Abstract
Many cyanobacteria produce microcystins, hepatotoxic cyclic heptapeptides that can affect animals and humans. The effects of photosynthetically active radiation (PAR) on microcystin production by Microcystis strain PCC 7806 were studied in continuous cultures. Microcystis strain PCC 7806 was grown under PAR intensities between 10 and 403 μmol of photons m−2 s−1 on a light-dark rhythm of 12 h -12 h. The microcystin concentration per cell, per unit biovolume and protein, was estimated under steady-state and transient-state conditions and on a diurnal timescale. The cellular microcystin content varied between 34.5 and 81.4 fg cell−1 and was significantly positively correlated with growth rate under PAR-limited growth but not under PAR-saturated growth. Microcystin production and PAR showed a significant positive correlation under PAR-limited growth and a significant negative correlation under PAR-saturated growth. The microcystin concentration, as a ratio with respect to biovolume and protein, correlated neither with growth rate nor with PAR. Adaptation of microcystin production to a higher irradiance during transient states lasted for 5 days. During the period of illumination at a PAR of 10 and 40 μmol of photons m−2 s−1, the intracellular microcystin content increased to values 10 to 20% higher than those at the end of the dark period. Extracellular (dissolved) microcystin concentrations were 20 times higher at 40 μmol of photons m−2 s−1 than at 10 μmol of photons m−2 s−1 and did not change significantly during the light-dark cycles at both irradiances. In summary, our results showed a positive effect of PAR on microcystin production and content of Microcystis strain PCC 7806 up to the point where the maximum growth rate is reached, while at higher irradiances the microcystin production is inhibited.
Cyanobacteria produce a wide range of bioactive and toxic substances (cyanotoxins), which can affect both animals and humans (3). Microcystins are the most widespread of the cyanobacterial toxins. They are hepatotoxic cyclic heptapeptides (1), with over 75 natural structural variants (24, 28), and are potent and specific inhibitors of protein phosphatases (17). Microcystins can be produced by Microcystis spp. (1), Anabaena spp. (12), Nostoc spp. (20), and Planktothrix spp. (16). Populations of these species are known to include both microcystin-producing and -nonproducing strains (25, 26, 33). Whether microcystins have a function in any physiological processes in the producer cells, or beyond at the ecological level for those species, is unknown so far. Case studies of the population dynamics of microcystin-producing cyanobacteria in relation to microcystin concentrations in water bodies have shown that changes in microcystin concentrations cannot be explained by changes in population density alone (5, 10, 36). Environmental factors may affect microcystin concentrations in two principal ways: by regulating the abundance of microcystin-producing strains within a population and by regulating microcystin production by the toxigenic strains. In culture experiments with single strains of different cyanobacteria, effects of photosynthetically active radiation (PAR); (2, 8, 27, 30), pH (10), iron (31), phosphorus (21), and nitrogen (14, 22), as well as interactive effects of phosphorus and nitrogen (34), on microcystin content have been found. Microcystin production was found to be positively correlated with growth rate under nitrogen (14, 22) and phosphorus (21) limitation, while such correlations were not reported for the other growth factors. Overall, there is no uniform pattern in the regulation mechanism of microcystin content and production that can be concluded from these studies. In fact, inconsistent results for effects of the same parameter were reported. As for PAR, it was found that microcystin content was enhanced with increasing irradiance (30) and with decreasing irradiance (27) and that it was not affected by PAR (2). These apparent contradictions may be due to a great extent to differences in methodology, since different species and strains with different microcystin variants were studied in batch, semibatch, or continuous cultures. Microcystin concentrations were determined using the mouse bioasay, enzyme-linked immunosorbent assay, protein phosphatase inhibition assays, or high-performance liquid chromatography analysis, and the concentrations were expressed as ratio of toxins to biovolume, protein, dry weight, chlorophyll a, or cell number, but few studies reported on microcystin content in relation to growth determinants. To date, there is no satisfactory understanding of the influence of PAR on microcystin production and content in cyanobacteria.
We have therefore carried out a comprehensive study of the effects of PAR on the growth and microcystin content of Microcystis strain PCC 7806 in continuous cultures. Variation in microcystin content and production was determined under steady-state conditions over a wide range of irradiances from PAR-limited to PAR-saturated growth conditions. Times for adaptation of microcystin production to changes in mean daily irradiance were estimated under transient-state conditions, and for the first time, diurnal changes in microcystin content under steady-state conditions were studied.
MATERIALS AND METHODS
Continuous cultures.
Unicellular cultures of Microcystis strain PCC 7806 were grown in continuous-culture systems to determine the microcystin content and growth of this strain under PAR limitation and saturation. Each culture system consisted of a flat glass culture vessel 27 by 18 by 5 cm, with a volume of 1.65 liters. The culture vessels were custom-made and are described in detail by Matthijs et al. (18). A glass cooling vessel of the same dimensions but thinner (1 cm) was positioned between the culture vessel and the light sources, which were white fluorescent tubes (Philips PL-L 24W/840/4P). Four of the 29-cm-long tubes were arranged parallel to each other so that they covered the front of a culture vessel. PAR was measured using a LI-COR quantum photometer (LI 250). The incoming irradiance, Iin, was measured at the back of the cooling vessel, and the outgoing light intensity, Iout, was measured at the back of the culture vessel (each at 10 points). The mean PAR, I, in the culture vessel was calculated as I = (Iin − Iout)/ln (Iin − Iout). Irradiance was varied by placing neutral-density filters on the back of the cooling vessel and adjusting the culture vessel at different distances from the light source. Other light sources were excluded by placing each culture system in a separate black box. Cultures were grown on a light-dark rhythm of 12 h-12 h at 22°C. They were supplied with nutrient-saturated mineral medium (O2-medium [32]). Medium flow was regulated with Gilson peristaltic pumps. The cultures were sparged with filtered air at 100 to 150 liters h−1 to provide mixing. By adding CO2 to the aeration system, cultures were provided with sufficient CO2. The CO2 pool was estimated indirectly by frequent measurements of the pH, which was adjusted in the range of pH 8.0 to 8.6. Cultures were run as turbidostats. The optical density of the culture at 750 nm was adjusted to 0.12 by regulating the medium flow. The flow rate was determined daily by measuring the medium inflow. The dilution rate (D) is a function of flow rate (f) and culture volume (V): D = f/V. Under steady-state conditions, the dilution rate is equivalent to the growth rate, μ. Steady-state conditions were established under PAR from 10 to 403 μmol of photons m−2 s−1.
Sampling, cell counts, and protein analysis.
During each steady state, the cultures were sampled on five individual days, 2 h after the beginning of the light period. Under transient-state conditions after increasing irradiance from 13 to 29 μmol of photons m−2 s−1 and from 63 to 126 μmol of photons m−2 s−1, the cultures were sampled daily until they reached steady-state conditions in order to estimate the adaptation time of the microcystin production to changes in irradiance. Diurnal changes in microcystin content were studied under steady-state conditions at irradiances of 10 and 40 μmol of photons m−2 s−1. At both irradiances, intracellular microcystin concentrations per cell and per unit biovolume and extracellular microcystin concentrations were measured on three different days. Sampling was started at the end of the dark period on each occasion. After the lights were switched on, samples were taken at 15-min intervals during the first 2 h in the light and subsequently at 2-h intervals. Aliquots of all samples were analyzed in triplicate for cell number, mean cell diameter, biovolume, protein concentration, and microcystin content. Cell number, mean cell diameter, and biovolume were measured with a cell counter (Casy 1 TTC, Schärfe System). For protein analysis, 5 ml of culture was centrifuged (10 min at 2,800 × g), the pellet was lyophilized and analysis was performed by the method of Lowry et al. (15).
Microcystin extraction.
Aliquots of 12 ml were filtered through glass microfiber filters (Whatman GF/C; 25 mm in diameter), which were placed in 2-ml vials, immediately frozen in liquid nitrogen, lyophilized, and stored at −20°C. From the samples of the diurnal study, the extracellular (dissolved) microcystin concentration was also analyzed from 2-ml aliquots of the filtrate, which was treated in the same way as the filters described above. According to Fastner et al. (4), microcystins were extracted with 75% (vol/vol) aqueous methanol. Each sample was extracted four times with 1.5 ml of 75% methanol. Cell disruption and filter dispersal were carried out with silica beads (0.5 mm in diameter) in a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) and additionally by ultrasonication for 5 min. Afterwards, samples were shaken for 30 min and centrifuged. The supernatants were pooled and blown to dryness with nitrogen.
Microcystin analysis.
Extracts were dissolved in 50% (vol/vol) aqueous methanol. Microcystins were analyzed by reverse-phase high-performance liquid chromatography with photodiode array detection using the following Kontron instruments: two model 422 pumps, an M 491 mixer, a 560 autosampler and a 440 photodiode array detector. Extracts were separated on a LiChrospher 100 ODS 5-μm LiChorCART 250-4 cartridge system (Merck), using a gradient of 30 to 70% aqueous acetonitrile (with 0.05% [vol/vol] trifluoroacetic acid) at a flow of 1 ml min−1 (13). Microcystins were identified by their characteristic UV spectra (13) and quantified using an external gravimetric standard of microcystin-LR (University of Dundee). For structural elucidation of microcystins, peaks showing characteristic UV spectra were collected manually and analyzed by matrix-assisted laser desorption time-of-flight mass spectrometry as detailed previously (6). Extracellular microcystin concentrations were analyzed using an enzyme-linked immunosorbent assay (19).
Data analysis.
Statistical analysis of data was performed with SPSS for Windows (SPSS Inc.).
RESULTS
Growth under steady-state conditions.
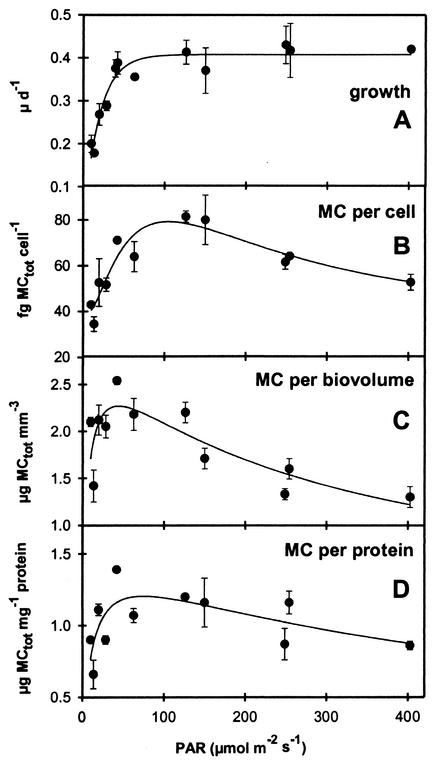
The relationship between the growth rate of Microcystis strain PCC 7806 and PAR is described by a saturation curve (Fig. 1A). Growth was limited by PAR below 80 μmol of photons m−2 s−1. Higher PAR had no effect on growth rate, which remained constant between 80 and 403 μmol of photons m−2 s−1.
FIG. 1.
Growth rate of Microcystis strain PCC7806 (A) and microcystin content per cell (B), per unit biovolume (C), and per unit protein (D) in relation to PAR. Mean values of five measurements under steady-state conditions of each irradiance are shown (error bars denote the standard deviation).
Microcystin content and production.
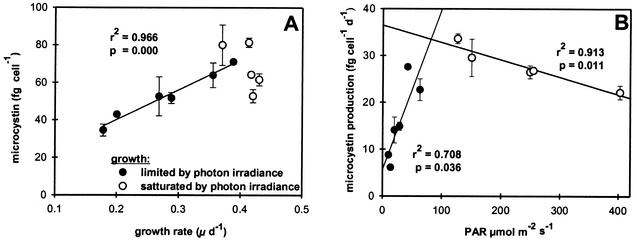
Microcystis strain PCC 7806 contained the microcystin variants MC-LR and [D-Asp3] MC-LR. Total microcystin content per cell, biovolume, and protein increased steeply with increasing PAR (Fig. 1B to D). The maximum microcystin content per cell was measured at a PAR of 126 μmol of photons m−2 s−1. Maximum microcystin concentrations per unit biovolume and unit protein were estimated at a PAR of 40 μmol of photons m−2 s−1. With further increasing irradiance, microcystin concentrations relative to cell number and protein concentration declined to values between the minimum estimated at the lowest irradiance and the maximum values. The microcystin concentration per unit biovolume reached minimum values at the highest PAR. The intracellular microcystin content showed a significant linear positive correlation with growth rate under PAR-limited growth conditions but was not correlated with growth rate under PAR-saturated growth conditions (Fig. 2A). No correlations were found between growth rate and microcystin concentration expressed per unit biovolume or protein. Microcystin production and PAR showed a significant positive correlation under PAR-limited growth conditions and a significant negative correlation under PAR-saturated growth conditions (Fig. 2B). The cell volume and protein content of Microcystis strain PCC 7806 increased with increasing PAR (Table 1).
FIG. 2.
Microcystin content of Microcystis strain PCC7806 in relation to growth rate (A) and microcystin production in relation to PAR (B). Mean values of five measurements under steady-state conditions of each irradiance are shown (error bars denote the standard deviation).
TABLE 1.
Variation of cell volume and protein content of Microcystis strain PCC 7806 at different irradiances
| PAR (μmol m−2 s−1) | Cell vol (μm3)a | Protein content (pg cell−1)a |
|---|---|---|
| 10 | 112.2 ± 4.3 | 5.1 ± 0.3 |
| 14 | 144.5 ± 2.4 | 5.0 ± 0.6 |
| 20 | 165.8 ± 16.9 | 4.7 ± 1.1 |
| 29 | 154.2 ± 21.8 | 5.3 ± 0.3 |
| 42 | 191.2 ± 10.1 | 5.2 ± 0.8 |
| 63 | 211.4 ± 13.6 | 5.9 ± 0.6 |
| 126 | 259.1 ± 13.7 | 6.8 ± 0.9 |
| 150 | 270.5 ± 6.2 | 6.9 ± 0.5 |
| 248 | 265.0 ± 9.0 | 6.7 ± 1.0 |
| 255 | 270.5 ± 6.2 | 6.1 ± 0.4 |
| 403 | 268.2 ± 11.5 | 6.2 ± 0.5 |
Mean values of five measurements during steady state ± standard deviation.
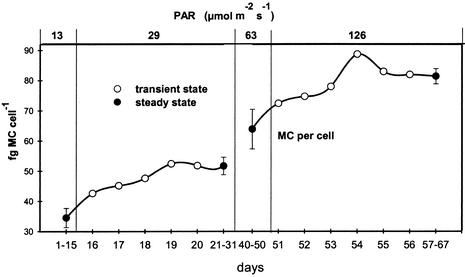
During the two investigated transient states, the intracellular microcystin content increased rapidly after PAR was increased over a period of 5 days and remained stable thereafter, when the culture reached steady-state conditions (Fig. 3).
FIG. 3.
Changes in the cellular microcystin content of Microcystis strain PCC7806 under transient states between 13 and 29 μmol of photons m−2 s−1 and between 63 and 126 μmol photons m−2 s−1 (error bars denote the standard deviation of the five measurements during steady states before and after transient state).
On a diurnal timescale under steady-state conditions at irradiances of 10 and 40 μmol of photons m−2 s−1, the intracellular microcystin content increased during the first 4 h of the light period (Fig. 4A and B). After that, it remained elevated by 10 to 20% compared to the end of the dark period. In addition, at 40 μmol of photons m−2 s−1, a maximum in intracellular microcystin content occurred 45 min after the beginning of the light period. Although the microcystin concentration at 45 min did not significantly differ from the values at 30 and 60 min, this maximum was measured on all three individual days.
FIG. 4.
Diurnal changes in the microcystin content of Microcystis strain PCC7806 per cell (A and B) and per unit biovolume (C and D) as well as the extracellular microcystin concentration (E and F) measured under steady-state conditions at 10 (A, C, and E) and 40 (B, D, and F) μmol of photons m−2 s−1. Mean values from three individual days are shown (error bars denote standard deviation).
Extracellular (dissolved) microcystin concentrations did not change significantly during the light-dark cycle at both irradiances. The mean concentration of dissolved microcystin, 16.73 μg liter−1 at 40 μmol of photons m−2 s−1, was 20 times higher than that, 0.83 μg liter−1, at 10 μmol of photons m−2 s−1. These concentrations are very low compared to the total microcystin concentrations (intra- plus extracellular), to which they contributed 2.47 and 0.22% at 10 and 40 μmol of photons m−2 s−1, respectively.
DISCUSSION
Our results clearly demonstrate that PAR affects the cellular production and content of microcystin in Microcystis strain PCC7806. Two trends were distinguished: (i) increasing the irradiance had a positive effect on microcystin production, resulting in an increased cellular microcystin content until the maximum growth rate was reached at 80 μmol of photons m−2 s−1, and (ii) further increasing the irradiance led to a decline in microcystin production and in the cellular microcystin content. Thus, saturating irradiances suppressed microcystin production and caused a decrease in cellular microcystin content, although irradiances of up to 402 μmol of photons m−2 s−1 showed no inhibitory effects on the growth rate. Hitherto, the effects of PAR on the microcystin content of cyanobacteria have been studied only under growth-limited conditions. In agreement with our findings, Utkilen and Gjølme (30) found an increase in microcystin concentration per unit protein in Microcystis aeruginosa CYA 228/1 continuous cultures when irradiance was increased from 20 to 75 μmol of photons m−2 s−1. However, for Planktothrix agardhii, Sivonen (27) reported a higher microcystin concentration per unit dry weight at lower (12 and 24 μmol of photons m−2 s−1) than at higher (50 and 95 μmol of photons m−2 s−1) irradiances. These findings need not necessarily be conflicting, considering the present results on the different effects of irradiance on microcystin production under PAR-limited and -saturated growth conditions, together with the fact that P. agardhii is a low-light-adapted species which can reach maximal growth rates at an irradiance of only 50 μmol of photons m−2 s−1 (7). Sivonen (27) did not report growth rates, but the negative effect of irradiance on the microcystin content of P. agardhii at 50 and 95 μmol of photons m−2 s−1 would be consistent with our findings if the maximum growth rate was reached at an irradiance below 50 μmol of photons m−2 s−1. In contrast to our findings, Böttcher et al. (2) found no changes in the microcystin content per unit biovolume or per cell of M. aeruginosa HUB 5-2-4 when grown in continuous cultures at growth-limiting irradiances from 5 to 75 μmol of photons m−2 s−1. However, they found a higher microcystin content per unit dry weight above 20 μmol of photons m−2 s−1. The finding by Böttcher et al. (2) on the unchanged microcystin-content-to-biovolume ratio also disagrees with the results of Hesse and Kohl (8), who, for the same strain (HUB 5-2-4) in semicontinuous cultures, found an increase in microcystin content per unit biovolume from 45 μmol of photons m−2 s−1 compared to 15 μmol of photons m−2 s−1 and an estimated maximum growth rate at 50 μmol of photons m−2 s−1. In addition, Hesse and Kohl (8) measured a decrease in the microcystin-LR content of M. aeruginosa W334, with a maximum growth rate at 80 μmol of photons m−2 s−1, but increases in microcystin-LR and -YR for M. aeruginosa W368, with a maximum growth rate at 100 μmol of photons m−2 s−1. These findings could indicate diverse effects of PAR on the microcystin content of Microcystis depending on the strain and on the microcystin variant. However, Hesse and Kohl (8) estimated the microcystin content only at four irradiances in a range from 15 to 125 μmol of photons m−2 s−1 with three Microcystis spp. strains that differed in their PAR demands for optimal growth, so that different trends under light-limited and -saturated growth conditions cannot be elucidated from their data. We suggest that the apparently inconsistent outcomes of these different studies are more likely to be due to differences in culture methods and the expression of cellular microcystin contents as ratios to a variety of different cell parameters.
Our data clearly show that both cellular biovolume and protein content in Microcystis strain PCC 7806 were themselves affected by irradiance during growth. Consequently, the ratios of microcystin concentration to biovolume, protein, and cell number differed over the studied range of PAR. Entirely opposite conclusions can be drawn from our data on diurnal changes in microcystin concentrations: a positive effect of PAR on microcystin production can be concluded on the basis of the increase in the cellular microcystin content during the light period. On the other hand, the decreasing microcystin concentrations per unit biovolume would suggest a negative effect of PAR on microcystin production. Similar findings on the variation in cell volume and cellular protein content were also made with M. aeruginosa MASH 01-A19 in nitrogen-limited cultures by Long et al. (14), who additionally found variations in cellular chlorophyll a content and dry weight. We agree with Long et al. (14) that microcystin concentrations should be determined as a ratio per cell, since potentially confusing interpretations can arise from determining the microcystin content as a ratio to cell components.
The changes in cellular microcystin content in our experiments ranged from 34.5 to 81.4 fg cell−1; thus, cell quotas in relation to irradiance varied by a factor of 2.4. Similar findings of 1.5- to 3-fold changes in the cellular microcystin content of different cyanobacteria were found under a variety of growth conditions (2, 8, 14, 21, 27, 30). Thus, microcystin production appears to be constitutive in the cyanobacteria investigated. Our findings support the suggestions that microcystin-producing strains always contain a minimum but do not exceed a maximum cellular microcystin content (14) and that toxigenic strains remain so under a variety of growth conditions (28).
Our results on the positive correlation between microcystin production and irradiance, as well as between the cellular microcystin content and growth rate under PAR-limited growth, agree with findings from studies on nutrient limitations. Positive correlations between growth rate and microcystin production were found under limitation of nitrogen (14, 22) and phosphorus (21). In addition, our results demonstrate that under PAR-saturated growth, the cellular microcystin content is not correlated with growth rate and the microcystin production is negatively correlated with irradiance. Thus, the microcystin content cannot in general be predicted by growth rate, as concluded earlier (14, 22). Of interest at this point are the findings that the growth rates of Microcystis strain PCC7806 and its mcyB mutant, which is deficient in microcystin biosynthesis, were similar under different irradiances and, consequently, that microcystins are not essential for growth (9). This conclusion is strengthened by our findings that there is no overall correlation between Microcystis strain PCC7806 growth rate and microcystin content.
Our data on diurnal changes show that the intracellular microcystin content increased by between 10 and 20% during the light period. These changes are small compared to those in microcystin content measured at various steady states over the whole range of PAR. Thus, for understanding the causes of the variation in microcystin concentration in natural waters, short-time changes in irradiance are of minor importance. However, our data do show that PAR affects short-term changes (minutes to hours) in microcystin production and suggest, from the shape of the plot (Fig. 4), that the regulation of these short-term responses might occur at the enzyme level. However, the longer-term changes in microcystin production in response to changes in the mean diurnal irradiance (Fig. 3), as measured under transient-state conditions, might be more likely to be due to changes at the genetic level. In fact, Kaebernick et al. (11) found an enhanced transcriptional response of the microcystin biosynthesis gene cluster of Microcystis strain PCC7806 in batch cultures which were exposed to high light levels after low-light adaptation. No information is available on the regulation at the molecular level of other processes in the biosynthesis of microcystins, including the translation of the mRNA and the kinetics of microcystin synthetase, and their impact on short-time and long-term changes in the microcystin content of cyanobacteria. However, our findings on short-term and long-time effects of PAR on the microcystin content of Microcystis strain PCC 7806 suggest that PAR is involved in the regulation of different processes in microcystin biosynthesis.
PAR was also found to affect extracellular dissolved microcystin concentrations. In contrast to the intracellular microcystin content, the extracellular microcystin concentration remained constant during the light-dark cycle. On average, extracellular concentrations were 20 times higher at 40 μmol of photons m−2 s−1 than at 10 μmol of photons m−2 s−1. It remains to be determined whether this results from increased extracellular release of microcystin by intact cells with a higher microcystin content and production or from increased cell lysis at higher irradiances. The extracellular microcystin concentrations at both irradiances accounted for only 2.47 and 0.22% of the total microcystin concentrations. Similarly, low relative proportions of extracellular microcystins were reported from other culture experiments (2, 23) and field studies (29, 36, 37). Higher concentrations of extracellular microcystin were measured only in very dense cultures (10, 23) or in natural waters after the breakdown of microcystin-producing cyanobacterial populations (35).
Finally, we conclude from our results that PAR has a positive effect on microcystin production and content in Microcystis strain PCC 7806 up to the point where the maximum growth rate is reached and that higher levels of PAR inhibit microcystin production. We suggest that PAR is involved in the regulation of microcystin biosynthesis in different processes that remain to be elucidated.
Acknowledgments
This study was supported by the TMR programme TOPIC (FMRX CT98-0246) and by CYANOTOX (ENV4-CT98-0802) within the Fourth Framework Programme of the European Union.
We thank Eveline Schnelder and Hans Balke for technical assistance.
REFERENCES
- 1.Botes, D. P., A. A. Tuinman, P. L. Wessels, C. C. Viljoen, H. Kruger, D. H. Williams, S. Santikarn, R. J. Smith, and S. J. Hamond. 1984. The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the cyanobacterium Microcystis aeruginosa. J. Chem. Soc. Perkin Trans. 1 1984:2311-2318. [Google Scholar]
- 2.Böttcher, G., I. Chorus, S. Ewald, T. Hintze, and N. Walz. 2001. Light limited growth and microcystin content of Planktothrix agardhii and Microcystis aeruginosa in turbidostats, p. 115-133. In I. Chorus (ed.), Cyanotoxins: occurrence, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 3.Carmichael, W. W., and I. R. Falconer. 1993. Diseases related to freshwater blue-green algal toxins, and control measures, p. 197-209. In I. R. Falconer (ed.), Algal toxins in seafood and drinking water. Academic Press, Ltd., London, England.
- 4.Fastner, J., I. Flieger, and U. Neumann. 1998. Optimised extraction of microcystins from field samples—a comparison of different solvents and procedures. Water Res. 32:3177-3181. [Google Scholar]
- 5.Fastner, J., M. Erhard, W. W. Carmichael, F. Sun, K. L. Rinehart, H. Rönicke, and I. Chorus. 1999. Characterization and diversity of microcystins in natural blooms and strains of the genera Microcystis and Planktothrix from German freshwaters. Arch. Hydrobiol. 145:147-163. [Google Scholar]
- 6.Fastner, J., M. Erhard, and H. von Döhren. 2001. Determination of oligopeptide diversity within a natural population of Microcystis spp. (cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 67:5069-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy, R. H. 1983. Interaction of temperature and light on the growth rates of two planktonic Oscillatoria species under a short photoperiod regime. Br. Phycol. J. 18:267-273. [Google Scholar]
- 8.Hesse, K., and J.-G. Kohl. 2001. Effects of light and nutrient supply on growth and microcystin content of different strains of Microcystis aeruginosa, p. 152-158. In I. Chorus (ed.), Cyanotoxins: occurrence, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 9.Hesse, K., E. Dittmann, and T. Börner. 2001. Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol. Ecol. 37:39-43. [Google Scholar]
- 10.Jähnichen, S., T. Petzoldt, and J. Benndorf. 2001. Evidence for control of microcystin dynamics in Bautzen Reservoir (Germany) by cyanobacterial population growth rates and dissolved inorganic carbon. Arch. Hydrobiol. 150:177-196. [Google Scholar]
- 11.Kaebernick, M., B. A. Neilan, T. Börner, and E. Dittmann. 2000. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 66:3387-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy, T., W. W. Carmichael, and E. W. Sarver. 1986. Toxic peptides from freshwater cyanobacteria (blue-green algae). I. Isolation, purification, and characterization of peptides from Microcystis aeruginosa and Anabaena flos-aquae. Toxicon 24:433-439. [DOI] [PubMed] [Google Scholar]
- 13.Lawton, L. A., C. Edwards, and G. A. Codd. 1994. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 119:1525-1530. [DOI] [PubMed] [Google Scholar]
- 14.Long, B. M., G. J. Jones, and P. T. Orr. 2001. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 67:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.Luukkainen, R., K. Sivonen, M. Namikoshi, M. Färdig, K. L. Rinehart, and S. Niemela. 1993. Isolation and identification of eight microcystins from thirteen Oscillatoria agardhii strains and structure of a new microcystin. Appl. Environ. Microbiol. 59:2204-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKintosh, C., K. A. Beattie, S. Klumpp, P. Cohen, and G. A. Codd. 1990. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 264:187-192. [DOI] [PubMed] [Google Scholar]
- 18.Matthijs, H. C. P., H. Balke, U. M. Hes, B. M. A. Kroon, L. R. Mur, and R. A. Binot. 1996. Application of light-emitting diodes in bioreactors: flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnol. Bioeng. 50:98-107. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf, J. S., S. G. Bell, and G. A. Codd. 2000. Production of novel polyclonal antibodies against the cyanobacterial toxin microcystin-LR and their application for the detection and quantification of microcystins and nodularin. Water Res. 34:2761-2769. [Google Scholar]
- 20.Namikoshi, M., K. L. Rinehart, R. Sakai, K. Sivonen, and W. W. Carmichael. 1990. Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue-green alga) Nostoc sp. strain 152. J. Org. Chem. 55:6135-6139. [Google Scholar]
- 21.Oh, H.-M., S. J. Lee, M.-H. Jang, and B.-D. Yoon. 2000. Microcystin production by Microcystis aeruginosa in a phosphorous-limited chemostat. Appl. Environ. Microbiol. 66:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orr, P. T., and G. J. Jones. 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 43:1604-1614. [Google Scholar]
- 23.Rapala, J., K. Sivonen, C. Lyra, and S. I. Niemelä. 1997. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 67:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinehart, K. L., M. Namikoshi, and B. W. Choi. 1994. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J. Appl. Phycol. 6:159-176. [Google Scholar]
- 25.Rohrlack, T., M. Henning, and J.-G. Kohl. 2001. Isolation and characterization of colony-forming Microcystis aeruginosa strains, p. 152-158. In I. Chorus (ed.), Cyanotoxins: occurrence, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 26.Shirai, M., A. Ohtake, T. Sano, S. Matsumoto, T. Sakamoto, A. Sato, T. Aida, K. Harada, T. Shimada, M. Suzuki, and M. Nakano. 1991. Toxicity and toxins of natural blooms and isolated strains of Microcystis spp. (Cyanobacteria) and improved procedure for purification of cultures. Appl. Environ. Microbiol. 57:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivonen, K. 1990. Effect of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl. Environ. Microbiol. 56:2658-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivonen, K., and G. Jones. 1999. Cyanobacterial toxins, p. 41-111. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water—a guide to their public health consequences, monitoring and management. E. & F. N. Spon, London, England.
- 29.Ueno, Y., S. Nagata, T. Tsutsumi, A. Hasewaga, F. Yoshida, M. Suttajit, D. Mebs, M. Pütsch and V. M. Vasconcelos. 1996. Survey of microcystins in environmental water by a highly sensitive immunoassay based on monoclonal antibody. Nat. Toxins 4:271-276. [DOI] [PubMed] [Google Scholar]
- 30.Utkilen, H., and N. Gjølme. 1992. Toxin production by Microcystis aeruginosa as a function of light in continuous cultures and its ecological significance. Appl. Environ. Microbiol. 58:1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utkilen, H., and N. Gjølme. 1995. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 61:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Liere, L., and L. R. Mur. 1978. Light-limited cultures of the blue-green alga Oscillatoria agardhii. Mitt. Int. Ver. Limnol. 21:158-167. [Google Scholar]
- 33.Vezie, C., L. Brient, K. Sivonen, G. Bertru, J. C. Lefeuvre, and M. Salkinoja- Salonen. 1998. Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France). Microb. Ecol. 35:126-135. [DOI] [PubMed] [Google Scholar]
- 34.Vezie, C., J. Rapala, J. Vaitomaa, J. Seitsonen, and K. Sivonen. 2002. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 43:443-454. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, M. F., K. Tsuji, Y. Watanabe, K. Harada and M. Suzuki. 1992. Release of heptapeptide toxins (microcystin) during the decomposition process of Microcystis aeruginosa. Nat. Toxins 1:48-53. [DOI] [PubMed] [Google Scholar]
- 36.Welker, M., C. Steinberg, and G. Jones. 2001. Release and persistence of microcystins in natural waters, p. 83-101. In I. Chorus (ed.), Cyanotoxins: occurrence, causes, consequences. Springer-Verlag KG, Berlin, Germany.
- 37.Wiedner, C., B. Nixdorf, R. Heinze, B. Wirsing, U. Neumann and J. Weckesser. 2002. Regulation of cyanobacteria and microcystin dynamics in polymictic shallow lakes. Arch. Hydrobiol. 155:383-400. [Google Scholar]