Abstract
Waterborne outbreaks associated with contamination of drinking water by Campylobacter jejuni are rather common in the Nordic countries Sweden, Norway, and Finland, where in sparsely populated districts groundwater is commonly used without disinfection. Campylobacters, Escherichia coli, or other coliforms have rarely been detected in potential sources. We studied three waterborne outbreaks in Finland caused by C. jejuni and used sample volumes of 4,000 to 20,000 ml for analysis of campylobacters and sample volumes of 1 to 5,000 ml for analysis of coliforms and E. coli, depending on the sampling site. Multiple samples obtained from possible sources (water distribution systems and environmental water sources) and the use of large sample volumes (several liters) increased the chance of detecting the pathogen C. jejuni in water. Filtration of a large volume (1,000 to 2,000 ml) also increased the rate of detection of coliforms and E. coli. To confirm the association between drinking water contamination and illness, a combination of Penner serotyping and pulsed-field gel electrophoresis (digestion with SmaI and KpnI) was found to be useful. This combination reliably verified similarity or dissimilarity of C. jejuni isolates from patient samples, from drinking water, and from other environmental sources, thus confirming the likely reservoir of an outbreak.
Campylobacters are the most common registered bacterial causes of human intestinal infections in many developed countries, including Finland (6, 20, 30). The most important species associated with these infections is Campylobacter jejuni (19). Most C. jejuni infections are sporadic, and in most cases the source of infection remains unknown (6, 26). In epidemiological studies, the commonly recognized risk factors for acquisition of campylobacter infection have been eating or handling poultry and drinking unpasteurized milk or untreated drinking water from private wells or groundwater sources (6, 13, 21). In waterborne epidemics associated with campylobacters, the drinking water source has been shown to be fecally contaminated either by runoff of surface water after rain or by leakage of a sewage pipe close to the drinking water pipeline (14, 31).
Campylobacters colonize a wide variety of hosts, from domestic animals to wild birds (14, 29), and thus the burden of campylobacters excreted via animal fecal material is extensive. Campylobacters are also common in natural water, such as streams, rivers, and lakes, due to discharges from wastewater treatment plants, runoff from pastures after rain, and direct contamination by wild birds (12). Campylobacters are able to survive for several days in moist, cool environments, including wells and groundwater (12). The low infectious dose increases the possibility of infection by drinking water containing only a few hundred viable campylobacters (19). In the Finnish population of 5.3 million people, between 1998 and 2000 21 waterborne outbreaks were registered. Ten of these outbreaks were caused by Norwalk-like caliciviruses, and nine were caused by campylobacters (18, 20).
Determination of fecal indicator organisms, such as total coliforms and especially Escherichia coli, has been used for more than 100 years for routine monitoring of the microbiological safety of drinking water. According to most regulations, a 100-ml sample of drinking water should not contain any coliform bacteria (27); a prerequisite for use of this volume is that the safety of the drinking water has been assessed by risk analysis, and any possibility of fecal contamination thus is minimized (27). Microbiological analysis is an additional tool for safety assessment. In confirmed waterborne outbreaks, however, when epidemiological studies have indicated that drinking water is the source of the infection, coliforms or E. coli has not always been detected either in source or net water samples (1, 5, 15, 17, 31, 32). One reason for the low detection rate may be too few samples combined with sample volumes that are too small (100 ml).
We studied three waterborne outbreaks caused by C. jejuni in Finland in 2000 and 2001 by using an intensified sampling procedure and analyzing both small and large water samples for indicator organisms and campylobacters. The campylobacter isolates from water, environmental, and patient samples were compared by typing for heat-stable Penner serotypes (Pen) and by genotyping with pulsed-field gel electrophoresis (PFGE) to evaluate any association between exposure to contaminated drinking water and illness.
MATERIALS AND METHODS
Outbreak 1.
During the last days of July and at the beginning of August 2000, in a small community in southern Finland with 5,500 inhabitants, approximately 400 people developed enteritis. All of these people used the same groundwater sources for drinking water. The first patient positive for C. jejuni was diagnosed on 8 August, and the highest numbers of symptomatic patients were found from 4 August through 10 August. Advice to boil water and discontinue use of the suspected groundwater sources began on 11 August. The epidemic lasted for approximately 2 weeks, and the last diagnostic stool samples positive for C. jejuni were taken on 22 and 23 August (18, 20).
In the conventional official monitoring program, no failures had been detected in the microbiological quality of water in samples taken approximately 1 month before the outbreak. Intensified sampling and analysis for campylobacters took place from 10 August through 6 September 2000, and the samples included samples from two groundwater wells in use and tap water samples from the distribution net. The volumes used for analysis of fecal indicator bacteria ranged from 100 to 1,000 ml.
Outbreak 2.
In August 2001, approximately 50 people in a small community in eastern Finland acquired diarrhea. The infections of individual patients were epidemiologically linked to a drinking water distribution net, and C. jejuni was isolated from five patients. The suspected source of infection was a groundwater well located close to a lake; the network served 600 to 800 users. Drinking water was distributed to the consumers after aeration, quartz sand filtration for iron removal, and lime alkalization for pH adjustment, but there was no chlorination. Prior to the outbreak a pipeline biofilm purification operation had been performed by a using pipeline internal gauging technique which also included (as the last step) spooling of the pipeline with a large amount of water from the groundwater source. Residents had been advised not to drink tap water from 30 July through 16 August. Most infections occurred from 21 August through 25 August. Advice to boil any drinking water from the pipeline was given on 27 August, and chlorination of the source was started on 29 August. No new patients with campylobacter infection associated with drinking water were seen after this.
Routine microbiological monitoring of source water and tap water samples in June and July 2001 had shown no abnormalities. After the pipeline purification operation, an intensified microbiological analysis program was started, which included sampling of lake water, raw water, and outlet water after sand filtration and sampling of tap water.
Outbreak 3.
From October through November 2001, an outbreak caused by C. jejuni occurred in a community located in southern Finland. Approximately 1,000 of the 18,000 users of the local drinking water distribution system acquired the infection. C. jejuni was detected in fecal samples from 56 patients. The last diagnosis associated with the outbreak was made on 6 November. Ten groundwater wells located in three different aquifers served as drinking water sources for the community. Water from these sources was combined in the net, and no chlorination was in use before the outbreak. Advice to boil water was given 24 October, and chlorination of the net began at the same time.
The first water samples (10,000 ml) were taken from the 10 groundwater sources and from two taps on 23 October. Additional 10,000-ml water samples from two groundwater sources found to be contaminated in the first sampling and from 15 additional points in the drainage area of the contaminated groundwater wells and surface water sources located close to the wells were taken from 26 October through 1 November. Fecal samples from 10 pigs and five ducks on a farm located close to the contaminated source were also studied. The last water samples were taken on 19 November from five sampling sites found to be positive in earlier studies.
Microbiological analyses of water samples for coliforms and E. coli.
Water samples examined for coliforms and for E. coli in outbreak 1 were studied at the Lahti Research Laboratory by using membrane filtration and Les Endo medium; typical colonies were confirmed to be E. coli colonies by indole and lactose fermentation at 44.5°C (3). Intensified monitoring of the water sources and distribution net by using 100- to 1,000-ml water samples continued until 6 September.
In outbreak 2, water samples used to examine indicator organisms were studied at the Municipal Food Laboratory Varkaus. Fecal coliforms in raw water, treated water, and tap water were studied on mFC media (3). Samples of lake water were also included. The volumes studied ranged from 10 to 5,000 ml. Colonies were confirmed to be E. coli colonies by the API 20E method (Biomerieux, Marcy l'Etoile, France).
Water samples in outbreak 3 were studied at the Department of Food and Environmental Hygiene, University of Helsinki, for E. coli and coliforms by using membrane filtration onto Chromocult Coliform medium (CM956; Oxoid Ltd., Basingstoke, United Kingdom) and for enterococci by using Slanetz-Bartley medium (3). A sample volume of 2,000 ml was used in the initial studies for coliforms and E. coli. In later studies100- and 1-ml samples were also used based on the level of contamination suspected.
Thermophilic campylobacters.
Thermophilic campylobacters (C. jejuni, Campylobacter coli, and Campylobacter lari) in outbreaks 1, 2, and 3 were studied by using 4,000- to 10,000-ml portions of suspected water samples sent to the laboratories. Within 2 to 8 h after sampling, the samples were filtered through 0.45-μm-pore-size membranes, which were incubated in a microaerobic atmosphere either in Preston broth (Oxoid) at 42°C for 24 h (22) (for outbreak 1) or in Bolton enrichment broth (Oxoid) for 24 and 48 h at 37°C (for outbreaks 2 and 3). After 24 and 48 h of microaerobic incubation a loopful of each enrichment broth was cultured on modified charcoal cefoperazone deoxycholate agar (mCCDA) (Oxoid). Organisms growing on mCCDA plates were confirmed to be campylobacters by Gram staining and motility analysis. Hippurate hydrolysis and catalase tests were used for preliminary identification of C. jejuni and C. coli.
MPN of C. jejuni in water samples (outbreak 3).
For campylobacter-positive well water and dike water samples, most-probable-number (MPN) counts were determined. A total of 1,500 ml of well water was analyzed by filtering 15 100-ml portions through a 0.45-μm-pore-size membrane. Each membrane was enriched in 20 ml of Bolton enrichment broth for 48 h, and after this each broth was subcultured onto an mCCDA plate, as described above. Similarly, 100 ml of dike water was distributed in 10-ml portions into 10 bottles with 10 ml of double-strength Bolton enrichment broth and cultured onto mCCDA plates. MPN counts were estimated from positive and negative tubes by using MPN tables (4).
Culture of human and animal fecal samples.
Human fecal samples were originally cultured at the local hospital laboratories on Campylobacter blood-free selective media (LabM or Oxoid), and organisms were identified as C. jejuni by using the tests described above. The isolates were further analyzed at the University of Helsinki. Fecal samples from pigs and ducks were cultured on mCCDA plates, and typical growth of campylobacters was analyzed after 48 h of incubation.
Penner serotyping and PFGE genotyping.
C. jejuni isolates from water and human fecal samples were analyzed further by Penner serotyping for heat-stable antigens and PFGE genotyping. In addition, all C. coli isolates were genotyped by PFGE. Penner serotyping for heat-stable antigens (24) was performed by using horse blood agar cultures of C. jejuni isolates and a commercially available antiserum set (Seiken campylobacter antiserum set; Denka Seiken, Tokyo, Japan) according to a procedure described previously (10). The agarose plugs for PFGE were prepared and the electrophoresis conditions used were as described previously (8, 9, 10). The restriction enzymes used for digestion were SmaI and KpnI.
RESULTS
Outbreak 1.
In outbreak 1, C. jejuni was detected in a 4,000-ml tap water sample collected on 10 August (Table 1). Among the 100-ml water samples studied, only one tap water sample from sampling point 2 was positive for indicator organisms. Neither campylobacters nor indicator bacteria were detected in the two groundwater wells used as water sources (Table 1). In later analyses, no coliforms were found in any of the 100-ml samples studied. Two separate 1,000-ml samples from wells 1 and 2 were found to be positive for E. coli at the end of August and at the beginning of September, respectively (Table 1).
TABLE 1.
Detection of coliforms, E. coli, and C. jejuni in water samples collected either from a groundwater source or tap water in waterborne outbreak 1
| Sampling site | Detection of bacteria (CFU)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coliformsa
|
E. colib
|
C. jejunic
|
||||||||
| Aug. 10 | Aug. 14 | Aug. 30 | Sept. 6 | Aug. 10 | Aug. 14 | Aug. 30 | Sept. 6 | Aug. 10 | Aug. 14 | |
| Groundwater source 1 (two wells) | − (100)d | − (100) | 1 (1,000) | 1 (1,000) | − (100) | − (100) | 1 (1,000) | − (1,000) | NDe | − (10,000) |
| Groundwater source 2 (two wells) | − (100) | − (100) | − (1,000) | − (1,000) | − (100) | − (100) | − (1,000) | − (1,000) | ND | − (10,000) |
| Sampling point 1, tap | − (100) | − (100) | ND | ND | − (100) | − (100) | ND | ND | + (4,000) | ND |
| Sampling point 2, tap | 1 (100) | − (100) | ND | ND | 1 (100) | − (100) | ND | ND | ND | ND |
Les Endo medium (100 or 1,000 ml) was used for the analysis.
The results were confirmed by using Les Endo medium and the API20E method.
The sample size was 4,000 or 10,000 ml.
−, not detected; +, detected; The values in parentheses are sample sizes (in milliliters).
ND, not done.
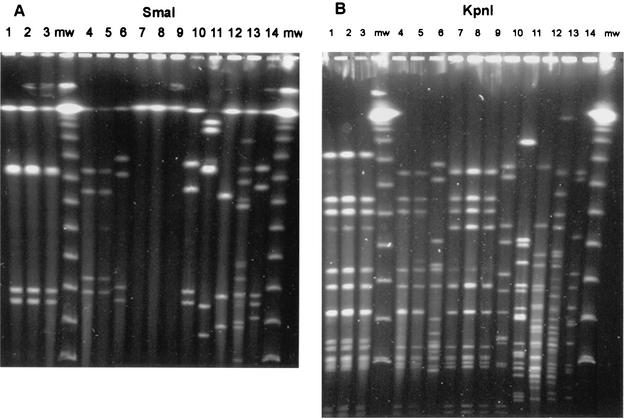
All 10 C. jejuni isolates analyzed from 10 patients, as well as the C. jejuni isolate from the tap water, were the same serotype, Pen 12, and produced identical SmaI and KpnI patterns in the PFGE analysis (Fig. 1, lanes 1 to 3).
FIG. 1.
SmaI (A) and KpnI (B) PFGE patterns of selected C. jejuni and C. coli strains isolated in association with waterborne outbreaks 1, 2, and 3. Lanes 1 and 2, patient isolates from outbreak 1; lane 3, drinking water isolate from outbreak 1; lanes 4 and 5, patient isolates from outbreak 2; lane 6, lake water isolate from outbreak 2; lanes 7, 8, and 9, patient isolates from outbreak 3; lane 10, C. jejuni isolate from well 2; lane 11, C. coli isolate from well 1; lane 12, C. coli isolate from dike; lane 13, C. coli isolate from a well at a farm; lane 14, C. jejuni isolate from duck pond; lanes mw, molecular weight markers.
Outbreak 2.
In outbreak 2, starting on 7 August, coliforms were recovered from several 100-ml samples taken from raw water, from outlet water at the plant, and from tap water (Table 2). None of the confirmed coliform colonies was an E. coli colony. During the outbreak, five E. coli colonies were obtained from a 5,000-ml tap water sample on 27 August. No campylobacters were detected when a 20,000-ml raw water sample collected on 28 August was examined. None of the 100-ml samples collected in September from the water distribution system contained coliforms. The five human C. jejuni isolates from five patients analyzed had identical SmaI and KpnI patterns, and each isolate was a serotype Pen 12 isolate. The PFGE genotype of a C. jejuni isolate from lake water was different from the PFGE genotypes of isolates obtained from the patients (Fig. 1, lanes 4 to 6).
TABLE 2.
Detection of coliforms and E. coli in water samples collected in association with waterborne outbreak 2
| Sampling site | No. of coliforms (CFU/100 ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| June 13 | Aug. 7 | Aug. 9 | Aug. 13 | Aug. 20 | Aug. 27 | Aug. 28 | Aug. 30 | Aug. 31 | Sept. 3 | |
| Lake water | NDa | ND | ND | ND | ND | 28 | ND | ND | ND | ND |
| Raw water | ND | 21 | 5 | 2 | 13 | 5 | 2 | 2 | 9 | 1 |
| Outlet water after treatment | <1 | 31 | 9 | 4 | 21 | 195b | 168b | <1 | 1 | <1 |
| Tap water, point 1 | ND | 4 | ND | 3 | 15 | 5c | ND | <1 | <1 | <1 |
| Tap water, point 2 | ND | ND | ND | ND | ND | 171 | ND | 16 | <1 | <1 |
ND, not done.
None of the colonies studied was confirmed to be E. coli.
The volume studied was 5,000 ml, and all five colonies were confirmed to be E. coli.
Outbreak 3.
In outbreak 3, the samples collected on 23 October included samples from all 10 groundwater sources and tap water from two sites, and they were studied for coliforms, E. coli, and campylobacters. One of the samples (well C) contained coliforms but no E. coli (results not shown). For another groundwater source, which included two wells (wells 1 and 2), one colony of E. coli was recovered from 2,000 ml from well 1, but no campylobacters were recovered from an 8,000-ml sample from this well (Table 3); C. jejuni was detected in an 8,000-ml sample from well 2 (Table 3). C. coli was isolated from an 8,000-ml sample from well 1 collected on 26 October (Table 3). The route and source of contamination of wells 1 and 2 were studied by sampling the the groundwater drainage area and a dike running from a nearby farm toward the wells. Several species, including pigs and ducks, were being raised on the nearby farm. Analyses of the dike water and water samples from a duck pond verified that there was fecal contamination and that C. jejuni and C. coli were present at both sampling sites (Table 3). It was possible to obtain an MPN count of C. jejuni only for a dike water sample, and this analysis resulted in a value of 9 per 100 ml. Samples obtained from wells 1 and 2 later, in November, showed that they were still fecally contaminated, but no campylobacters were isolated from these samples (Table 3).
TABLE 3.
Detection of coliforms, E. coli, enterococci, and campylobacters in selected water samples in waterborne outbreak 3
| Sampling site | Date | CFU
|
Campylobacter(s) detected | ||
|---|---|---|---|---|---|
| Coliforms | E. coli | Enterococci | |||
| Groundwater, well 1 | Oct. 23 | 1 (2,000)a | 1 (2,000) | 3 (2,000) | −c (8,000) |
| Oct. 26 | NDb | ND | ND | C. coli (10,000) | |
| Nov. 1 | ∞ (5,000) | 200 (5,000) | ND | − (5,000) | |
| Nov. 19 | 66 (100) | 1 (100) | ND | − (10,000) | |
| Groundwater, well 2 | Oct. 23 | 10 (2,000) | − (2,000) | − (2,000) | C. jejuni (8,000) |
| Nov. 1 | − (5,000) | − (5,000) | ND | − (5,000) | |
| Nov. 19 | − (100) | − (100) | ND | − (10,000) | |
| Tap water sample 1 | Oct. 23 | − (100) | − (100) | ND | − (10,000) |
| Tap water sample 2 | Oct. 23 | − (100) | − (100) | ND | − (10,000) |
| Dike 1 close to wells 1 and 2 | Oct. 31 | ND | 100 (100) | 360 (100) | C. jejuni (140) |
| Dike 2 | Oct. 31 | ND | 630 (100) | 1,080 (100) | C. jejuni and C. coli (100) |
| Duck pond | Oct. 31 | ND | (1) | ND | C. jejuni (100) |
The values in parentheses are sample sizes (in milliliters).
ND, not done.
−, not detected.
All 10 C. jejuni isolates from 10 patients were serotype Pen 12 isolates and had identical PFGE KpnI patterns (Fig. 1B, lanes 7 to 9). Their DNA could not be digested with SmaI (Fig. 1A, lanes 7 to 9). The C. jejuni isolate from well 2 was a serotype Pen 55 isolate, and it also had a different genotype than the patient isolates (Fig. 1, lanes 10). The SmaI and KpnI PFGE patterns of the C. coli isolate from well 2 are shown in Fig. 1 (lanes 11). Two different genotypes were identified among the C. coli isolates from dike water (lanes 12) and well water from a farm (lanes 13), and the PFGE pattern of the pond water C. jejuni isolate was also unique (Fig. 1, lanes 14).
DISCUSSION
In the three waterborne outbreaks caused by C. jejuni that we studied, it was evident that multiple samples and analysis of rather large sample volumes improved the chances of detecting the pathogen. Similarly, larger volumes (1,000 to 2,000 ml) were needed to detect the fecal indicator organism, E. coli. A combination of serotyping and PFGE genotyping was useful for confirming the association between drinking water and illness. In outbreaks 1 and 3 the cause of contamination was probably runoff of surface water into groundwater wells after heavy precipitation. The source of contamination in outbreak 2 remained unresolved.
Waterborne outbreaks caused by campylobacters have been reported especially in countries where groundwater sources that are not chlorinated are commonly used as the drinking water supply (2, 15, 16, 17, 18, 25, 31). In Finland, almost 1,500 small drinking water plants use groundwater as a raw water source, and they distribute approximately 45% of the total amount of drinking water consumed (18). In normal situations, the hygienic and chemical quality of groundwater is good, and only minor treatment procedures are needed to improve the quality. Due to free carbonate activity and rather high Fe and Mn contents, the most common treatment procedures include removing these compounds by aeration and sand filtration and increasing the pH with NaOH (http://www.vyh.ymparisto.fi). If no disinfection procedure is used, there is no barrier against fecal contamination.
In waterborne outbreaks caused by C. jejuni, epidemiological studies often indicate that there is a strong association between consumption of drinking water and human illness, but neither fecal indicators nor campylobacters have been detected in the water samples studied, or coliforms but no campylobacters have been detected (15, 17, 23, 31, 32). There are multiple reasons for these negative results. The biology and pathogenesis of human campylobacter infections result in a lag of approximately 2 weeks between exposure and recognition of waterborne transmission in a campylobacter outbreak in a community. First, the incubation time of the illness ranges from a few days to 1 week (6). Then diagnosis of fecal samples takes a few days. Finally, it takes a few days before the number of patients with gastrointestinal symptoms is high enough to make health authorities suspect a common source of infection. Water samples are usually taken for analysis when first suspicions of waterborne transmission are presented, and this may be too late to perform water analysis, especially if the drinking water has been contaminated only transiently.
In our studies, outbreaks 1 and 3 were more long-lasting than outbreak 2, and the contamination source implicated was groundwater wells. In outbreak 1, C. jejuni was detected in a tap water sample but not in a source water sample. In outbreak 3, two wells were continuously contaminated by floodwater from a dike which had been contaminated by runoff, probably originating from animal sources. Contamination of the dike water and the groundwater well was verified with several samples by detection of C. jejuni, C. coli, or E. coli in samples. All tap water samples were negative, even when 8,000-ml samples were studied for campylobacters. The contamination source in outbreak 2 remained unverified. It was either the groundwater well in which water was suspected to be contaminated by lake water when the net was rinsed with a large volume of groundwater after a biofilm purification operation or tap water contaminated in the distribution system by leakage in a pipe. The hygienic quality of the lake water was known to be good, because the microbiological quality at a local beach, located close to the well, was regularly checked during the summer. Detection of coliforms in several tap water samples and especially detection of a few E. coli colonies in a 5,000-ml tap water sample obtained in the area where several ill patients were identified suggest that there was fecal contamination of the pipeline.
As shown in our studies, the volume of water studied either for fecal indicator microbes or for suspected pathogens is crucially important. With the exception of one 100-ml tap water sample (outbreak 1) that contained one colony of E. coli, the 100-ml samples were negative for E. coli. When larger volumes (1,000 to 2,000 ml) were used for studies of E. coli in outbreaks 1 and 3, some samples were positive, and evidence of fecal contamination was obtained. In outbreak 1, the contamination by surface runoff was not continuous but occurred after rain. In outbreak 2, the only sample positive for E. coli was obtained from a 5,000-ml tap water sample. This result confirmed that drinking water in the drinking water pipeline had been fecally contaminated and probably also had been the source of C. jejuni.
It has been proposed that the ratio of enterococci to fecal coliforms in a water sample is an indicator of the contamination source. A value of <0.7 indicates that there was fecal contamination from animal sources, and a value of >4 indicates that there was a human source (7). The index value, calculated by using the ratio of enterococci to E. coli, was 0.27 in the dike water in outbreak 3, indicating that there was contamination from animal sources. This was also consistent with the fact that the dike drained a duck pond at the farm. A sample of pond water contained both E. coli and C. jejuni.
As indicated by the findings described here, the volume used for detection of the suspected pathogen C. jejuni in drinking water should be several liters. In outbreak 1 C. jejuni was detected by using a 4,000-ml tap water sample, and in outbreak 3 campylobacters were detected in a drinking water source when 8,000- to 10,000-ml samples were used but not when a 5,000-ml sample was used. Savill et al. (28) showed that low campylobacter counts (maximum, 0.3 CFU/100 ml) occurred in their drinking water samples collected in nonepidemic situations. In our study, campylobacters were also detected by enrichment from a 100-ml sample of dike water, and an MPN count of 9 CFU/100 ml was detectable. Our studies suggest that sample volumes of 100 to 1,000 ml, as proposed in the International Standardisation Organisation draft for detection of campylobacters from drinking water (11), are too small for detection of campylobacters in waterborne outbreaks.
Typing of pathogens associated with an outbreak has been shown to be a useful tool for confirming the similarity or dissimilarity of the isolates from patients and from the suspected source. In outbreak 1, the serotype (Pen 12) and the PFGE genotype of the isolates from the patients and the isolate from water were identical, confirming the results of epidemiological studies. In outbreak 2, all patient isolates had the same serotype (Pen 12) and the same PFGE genotype. Interestingly, in outbreak 3, although all patient isolates obtained from 19 October through 29 October had the same serotype (Pen 12) and the same PFGE genotype, the C. jejuni isolate from contaminated well 2, sampled on 23 October, was a serotype Pen 55 isolate, and its PFGE genotype was also different. The isolate from well 1 was C. coli. A variety of unique genotypes were also identified by PFGE analysis among the isolates from dike water, the duck pond, and a well at the farm. It is possible that wells 1 and 2 were also contaminated by the same C. jejuni serogenotype that was identified in patients, but either its level was below the threshold of detection with the method which we used or our enrichment methods suppressed the growth of this particular type. It is also possible that the C. jejuni strain associated with the outbreak had already disappeared from the wells by the time of sampling. The presence of both C. jejuni and C. coli and the variety of C. jejuni and C. coli genotypes in the wells and in the surrounding water sources indicate that there was a continuous inflow of campylobacters from the surrounding environment into the wells after precipitation and flooding in late September. Several animal species were raised on the farm, including ducks and pigs, which were sampled and were studied for campylobacters during the outbreak. C. coli was found in fecal samples from all 10 pigs. Ducks are known avian sources of C. jejuni and C. coli, and C. coli commonly colonizes the porcine gut (28). Continuous fecal contamination of a drinking water source by several serotypes that are different from those identified in patients has also been seen in other waterborne campylobacter outbreaks (16).
All three outbreaks were caused by the same serotype, Pen 12, and the SmaI and KpnI patterns of the isolates from outbreaks 1 and 2 were closely related. The DNA of the human isolates from outbreak 3 was not digested by SmaI, but the KpnI patterns were closely related to those found in outbreaks 1 and 2. In our follow-up studies of human domestically acquired campylobacter infections since 1995, serotype Pen 12 with the same three variants of PFGE genotypes that were found among waterborne isolates in the present study has been among the most common serogenotypes identified in patients (8, 9, 10).
In conclusion, our studies of three waterborne outbreaks of disease caused by C. jejuni showed that large volumes of water and multiple samples from groundwater sources and several sites in the drinking water distribution system over a longer period of time improved campylobacter detection rates. In addition, larger water volumes also allowed detection of coliform bacteria and E. coli as indicators of environmental contamination, which allowed the route and source of contamination to be determined more definitely. The combination of serotyping and PFGE typing of patient and water isolates further confirmed the source of infection.
Acknowledgments
This study was supported by The Helsinki University's Research Funds and the Finnish Cultural Foundation, Helsinki, Finland.
REFERENCES
- 1.Aho, M., M. Kurki, H. Rautelin, and T. U. Kosunen. 1989. Waterborne outbreak of campylobacter enteritis after outdoors infantry drill in Utti, Finland. Epidemiol. Infect. 103:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, Y., and T. A. Stenstöm. 1987. Waterborne outbreaks in Sweden—causes and etiology. Water Sci. Technol. 19:575-580. [Google Scholar]
- 3.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed., p 956-976. American Public Health Association, Washington, D.C.
- 4.deMan, J. C. 1975. The probability of most probable numbers. Eur. J. Appl. Microbiol. 1:67-78. [Google Scholar]
- 5.Engberg, J., P. Gerner-Smidt, F. Scheutz, E. Moller-Nielsen, S. L. On, and K. Molbak. 1998. Water-borne Campyloabcter jejuni infection in a Danish town—a 6-week continuous source outbreak. Clin. Microbiol. Infect. 4:648-656. [DOI] [PubMed] [Google Scholar]
- 6.Friedman, J., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology; Washington, D.C.
- 7.Gelreich, E. E., and B. A. Kenner. 1969. Concepts of fecal streptococci in stream pollution. J. Water Pollut. Control Fed. 41:R355-R352. [PubMed] [Google Scholar]
- 8.Hänninen M.-L., S. Pajarre, M.-L. Klossner, and H. Rautelin. 1998. Typing of human Campylobacter jejuni isolates in Finland by pulsed-field gel electrophoresis. J. Clin. Microbiol. 36:1787-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hänninen M.-L., P. Perko-Mäkelä, A. Pitkälä, and H. Rautelin. 2000. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J. Clin. Microbiol. 38:1998-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hänninen M.-L., P. Perko-Mäkelä, H. Rautelin, B. Duim, and J. A. Wagenaar. 2001. Genomic relatedness within five common pulsed-field gel electrophoresis genotypes studied by amplified fragment length polymorphism analysis, ribotyping, and serotyping. Appl. Environ. Microbiol. 67:1581-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Standardisation Organisation. 2002. Water quality—detection and enumeration of thermophilic campylobacter. Committee Draft ISO/CD 17995. International Standardisation Organisation, Geneva, Switzerland.
- 12.Jones, K. 2001. Campylobacters in water, sewage and environment. J. Appl. Microbiol. 90:68S-79S. [DOI] [PubMed]
- 13.Kapperud, G., E. Skjerve, L. Vik, K. Hauge, A. Lysaker, I. Aalmen, S. M. Ostroff, and M. Potter. 1992. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J. Clin. Microbiol. 30:3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenraad, P. M. F. J., F. M. Rombouts, and S. H. W. Notermans. 1997. Epidemiological aspects of thermophilic Campylobacter in water-related environments: a review. Water Environ. Res. 69:52-63. [Google Scholar]
- 15.Kramer, M. H., B. L. Herwaldt, F. C. Craun, R. L. Calderon, and D. D. Juranek. 1996. Surveillance for waterborne-disease outbreaks. United States 1993-1994. Morbid. Mortal. Wkly. Rep. 45:1-33. [PubMed] [Google Scholar]
- 16.Melby, K. K., B. Gondrosen, S. Gregusson, H. Ribe, and O. P. Dahl. 1991. Waterborne campylobacteriosis in northern Norway. Int. J. Food Microbiol. 12:151-156. [DOI] [PubMed] [Google Scholar]
- 17.Melby, K. K., J. G. Svenby, T. Eggebo, L. A. Holmen, B. M. Andersen, L. Lind, E. Sjögren, and B. Kaijser. 2000. Outbreak of campylobacter infection in a subarctic community. Eur. J. Clin. Microbiol. Infect. Dis. 19:542-544. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen, I. T., O. Zacheus, C.-H. von Bonsdorff, and T. Vartiainen. 2001. Waterborne epidemics in Finland 1998-1999. Water Sci. Technol. 43:67-71. [PubMed] [Google Scholar]
- 19.Nachamkin, I. 1997. Campylobacter jejuni, p. 159-170. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. American Society for Microbiology, Washington, D.C.
- 20.National Public Health Institute. 2001. Infectious diseases in Finland 2000. Publication KTL B10/2001. [Online.] National Public Health Institute, Helsinki, Finland. http://www.ktl.fi/ttr/tt_en_2000.pdf.
- 21.Niemann, J., J. Engberg, K. Molbak, and H. C. Wegener. 1998. Foodborne risk factors associated with sporadic campylobacteriosis in Denmark. Dan. Veterinaertidssk. 81:702-705. (In Danish.)
- 22.Nordic Committee on Food Analysis. 1990. Campylobacter jejuni/coli. Detection in foods. Publication 119. Nordic Committee on Food Analysis, Helsinki, Finland.
- 23.Pebody, R. G., M. J. Ryan, and P. G. Wall. 1997. Outbreaks of campylobacter infection: rare events for a common pathogen. Commun. Dis. Rep. 7:R33-R37. [PubMed] [Google Scholar]
- 24.Penner, J. L., and J. N. Hennessy. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J. Clin. Microbiol. 12:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rautelin, H., K. Koota, R. von Essen, M. Jahkola, A. Siitonen, and T. U. Kosunen. 1990. Waterborne Campylobacter jejuni epidemic in a Finnish hospital for rheumatic diseases. Scand. J. Infect. Dis. 22:321-326. [DOI] [PubMed] [Google Scholar]
- 26.Rautelin, H., and M.-L. Hänninen. 2000. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann. Med. 32:440-445. [DOI] [PubMed] [Google Scholar]
- 27.Sartory, D. P., and J. Watkins. 1999. Conventional culture for water quality assessment: is there a future? J. Appl. Microbiol. Symp. Suppl. 85:225S-233S. [DOI] [PubMed]
- 28.Savill, M. G., J. A. Hudson, A. Ball, J. D. Klena, P. Scholes, R. J. Whyte, R. E. McCormick, and D. Jankovic. 2001. Enumeration of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 91:38-46. [DOI] [PubMed] [Google Scholar]
- 29.Skirrow, M. B. 1994. Diseases due to Campylobacter, Helicobacter and related bacteria. J. Comp. Pathol. 111:113-149. [DOI] [PubMed] [Google Scholar]
- 30.Swedish Institute for Infectious Disease Control. 2001. Communicable diseases in Sweden 2001. Annual report of the Department of Epidemiology. [Online.] Swedish Institute for Infectious Disease Control, Solna, Sweden. http://www.smittskyddsinstitutet.se/download/pdf/rapp01.pdf.
- 31.Thomas, C., H. Gibson, D. J. Hill, and M. Mabey. 1999. Campylobacter epidemiology: an aquatic perspective. J. Appl. Microbiol. Symp. Suppl. 85:168S-177S. [DOI] [PubMed]
- 32.Vogt, R. L., H. E. Sours, T. Barrett, R. A. Feldman, R. J. Dickinson, and L. Witherell. 1982. Campylobacter enteritis associated with contaminated water. Ann. Intern. Med. 96:292-296. [DOI] [PubMed] [Google Scholar]