (F) and are prominent in stable v-Src/Δp85α transfectants (D). Bars, 10 μm.
Figure 6.
Constitutive macropinocytosis is abrogated by cytochalasin E in transformed fibroblasts. Control (white bars), v-Src (black bars), and K-Ras (hatched bars) fibroblasts were preincubated for 30 min in DMEM without serum supplemented with the indicated concentrations of cytochalasin E and then incubated for another 30 min in the same medium supplemented with 4 mg/ml HRP. Peroxidase accumulation is calculated as in Figure 1. Values are means ± SD of three dishes.
Constitutive Macropinocytosis in Transformed Fibroblasts Depends on PI3K Activity
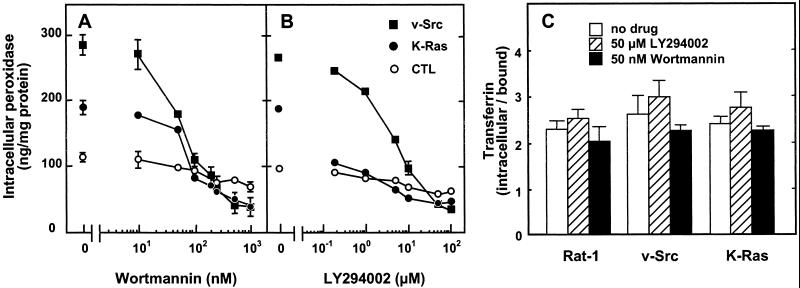
The difference in intracellular peroxidase accumulation between v-Src– or K-Ras–transformed fibroblasts and their nontransformed counterpart was abrogated within less than 1 h upon pharmacological inhibition of PI3K by wortmannin and LY294002 (Figure 7, A and B). The inhibition was dose-dependent and complete at 100 nM wortmannin and 20 μM LY294002. Although receptor-mediated endocytosis of transferrin was reported to be sensitive to wortmannin in various cell types (Spiro et al., 1996), it was not appreciably affected by the two PI3K inhibitors in the three cell lines analyzed in this study (Figure 7C).
Figure 7.
Constitutive macropinocytosis, but not receptor-mediated endocytosis, is abrogated by pharmacological inhibition of PI3K in transformed fibroblasts. Control (○), v-Src (▪), and K-Ras (●) fibroblasts were preincubated for 30 min in DMEM without serum supplemented with the indicated concentrations of wortmannin or LY294002 and then incubated for another 30 min in the same medium with 4 mg/ml added HRP (A and B) or for 10 min with 50 nM added 125I-transferrin and 1% BSA (C). Intracellular tracer accumulation is calculated as in Figure 1. Values are means ± SD of three dishes. The concentration-dependence of the effects of PI3K inhibitors on control and v-Src–transformed fibroblasts was reproduced at least three times.
Effect of Dominant-Positive and Dominant-Negative p85α
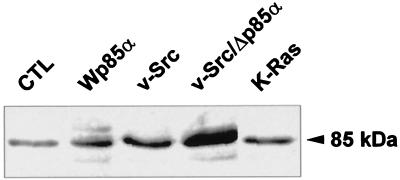
Because bovine regulatory subunit p85α can interact with the p110 PI3K catalytic subunit from various species, we stably transfected the parental Rat-1 fibroblasts with a plasmid coding for wild-type bovine p85α to generate dominant-positive cells (referred to as Wp85α). We also stably transfected v-Src–transformed Rat-1 fibroblasts with an expression vector for a truncated bovine p85α subunit whose domain of interaction with the catalytic PI3K subunit was deleted to generate dominant-negative cells (v-Src/Δp85α). As a result, the intensity of p85 demonstrated by Western blotting increased ∼2.5-fold both in Wp85α cells compared with nontransfected control fibroblasts and in v-Src/Δp85α cells compared with nontransfected v-Src fibroblasts (Figure 8). These values suggest a moderate overexpression of exogenous p85 in the stable transfectants, as anticipated for viable constructs, but do not allow us to accurately establish the relative level of exogenous and endogenous p85, because it cannot be ascertained whether antibodies raised against endogenous (rat) p85 recognize with equivalent efficiency exogenous (bovine) p85. Interestingly, transformation by Src and Ras enhanced p85 expression by itself (∼2.3- and 1.5-fold, respectively).
Figure 8.
p85 expression. Equal loads of the indicated cell lysates were analyzed by Western blotting with a polyclonal antiserum against rat p85.
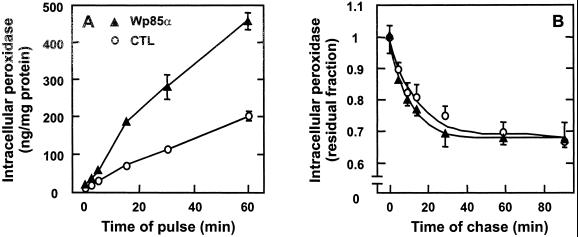
Both stable transfectants showed dramatic phenotypic alterations. Rat-1 cells stably transfected for wild-type bovine p85α expression (Wp85α) assumed a round cell body, devoid of stress fibers (Figure 5C), but showing one major or several cellular extensions, with extensive ruffling (Figure 2, D and H) and active macropinosome generation, even more than in v-Src–transformed cells (Figure 4C). This was reflected by a strong increase of peroxidase accumulation without a change in peroxidase efflux (Figure 9).
Figure 9.
Stable Wp85α transfection of control Rat-1 fibroblasts accelerates fluid-phase endocytosis. Control (○) or Wp85α fibroblasts (▴) were preincubated for 30 min in DMEM without serum, further incubated for the indicated times of pulse in the same medium containing HRP (A) or for 30 min with HRP, washed, and then chased for the indicated times in DMEM (B). Intracellular tracer accumulation is calculated as in Figure 1. Values are means ± SD of four dishes.
In contrast, v-Src–transformed Rat-1 fibroblasts stably transfected for expression of truncated bovine p85α (v-Src/Δp85α) assumed an abnormally flat and spread-out appearance, rigidified by an extensive array of parallel stress fibers (Figure 5D), and no longer showed ruffling (Figure 2E) and macropinosome formation (Figure 4D). This was reflected by a similar level of peroxidase accumulation as in the original Rat-1 fibroblasts (see Figure 11A).
Figure 11.
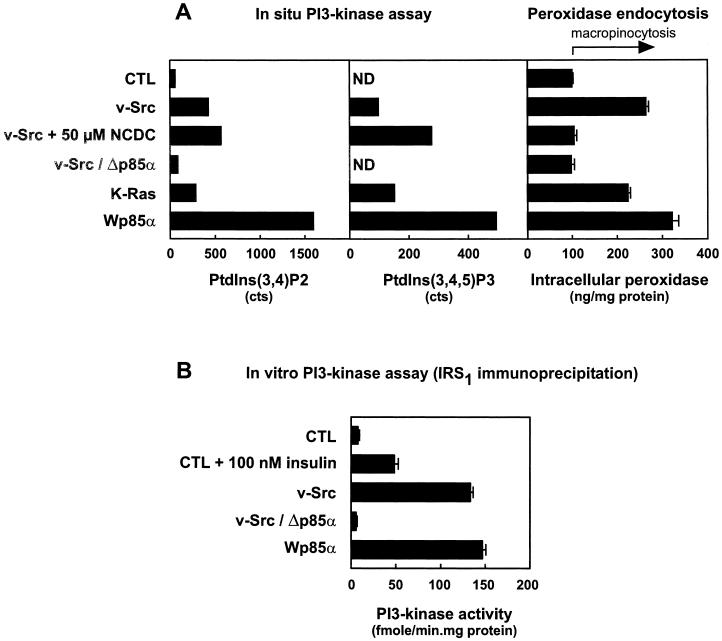
PI3K activity correlates with constitutive macropinocytosis. (A) PI3K was assayed in situ and compared with peroxidase accumulation. The indicated cells were incubated for 6 h in phosphate-free DMEM containing 0.5% dialyzed FCS and 250 μCi/ml [32P]orthophosphate. Inhibitors were added 30 min before this incubation and maintained throughout. After a brief wash, cell lipids were extracted and phosphoinositides were analyzed by HPLC and expressed in absolute counts. ND, not detectable. Data for PtdIns(3,4)P2 levels are from one experiment representative of two to five experiments. Counts of PtdIns(3,4,5)P3 are from the same experiment, which provided the best resolution of this minor compound. These values are compared with macropinocytosis, measured after cells were incubated for 30 min in DMEM without serum in the presence or absence of the inhibitors and then for another 30 min with HRP, as in Figure 1. Values are means ± SEM of 16 to 89 dishes compiled from all experiments performed for this paper. (B) PI3K was assayed in vitro in the indicated cell lines after IRS-1 immunoprecipitation. Values are means ± SEM of five to nine dishes per condition.
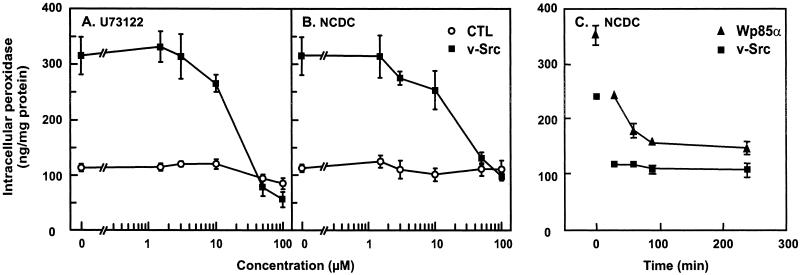
Interestingly, the effect of Src and dominant-positive p85α transfection on the actin cytoskeleton could be reversed within 30–60 min by pharmacological inhibitors of either PI3K (100 nM wortmannin; Figure 5F) or PI-PLC (100 μM NCDC; our unpublished results). This was paralleled by a strong decrease of the population of cells showing active membrane ruffling upon wortmannin (16 ± 8% of the fraction observed in untreated v-Src cells, 2 ± 4% for Wp85α cells) or NCDC (32 ± 14% of the fraction in untreated v-Src cells, 10 ± 8% for Wp85α cells) administration. In addition, the PI-PLC inhibitors NCDC and U73122 suppressed macropinocytosis in v-Src–transformed fibroblasts and in Wp85α cells (Figure 10, A–C), without detectable effect on receptor-mediated endocytosis of transferrin. These rapid inhibitions indicated that active cytoskeleton remodeling, membrane ruffling, and macropinocytosis did not depend on (indirect) changes in gene expression but depended directly on permanent activation of PI3K and PI-PLC in these cells. This prediction was tested directly by corresponding enzyme assays.
Figure 10.
PI-PLC inhibitors abrogate constitutive macropinocytosis in v-Src–transformed and Wp85α stably transfected fibroblasts. (A and B) Control or Src fibroblasts were preincubated for 30 min in DMEM without serum supplemented with the indicated concentrations of inhibitors and then incubated for another 30 min in the same medium with 4 mg/ml added HRP. (C) Rat-1/v-Src and Rat-1/Wp85α fibroblasts were preincubated for the indicated times with 100 μM NCDC, and HRP was added for the last 30 min of incubation. Values are means ± SD of four dishes. The concentration-dependence of the effects of PI-PLC inhibitors on control and v-Src–transformed fibroblasts was reproduced at least three times.
Assays of PI3K
PI3K activity was first measured by an in situ assay, based on incorporation of 32P, lipid extraction, and phosphoinositide analysis by HPLC, and compared with macropinocytosis, measured by the difference in peroxidase accumulation with control cells (Figure 11A). In Rat-1 and the dominant-negative construct v-Src/Δp85α, 32P incorporation into PtdIns(3,4)P2 was low, and no incorporation could be detected at the position of PtdIns(3,4,5)P3. In contrast, incorporation into PtdIns(3,4)P2 was increased ∼7-fold upon v-Src transformation, 4-fold upon K-Ras transformation, and >25-fold in the dominant-positive construct Wp85α, and there was a signal of corresponding relative intensity at the position of PtdIns(3,4,5)P3 in these cells. We have verified that 32P incorporation into PtdIns(3,4)P2 was abrogated by wortmannin and LY294002 in Src-transformed cells and by wortmannin in Wp85α cells (all <5% of untreated cells). In contrast, this assay in Src cells was not affected by NCDC, a PI-PLC inhibitor that also abrogates macropinocytosis (Veithen et al., 1998; see below). In addition, NCDC had no effect in an in vitro PI3K assay on two recombinant isoforms (the class IA member p110α/p85α and the class IB member p110γ; R. Stein, personal communication). This finding indicates that PI3K and PI-PLC are both involved in the signaling pathway leading to actin cytoskeleton remodeling in transformed cells.
Because Rat-1 fibroblasts respond to insulin, and rodent and bovine p85α interact similarly with insulin-responsive substrate-1 (IRS-1), activation of PI3K was also explored by immunoprecipitation of IRS-1 followed by measurement of PI3K activity in the immunoprecipitate. This alternative in vitro assay, presented at Figure 11B, shows that PI3K recruitment at the plasma membrane, as retrieved by coimmunoprecipitation with IRS-1, was increased ∼17-fold upon transformation with v-Src and 20-fold in the dominant-positive construct Wp85α, but it was abolished in v-Src–transformed cells by the dominant-negative construct v-Src/Δp85α.
Activation of PI-PLC and Its Relation with PI3K
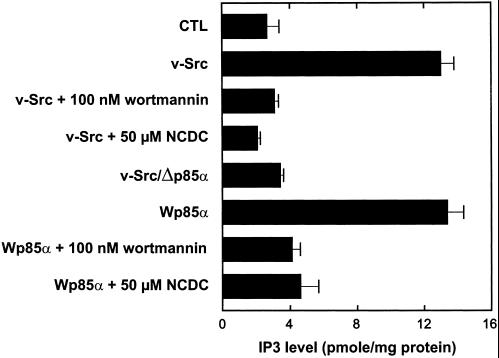
The activity of PI-PLC was also measured in situ by quantification of intracellular levels of its product IP3. In addition, because PDGF signaling involves PI3K and PI-PLC in sequential order (Falasca et al., 1998) and inhibitors of both enzymes abrogate constitutive macropinocytosis in transformed fibroblasts (Veithen et al., 1998), we finally explored their relation in the signaling cascade with the use of this assay. Activation of PI-PLC in v-Src–transformed fibroblasts and Wp85α cells was demonstrated directly by a fivefold increase in IP3 level, as measured by a radioreceptor assay (Figure 12). In these two cell lines, PI-PLC activation was suppressed not only by NCDC but also by wortmannin. PI-PLC activation was also suppressed by the dominant-negative construct of PI3K. These data demonstrate that PI-PLC is activated upon recruitment on PI3K products and thus place PI-PLC downstream of PI3K in the same signaling cascade of oncogene-induced constitutive macropinocytosis.
Figure 12.
PI-PLC is downstream of PI3K in the same signaling pathway to constitutive macropinocytosis. In situ phospholipase activity was determined by intracellular IP3 levels with the use of a competitive radioligand-binding assay. The indicated cells were preincubated at 37°C for 1 h with DMEM without serum supplemented with the indicated concentrations of inhibitors. All values are means ± SEM of four dishes pooled from two separate experiments.
DISCUSSION
We have previously reported that v-Src causes constitutive macropinocytosis in Rat-1 fibroblasts (Veithen et al., 1996, 1998). In their pioneering study, Bar-Sagi and Feramisco (1986) had shown that microinjection of the constitutively activated oncoprotein H-Ras, a palmitoylated and farnesylated molecule that partitions into rafts (Roy et al., 1999), produces lasting membrane ruffling and the appearance of large pinocytic vacuoles. The present study extends their observations to another activated oncogene, K-Ras. Because the latter, like v-Src, is rich in polybasic stretches and is not recruited on caveolar domains (Roy et al., 1999), signaling through caveolae does not appear to be required for the stimulation of ruffling and pinocytosis. Our data further clarify the significance of their observations by demonstrating that K-Ras enhances pinocytosis as a result of constitutive macropinocytosis. The analysis of its signaling pathway also provides a molecular linkage with v-Src–induced constitutive macropinocytosis (Veithen et al., 1996, 1998).
Macropinocytosis, an integrated end point of membrane–cytoskeleton interactions, is currently receiving considerable attention as a tool to decipher their molecular regulations. A common theme in signaling cascades is the recruitment of various regulatory or effector proteins at the cytoplasmic leaflet of the plasma membrane, either by protein–lipid interactions on D3 phosphoinositides via pleckstrin-homology, lysine/arginine-rich, or SH2 domains (Martin, 1998; Rameh and Cantley, 1999), or by protein–protein interactions via SH2, SH3, or proline-rich domains (Pawson, 1995). By analogy with the signaling pathway downstream of PDGF (Falasca et al., 1998), we tested the hypothesis that v-Src and K-Ras similarly recruit and activate PI3K and PI-PLC.
This interpretation was supported by several lines of evidence from other experimental systems. First, PI3K functions upstream of Rac, because stimulation by PDGF and insulin of the Rac exchange factor depends on PI3K (Hawkins et al., 1995), and because PI3K inhibition by wortmannin or LY294002 prevents induction of membrane ruffling by PDGF or activated Ras but not by activated Rac (Kotani et al., 1994; Wennström et al., 1994; Nobes and Hall, 1995; Rodriguez-Viciana et al., 1997). Second, Src and Ras interact directly with PI3K. Third, the D3 subgroup of PI3K products serves as site-specific membrane signals to directly recruit various cytosolic proteins and generate protein complexes at the interface with the cytoplasm. Among the many proteins that preferentially bind to the D3 subgroup are PLCγ1, some guanine nucleotide exchange factors, and various regulators of the actin cytoskeleton (Falasca et al., 1998; Martin, 1998). All of these constituents are of potential importance for macropinocytosis.
Our study demonstrates that v-Src and K-Ras produce constitutive macropinocytosis by permanently activating PI3K, resulting in a manyfold enrichment of 3-phosphoinositides at the total cellular level, which may reflect much higher concentrations in active membrane domains. This increase closely correlates with a profound reorganization of the actin cytoskeleton, active ruffling, and macropinocytosis, the most dramatic response being found for the dominant-positive Wp85α fibroblasts. Conversely, cytoskeleton reorganization and macropinocytosis were abrogated by wortmannin and LY294002 and by the dominant-negative expression of the PI3K adaptor subunit p85α, which is competent for being recruited at the plasma membrane but was unable to recruit the PI3K catalytic subunit p110. In contrast to its direct role in the constitutive formation of macropinosomes in oncogene-transformed fibroblasts, Murray et al. (2000) recently reported that PI3K was not required for the accelerated formation of endocytic vesicles in M-CSF–stimulated macrophages but was necessary for their subsequent centripetal movement and increase of size, presumably by homotypic fusion. This discrepancy emphasizes that different machineries may operate in constitutive versus transiently stimulated macropinocytosis.
The signaling pathway downstream of PI3K involves multiple arms. Among these, PLCγ1, but not PLCγ2, is recruited directly at plasma membrane ruffles, and a sequential signaling via PI3K lipid products to PLCγ1 has been demonstrated for the response of mast cells to stimulation by immunoglobulin E receptor cross-linking (Barker et al., 1998). In addition, recruitment by protein–protein interactions is also possible, as indicated by coimmunoprecipitation of PI3K and PI-PLC. Plasma membrane translocation would allow PLCγ1 to be intimately associated with the signal transduction complexes and to be activated by phosphorylation as well as by recruitment close to its substrate PtdIns(4,5)P2. However, independent PI3K and PLCγ1 pathways in response to PDGF receptor activation have been suggested (Rönnstrand et al., 1999).
We first report in this work that PI-PLC is constitutively activated in v-Src–transformed fibroblasts and upon PI3K activation by dominant-positive Wp85α transfection. This was shown by a major increase in IP3 levels and its abrogation by the PI-PLC inhibitor NCDC. Second, PI-PLC activation depends on PI3K products, because it was suppressed by wortmannin and upon dominant-negative Δp85α transfection. Third, PI3K and PI-PLC are both mandatory intermediates in constitutive ruffling and macropinocytosis, because both activities can be suppressed by pharmacological inhibitors of each of these enzymes. In conclusion, early signaling depends on the sequential activation of PI3K, which is blocked by wortmannin, and then of PI-PLC, which is inhibited by NCDC. Once activated upon plasma membrane recruitment, PI-PLC produces the cytosolic messenger IP3, which triggers Ca2+ release from intracellular stores and thereby affects the actin cytoskeleton.
Actin cytoskeleton dynamics is controlled by the small GTPases Rac, Rho, and Cdc42 (Schmidt and Hall, 1998). Like the plasma membrane recruitment and activation of PI-PLC, conversion of Rac from its inactive, GDP-bound form to its active, GTP-bound form upon PI3K activation (Leevers et al., 1999) is attributed to the recruitment on D3 phosphoinositides of a guanine nucleotide exchange factor (Carpenter et al., 1997; Hall, 1998), possibly through the pleckstrin-homology domain of the guanine nucleotide exchange factor Vav (Han et al., 1998). In addition, Rac in its activated form can physically associate with PI3K, an association enhanced upon PDGF exposure. In turn, Rac activates PtdIns(4)P 5-kinase to produce PtdIns(4,5)P2, which competes with gelsolin, resulting in F-actin uncapping (Carpenter et al., 1997).
Our results illustrate the striking inverse relationship between cortical actin recruitment supporting ruffling and macropinocytosis, which presumably depend on Rac, and the formation of stress fibers, which depends on Rho (Hall, 1998). Two mechanisms have been proposed to explain how Src leads to the rapid disappearance of actin stress fibers, one linked to its tyrosine kinase activity and the other involving a PI3K relay. In the first mechanism, Rho can be rapidly inactivated by Src as a result of tyrosine phosphorylation of p190, which triggers its association with its inactivator p120RasGAP (Fincham et al., 1999). Alternatively, PI3K activation, demonstrated directly in the present study, was reported to be necessary and sufficient for insulin-stimulated stress fiber breakdown, as shown by wortmannin inhibition and expression of constitutively active p110 PI3K subunit (Martin et al., 1996). How PI3K products control Rho is currently unknown.
Finally, 3-phosphoinositides can also directly recruit and modulate various regulators of the actin cytoskeleton through interaction via their domains rich in basic and hydrophobic sequences, including gelsolin, profilin, cofilin, and vinculin (Martin, 1998). In knockout mice, gelsolin was found to be necessary for the growth factor–stimulated macropinocytosis in fibroblasts (Azuma et al., 1998) but was not essential for phorbol myristyl acetate–stimulated and constitutive macropinocytosis in dendritic cells (West et al., 1999). Increasing the number of free barbed ends available for filament elongation would support membrane ruffling. In addition, because Rho rigidifies nontransformed cells by promoting the formation of actin stress fibers (Hall, 1998), its inactivation should both decrease physical constraints against membrane movement and increase actin availability for the cytocortex. Interestingly, lamellipodia extension triggered by PDGF correlates with a decrease in membrane tension, and this response is blocked by the PI-PLC inhibitor U73122 (Raucher and Sheetz, 2000).
Like phagocytosis, macropinocytosis in mammalian cells clearly depends on actin polymerization at the plasma membrane. Direct dependence of macropinocytosis on actin is in marked contrast with clathrin-dependent micropinocytosis, which was affected neither by cytochalasins (Figure 6) (Gaidarov et al., 1999) nor by PI3K inhibitors in our experimental system. However, whereas it is clear that PI3K controls pseudopod extension, its requirement for their fusion is still controversial, and it may vary with the particle size (Araki et al., 1996; Cox et al., 1999; Swanson et al., 1999). In addition, whereas PI3K inhibition suppresses macropinocytosis in Dictyostelium (Seastone et al., 1998; Zhou et al., 1998), it does not affect phagocytosis in these cells (Seastone et al., 1999), suggesting that these two processes requiring cortical actin polymerization could be differentially regulated.
ACKNOWLEDGMENTS
We are particularly grateful to Dr. W. Ogawa (Kobe, Japan) for providing Wtp85α and Δp85α plasmids, to Dr. R. Stein and Ms F. Savoy (Ludwig Institute for Cancer Research, London, United Kingdom) for testing the absence of interaction of NCDC on recombinant PI3Ks in an in vitro assay, and to Dr. B. Vanhaesebroeck (Ludwig Institute for Cancer Research, London, United Kingdom) for helpful discussions. The outstanding technical help and secretarial assistance of F. N′Kuli, M. Leruth, and Y. Marchand are greatly appreciated. This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) and Concerted Research Actions (Communauté Française de Belgique) as well as from Interuniversity Attraction Poles (Belgian State). M.A. holds a Ph.D. student fellowship of the FNRS.
REFERENCES
- Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of Rac for fibroblast motility. EMBO J. 1998;17:1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Pfeiffer JR, Wilson BS. Wortmannin-sensitive phosphorylation, translocation, and activation of PLCγ1, but not PLCγ2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, McCormick F, Milley RJ, Feramisco JR. Inhibition of cell surface ruffling and fluid-phase pinocytosis by microinjection of anti-ras antibodies into living cells. J Cell Physiol. 1987;5(suppl):69–73. doi: 10.1002/jcp.1041330414. [DOI] [PubMed] [Google Scholar]
- Boschek CB, Jockusch BM, Friis RR, Back R, Grundman E, Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981;24:175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Tolias KF, Couvillon AC, Hartwig JH. Signal transduction pathways involving the small G proteins Rac and CDC42 and phosphoinositide kinases. Adv Enzyme Regul. 1997;37:377–390. doi: 10.1016/s0065-2571(96)00005-2. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- Cupers P, Veithen A, Kiss A, Baudhuin P, Courtoy PJ. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J Cell Biol. 1994;127:725–735. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C, Prévost M-C, Sansonetti J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signaling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson LE, Ring KM, Öfverholm T. Selective macropinocytosis of thyroglobulin in rat thyroid follicles. Endocrinology. 1983;113:1746–1753. doi: 10.1210/endo-113-5-1746. [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- Gratacap MP, Payrastre B, Viala C, Mauco G, Plantavid M, Chap H. Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-g2 is an early key event in FcgRIIA-mediated activation of human platelets. J Biol Chem. 1998;273:24314–24321. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Murali Krishna U, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hara K, et al. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc Natl Acad Sci USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PT, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Ketelbant-Balasse P, Rodesch F, Neve P, Pasteels JM. Scanning electron microscope observations of apical surfaces of dog thyroid cells. Exp Cell Res. 1973;79:111–119. [PubMed] [Google Scholar]
- Kotani K, Hara K, Kotani K, Yonezawa K, Kasuga M. Phosphoinositide 3-kinase as an upstream regulator of the small GTP-binding protein Rac in the insulin signaling of membrane ruffling. Biochem Biophys Res Commun. 1995;208:985–990. doi: 10.1006/bbrc.1995.1431. [DOI] [PubMed] [Google Scholar]
- Kotani K, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause U, Rider MH, Hue L. Protein kinase signaling pathway triggered by cell swelling and involved in the activation of glycogen synthase and acetyl-CoA carboxylase in isolated rat hepatocytes. J Biol Chem. 1996;271:16668–16673. doi: 10.1074/jbc.271.28.16668. [DOI] [PubMed] [Google Scholar]
- Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signaling through phosphoinositide 3-kinases: the lipids take center stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- Lewis WH. Pinocytosis. Johns Hopkins Hosp Bull. 1931;49:17–27. [Google Scholar]
- Liu X, Marengere LEM, Koch CA, Pawson T. The v-Src SH3 domain binds phosphatidylinositol 3′-kinase. Mol Cell Biol. 1993;13:5225–5232. doi: 10.1128/mcb.13.9.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Martin SS, Rose DW, Saltiel AR, Klippel A, Williams LT, Olefsky JM. Phosphatidylinositol 3-kinase is necessary and sufficient for insulin-stimulated stress fiber breakdown. Endocrinology. 1996;137:5045–5054. doi: 10.1210/endo.137.11.8895379. [DOI] [PubMed] [Google Scholar]
- Martin TFJ. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- McFarlane AS. Efficient trace labeling of proteins with iodine. Nature. 1958;183:53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Meijne AML, Ruuls-Van Stalle L, Feltkamp CA, McCarthy JB, Roos E. v-Src-induced cell shape changes in rat fibroblasts require new gene transcription and precede loss of focal adhesions. Exp Cell Res. 1997;234:477–485. doi: 10.1006/excr.1997.3637. [DOI] [PubMed] [Google Scholar]
- Murray J, Wilson L, Kellie S. Phosphatidylinositol-3′ kinase-dependent vesicle formation in macrophages in response to macrophage colony stimulating factor. J Cell Sci. 2000;113:337–348. doi: 10.1242/jcs.113.2.337. [DOI] [PubMed] [Google Scholar]
- Nakai J. Dissociated dorsal root ganglia in tissue culture. Am J Anat. 1956;99:81–99. doi: 10.1002/aja.1000990105. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Ojcius DM, Bravo de Alba Y, Kanellopoulos JM, Hawkins RA, Kelly KA, Rank RG, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- Pawson T. Protein modules and signaling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Cantley LG. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cγ-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L, Siegbahn A, Rorsman C, Johnell M, Hansen K, Heldin CH. Overactivation of phospholipase C-γ1 renders platelet-derived growth factor β-receptor-expressing cells independent of the phosphatidylinositol 3-kinase pathway for chemotaxis. J Biol Chem. 1999;274:22089–22094. doi: 10.1074/jbc.274.31.22089. [DOI] [PubMed] [Google Scholar]
- Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF, Parton RG. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Seastone DJ, Lee E, Bush J, Knecht D, Cardelli J. Overexpression of a novel Rho family GTPase, RacC, induces unusual actin-based structures and positively affects phagocytosis in Dictyostelium discoideum. Mol Biol Cell. 1998;9:2891–2904. doi: 10.1091/mbc.9.10.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seastone DJ, Zhang L, Buczynski G, Rebstein P, Weeks G, Spiegelman G, Cardelli J. The small Mr Ras-like GTPase Rap1 and the phospholipase C pathway act to regulate phagocytosis in Dictyostelium discoideum. Mol Biol Cell. 1999;10:393–406. doi: 10.1091/mbc.10.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölander A, Yamamoto K, Huber BE, Lapetina EG. Association of p21ras with phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1991;88:7908–7912. doi: 10.1073/pnas.88.18.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N. A contractile activity that closes phagosomes in macrophages. J Cell Sci. 1999;112:307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Thilo L. Selective internalization of granule membrane after secretion in mast cells. Proc Natl Acad Sci USA. 1985;82:1711–1715. doi: 10.1073/pnas.82.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J Cell Sci. 1998;111:2329–2335. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- Veithen A, Cupers P, Baudhuin P, Courtoy PJ. v-Src induces constitutive macropinocytosis in rat fibroblasts. J Cell Sci. 1996;109:2005–2012. doi: 10.1242/jcs.109.8.2005. [DOI] [PubMed] [Google Scholar]
- Wennström S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- West MA, Antoniou AN, Prescott AR, Azuma T, Kwiatkowski DJ, Watts C. Membrane ruffling, macropinocytosis and antigen presentation in the absence of gelsolin in murine dendritic cells. Eur J Immunol. 1999;29:3450–3455. doi: 10.1002/(SICI)1521-4141(199911)29:11<3450::AID-IMMU3450>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wilde A, Beattie EC, Lem L, Riethof DA, Liu S-H, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. The endocytotic rate constant: a cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982;257:4222–4229. [PubMed] [Google Scholar]
- Zhou K, Pandol S, Bokoch G, Traynor-Kaplan AE. Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J Cell Sci. 1998;111:283–294. doi: 10.1242/jcs.111.2.283. [DOI] [PubMed] [Google Scholar]