Abstract
We tested the effects of solar radiation, and UV-B in particular, on the growth of Antarctic terrestrial fungi. The growth responses to solar radiation of five fungi, Geomyces pannorum, Phoma herbarum, Pythium sp., Verticillium sp., and Mortierella parvispora, each isolated from Antarctic terrestrial habitats, were examined on an agar medium in the natural Antarctic environment. A 3-h exposure to solar radiation of >287 nm reduced the hyphal extension rates of all species relative to controls kept in the dark. Pythium sp. cultures exposed to solar radiation for 1.5 h on five consecutive days were most sensitive to radiation of >287 nm, but radiation of >313 nm also inhibited growth to a lesser extent. Radiation of >400 nm had no effect on hyphal growth relative to controls kept in the dark. Short-wave solar UV-B radiation of between 287 and 305 nm inhibited the growth of Pythium sp. hyphae on and below the surface of the agar medium after 24 h, but radiation of ≥345 nm only reduced the growth of surface hyphae. Similar detrimental effects of UV-B on surface and, to a lesser extent, submerged hyphae of all five fungi were shown in the laboratory by using artificial UV-B from fluorescent lamps. A comparison of growth responses to solar radiation and temperature showed that the species that were most resistant to UV radiation grew fastest at higher temperatures. These data suggest that solar UV-B reduces the growth of fungi on the soil surface in the Antarctic terrestrial environment.
Fungi are important components of terrestrial ecosystems because they mineralize nutrients from soil organic matter (15). Abiotic factors, including temperature, water, and nutrient availability, influence their growth in the natural environment (3). Exposure to solar radiation, particularly biologically damaging UV-B (280 to 315 nm) radiation, may also limit fungal growth (6, 11). Previous studies have shown deleterious effects of short-wavelength radiation on the growth of sub-Arctic and temperate soil fungi (5, 10, 11), but little is known about the influence of radiation on fungi on the surface of Antarctic soils. These organisms are exposed to wide fluctuations in incident solar radiation owing to seasonal changes in snow and ice cover, solar zenith angle, and surface albedo (16). Stratospheric ozone depletion in the late austral spring and early summer also increases the UV-B radiation reaching Antarctic terrestrial habitats by up to 50% as well as increasing the penetration of shorter wavelengths of UV-B through the atmosphere (4).
Anthropogenic increases in UV-B radiation, together with natural increases in UV-B, UV-A (315 to 400 nm), and photosynthetically active radiation (400 to 700 nm) arising from snow and ice melt, may influence the growth of fungi inhabiting the surface of Antarctic soils (17), although little empirical evidence exists to support this view. Our objectives in this study were to determine the effect of solar radiation on fungi isolated from Antarctic terrestrial habitats and to determine if solar radiation sensitivity is associated with growth rate at different temperatures.
MATERIALS AND METHODS
Experiments were carried out at Rothera Research Station (Rothera Point, Adelaide Island, Antarctica Peninsula, 67° 34′S, 68° 08′W) during the austral summer between December 2000 and January 2001.
Isolation of fungi.
Four fungi were isolated from colonies of the leafy liverwort Cephaloziella varians (Gottsche) Steph., collected from Rothera Point. The uppermost 1 to 2 mm of foliage was washed for 2 min in 20 aliquots of 15 ml of sterile distilled water with a wrist action shaker (Griffin & George Ltd., London, United Kingdom) set to 60 beats s−1. Water (100 μl) from each wash was then spread on the surface of 20 ml of Czapek Dox agar medium in two 90-mm petri dishes. Rose bengal (1:15,000) was added to the medium to slow the growth of faster-growing species of fungi. Forty petri dishes were prepared and incubated at 15°C. This temperature is frequently attained at soil surfaces during the Antarctic summer. Single colonies of fungi were isolated after 35 days.
A fungus was also cultured from a cryptoendolithic community in a gypsum crust on the surfaces of boulders at Two Step Cliffs (Alexander Island, Antarctic Peninsula, 71o 54′S, 68o 13′W). Gypsum crust was aseptically sampled from the surface of five boulders and placed in sterile polyethylene bags. In the laboratory, the crust material was aseptically broken into small fragments (5 by 5 mm) and washed for 5 min in 10 changes of sterile distilled water. Following gentle crushing, the crust material was placed onto potato dextrose agar medium (PDA; Oxoid; Unipath Ltd., Basingstoke, United Kingdom) and incubated at 15°C. Single colonies of a fungus were isolated after 28 days.
Following isolation, all strains were routinely grown on potato dextrose agar in 90-mm diameter petri dishes. Taxonomic identification to species level, where possible, was done through morphological features. The fungi isolated from Rothera Point were identified as Geomyces pannorum (Link) Sigler & J. W. Carmich., Mortierella parvispora Linnem., Phoma herbarum Westend., and a Pythium sp. The fungus isolated from Two Step Cliffs was identified as a Verticillium sp.
Responses of fungi to solar radiation and temperature.
The response of fungal isolates to solar radiation and temperature was examined in five separate experiments. A range of optical filters was used to provide different radiation treatments. Aluminum foil was used to prevent penetration of all radiation. OXO2 Perspex (3 mm; Ineos Acrylics, Darwen, Lancashire, United Kingdom), OXO2 Perspex plus Mylar polyester (125 μm; Dupont Co., Wilmington, Del.), and VE (3 mm; Ineos Acrylics, Darwen, Lancashire, United Kingdom) prevented transmission of radiation of <287 nm, <313 nm, and <400 nm, respectively. The UV waveband was further divided with long-pass filters with nominal cutoffs at 280, 295, 305, 320, 335, 345, and 360 nm (Schott WG series, Mainz, Germany).
Hyphal extension analyses were carried out in all experiments on three or four replicate petri dishes of each fungus per treatment, with a mean value per petri dish derived from six measurements made with sliding calipers to an accuracy of 0.1 mm. Radiation measurements were made with a double monochromator spectroradiometer (DM150; Bentham Instruments, Reading, United Kingdom) with a scan range of 280 to 600 nm (step, 0.5 nm). The radiometer was calibrated to a 1-kW quartz-halogen lamp traceable to a National Institute of Standards and Technology standard. Irradiances were weighted according to the DNA action spectrum of Setlow (13), normalized to 1 at 300 nm.
(i) Experiment 1: effects of solar radiation.
For all five fungi, plugs of agar (4.5-mm diameter) cut from the margins of colonies were cultured on PDA until hyphal extension was >2 mm. They were subjected to solar radiation for 3 h, 90 min on either side of solar noon (13:30 local time) on 4 January (Table 1). Ambient temperatures in the petri dishes during these exposures were between 2 and 5°C. Foil and OXO2 were used to cover cultures. After exposure, cultures were incubated at 15°C, and hyphal extension was recorded at 0, 12, 24, 48, and 192 h. Extension rates between 0 and 192 h were calculated by linear regression.
TABLE 1.
Solar radiation doses under different treatments in experiments 1 to 3a
| Expt | Treatment | Solar exposure period (h) | UV-BDNAb (kJ m−2) | UV-B (W m−2) | UV-A (105 W m−2) | Photosynthetically active radiationc (106 W m−2) |
|---|---|---|---|---|---|---|
| 1 | Foil | 3 | 0 | 0 | 0.00 | 0.00 |
| OXO2 | 3 | 2.14 | 10,400 | 4.48 | 2.36 | |
| 2 | Foil | 7.5 | 0 | 0.00 | 0.00 | 0.00 |
| OXO2 | 7.5 | 4.63 | 22,300 | 9.35 | 4.66 | |
| Mylar | 7.5 | 0.14 | 144 | 8.05 | 4.37 | |
| VE | 7.5 | 0 | 0.00 | 0.00 | 4.33 | |
| Ambient | 7.5 | 5.25 | 24,900 | 10.01 | 4.99 | |
| 3 | WG 280 | 3 | 1.88 | 9,000 | 3.76 | 2.29 |
| WG 295 | 3 | 1.36 | 7,450 | 3.75 | 2.30 | |
| WG 305 | 3 | 1.10 | 6,450 | 3.68 | 2.29 | |
| WG 320 | 3 | 0.07 | 420 | 3.38 | 2.26 | |
| WG 335 | 3 | 0.03 | 0.9 | 3.20 | 2.28 | |
| WG 345 | 3 | 0.01 | 0.00 | 2.13 | 2.21 | |
| WG 360 | 3 | 0.00 | 0.00 | 1.04 | 2.18 | |
| VE | 3 | 0.00 | 0.00 | 0.00 | 2.14 |
Data are radiation doses during the entire exposure period. Data for experiment 2 are total doses over the five 1.5-h exposure periods.
UV-BDNA is the UV-B dose weighted with the DNA damage action spectrum of Setlow (13).
400 to 600 nm.
(ii) Experiment 2: effects of daily solar radiation on Pythium sp.
The Pythium sp. was used for experiments 2 and 3 because of its rapid growth rate relative to the other isolates. Plugs of hyphae newly inoculated onto PDA were exposed to solar radiation daily for 45 min either side of solar noon (Table 1). OXO2, OXO2 plus Mylar, VE, and foil were used to cover cultures. The 90-min exposures continued for five consecutive days (21 to 25 December), and hyphal extension was recorded every 24 h during this period. Petri dishes were incubated at 15°C in the dark when not exposed to solar radiation.
(iii) Experiment 3: effects of solar UV on Pythium sp.
Plugs of agar cut from the margins of the Pythium sp. colonies were inoculated onto PDA. After 16 h of growth in the dark at 15°C, developing colonies were placed under long-pass filters (Schott WG and VE Perspex) and exposed to solar radiation for 90 min on either side of solar noon (Table 1). After exposure, the colonies were incubated at 15°C in the dark, and surface hyphal extension was measured at 0, 12, and 24 h after exposure. The extension of hyphae submerged in the agar medium, which were differentiated from surface hyphae by holding petri dishes up to the light, was measured at 24 h.
(iv) Experiment 4: effects of artificial UV-B.
All five fungi were cultured on PDA until hyphal extension was >5 mm and then placed into a plant growth chamber (Sanyo-Gallenkamp model SGC 097.PPX.FS), held at 15°C, containing eight Philips TL12 UV-B fluorescent lamps (Starna Ltd., Romford, United Kingdom) at a position 1.15 m below the lamps. This position provided a DNA-weighted flux of 6.23 × 10−4 kJ m−2 s−1, which is similar to that encountered in the natural environment (6.01 × 10−4 kJ m−2 s−1, recorded at Rothera Point under an ozone column of 133 Dobson units (DU) on 17 October 2000 at 16:00 h Greenwich mean time). OXO2 or foil was placed over each petri dish. Hyphal extension was measured at 0, 24, 48, 72, and 312 h, and extension rates between 0 and 312 h were calculated by linear regression.
(v) Experiment 5: effects of temperature.
Agar plugs taken from the colony margins of all five fungi were inoculated onto PDA and incubated in the dark at −2, 4, 15, 25, and 37°C. Hyphal extension was measured at 3, 6, 16, 27, and 35 days. Hyphal extension rates between 3 and 35 days were calculated by linear regression.
Statistical analyses.
Mean hyphal extension values were compared at each time point by one-way analysis of variance with Tukey's test to make a posteriori comparisons. Differences in hyphal extension rates were compared by determining slopes and standard errors of growth curves from linear regression. A Spearman's rank correlation test was used to determine associations between the percentage reductions in growth rates under solar UV in experiment 1 and the relative responses of fungi to temperature in experiment 5. Statistical analyses were made in the MINITAB 13.3 package.
RESULTS
Experiment 1.
A 3-h exposure to solar radiation of >287 nm reduced mean hyphal extension rates between 0 and 192 h after exposure by 100% (M. parvispora), 63% (Verticillium sp.), 48% (G. pannorum), 41% (Pythium sp.), and 15% (P. herbarum) relative to controls kept in the dark (Table 2).
TABLE 2.
Hyphal extension rates measured in experiments 1 and 4a
| Fungus | Treatment | Expt 1
|
Expt 4
|
||
|---|---|---|---|---|---|
| Slope | Standard error of slope | Slope | Standard error of slope | ||
| Pythium sp. | Dark control | 0.335 x | 0.006 | 0.370 x | 0.010 |
| UV-B surface | 0.197 y | 0.039 | 0.030 y | 0.011 | |
| UV-B submerged | 0.330 z | 0.010 | |||
| P. herbarum | Dark control | 0.067 x | 0.001 | 0.086 x | 0.001 |
| UV-B surface | 0.057 y | 0.005 | 0.058 y | 0.003 | |
| UV-B submerged | 0.076 z | 0.001 | |||
| M. parvispora | Dark control | 0.080 x | 0.003 | 0.091 x | 0.001 |
| UV-B surface | 0.000 y | 0.000 | 0.000 y | 0.001 | |
| UV-B submerged | 0.077 z | 0.003 | |||
| G. pannorum | Dark control | 0.022 x | 0.001 | 0.020 x | 0.001 |
| UV-B surface | 0.012 y | 0.002 | 0.001 y | 0.001 | |
| UV-B submerged | 0.012 z | 0.001 | |||
| Verticillium sp. | Dark control | 0.025 x | 0.001 | 0.026 x | 0.001 |
| UV-B surface | 0.009 y | 0.001 | 0.004 y | 0.001 | |
| UV-B submerged | 0.019 z | 0.001 | |||
Data were derived from three replicates (experiment 1) and four replicates (experiment 4) of each species per treatment. Slopes with different letters (x, y, and z) are significantly different (P < 0.05). Values for dark-held controls are means for surface and submerged hyphae. One-way analysis of variance indicated that these values did not differ (P > 0.05).
Experiment 2.
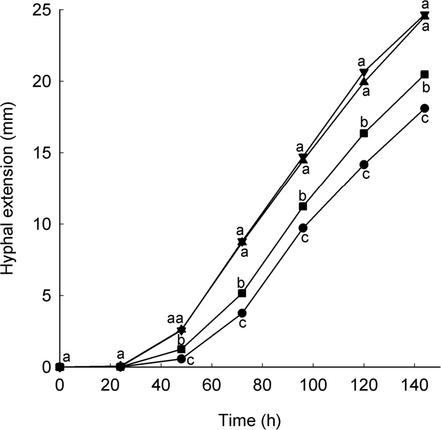
Hyphal extension of the Pythium sp. was inhibited by five consecutive daily 1.5-h exposures to solar UV-B, relative to that measured with UV-A, photosynthetically active radiation, and in the dark-held control, between 48 and 144 h after initial exposure (Fig. 1). UV-A was less inhibitory than UV-B radiation but also reduced the growth of the fungus relative to that measured with photosynthetically active radiation and in the dark-held control between 48 and 144 h. Photosynthetically active radiation alone did not have a significant effect on hyphal extension compared to the dark-held control.
FIG. 1.
Hyphal extension of Pythium sp. in experiment 2. Solar radiation treatments: >287 nm (•); >313 nm (▪); >400 nm (▴); dark (▾). Values are means of four replicates. Values with different letters at each time point are significantly different (P < 0.05).
Experiment 3.
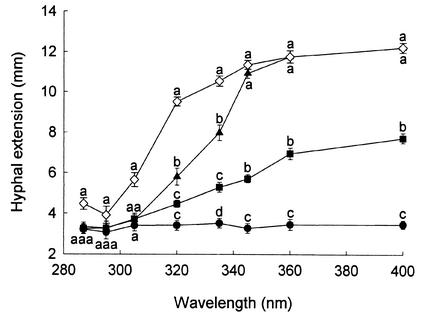
Wavelengths of solar UV radiation of ≤305 nm halted surface hyphal extension of the Pythium sp. up to 24 h after exposure (Fig. 2). This effect was also observed for hyphae submerged in the agar medium. At 12 h, radiation of ≥320 nm inhibited the growth of surface hyphae but had no effect on submerged hyphal growth. Longer wavelengths of UV-A (>345 nm) did not influence hyphal extension of the Pythium sp. (Fig. 2).
FIG. 2.
Hyphal extension of Pythium sp. after exposure to solar radiation through a range of WG Schott filters in experiment 3. Surface hyphal extension after 0 (•), 12 (▪), and 24 h (▴), and submerged hyphal extension after 24 h (◊). Values are means of four replicates ± standard error of the mean. Values with different letters at each time point are significantly different (P < 0.05).
Experiment 4.
The effects of artificial UV-B on hyphal extension rates of fungi are shown in Table 2. UV-B inhibited surface hyphal extension rates of all fungi compared with submerged hyphae and the dark-held control. Surface hyphal extension rates were reduced by 100% (M. parvispora), 94% (G. pannorum), 92% (Pythium sp.) 87% (Verticillium sp.), and 33% (P. herbarum) compared with the dark-held control. The extension rates of submerged hyphae were also reduced by exposure to UV-B radiation relative to the controls kept in the dark. Extension rates of submerged hyphae were reduced by 42% (G. pannorum), 29% (Verticillium sp.), 16% (M. parvispora), 12% (P. herbarum), and 11% (Pythium sp.) compared with the dark-held control. P. herbarum produced a brown pigment within 24 h of exposure to artificial UV-B which was accompanied by production of conidiomata and conidia.
Experiment 5.
All fungi showed slow hyphal extension rates at −2°C and no growth at 37°C (Table 3). M. parvispora grew fastest at −2 and 4°C, while Pythium sp. grew fastest at 15 and 25°C. G. pannorum and Verticillium sp. were found to have similar but comparatively slow growth rates. Both of these fungi showed little growth at 25°C, and M. parvispora did not grow at this temperature. Rank correlation analysis indicated a negative association between reduction in hyphal extension rates after exposure to solar UV radiation and growth rate at 25°C (rs= −0.90, P = 0.037), indicating that the most UV-resistant fungi were able to grow fastest at higher temperatures.
TABLE 3.
Fungal hyphal extension rates at different temperaturesa
| Temp (°C) | Hyphal extension rate (mm day−1)
|
||||
|---|---|---|---|---|---|
| Pythium sp. | P. herbarum | M. parvispora | G. pannorum | Verticillium sp. | |
| −2 | 0.06*** | 0.05* | 0.07* | 0.05** | 0.03* |
| 4 | 0.65*** | 0.62*** | 0.89* | 0.20** | 0.22** |
| 15 | 5.20*** | 1.36*** | 2.23* | 0.52*** | 0.51*** |
| 25 | 5.69*** | 1.26** | 0.00*** | 0.02* | 0.01** |
| 37 | 0.00*** | 0.00*** | 0.00*** | 0.00*** | 0.00*** |
Values are slopes of growth curves between 3 and up to 35 days and are means of four replicates. Significance of correlation coefficients for slopes are denoted by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
DISCUSSION
Our study indicated that exposure to solar and artificial radiation of >287 nm on an artificial medium inhibited the hyphal growth of five fungi isolated from Antarctic terrestrial habitats. Solar radiation halted the hyphal growth of M. parvispora on the surface of the medium, and artificial radiation, applied from fluorescent lamps, led to substantial inhibitory effects on the surface hyphal growth of G. pannorum, Pythium sp., and Verticillium sp. in the laboratory. Further experiments with the Pythium sp. indicated that the inhibitory effect of solar radiation on hyphal growth increased with decreasing wavelength and that UV-B radiation was most inhibitory to growth. Our data broadly corroborate those from studies in temperate and tropical regions that have shown deleterious effects of UV-B radiation on the abundances of fungi on natural substrates, such as attached leaves or plant litter (6, 11, 12). They support the observation that short-wavelength UV radiation is the most biologically damaging portion of the electromagnetic spectrum to a range of plants, algae, and fungi (2).
A previous study has shown that hyphal growth of 14 out of 16 litter or phylloplane fungi on PDA medium is reduced by UV-BDNA doses of ≤ 1.7 kJ m−2 day−1 (10). Our data confirm that similar doses of UV-B also inhibit the growth of Antarctic fungi and that little resistance to UV-B has apparently developed in these organisms. In common with the results obtained by Moody et al. (10), our study demonstrated that fungi responded to UV-B and, to a lesser extent, short-wave UV-A radiation by growing submerged within the PDA medium, where the flux of UV-B radiation is reduced by up to 99% (10). Elevated UV-B radiation elicited the production of a brown pigment, most probably melanin, by P. herbarum within 24 h of exposure in the present study. Melanins are a common group of pigments among fungi, including the genus Phoma (14), and are thought to protect cells from UV damage owing to their strong absorption in the UV region of the spectrum (1). P. herbarum also produced conidia in response to UV-B radiation. It is widely recognized that both UV-B and UV-A radiation trigger sporogenesis in many fungi, and action spectra constructed for fungal sporogenesis typically have peaks at 280 to 290 nm (7, 8)
Organisms living at or close to the surfaces of Antarctic soils and vegetation are frequently exposed to temperatures of up to 30°C during cloudless periods (9), and because of the strong positive relationship between radiative flux and temperature in the natural environment, these organisms will also be exposed to increased UV-B irradiances. Consequently, fungi occupying soil surfaces are likely to be selected for resistance to both high UV irradiances and high temperatures. Our study suggests that this may occur in the natural environment: we found that the fungi that grew fastest at 25°C were also the least inhibited by UV-B radiation.
Our study indicates that increases in short-wavelength UV-B irradiances that occur in Antarctica during periods of ozone depletion are likely to exacerbate the inhibitory effects of solar radiation of on the growth of terrestrial fungi. In view of the pivotal role played by fungi in soil nutrient cycling, exposure to solar radiation thus has the potential to affect biogeochemical cycles, particularly during periods of ozone depletion, with possible consequences for higher-order interactions.
Acknowledgments
This work was supported by the British Antarctic Survey's Biomolecular Responses to Environmental Stresses in Antarctica and Terrestrial and Freshwater Biodiversity projects.
We thank P. Geissler, M. Nicolson, and H. Peat for technical assistance, H. de Gruyter and D. Minter for identification of the P. herbarum and Verticillium sp. isolates, and P. Convey and three anonymous reviewers for comments on the manuscript.
REFERENCES
- 1.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 2.Caldwell, M. M. 1971. Solar UV radiation and the growth and development of higher plants, p. 131-177. In A. C. Giese (ed.), Photophysiology, vol. 6. Academic Press, New York, N.Y.
- 3.Dix, N. J., and J. Webster. 1995. Fungal ecology. Chapman & Hall, London, United Kingdom.
- 4.Frederick, J. E., and D. Lubin. 1994. Solar ultraviolet irradiance at Palmer Station, Antarctica, p. 43-52. In C. S. Weiler and P. A. Penhale (ed.), Ultraviolet radiation in Antarctica: measurements and biological effects. Antarct. Res. Ser. no. 62. American Geophysical Union, Washington, D.C.
- 5.Gehrke, C., U. Johanson, T. V. Callaghan, D. Chadwick, and C. H. Robinson. 1995. The impact of enhanced ultraviolet-B radiation on litter quality and decomposition processes in Vaccinium leaves from the sub-Arctic. Oikos 72:213-222. [Google Scholar]
- 6.Gunasekera, T. S., N. D. Paul, and P. G. Ayres. 1997. The effects of ultraviolet-B (UV-B: 290-320 nm) radiation on blister blight disease of tea (Camellia sinensis). Plant Pathol. 46:179-185. [Google Scholar]
- 7.Leach, C. M. 1971. A practical guide to the effects of visible and ultraviolet light on fungi, p. 609-664. In C. Booth (ed.) Methods in microbiology, vol. 4. Academic Press, London, United Kingdom.
- 8.Leach, C. M., and E. J. Trione. 1966. Action spectra for light-induced sporulation of Pleospora herbarum and Alternaria dauci. Photochem. Photobiol. 5:621-630. [Google Scholar]
- 9.Longton, R. E., and M. W. Holdgate. 1967. Temperature relationships of Antarctic vegetation. Phil. Trans. R. Soc. B 252:237-250. [Google Scholar]
- 10.Moody, S. A., K. K. Newsham, P. G. Ayres, and N. D. Paul. 1999. Variation in the responses of litter and phylloplane fungi to UV-B radiation (290-315 nm). Mycol. Res. 103:1469-1477. [Google Scholar]
- 11.Newsham, K. K., A. R. McLeod, J. D. Roberts, P. D. Greenslade, and B. A. Emmett. 1997. Direct effects of elevated UV-B radiation on the decomposition of Quercus robur leaf litter. Oikos 79:592-602. [Google Scholar]
- 12.Newsham, K. K., M. N. R. Low, A. R. McLeod, P. D. Greenslade, and B. A. Emmett. 1997. Ultraviolet-B radiation influences the abundance and distribution of phylloplane fungi on pedunculate oak (Quercus robur). New Phytol. 136:287-297. [Google Scholar]
- 13.Setlow, R. B. 1974. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc. Natl. Acad. Sci. USA 71:3363-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterflinger, K. 2000. Fungi as geological agents. Geomicrobiol. J. 17:97-124. [Google Scholar]
- 15.Swift, M. J., O. W. Heal, and J. M. Anderson. 1979. Decomposition in terrestrial ecosystems. Blackwell, Oxford, United Kingdom.
- 16.Walton, D. W. H. 1984. The terrestrial environment, p. 1-60. In R. M. Laws (ed.), Antarctic ecology, vol. 1. Academic Press, London, United Kingdom.
- 17.Wynn-Williams, D. D. 1996. Response of pioneer soil microalgal colonists to environmental change in Antarctica. Microb. Ecol. 31:177-188. [DOI] [PubMed] [Google Scholar]