Abstract
During inactivation of poliovirus type 1 (PV-1) by exposure to UV, hypochlorite, and heat (72°C), the infectivity of the virus was compared with that of its RNA. DEAE-dextran (1-mg/ml concentration in Dulbecco's modified Eagle medium buffered with 0.05 M Tris, pH 7.4) was used to facilitate transfecting PV-1 RNA into FRhK-4 host cells. After interaction of PV-1 RNA with cell monolayer at room temperature (21 to 22°C) for 20 min, the monolayers were washed with 5 ml of Hanks balanced salt solution. The remainder of the procedure was the same as that for the conventional plaque technique, which was also used for quantifying the PV-1 whole-particle infectivity. Plaque formation by extracted RNA was approximately 100,000-fold less efficient than that by whole virions. The slopes of best-fit regression lines of inactivation curves for virion infectivity and RNA infectivity were compared to determine the target of inactivation. For UV and hypochlorite inactivation the slopes of inactivation curves of virion infectivity and RNA infectivity were not statistically different. However, the difference of slopes of inactivation curves of virion infectivity and RNA infectivity was statistically significant for thermal inactivation. The results of these experiments indicate that viral RNA is a primary target of UV and hypochlorite inactivations but that the sole target of thermal inactivation is the viral capsid.
Leading causes of food-borne, and probably water-borne, disease in the United States are the Norwalk-like viruses (NLVs) of the family Caliciviridae and the hepatitis A virus (HAV) of the family Picornaviridae (11). Poliovirus (PV) is the type species of the genus Enterovirus in the Picornaviridae family (19). PV has the same genomic structure and gene organization as that of HAV and has a close phylogenetic relationship with the NLVs (26). We have studied the inactivation of PV, HAV, and feline calicivirus (FCV, which is often used as a surrogate for NLVs because NLVs have no laboratory host cell line). Inactivating agents used in these studies were UV, hypochlorite, and heat (72°C), all of which are commonly used in food processing or preparation and in water disinfection.
These simple viruses comprise only a single strand of RNA coated with protein. The RNA contains the genetic information by which intracellular infection results in production of progeny virus, so infectivity ultimately resides in the RNA. The coat protein (capsid) performs three functions: (i) protection of the RNA against environmental degradation and in transit down the digestive tract until susceptible cells are reached; (ii) attachment to the receptor of a susceptible cell, whereby the viral particle is engulfed and the capsid removed, to initiate the infection; and (iii) antigenic activity that evokes an immune response by the host and reacts with the antibody that has been produced. We have shown that capsids of HAV, vaccine PV type 1 (PV-1), and FCV inactivated (from an initial titer of ∼1,000 PFU/ml) by UV, hypochlorite, or high temperature (HT) lose function (i) (13). We have also shown loss of function (ii), except for that of UV-inactivated HAV, and of function (iii) only for PV inactivated by all three treatments (14). This afforded considerable information about the effects of inactivating agents on enteric viruses, but it did not determine the extent to which inactivation affected the functional state of the viral RNA. The present study was undertaken to determine whether the RNA in viruses inactivated by various means had retained its infectious potential. Only PV was used in these experiments, because we were unable to infect cells with RNA from the other two viruses under the conditions shown to work with PV.
The UV dose to inactivate 90% of PFU (1 log10 of inactivation) of PV/milliliter is about 0.96 mW · s/cm2 (9.6 J/m2) in clear suspending medium (29) and is between 0.377 and 0.745 mW · s/cm2 (3.77 and 7.45 J/m2) in estuarine water and seawater, respectively (6, 7); a decimal inactivation dose of 24.1 mW · s/cm2 (241 J/m2) for PV-1 in phosphate-buffered saline (PBS) was recently reported (16). For hypochlorite inactivation at pH ∼7.0, the C×T (concentration × time) values (in milligrams per liter per minute) to inactivate 90% of PV-1 are ca. 0.717 (21, 30). At 72°C, inactivating 90% of PV-1 PFU/milliliter requires only 6 s, whereas HAV requires up to 19 s; therefore, HAV is more heat resistant than PV-1 (10).
The mechanisms of these inactivations have been studied for both PV and diverse groups of viruses. For UV inactivation, loss of infectivity is associated with formation of photoproducts (photodimer or photohydrate) or loss of function of the viral genomes with doses between 0.8 and 140 mW · s/cm2 (8 and 1,400 J/m2) (5, 12, 24, 27), whereas doses of >1,000 mW · s/cm2 (>10,000 J/m2) affect the capsid protein and also generate RNA-protein linkages (3, 23, 28). The targets of UV on the virion are apparently dose dependent. In most cases no definite conclusions regarding the mechanisms of inactivation can be made, since the results vary depending on the types of viruses tested and how the authors measure the effect of inactivation upon the viral capsids and genomes. Inactivation of PV with chlorine as sodium hypochlorite (0.8 to 1.0 mg/liter at 25°C and pH 6.0 to 7.0) is due to the degradation of PV RNA without major capsid conformational changes (1, 16), whereas the isoelectric points of chlorine-inactivated echovirus type 1 (Farouk) are shifted permanently from two interconvertible pIs of 5.7 (A form) and 5.1 (B form) to a single isoelectric point of 5.1 (B* form) (30). Temperatures in the range of 45 to 55°C change the native antigenicity of the PV capsid (2, 20).
The objective of the present study was to compare the virion infectivity and RNA infectivity of PV-1 that had been inactivated by exposure to UV, hypochlorite, and heat (72°C). PV-1 was chosen as the model virus due to the availability of an RNA infectivity assay, whereas we were not successful in applying this assay procedure to HAV and FCV.
MATERIALS AND METHODS
Virus and cell culture.
PV-1, strain CHAT, was obtained from the American Type Culture Collection (ATCC VR-192; Manassas, Va.). FRhK-4, a continuous line of fetal rhesus monkey kidney cells, was contributed by Marylynn Yates (University of California, Riverside). The cell line was grown in a medium composed of Dulbecco's modified Eagle medium powder containing 4,500 mg of d-glucose/liter, 4,500 mg of l-glutamine/liter, 110 mg of sodium pyruvate/liter, and 110 mg of pyridoxine hydrochloride/liter (Gibco BRL, Life Technologies, Grand Island, N.Y.) and supplemented with a solution containing 10% fetal bovine serum (Sigma, St. Louis, Mo.), 0.1 mM nonessential amino acids (Gibco BRL), and 44 mM NaHCO3 (Mallinckrodt AR, Paris, Ky.). The maintenance medium was like the growth medium but contained only 5% fetal bovine serum. Cells for virus propagation and assay were grown in polystyrene flasks (Corning Glass Works, Corning, N.Y.).
Virus preparation.
The viruses were prepared as previously described (13). Briefly, the PV-1 was propagated in FRhK-4 cells. After observation for cytopathic effect, the harvested fluids were centrifuged to separate the cell sediment from the fluid medium. The sediments were mixed with the cell monolayers, which had been treated with sodium dodecyl sulfate. The supernatant and treated cell monolayer were pooled, filtered, and kept at −70°C until used.
Virus assay (plaque technique).
Tenfold virus dilutions were inoculated (0.5 ml) on confluent monolayers of FRhK-4 cells in 25-cm2 flasks. The control flasks were inoculated with 0.5 ml of viral diluent. The viral diluent was PBS (Sigma) containing 137 mmol of sodium chloride, 2.7 mmol of potassium chloride, and 10 mmol of phosphate buffer. Flasks were incubated and rocked at 37°C for 1 h. Without pipetting off the inoculum, 5 ml of overlay medium at 45°C was added. The overlay medium was the maintenance medium plus a final concentration of 0.75% agarose (Agarose Type II Medium EEO; Sigma). All flasks were incubated cell side up at 37°C for 3 days. Following the incubation period, 10 ml of a 1:1 mixture of 37% (wt/wt) formaldehyde solution and deionized water was added to fix the cell monolayers at room temperature for more than 20 min. Both overlay medium and formaldehyde solution were shaken out of the flasks, and the cell monolayers were rinsed twice with water. The plaques in each flask were made visible by staining with 2 ml of 0.5% crystal violet solution at room temperature for 5 to 10 min. The crystal violet solution was poured off, and the cell monolayer was rinsed twice with excess water or until the water was clear. The flasks were dried in a hot air oven at 70°C for 2 h or at room temperature overnight. Virus titer was recorded as the number of PFU per milliliter of virus suspension inoculated.
UV inactivation.
A low-pressure mercury vapor discharge lamp (a germicidal lamp, Phillips TUV 30W) was used for this study. The germicidal lamp, with tubular glass envelope, emits short-wavelength UV radiation with the peak (monochromatic) at 253.7 nm. The intensity measured by a digital UVX radiometer (UVPR, San Gabriel, Calif.) was 7.77 mW · s/cm2 (77.7 J/m2). The UV doses used in the experiment were 0, 38.85, 77.7, and 116.55 mW · s/cm2 (0, 388.5, 777, and 1165.5 J/m2, respectively). The UV lamp was warmed up for 20 min before the experiments were started. The continuous ventilation in the biosafety cabinet and the glass of the lamp tube filtering out 185-nm-wavelength radiation prevented ozone formation in the air between the UV source and sample. The stock virus suspensions were diluted with PBS to eliminate UV absorption by any proteins left over from the cell culture maintenance medium. It has been shown that phosphate buffer solution has only slight absorption of wavelengths of >220 nm, even with solutions 1 cm deep (8). Immediately after UV exposures of the virus suspensions were complete, the samples were serially diluted 10-fold and were quantitated by plaque assay to determine the residual titers of UV-irradiated virus. Photoreactivation of PV-1 was found not to occur in this system (15).
Hypochlorite inactivation.
The free chlorine (FC) concentration was measured by the N,N-diethyl-p-phenylene diamine colorimetric method by using a portable microprocessor chlorine colorimeter HI 93701 plus FC reagent HI 93701-0 (Hanna Instruments, Woonsocket, R.I.). The stock solution of 5% sodium hypochlorite (Sigma) was added directly to the working virus suspension, giving a final concentration of FC of 4.8 mg/liter (ppm). The concentration of FC was measured directly from the suspension. The amount of sodium hypochlorite to be added is dependent upon the chlorine demand of the virus suspension and container. Inactivation was done at 5°C. To neutralize the FC activity, 0.1 ml of 4.80-g/liter (0.48%) sodium thiosulfate (Sigma) was added to 10 ml of PV suspension to a final concentration of 48 mg/liter (ppm), whereby the neutralizer concentration was 10 times the initial FC concentration. At approximately pH 7.0, the C×T value (in milligrams per liter per minute) to inactivate 90% of PV-1 is 0.717 (18, 21).
Thermal inactivation.
The HT inactivation was done at 72°C to avoid complete destruction of the viral coat protein. PBS as diluent was preheated in the water bath at 72°C, and the stock virus suspension was diluted 10-fold into the preheated diluent and was incubated for 5, 10, and 15 s. When the selected time had elapsed, the treated virus suspension was diluted 10-fold in prechilled diluent. This method minimized the time spent by the virus at temperatures other than what was selected.
RNA extraction.
Half of each sample in an inactivation experiment was assayed directly for PFU, and RNA was extracted from the other half for infectivity assay. The QIAamp viral RNA mini kit (Qiagen, Valencia, Calif.) was used to extract the viral RNA from the virus suspensions after digestion according to the manufacturer's directions. Briefly, the process entails extracting the RNA chemically from the virus and loading it on a small chromatographic column in a microcentrifuge tube. After two washings, the RNA is eluted and ready for analysis or reverse transcription-PCR. The extracted viral RNA is stable for up to 1 year when stored at or below −70°C.
RNA infectivity.
The DEAE-dextran (Sigma) polymer chloride form, which was prepared from dextran of an average molecular weight of 5 × 105, was used to facilitate the entry process of viral RNA into the host cell (17). The most effective concentration of DEAE-dextran was 1 mg/ml (data not shown) in Dulbecco's modified Eagle medium (Gibco BRL) buffered with 0.05 M Tris, pH 7.4. The medium was not supplemented with serum in this application. The confluent FRhK-4 cell monolayers were washed once with 5 ml of PBS, aspirated free of PBS, and inoculated with 0.5 ml of PV-1 RNA suspension medium. After interaction of PV-1 RNA with the cell monolayer at room temperature (21 to 22°C) for 20 min, the monolayers were washed with 5 ml of Hanks balanced salt solution (Gibco BRL). The remainder of the procedure was the same as that of the plaque technique described above.
Statistical analysis.
Regression analyses of single-replicate inactivations were used to determine the best-fitting regression lines by the least-squares method (Microsoft Excel 2000). Comparison of two fit-curve regression lines was done by testing for parallelism of the two straight-line regression slopes by using separate regression fits (9).
RESULTS AND DISCUSSION
Best-fitting regression lines were derived from UV (Fig. 1), hypochlorite (Fig. 2), and HT inactivations (Fig. 3). Plaque formation by extracted RNA was approximately 100,000-fold less efficient than that by whole virions, but the assay still allowed comparison between loss of virion infectivity and of RNA infectivity. The slopes of all regression lines were negative (inverse and linear correlation), so the decrease of virus infectivities was related to the progressive effects of the inactivating agents. The causality of these relationships was substantiated by the fact that the sample correlation coefficients (r) were close to 1.0, except for that for RNA infectivity of PV-1 inactivated by HT.
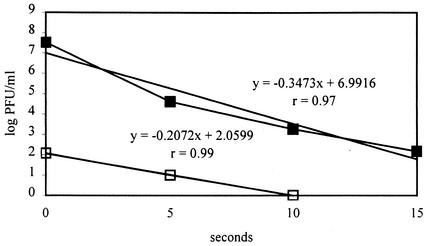
FIG. 1.
Infectivity of PV-1 whole particle (closed squares) and PV-1 RNA (open squares) inactivated by UV. The regression equations with sample correlation coefficients (r) are also shown, where y is log PFU/milliliter and x is seconds. The UV doses at the respective time points were 0, 38.85, 77.7, and 116.55 mW · s/cm2 (0, 388.5, 777, and 1165.5 J/m2, respectively).
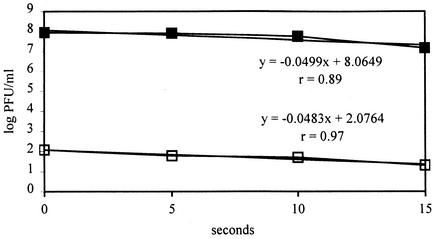
FIG. 2.
Infectivity of PV-1 whole particle (closed squares) and PV-1 RNA (open squares) inactivated by hypochlorite. The regression equations with sample correlation coefficients (r) are also shown, where y is log PFU/milliliter and x is seconds.
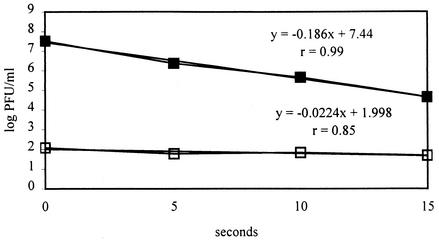
FIG. 3.
Infectivity of PV-1 whole particle (closed squares) and PV-1 RNA (open squares) inactivated by heat. The regression equations with sample correlation coefficients (r) are also shown, where y is log PFU/milliliter and x is seconds.
Inactivation curves did not show any significant deviation from one-hit kinetics (linearity), e.g., shoulder, rapid initial inactivation, or tailing off (25). It could be concluded that, in this experiment, PV-1 was appropriately inactivated and free from resistant subpopulations or aggregated virus particles. A deviation of the inactivation curve from linearity could confound the interpretation of inactivation. The strong linearity of hypochlorite inactivation curves in this experiment is consistent with the results of some previous experiments that used PV-1 strains Mahoney and Brunhilde and the PV-2 strain Lansing (4, 22, 30); however, the hypochlorite inactivation curves of echovirus and coxsackievirus strains B5 and A9 are not linear (4, 30). The linearity or one-hit kinetics of hypochlorite inactivation of PV might be species specific rather than family specific.
The PV-1 virion has two major components: viral capsid and RNA. If the slope of the inactivation curve of the whole virion is equivalent to that of RNA, then presumably the target of inactivation would be the viral RNA; however, this does not rule out concurrent degradation of the coat protein. This statement seems to be applicable to UV and hypochlorite inactivation of PV-1. For UV, the inactivation curve was linear, as in a previous study with PV-1 strain Mahoney (5). The test of parallelism of two slopes of best-fitting regression lines revealed that the UV inactivation curve of PV-1 and that of corresponding inactivated RNA are not significantly different (P > 0.1). With low UV doses (<1,000 mW · s/cm2 [<10,000 J/m2]) from this experiment, the target site of UV inactivation appeared to be the viral RNA, which agreed with some previous findings (5, 12, 24, 27). However, capsid denaturation was also seen (13, 14).
The test of parallelism of two slopes of best-fitting regression lines, which used separated regression fits, indicated that the inactivation curves of PV-1 (whole virion particle) inactivated by hypochlorite and its corresponding inactivated RNA were not significantly different (P > 0.1). Thus, hypochlorite inactivated the RNA of PV-1; effects on the capsid have also been shown (13). Previously, PV-1 strain Mahoney was tested and the target component was RNA (1, 16), whereas the capsid of echovirus was the target of hypochlorite inactivation (30). The apparent hypochlorite inactivation target is virus species dependent; however, when comparing results from different laboratories, some other factors should also be taken into consideration, e.g., pH, contact time, temperature, ionic strength, and buffer (22).
When the infectivity of inactivated RNA is not inversely and linearly correlated (i.e., slope of regression line is close to zero and r is low) with a large degree of inactivation, then presumably the target of inactivation would be the viral capsid. For HT inactivation, the slope of the corresponding RNA infectivity regression line was ca. −0.0224 and the sample correlation coefficient was only 0.85, whereas the sample correlation coefficient of HT inactivation of PV-1 was almost 1.0. The slope of PV-1 RNA from virus inactivated by HT was not significantly different from zero (0.05 < P < 0.10), so the loss of RNA infectivity of PV-1 and degree of HT inactivation were not inversely and linearly correlated. Furthermore, the test of parallelism of these two regression slopes showed a significant difference (0.01 < P < 0.025). Therefore, the HT inactivation did not cause the loss of RNA infectivity of PV-1, and one might conclude that the target of HT inactivation upon PV-1 is the capsid, not the RNA. The result was consistent with those of some previous studies (2, 20) showing that heat inactivation caused conformational changes of capsid, which led to loss of infectivity and of antigenicity. Although the present data set derives only from PV-1, we have shown that effects of UV, hypochlorite, and HT on HAV and on FCV (serving as a surrogate for the NLVs) were highly comparable (13, 14), so it may be that the fate of the RNA in these viruses is similar to that of RNA in PV-1.
Acknowledgments
We thank Tadesse Mariam for technical assistance.
REFERENCES
- 1.Alvarez, M. E., and R. T. O'Brien. 1982. Effects of chlorine concentration on the structure of poliovirus. Appl. Environ. Microbiol. 43:237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breindl, M. 1971. The structure of heated poliovirus particles. J. Gen. Virol. 11:147-156. [DOI] [PubMed] [Google Scholar]
- 3.De Sena, J., and D. L. Jarvis. 1981. Modification of the poliovirus capsid by ultraviolet light. Can. J. Microbiol. 27:1185-1193. [DOI] [PubMed] [Google Scholar]
- 4.Engelbrecht, R. S., M. J. Weber, B. L. Salter, and C. A. Schmidt. 1980. Comparative inactivation of viruses by chlorine. Appl. Environ. Microbiol. 40:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helentjaris, T., and E. Ehrenfeld. 1977. Inhibition of host cell protein synthesis by UV-inactivated poliovirus. J. Virol. 21:259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill, W. F., Jr., F. E. Hamblet, and W. H. Benton. 1969. Inactivation of poliovirus type 1 by the Kelly-Purdy ultraviolet seawater treatment unit. Appl. Microbiol. 17:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill, W. F., Jr., F. E. Hamblet, W. H. Benton, and E. W. Akin. 1970. Ultraviolet devitalization of eight selected enteric viruses in estuarine water. Appl. Microbiol. 19:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagger, J. 1967. Introduction to research in ultra-violet photobiology. Prentice-Hall, Englewood Cliffs, N.J.
- 9.Kleinbaum, D. G., L. L. Kupper, and K. E. Muller. 1988. Applied regression analysis and other multivariable methods, 2nd ed. PWS-Kent Pub. Co., Boston, Mass.
- 10.Mariam, T. W., and D. O. Cliver. 2000. Small round coliphages as surrogates for human viruses in process assessment. Dairy Food Environ. San. 20:684-689. [Google Scholar]
- 11.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, R. L., and P. G. Plagemann. 1974. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J. Virol. 13:729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuanualsuwan, S., and D. O. Cliver. 2002. Pretreatment to avoid positive RT-PCR results with inactivated viruses. J. Virol. Methods 104:217-225. [DOI] [PubMed] [Google Scholar]
- 14.Nuanualsuwan, S., and D. O. Cliver. 2003. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl. Environ. Microbiol. 69:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuanualsuwan, S., T. Mariam, S. Himathongkham, and D. O. Cliver. 2002. Ultraviolet inactivation of feline calicivirus, human enteric viruses, and coliphages. Photochem. Photobiol. 76:406-410. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien, R. T., and J. Newman. 1979. Structural and compositional changes associated with chlorine inactivation of polioviruses. Appl. Environ. Microbiol. 38:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagano, J. S., J. H. McCutchan, and A. Vaheri. 1967. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J. Virol. 1:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poduska, R. A., and D. Hershey. 1972. Model for virus inactivation by chlorination. J. Water Pollut. Control Fed. 44:738-745. [PubMed] [Google Scholar]
- 19.Racaniello, V. R., and D. Baltimore. 1981. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl. Acad. Sci. USA 78:4887-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rombaut, B., B. Verheyden, K. Andries, and A. Boeye. 1994. Thermal inactivation of oral polio vaccine: contribution of RNA and protein inactivation. J. Virol. 68:6454-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarpino, P. V., M. Lucas, D. R. Dahling, G. Berg, and S. L. Chang. 1974. Effectiveness of hypochlorous acid and hypochlorite ion in destruction of viruses and bacteria, p. 359-368. In A. J. Rubin (ed.), Chemistry of water supply, treatment, and distribution. Ann Arbor Science Publisher, Inc., Ann Arbor, Mich.
- 22.Sharp, D. G., and J. Leong. 1980. Inactivation of poliovirus I (Brunhilde) single particles by chlorine in water. Appl. Environ. Microbiol. 40:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smirnov, Y. A., M. P. Rodrigues-Molto, and M. T. Famadas. 1983. Protein-RNA interaction in encephalomyocarditis virus as revealed by UV light-induced covalent linkages. J. Virol. 45:1048-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smirnov, Y. A., S. P. Kapitulets, N. N. Amitina, V. A. Ginevskaya, and N. V. Kaverin. 1991. Effect of UV-irradiation on rotavirus. Acta Virol. 35:1-6. [PubMed] [Google Scholar]
- 25.Thurman, R. B., and C. P. Gerba. 1988. Molecular mechanisms of viral inactivation by water disinfectants. Adv. Appl. Microbiol. 33:75-105. [DOI] [PubMed] [Google Scholar]
- 26.van Regenmortel, M.H.V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.). 2000. Family Picornaviridae, p. 657-678. In Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 27.Wang, C. H., S. Y. Tschen, and B. Flehmig. 1995. Antigenicity of hepatitis A virus after ultra-violet inactivation. Vaccine 13:835-840. [DOI] [PubMed] [Google Scholar]
- 28.Wetz, K., and K. O. Habermehl. 1982. Specific cross-linking of capsid proteins to virus RNA by ultraviolet irradiation of poliovirus. J. Gen. Virol. 59:397-401. [DOI] [PubMed] [Google Scholar]
- 29.Wetz, K., H. Zeichhardt, P. Willingmann, and K. O. Habermehl. 1983. Dense particles and slow sedimenting particles produced by ultraviolet irradiation of poliovirus. J. Gen. Virol. 64:1263-1275. [DOI] [PubMed] [Google Scholar]
- 30.Young, D. C., and D. G. Sharp. 1985. Virion conformational forms and the complex inactivation kinetics of echovirus by chlorine in water. Appl. Environ. Microbiol. 49:359-364. [DOI] [PMC free article] [PubMed] [Google Scholar]