Abstract
Seventy-five aerobic heterotrophs have been isolated from a packed-column bioreactor inoculated with soil from Antarctica. The column was maintained at 10°C and continuously fed with a casein-containing medium to enrich protease producers. Twenty-eight isolates were selected for further characterization on the basis of morphology and production of clearing zones on skim milk plates. Phenotypic tests indicated that the strains were mainly psychrotrophs and presented a high morphological and metabolical diversity. The extracellular protease activities tested were optimal at neutral pH and between 30 and 45°C. 16S ribosomal DNA sequence analyses showed that the bioreactor was colonized by a wide variety of taxons, belonging to various bacterial divisions: α-, β-, and γ-Proteobacteria; the Flexibacter-Cytophaga-Bacteroides group; and high G+C gram-positive bacteria and low G+C gram-positive bacteria. Some strains represent candidates for new species of the genera Chryseobacterium and Massilia. This diversity demonstrates that the bioreactor is an efficient enrichment tool compared to traditional isolation strategies.
In the laboratory, microorganisms are generally cultivated as cell suspensions in liquid media. However, their natural environments are often more complex, and most microorganisms are members of mixed populations, growing frequently in a nearly static situation (e.g., embedded in soil or as biofilms) resulting from the passage of an aqueous stream of dissolved gases and nutrients over them. A wide variety of surfaces are available in natural environments for attachment and colonization by microorganisms, and these attached bacteria are often more active than free cells (29).
Developing new strategies to recover the microbial population that is not accessible by using classical enrichment techniques has become a real issue for microbiologists. Molecular studies of microbial diversity have shown that these “not yet cultivated” microorganisms can correspond to 99% of the total microbial population (2). Furthermore, access to this reservoir of genetic and metabolic diversity is of interest in areas such as food production, medicine, bioremediation of waste materials, and agriculture. In particular, there is an important biotechnological interest in the isolation of extremophilic microorganisms that are adapted to growth under extreme conditions of temperature, pH, salinity, pressure, and/or in the presence of radioactivity or high concentrations of metal. These extremophiles present a commercial potential for various industries and products, including agricultural applications, chemical synthesis, laundry detergents, and pharmaceuticals (22).
In the present study, microrganisms were enriched by using a reactor designed to mimic natural ecosystems. This reactor contained a column filled with packing to allow microbial colonization and was fed by culture medium in a slow upflow mode. It was used in an attempt to isolate microorganisms growing at low temperatures and producing cold-active enzymes. Psychrophiles are defined as microorganisms whose cardinal (i.e., minimum, optimum, and maximum) growth temperatures are less than 0, 15, and 20°C, respectively. In comparison, microorganisms that have cardinal temperatures of 0 to 5, >15, and >20°C are regarded as psychrotrophic (19). These bacteria have considerable biotechnological potential due to their high level of cold adaptation (23). The specific industrial targets of our project were cold-active proteases, which are stable at room temperature and which therefore might have uses in various cleaning products. We present here the isolation, by using a packed column, of protease producers from Antarctica and their characterization by using both phenotypic and 16S ribosomal DNA (rDNA) sequence analyses.
MATERIALS AND METHODS
Collection of samples.
The environmental sample, collected in December 2000 at Cape Evans on Ross Island, Antarctica, consisted of moist soil, volcanic in nature, 100 m from Scott's hut. This location is a seasonally cold environment, and the temperature and pH measured in situ were 5°C and pH 9, respectively. The samples, originally frozen, were kept at temperatures below 5°C during transport to England. They were then stored at −20°C.
Description of the bioreactor.
The flow unit comprised a glass column 250 mm in length and 25 mm in diameter, operated in upflow mode, and filled with nonporous glass beads 1.5 to 2 mm in diameter (Potters Europe, Bury St. Edmunds, United Kingdom). The system was designed to function in two successive modes. A circulating mode, during which the column was fed with the same medium circulating through the system, enabled microbial colonization. The bioreactor was then run in a washing mode, during which the column was continuously fed with fresh medium. A column jacket, supplied with refrigerated water, maintained the reactor at 10°C. The medium feed was cooled by using a condensor located before the column, and the reservoir bottle was cooled during the circulating mode. Tubing, fittings, connectors, valves, source reservoir bottles, and the jacketed-glass column were supplied by Omnifit, Ltd. (Cambridge, United Kingdom). Two sampling modules enabled the analysis of the liquid phase before and after the glass bead packing. A peristaltic pump delivered a low flow rate through the system of 0.2 to 0.4 ml min−1. The bottles of medium were aerated by using spargers connected with air filtered to 0.22 μm.
Enrichment medium.
The enrichment medium is based on that used by Brock and Freeze (6), in which tryptone and yeast extract were replaced by 0.01% (wt/vol) casein. This defined minimal medium contained Nitsch's trace elements solution and Castenholtz basal salts (25). After solubilization of casein at high pH, the medium was adjusted to pH 7.6 and sterilized by filtration through 0.22-μm (pore-size) filters.
Preparation of the bioreactor and inoculation.
After a wash with distilled water, the system was sterilized by autoclaving and then filled with the medium by using a peristaltic pump under sterile conditions. Once the aeration and cooling conditions were set up, the bioreactor was inoculated. A total of 5 g of the environmental sample was defrosted on ice, and 15 ml of minimal medium was added. After being mixed by inversion, the liquid phase was collected. This operation was repeated with stronger manual shaking conditions, and the two liquid phases were pooled and used for the inoculation with a syringe inserted in a female fitting at the bottom of the column. The direction of the flow was changed several times during the inoculation to prevent any blockage of the valve.
Analyses performed during the bioreactor run.
Using the sampling modules placed before and after the column, the following parameters were analyzed: flow rate, pH, ATP levels, cellular concentration, and morphological changes. The cellular concentration and morphological changes were examined by using a Helber counting chamber and a Nikon E200 microscope. The ATP concentration was determined in relative light units by using the luminometric assay SystemSURE Hygiene Monitoring kit (Celsis, Cambridge, United Kingdom) as previously reported (3). From each sample taken, aliquots were fixed in formaldehyde (10%, vol/vol) and kept at 4°C; for some samples, aliquots were also stored at −80°C in 10% (vol/vol) glycerol.
Access to the attached and nonattached microbial populations.
After dismantling the bioreactor at the end of the run, beads located at the bottom, middle, and top of the column were sampled, as were the contents of the condensor. For each sample, one aliquot was fixed in 10% (vol/vol) formaldehyde, one was used for plating to isolate microorganisms, and the rest was stored at −20°C in 10% (vol/vol) glycerol. Samples containing beads were sequentially treated to access their microbial population. First, the beads were washed with fresh minimal medium, the liquid phase was then collected, and fresh medium was added to the beads. The sample was next vortexed for 2 min, keeping it on ice for 1 min every 30 s. After removal of the liquid phase and the addition of fresh medium, the sample was finally sonicated for 1 min and the liquid phase was removed.
Scanning electron microscopy.
Samples were fixed in formaldehyde, rinsed in phosphate-buffered saline, and freeze-dried in an Edwards-Pearse freeze-drier (BOC Edwards, Crawley, United Kingdom). The samples were then mounted on aluminum planchettes by using adhesive carbon tabs, sputter coated with gold, and viewed in a JEOL JSM6310 scanning electron microscope at 10 kV (JEOL, Tokyo, Japan).
Isolation of aerobic heterotrophs producing extracellular proteases.
The samples collected during the run and after dismantling the bioreactor were used for isolating microorganisms. All the cultures were incubated at 10°C without shaking. The skim milk medium used for screening protease producers on plates was composed of 10 g of skim milk powder (Oxoid, Basingstoke, United Kingdom), 1 g of yeast extract (Sigma-Aldrich, Poole, United Kingdom), and 15 g of agar (Sigma-Aldrich) liter−1. The production of extracellular proteases induced the formation of clearing zones around the colonies. The strains were isolated by repeated streaking on plates and were maintained in Luria-Bertani (LB) liquid medium and on skim-milk plates at pH 7.5.
Storage.
The isolates were maintained at 10°C on solid medium, with plating every 2 months. For long-term storage, cultures in exponential growth phase were stored at −80°C and in liquid nitrogen after the addition of glycerol (10%, vol/vol) as cryoprotectant.
Phenotypic description of the strains.
The following phenetic characteristics were recorded: cellular morphology; Gram staining; colony morphology and pigmentation; the production of catalase, oxidase, and β-lactamase; APIZYM test; nitrate and nitrite reduction; and growth temperature. Oxidase and β-lactamase production were determined by using identification sticks inoculated with fresh colonies (Oxoid). The nitrite and nitrate reductions were tested as described by Cowan (8). Unless indicated otherwise, tests were performed after cultivation at 10°C. Cultivation in nonagitated flasks enabled the determination of the growth pattern of each strain (sedimentation, formation of a pellicule at the surface of the medium, or uniform growth). To determine the growth temperature range, plates were incubated at 4, 10, 25, 37, and 45°C. The formation of clearing zones around the colonies was checked for each plate. The APIZYM test (Biomerieux, Lyon, France) was used to screen the isolates for a range of hydrolytic enzymatic activities. The strains were grown on LB medium until stationary phase and, after cell counting and centrifugation, the pellets were resuspended in the solution delivered by the APIZYM manufacturer. After inoculation, the APIZYM strips were incubated for 24 h in a humid chamber at 10°C before the results were recorded.
Detection and analysis of the protease activities.
After being screened on skim milk plates, the protease activities were confirmed and analyzed by fluorescence assay by using the EnzCheck green fluorescence protease assay kit from Molecular Probes Europe BV (Leiden, The Netherlands) (28). The activities were monitored in the supernatant by using a 96-well microplate fluorimeter (excitation, 485 nm; emission, 535 nm). The temperature and pH profiles were determined for some isolates. The protease pH profiles were determined using the following buffers: 50 mM MES-NaOH (pH 5.5 to 6.5), 50 mM MOPS-KOH (pH 6.5 to 7.5), 50 mM EPPS-NaOH (pH 7.5 to 8.5), 100 mM glycine (pH 8.5 to 9.5), and 100 mM Na2CO3-NaHCO3 (pH 9.5 to 10.5).
Genomic DNA extraction.
The amplification of the 16S rDNA genes was performed directly on cells lysed by treatment at −80°C for 10 min, followed by 3 min at 100°C. Lysis efficiency was checked under the microscope, and genomic DNA extraction efficiency was checked on 0.8% (wt/vol) agarose TAE (0.04 M Tris-acetate, 1 mM EDTA) gels containing 0.8 μg of ethidium bromide ml−1.
16S rRNA gene amplification.
16S rDNA was selectively amplified from genomic DNA by PCR with oligonucleotide primers designed to anneal to conserved positions in the 3′ and 5′ regions of the 16S rRNA genes. Primers specific for the domain Bacteria were used, and for all the isolates tested the forward primer 27f (5′-AGAGTTTGATCATGGCTCAG-3′) and the reverse primer 1492r (5′-TACGGTTACCTTGTTACGACT-3′) modified from Lane (16) enabled the amplification of ca. 1,450 bp of the 16S rRNA gene. The following reagents were combined in a total volume of 50 μl: 100 ng of template DNA or 1 μl of lysed cell solution, 2 U of Vent DNA polymerase (New England BioLabs, Hitchin, United Kingdom), 5 μl of 10× ThermoPol reaction buffer (BioLabs), 200 μM concentrations of each deoxynucleoside triphosphate, a 2 mM concentration of the forward primer, and a 2 mM concentration of the reverse primer. The complete reaction mixture was incubated in a Mastercycler (Eppendorf UK, Ltd., Cambridge, United Kingdom). The PCR temperature profile used was as follows: 94°C for 3 min, followed by 30 cycles of 94°C for 1 min, 52°C for 1 min 30 s, and 72°C for 2 min; and finally an extension step at 72°C for 6 min. PCR products were analyzed by electrophoresis on 0.8% (wt/vol) agarose TAE gels, containing 0.8 μg of ethidium bromide ml−1. PCR products were then purified using the QIAquick PCR purification kit from Qiagen, Ltd. (Crawley, United Kingdom), followed if necessary by ethanol precipitation for salt removal.
Sequence analysis.
In a first step, 300 bp of the 16S rRNA gene were sequenced using the primer 27f to determine the phylogenetic affiliation of the strain and to compare to the sequences previously obtained in the collection. For each new phylogenetic positioning, the complete 16S rRNA gene was sequenced and compared to sequences available in GenBank and EMBL by using BLAST program (www.ncb.nlm.nih.gov/blast/blast.cgi, [1]) and the RDPII analysis software (www.cme.msu.edu/RDP/html/analyses.html). Alignments of the different 16S rDNA sequences were obtained by the CLUSTALW method with weighted residues (27). Nucleotide percent identities were then determined using the program distances under GCG. Phylogenetic reconstruction was produced using PHYLO_WIN (12) with the following setup: Kimura distance matrix and, successively, the neighbor-joining (24), maximum-parsimony (15), and maximum-likelihood (11) methods. Bootstrap values were determined according to the method of Felsenstein (10). The 16S rDNA nucleotide sequences obtained have been deposited in the EMBL database under the following accession numbers (isolate): AJ495802 (P20H), AJ495803 (P22P), AJ495804 (P1A), AJ495805 (P3C), AJ495806 (P22D), AJ495807 (P13F2), AJ495808 (P20G), AJ496037 (P11E), AJ496038 (P8E), and AJ496039 (P16X).
RESULTS
Colonization of the bioreactor.
The bioreactor was run for 25 days, comprising 10 days in circulating mode, followed by 15 days of continuous feed (washing mode). The cellular concentration increased during the circulating mode to achieve 108 cells ml−1 in liquid phase. The progressive colonization of the system during the first 10 days was also shown by an increase in ATP level from 200 to 30,000 relative light units, as measured by a luminometric assay. In the flowthrough, round cells, short motile rods, and long rods could be observed. After 7 days of circulating mode, a decrease of medium viscosity was visually observed, indicating the hydrolysis of most of the casein in the minimal medium. After this observation and the increasing cellular concentration, the bioreactor was configured to the washing mode. The concentration of cells recorded post-column was then stabilized at 108 cells ml−1. The concentration before the column also remained stable, showing that parts of the system before the column were also colonized. After 10 days of washing mode, white aggregates were collected in the sampling system before the column and appeared to be mainly composed of small cocci and short rods. After the system was dismantled, white and yellow biofilms were observed at the bottom of the column, and at different locations of the condensor placed before the column. The biofilms inside the condensor and beads from the top, middle, and bottom parts of the column were sampled. The microbial concentration after the beads were vortexed and sonicated did not significantly increase. Nevertheless, in some cases, aggregates with different cellular morphologies were recovered after sonication, suggesting that the treatment was efficient in the recovery of microorganisms attached to glass bead surfaces. No significant differences in colony morphologies were observed on plates between the microorganisms recovered from different parts of the column.
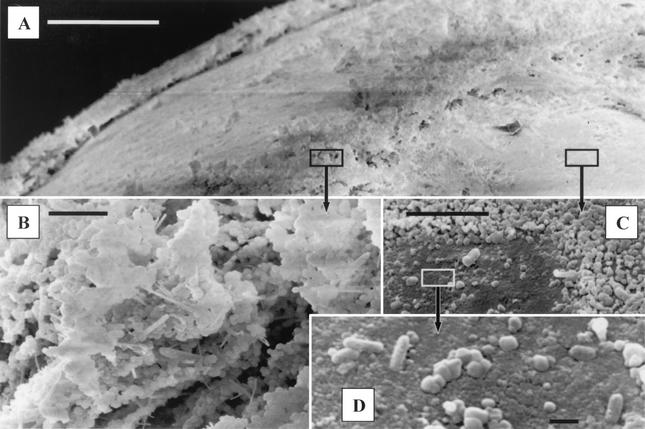
Scanning electron microscopy of the glass beads confirmed the effective colonization of their surfaces by a mixed microbial population. The biofilm showed a stratification in several layers (Fig. 1A and C). The major structure of the biofilm was composed of round cells with a diameter of ≤1 μm (Fig. 1B). Several types of morphologies of rods were included in the aggregates of round cells (Fig. 1B) and could also be observed on the first layer of the biofilm directly on the bead surface (Fig. 1D). Similar results were obtained with beads from the top, middle, and bottom parts of the bioreactor.
FIG. 1.
Scanning electron microscopy of glass beads. (A) Biofilm at the surface of one bead; (B through D) round cells and rods included in the biofilm in the upper layers (B) and on the first layer directly on the surface of the bead (C and D). Bars, 100 μm (A), 5 μm (B and C), and 1 μm (D).
Selection of protease producers by using the bioreactor.
During the bioreactor run, samples before and after the column were used to inoculate skim milk and LB plates. After 2 weeks of incubation, despite growth on both media for all samples, no clearing zones could be observed on the skim milk plates inoculated with the samples from the first 10 days of the run. During this period, visual observation of the colony morphologies on LB and skim milk plates indicated that the diversity decreased by approximately fivefold. Clearing zones appeared first on the plates inoculated with the samples from the 11th day, 1 day after we started to run the system in the washing mode. All samples obtained during the following days produced clearing zones after a few days of incubation, whether during the washing mode or after dismantling the system, including beads from different parts of the column. These results show that protease producers were successfully enriched from the environmental sample by using the bioreactor.
Phenotypic characterization of the isolates.
From the 75 isolates obtained from the bioreactor and subsequently grown on plates, 28 were selected for further characterization; these included all the strains producing clearing zones on skim milk plates, and a selection of other isolates exhibiting a variety of morphological characteristics. These 28 isolates were found to fall into 13 groups, according to colony and cell morphologies, enzyme activities, and APIZYM profiles. Table 1 presents these characteristics for one representative of each group. Cellular morphologies obtained after isolation included rods of various lengths (some of them motile), nonmotile coccobacilli, and motile coccoid cells. Some isolates produced pigmented colonies, from pale yellow to pink or shiny red, and most isolates formed aggregates or flocks in old cultures. All were aerobic psychrotrophs that could grow at 10 and 25°C. The isolates P3C and P20G were the only ones growing more rapidly at 10 than at 25°C. The isolates could be distributed into different APIZYM groups, depending on the type of enzymes they produce, with the protease producers presenting only two metabolic patterns. The first group produced high levels of lipases, phosphatases, and proteases, but only a few strains showed glycosyl-hydrolase activities, and at very low levels (isolates P1A, P20C, P8H, P8E, and P20H). In contrast, the strains of the second group (P16X and P22Z) produced all four types of enzymes but only lipases and glycosyl-hydrolases at a high level (β-galactosidase, α-glucosidase, and β-glucosidase). The hydrolysis of casein indicated by clearing of skim milk plates was much weaker in this group. The non-protease producers could also be divided into two groups, depending on the production levels of glycosyl-hydrolases.
TABLE 1.
Phenotypic characteristics of isolates from Antarctic soil
| Parameter | Characteristics of strain:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1A | P20C | P8H | P8E | P20H | P16X | P22Z | P11D | P11E | P3C | P13F2 | P20G | P22P | |
| Colony morphology | Circular | Circular | Circular | Punctiform | Circular | Circular | Irregular | Irregular | Irregular | Punctiform | Irregular | Circular | Round |
| Texture | Moist | Moist | Moist | Rough | Moist | Dry | Mucoid | Mucoid | Mucoid | Moist | Rough | Rough | Rough |
| Color | Yellow | Yellow | Clear yellow | Yellow | Yellow clear | Red | White translucent | White translucent | White translucent | White | White | Pale yellow | Orange |
| Cell morphologya | Short thin rods | Long thin rods | Short rods | Short rods | Short rods | Cocci | Long rods, sporulating | Long rods | Curved rods | Coccobacilli | Small round | Short rods | Short rods |
| Motility | + | + | ++ | + | None | + | + | + | None | None | None | + | ++ |
| Arrangements | Chains | Chains | Clusters | Clusters | None | Clusters | Clusters | None | None | None | Clusters | Clusters | Clusters |
| Growth pattern | Uniform | Pellic | Pellic | Pellic | Uniform | Sedim | Sedim | Sedim | Uniform | Pellic | Pellic | Sedim | Pellic |
| Gram stain | Neg | Neg | Neg | Neg | Neg | Pos | Pos | Neg | Neg | Neg | Pos | Pos | Neg |
| Growthb | |||||||||||||
| Growth at 4°C | None | None | + | +/P | +/P | +/P | None | +/P | None | ++ | None | + | + |
| Growth at 37°C | ++/P | +/P | None | None | + | None | +/P | + | + | + | None | None | None |
| Enzymatic activitiesc | |||||||||||||
| Catalase | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Oxidase | √ | √ | √ | − | √ | √ | − | − | √ | √ | − | √ | ND |
| β-Lactamase | − | − | − | − | √ | − | − | − | − | − | − | − | − |
| Nitrate reduction | − | − | − | − | − | − | − | − | − | √ | √ | √ | − |
| Nitrite reduction | − | − | − | − | √ | − | − | − | − | − | − | − | − |
| Protease production | |||||||||||||
| CZ on SMd | √ | √ | √ | √ | √ | √ | √ | √ | − | − | − | − | − |
| Topt protease (°C) | 30-45 | ND | ND | ND | ND | 30-45 | 30-45 | ND | |||||
| pHopt protease | 7-8 | ND | ND | ND | ND | 8 | 7-8 | ND | |||||
| APIZYM teste | |||||||||||||
| Lipases | + | ++ | + | + | +++ | + | +++ | ++ | + | + | + | + | ++ |
| Phosphatases | +++ | +++ | +++ | +++ | +++ | + | + | − | +++ | − | + | + | +++ |
| Proteases | + | + | + | ++ | +++ | + | + | + | +++ | + | + | ++ | ++ |
| α-Galactosidase | − | − | − | − | − | − | + | + | + | − | − | + | − |
| β-Galactosidase | − | − | − | + | − | + | +++ | +++ | + | − | − | − | − |
| α-Glucosidase | − | − | + | − | − | +++ | + | + | +++ | − | − | − | + |
| β-Glucosidase | + | − | − | − | − | + | +++ | +++ | + | − | − | − | − |
| Chitinase | − | − | − | + | − | − | − | + | +++ | − | − | − | − |
Growth pattern in broth: Sedim, sedimentation; Pellic, pellicule. Gram stain; Neg, negative; Pos, positive.
All isolates were able to grow at 10 and 25°C; P indicates the presence of clearing zones around colonies on skim milk plates.
Enzymatic activities are recorded as present (√) or absent (−).
That is, clearing zones (CZ) on skim milk (SM) plates.
That is, enzymatic activities as revealed by APIZYM tests, scored on an arbitrary scale from “+” to “+++”. ND, not determined.
Phylogenetic positioning by using 16S rDNA sequences.
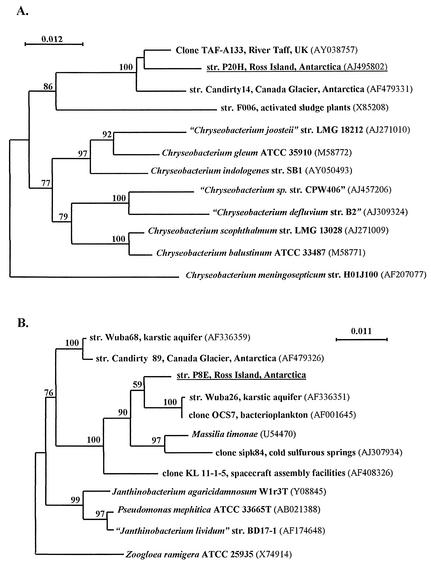
The 16S rRNA gene of one representative of each of the 13 groups obtained by phenotypic analyses was sequenced (Table 2). The 13 isolates belong to the genera Brevundimonas (α-Proteobacteria), Massilia (β-Proteobacteria), Stenotrophomonas, Psychrobacter and Acinetobacter (γ-Proteobacteria), Chryseobacterium and Sphingobacterium (the Cytophagales-Flavobacterium-Bacteroides group), Paenibacillus and Planococcus (low G+C gram-positive bacteria), and Arthrobacter (high G+C gram-positive bacteria). This confirms the taxonomic diversity of the microbial population colonizing the bioreactor. The isolates belonging to the genera Brevundimonas, Arthrobacter, Planococcus, Acinetobacter, and Sphingobacterium showed growth at 4°C but not at 37°C, whereas the opposite could be observed for strains belonging to the genera Stenotrophomonas and Paenibacillus. Table 2 presents the closest phylogenetic relatives by using 16S rDNA sequence comparisons. For all isolates, sequences showing similarities greater than 97% were obtained. In some cases, the closest relatives corresponded to clones produced by studies of microbial diversity based on 16S rDNA libraries and not to cultivated species. In particular, the 16S rDNA percent identity between the isolate P20H and its closest described species, Chryseobacterium scophthalmum, was only 94.1%, indicating that it could belong to a new species of the genus Chryseobacterium. Isolate P8E had 97% sequence identity with the species Massilia timonae, and phenotypic differences between the two strains indicated that P8E could belong to a new species of β-Proteobacteria. The taxonomic originality of the isolates P8E and P20H was confirmed by the 16S rDNA phylogenetic trees that include cultivated but nondescribed isolates and the closest environmental clones, some of which were recovered from other Antarctic sites or marine environments (Fig. 2). In both cases, our isolates clustered with environmental sequences distinct from the clusters formed by the described species, which confirmed their novelty.
TABLE 2.
Analysis of the closest relatives on the basis of 16S rDNA sequences
| Phylogenetic affiliation (division, subdivision, genus) | Isolate | Sequence length (bp) | Top matches, sourcea | EMBL accession no. | Nucleotide identity (%)b |
|---|---|---|---|---|---|
| Flexibacter-Cytophaga-Bacteroides, Chryseobacterium | P20H | 1,450 | Strain CanDirty14, ice from Antarctica Chryseobacterium scophthalmum Str. LMG-13028 | AF479331AJ271009 | 97.4 94.1 |
| Flexibacter-Cytophaga-Bacteroides, Sphingobacterium | P11E | 1,395 | Env. OS1L-21, activated sludge Sphingobacterium multivorum Str. OM-A8 | AB076874AB020205 | 98.7 98.5 |
| Proteobacteria, α-subclass, Brevundimonas | P22P | 1,386 | Env. 4-8, Lake Baikal Brevundimonas vesicularis Str. IAM-12105T | AJ222832AJ021414 | 98.7 98.5 |
| Proteobacteria, β-subclass | P8E | 1,472 | Env. OCS7, bacterioplankton Massilia timonae | AF001645U54470 | 98.6 97.0 |
| Proteobacteria, γ-subclass, Stenotrophomonas | P1A | 1,442 | Stenotrophomonas maltophila Str. LMG 10877 | AJ131784 | 99.8 |
| Proteobacteria, γ-subclass, Psychrobacter | P3C | 1,429 | Psychrobacter glacincola Str. DSM 12194T, marine environments Psychrobacter glacincola Str. IC084, Antarctic sea ice | AJ312213 U85876 | 99.4 97.6 |
| Proteobacteria, γ-subclass, Acinetobacter | P13F2 | 1,441 | “Anoxic,” batch reactor Env. HTA554, Mariana Trench Acinetobacter johnsonni Str. DSM6963 | AY055373AB002656X81663 | 99.4 99.2 99.2 |
| Low G+C gram-positive bacteria, Firmicutes, Paenibacillus | P22Z | 1,469 | Paenibacillus amylolyticus Str. NRRL NRS-290T Paenibacillus sp. Str. V22, Lake Vostok, Antarctica | D85396AF324200 | 98.6 98.5 |
| Low G+C gram-positive bacteria, Firmicutes, Planococcus | P16X | 1,442 | SOS orange, Antarctica Planococcus okeanokoites Str. IFO 12536T | AF242541D55729 | 98.6 97.3 |
| High G+C gram-positive bacteria, Arthrobacter | P20G | 1,456 | Wuba 49, karstic aquifer AH13, bacterioplankton Arthrobacter sulfureus Str. DSM 20167 | AF336357AJ289962X83409 | 98.7 98.6 98.3 |
Top match as determined by the BLAST method using the EMBL and GenBank databases (1). Str., 16S rRNA gene sequences from cultivated bacteria; Env., sequences of PCR-amplified products in environmental samples.
Nucleotide identity as determined by the program distances under GCG. The 16S rDNA sequences of isolates P20C, P8H, and P11D were identical to those of P1A, P22P, and P22Z, respectively.
FIG. 2.
Phylogenetic positions of the strains P20H and P8E within Chryseobacterium (A) and Massilia (B) genera. A total of 1,067 (A) and 1,173 (B) sites were used for the phylogenetic analysis. Chryseobacterium meningosepticum (A) and Zooglea ramigera (B) were, respectively, used as outgroups. The topology shown is an unrooted tree obtained by a neighbor-joining algorithm (with Kimura corrections) established using PHYLO_WIN. Significant bootstrap values (calculated from 900 trees) are indicated as percentages at the branching points. Bars indicate 1.2 (A) and 1.1 (B) nucleotide substitutions per 100 nucleotides. The accession numbers of the sequences are given in brackets.
The clusters obtained by phenotypic and phylogenetic data were congruent. However, morphological differences were observed for strains showing high levels of identity of 16S rDNA sequences, indicating an intracluster diversity not revealed by the molecular analysis. For example, the two strains P1A and P20C belonging to Stenotrophomonas maltophila presented very different cell lengths when grown under the same conditions. P8H and P22P belonging to Brevundimonas sp. exhibited different colony colors.
Characterization of the proteases.
To study the protease activities of the strains producing clearing zones on skim milk plates, a fluorimetric assay was used (28) that was far more sensitive than the azocasein method (7, 30) and was suitable for continuous assays. Protease activity was detected for all the strains tested. The protease producers isolated belong to the genera Brevundimonas, Massilia, Chryseobacterium, Planococcus, Stenotrophomonas, and Paenibacillus. The strains with the highest protease activities belonged to Stenotrophomonas maltophila. Other representatives of this species have been isolated from Antarctica and from alpine environments, and their protease activities have been reported (18). The activities of the extracellular proteases excreted by Stenotrophomonas sp. (P1A), Planococcus sp., and Paenibacillus sp. (P22Z) were tested at different temperatures and pH values. The pH ranges of the proteases produced by Chryseobacterium sp. and Planococcus sp. (P16X) were also tested. In all cases, the apparent maximum protease activities were at neutral pH and at temperatures between 30 and 45°C, although the enzymes remained active at low temperatures. This finding was consistent with the fact that the strains were mainly psychrotrophs and were enriched in neutral pH conditions.
DISCUSSION
The bioreactor was colonized in only a few days by a microbial population presenting a high level of taxonomic diversity, and protease producers were successfully enriched from the environmental sample. However, the original environmental sample that was used to inoculate the bioreactor did not result in colonies showing clearing zones when plated directly on skim milk plates, even after 3 weeks of incubation at 10°C. Therefore, the conditions inside the bioreactor were favorable to the emergence of protease producers not recovered on plates, demonstrating the bioreactor's potential to enrich microorganisms with particular metabolic characteristics.
All the isolates obtained belong to the domain Bacteria and, even with a cultivation-orientated approach, the bacterial diversity generated in the bioreactor was very high. Strains belonging to at least 10 different genera, within various bacterial lineages (the Cytophagales-Flavobacterium-Bacteroides group, Proteobacteria, and low G+C and high G+C gram-positive bacteria) have been isolated. Some strains, such as P20G and P11E, belong to ubiquitous genera already described in Antarctica and are more generally widespread in diverse environments (Arthrobacter and Sphingobacterium spp., respectively) (14). Others obtained in the present study belong to genera specifically colonizing cold environments, such as P3E and P16X, belonging to the genera Psychrobacter and Planococcus, respectively. New species of these four bacterial genera have been previously isolated from Antarctic samples and described (4, 5, 13, 20, 21, 26). However, according to 16S rDNA sequence analyses, most of the strains in the current study do not belong to the species previously isolated from Antarctica but are close to other species of these genera isolated from different environments. For other isolates, such as P8E or P20H, the highest 16S rDNA sequence identity with cultured microorganisms was <97%, indicating good candidates for new species of psychrotrophs. Finally, some taxons recovered in the present study, such as Chryseobacterium sp. or Brevundimonas sp., have never been characterized in Antarctic samples. Therefore, the use of a packed-column bioreactor to enrich microorganisms has allowed access not only to a high bacterial diversity but also to taxonomic originality. Further characterization of the corresponding strains is required to confirm their novelty.
The packed column offers growth conditions different from classical growth vessels such as agitated flasks, allowing the enrichment of microorganisms growing in biofilm after attachment on a surface, as confirmed by the scanning electron microscopy observations. The stratified biofilm gathered different microenvironments and therefore offered the opportunity to grow various microorganisms. From the same column, 10 different genera belonging to various bacterial lineages were indeed obtained due to these favorable growth conditions. Previous studies comparing attached and unattached populations in a simulated aquifer under porous flow conditions have shown that they constitute two metabolically different microbial populations that are strongly interacting. Lehman et al. (17) found that attached bacteria exhibited higher extracellular enzymatic activities than their free-living counterparts. Furthermore, unattached bacteria had higher hydrolysis rates for polysaccharides and lower rates for polypeptides than the attached cells. In our study, the differences observed in the metabolic patterns revealed by APIZYM and the fact that some microorganisms were not producing clearing zones on skim-milk plates are indicative of a complex microbial population with possible strong interactions between the different components of the biofilm. Peptides and amino acids produced by hydrolysis of the casein by the protease producers could constitute possible substrates for other bacteria that are not producing clearing zones on skim milk plates.
In the present study, our efforts were focused on protease activities, and the pH and temperature profiles determined enabled us to select isolates for further characterization of the proteases. However, psychrotrophs have biotechnological interest as producers of other enzymes with industrial potential, such as α-amylases, β-galactosidases, chitinases, or lipases (9). Preliminary results have shown the production of some of these enzymes by the isolates obtained in the present study.
In conclusion, the packed-column bioreactor was colonized by diverse bacterial species, and protease producers were successfully enriched. The collection of microorganisms produced has potential in terms of biotechnological applications and in terms of taxonomic originality.
Acknowledgments
We are indebted to R. Farrell, University of Waikato, Hamilton, New Zealand, for leading Antarctic NZ Event KO21, during which the microbial sample was collected. We are grateful to Ursula Potter (University of Bath) for the scanning electron microscopy analyses, Fernando Acosta (University of Bath) for advice on the construction of the bioreactor, and Russell Chedgy (University of Bath) for help with the maintenance of the microbial collection. We also thank R. Sharp and N. Raven, CAMR, Porton Down, United Kingdom, for helpful discussions and expert advice.
Generous financial support was provided by the UK Biotechnology and Biological Sciences Research Council; Reckitt Benckiser plc.; Glaxo Wellcome Operations; EHC Viridian, Ltd. (LINK research grant 86/ABC11254); the Society for General Microbiology; Avecia LifeScience Molecules; and GeneSys, Ltd.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K. H. Schleiffer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson, T., S. Cairns, D. A. Cowan, M. J. Danson, D. W. Hough, D. B. Johnson, P. R. Norris, N. Raven, C. Robinson, R. Robson, and R. J. Sharp. 2000. A microbiologicl survey of Montserrat Island hydrothermal biotopes. Extremophiles 4:305-313. [DOI] [PubMed] [Google Scholar]
- 4.Bowman, J. P., D. S. Nichols, and T. A. McMeekin. 1997. Psychrobacter glacincola sp. nov., a halotolerant, psychrophilic bacterium isolated from Antarctic sea ice. Syst. Appl. Microbiol. 20:209-215. [Google Scholar]
- 5.Bowman, J. P., M. S. A., M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock, T. D., and H. Freeze. 1969. Thermus aquaticus gen. n. and sp. n., a non-sporulating extreme thermophile. J. Bacteriol. 98:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charney, J., and R. M. Tomarelli. 1947. A colorimetric method for the determination of the proteolytic activity of duodenal juice. J. Biol. Chem. 171:501-507. [PubMed] [Google Scholar]
- 8.Cowan, S. T. 1974. Cowan and Steel's manual for the identification of medical bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 9.Deming, J. W. 2002. Psychrophiles and polar regions. Curr. Opin. Microbiol. 5:301-309. [DOI] [PubMed] [Google Scholar]
- 10.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 30:783-791. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Biol. 17:368-376. [DOI] [PubMed] [Google Scholar]
- 12.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12:543-548. [DOI] [PubMed] [Google Scholar]
- 13.Junge, K., J. J. Gosink, H. G. Hoppe, and J. T. Staley. 1998. Arthrobacter, Brachybacterium and Planococcus isolates identified from Antarctic sea ice brine. Description of Planococcus mcmeekinii sp. nov. Syst. Appl. Microbiol. 48:1083-1084. [DOI] [PubMed] [Google Scholar]
- 14.Kisand, V., R. Cuadros, and J. Wikner. 2002. Phylogeny of culturable estuarine bacteria catabolizing riverine organic matter in the Northern Baltic Sea. Appl. Environ. Microbiol. 68:379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lake, J. A. 1987. A rate-independent technique for analysis of nucleic acid sequences: evolutionary parsimony. Mol. Biol. Evol. 4:167-191. [DOI] [PubMed] [Google Scholar]
- 16.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 130-141. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Wiley Press, Chichester, United Kingdom.
- 17.Lehman, R. M., F. S. Colwell, and G. A. Bala. 2001. Attached and unattached microbial communities in a simulated basalt aquifer under fracture- and porous-flow conditions. Appl. Environ. Microbiol. 67:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margesin, R., and F. Schinner. 1991. Characterization of a metalloprotease from psychrophilic Xanthomonas maltophilia. FEMS Microbiol. Lett. 79:257-262. [Google Scholar]
- 19.Morita, R. Y. 1975. Psychrophilic bacteria. Bacteriol. Rev. 39:144-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy, G. S. N., R. K. Aggarwal, G. I. Matsumoto, and S. Shivaji. 2000. Arthrobacter flavus sp.nov, a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley, Antarctica. Int. J. Syst. E vol. Microbiol. 50:1553-1561. [DOI] [PubMed] [Google Scholar]
- 21.Reddy, G. S. N., J. S. S. Prakash, G. I. Matsumoto, and E. Stackebrandt. 2002. Arthrobacter roseus sp. nov., a psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int. J. Syst. E vol. Microbiol. 52:1017-1021. [DOI] [PubMed] [Google Scholar]
- 22.Rothschild, L. J., and R. L. Mancinelli. 2001. Life in extreme environments. Nature 409:1092-1101. [DOI] [PubMed]
- 23.Russell, N. J. 2000. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83-90. [DOI] [PubMed] [Google Scholar]
- 24.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 25.Sharp, R., D. Cossar, and R. Williams. 1995. Physiology and metabolism of Thermus, p. 67-91. In R. Sharp and R. Williams (ed.), Thermus species, vol. 9. Plenum Press, New York, N.Y.
- 26.Shivaji, S., M. K. Ray, N. S. Rao, L. Saisree, M. V. Jagannadham, G. S. Kumar, G. S. N. Reddy, and P. M. Bhargava. 1992. Sphingobacterium antarcticus sp. nov., a psychrotrophic bacterium from the soils from Schirmacher Oasis, Antarctica. Int. J. Syst. Bacteriol. 42:102-106. [Google Scholar]
- 27.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson, V. F., S. Saldana, J. Cong, and D. E. Goll. 2000. A BODIPY fluorescent microplate assay for measuring activity of calpains and other proteases. Anal. Biochem. 279:107-178. [DOI] [PubMed] [Google Scholar]
- 29.Van Loosdrecht, M. C. M., J. Lyklema, W. Norde, and A. J. B. Zehnder. 1990. Influence of interfaces on microbial activity. Microbiol. Rev. 54:75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasquez, S. C., L. N. Rios Merino, W. P. MacCormack, and E. R. Fraile. 1995. Protease-producing psychrotrophic bacteria isolated from Antarctica. Polar Biol. 15:131-135. [Google Scholar]