Abstract
The antimicrobial effect obtained upon combining the prokaryotic antimicrobial peptides (AMPs; more commonly referred to as bacteriocins) pediocin PA-1, sakacin P, and curvacin A (all produced by lactic acid bacteria [LAB]) with the eukaryotic AMP pleurocidin (from fish) has been investigated. The three LAB AMPs alone were active against gram-positive Listeria ivanovii bacteria at nanomolar concentrations, whereas they were inactive against gram-negative Escherichia coli bacteria. Pleurocidin alone was active against both of these types of bacteria at micromolar concentrations. Little if any synergy between the LAB AMPs and pleurocidin against the gram-positive L. ivanovii strain was obtained. In contrast, the LAB AMPs and pleurocidin acted highly synergistically against the gram-negative E. coli strain. Nanomolar concentrations of LAB AMPs increased the growth inhibitory potency of pleurocidin by about fourfold. When micromolar concentrations of LAB AMPs were combined with 2 μg of pleurocidin/ml, 100% growth inhibition was attained, whereas pleurocidin alone at a concentration of 2 μg/ml gave no growth inhibition. Most noteworthy, when high concentrations (128 μg/ml) of pleurocidin in the absence of LAB AMPs were used over a long period of incubation (1 week), some growth of E. coli was observed, whereas 16 μg of pleurocidin/ml completely abolished growth in the presence of 64 to 128 ng of LAB AMPs/ml over the same period of time. The results clearly demonstrate that combining eukaryotic and prokaryotic AMPs can greatly increase the specific activity and broaden the target-cell range of these peptides.
Gene-encoded, ribosomally synthesized antimicrobial peptides (AMPs) are widely produced by various cellular life forms—microorganisms (8, 17), plants (4), and animals (1), including humans. The peptides are often small in size (20 to 60 amino acid residues), cationic, and amphiphilic and/or hydrophobic. Killing of susceptible microorganisms is often correlated with permeabilization of the target-cell membrane (18), but other mechanisms have also been proposed (19, 23). More than 750 different AMPs have been identified in eukaryotes (Antimicrobial Sequences Database, http://www.bbcm.univ.trieste.it/∼tossi/pag1.htm), where the AMPs constitute an important part of the host defense system (10, 12). In prokaryotes, AMPs are found most frequently in gram-positive bacteria, and more than 50 AMPs, most commonly referred to as bacteriocins, have been identified in lactic acid bacteria (LAB).
AMPs may be developed into new and useful antimicrobial additives and drugs. Examples of this are the LAB AMPs nisin and lacticin 3147, both of which are used as food preservatives (7, 14). The latter is also used for preventing bovine mastitis (20). Several eukaryotic AMPs have been tested for use in human medicine, but none are currently in use (24).
There are marked differences in the antimicrobial activities of eukaryotic and prokaryotic AMPs. While AMPs from eukaryotic cells often show activity against both gram-negative and gram-positive bacteria, and sometimes also against other microorganisms, their prokaryotic counterparts often have relatively narrow inhibitory spectra; i.e., most of them are active against only species and genera related to the AMP-producing bacteria (13). Another feature that differentiates the eukaryotic and prokaryotic AMPs is the much higher antimicrobial potency of the latter. While many prokaryotic AMPs are able to kill or inhibit the growth of bacteria at nanomolar concentrations, eukaryotic AMPs require micromolar concentrations to achieve similar killing efficacy.
The broad inhibitory spectra of the eukaryotic AMPs and the high potency of prokaryotic AMPs prompted us to examine possible synergy between these AMPs. The eukaryotic AMP pleurocidin (6) and the LAB AMPs curvacin A (21), pediocin PA-1 (11, 16), and sakacin P (21) were chosen for this study. Pleurocidin is an amphipathic α-helical 25-residue AMP which has been isolated from fish (6). It is active against several gram-negative and gram-positive bacteria at micromolar concentrations. The three LAB AMPs all belong to the pediocin-like family of bacteriocins (15). Today, at least 20 AMPs that belong to this family have been characterized (9). All of these AMPs contain 37 to 48 residues, have similar amino acid sequences, and kill cells by permeabilizing the target-cell membranes. These AMPs have very potent antilisterial activity but have no activity against gram-negative bacteria (15).
MATERIALS AND METHODS
Antimicrobial peptides.
Synthetic pleurocidin was obtained from J. Gray of the Molecular Biology Unit of the University of Newcastle upon Tyne, Newcastle upon Tyne, United Kingdom.
The bacteriocins curvacin A and pediocin PA-1 were purified from Lactobacillus curvatus LTH1174 and Pediococcus acidilactici LMG 2351, respectively, by using a recently developed method (22), while sakacin P was produced in Lactobacillus sakei by a bacteriocin expression system (2) and purified by the same method.
Microorganisms.
Escherichia coli ATCC 14763 and Listeria ivanovii Li4 were grown overnight at 25°C in Trypticase soy broth (TSB; Difco). The cultures were diluted to an optical density at 600 nm of approximately 0.2 in TSB with 15% (vol/vol) glycerol, aliquoted (0.2 ml), and stored at −80°C.
Upon use, 1 ml of TSB was added to one vial of frozen bacteria, which was incubated at 37°C for 30 min followed by 3 h at 25°C. This culture was diluted 1:100 with TSB prior to use in the antimicrobial assay, giving a concentration of approximately 5 × 105 CFU/ml for E. coli and approximately 1 × 106 CFU/ml for L. ivanovii.
Antimicrobial and bactericidal assays.
A checkerboard assay was used to measure the antimicrobial activity for pairs of peptides. Serial twofold dilutions of each peptide were made in 0.01% acetic acid-0.2% bovine serum albumin (total volume, 50 μl) in 96-well polystyrene microtiter plates (tissue culture treated), and 50 μl of bacteria in TSB was added to each well. Controls of each peptide alone, as well as controls without peptide (growth control) and without bacteria (sterility control), were included. The plates were incubated at 25°C for 16 to 18 h. MICs were taken as the lowest concentration in each row without visible growth, measured by reading the optical density at 620 nm in a microtiter plate reader.
To determine whether or not the observed growth inhibition was due to bacterial killing, 10-μl aliquots of each well from the antimicrobial assay were plated on Trypticase soy agar (1 ml in 24-well plates) at 0, 3, and 16 to 18 h after mixing. The plates were incubated at 25°C overnight. Bactericidal activity was observed as the absence of growth.
RESULTS AND DISCUSSION
Synergistic activity is often expressed as the FIC (fractional inhibition concentration) index, calculated as follows: FIC index = ([A]/MICA) + ([B]/MICB), where MICA and MICB are the MICs of the peptides alone and [A] and [B] are the MICs of A and B when used together. Synergism is defined as a FIC index of 0.5 or less (3).
The antimicrobial effect obtained upon combining one of the LAB AMPs (pediocin PA-1, sakacin P, or curvacin A) with pleurocidin was measured by using gram-positive L. ivanovii and gram-negative E. coli bacteria as target cells. The three LAB AMPs alone were very active against L. ivanovii, with pediocin PA-1 and sakacin P being more active (MICs of 0.25 and 0.064 ng/ml, respectively [Table 1 ]) than curvacin A (MIC of 4 to 8 ng/ml [Table 1]). As expected, these LAB AMPs were all inactive against the gram-negative E. coli target strain (MICs of >512 μg/ml [Table 2 ]). Pleurocidin alone was active against both target strains, but it was more active against E. coli (MIC of 16 μg/ml [Table 2]) than against L. ivanovii (MIC of 64 μg/ml).
TABLE 1.
MICs of LAB AMPs for L. ivanovii at different pleurocidin concentrations
| AMP | MIC (ng/ml) at pleurocidin concn (μg/ml) of:
|
FIC indexb | ||||
|---|---|---|---|---|---|---|
| 64a | 32 | 8 | 0.5 | 0 | ||
| Curvacin A | NA | 2-8 | 2-4 | 2-4 | 4-8 | 0.5 |
| Pediocin PA-1 | NA | 0.25 | 0.25 | 0.13 | 0.25 | 0.5 |
| Sakacin P | NA | 0.064 | 0.064 | 0.064 | 0.064 | 1 |
The MIC of pleurocidin for L. ivanovii is 64 μg/ml. NA, not applicable.
FIC indexes were calculated as described in the text with a pleurocidin concentration of 0.5 μg/ml and corresponding MICs of LAB AMPs.
TABLE 2.
MICs of LAB AMPs for E. coli at different pleurocidin concentrationsa
| AMP | MIC at pleurocidin concn of:
|
FIC index | |||||
|---|---|---|---|---|---|---|---|
| 16b | 4 | 2 | 1 | 0.5 | 0 | ||
| Curvacin A | NA | 0.008 | 16-32 | 64-128 | 256 | >512 | <0.19 |
| Pediocin PA-1 | NA | 0.002 | 16-32 | 64-128 | 128 | >512 | <0.19 |
| Sakacin P | NA | 0.001 | 32-64 | 128-256 | 256 | >512 | <0.25 |
All concentrations are given in micrograms per milliliter.
The MIC of pleurocidin for E. coli is 16 μg/ml. NA, not applicable.
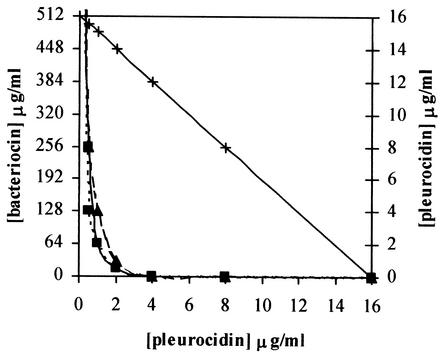
Only a small additional effect (FIC index, 0.5 to 1) was seen upon exposing L. ivanovii to both pleurocidin and LAB AMPs (Table 1), and thus little if any synergy against this gram-positive target bacterium was obtained. A remarkably high synergy (FIC index, 0.19 to 0.25 [Table 2]) was, however, observed when the combination was used against the gram-negative E. coli target bacterium, which is resistant to the LAB AMPs alone. Very small amounts of the LAB AMPs (1 to 8 ng/ml) increased the potency of pleurocidin by about fourfold, and an increase of about 30-fold was obtained upon the addition of 100 to 250 μg of LAB AMPs/ml (Table 2). Moreover, exposure to LAB AMPs at concentrations of 16 to 32 μg/ml together with 2 μg of pleurocidin/ml resulted in 100% growth inhibition after incubation for about 18 h, whereas pleurocidin alone at a concentration of 2 μg/ml gave no growth inhibition (data not shown). It is noteworthy that upon longer incubation (1 week), growth did occur even with quite high pleurocidin concentrations (128 μg/ml) in the absence of LAB AMPs, whereas 16 μg of pleurocidin/ml was enough to completely abolish growth in the presence of 64 to 128 ng of LAB AMPs/ml (results not shown). The synergistic behavior is clearly seen in Fig. 1; an additive effect is shown as a straight line (as seen for pleurocidin plotted against pleurocidin), whereas lines representing synergistic effects fall below the straight line (3).
FIG. 1.
Minimum concentrations of LAB AMPs curvacin A (—▪—), pediocin PA-1 (· · · · ▪ · · · ·), and sakacin P (- - ▴ - -) needed at various pleurocidin concentrations for growth inhibition of E. coli. Pleurocidin (—×—) is plotted against pleurocidin to show additive effect.
The outer membranes (present in gram-negative but not gram-positive bacteria) may prevent the LAB AMPs from being effective against gram-negative bacteria, such as E. coli. Pleurocidin, however, active against gram-negative bacteria, is apparently able to penetrate the outer membranes and thereby reach the targets, which are most likely the inner membranes of these bacteria. A possible explanation for the strong synergy between pleurocidin and the LAB AMPs may, consequently, be that pleurocidin renders the outer membranes permeable for the bacteriocins and thereby makes the inner membranes accessible to the LAB AMPs. The LAB AMPs are very potent against many gram-positive bacteria, and it has been shown that they act on the membranes (5). The strong synergy between pleurocidin and LAB AMPs against the gram-negative target bacterium clearly demonstrates that in the search for efficient antimicrobial agents the use of different AMPs in combination may be a promising approach. Combining different AMPs can greatly increase the specific activity and broaden the target-cell range of these peptides.
The mode of action has not been properly established, but we have observed some killing by using this combination of prokaryotic and eukaryotic AMPs. It seems that the survival frequencies of target bacteria are dose dependent, which is not unlike what has frequently been seen with a single AMP. However, further studies are required to understand in detail what peptide concentrations are needed to obtain a killing or growth-inhibiting mode of action.
Acknowledgments
L. ivanovii Li4 bacteria were obtained from the Norwegian Food Research Institute.
This work was supported by grants from the Norwegian Research Council and EU (project QLK2-CT-2000-00422).
REFERENCES
- 1.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers (Pept. Sci.) 47:415-433. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson, L., T. Katla, M. Bjornslett, V. G. Eijsink, and A. Holck. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol. Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1981. Criteria for analyzing interactions between biologically active agents. Adv. Cancer Res. 35:269-335. [DOI] [PubMed] [Google Scholar]
- 4.Cammue, B. P. A., M. F. C. De Bolle, H. M. E. Schoffs, F. R. G. Terras, K. Thevissen, R. W. Osborn, S. B. Rees, and W. F. Broekaert. 1994. Gene encoded antimicrobial peptides from plants, p. 91-106. In H. G. Bomam, J. Marsh, and J. A. Goode (ed.), Antimicrobial peptides. Wiley, New York, N.Y. [DOI] [PubMed]
- 5.Chikindas, M. L., M. J. Garcia Garcera, A. M. Driessen, A. M. Lederboer, J. Nissen-Meyer, I. F. Nes, T. Abee, W. N. Konings, and G. Venema. 1993. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl. Environ. Microbiol. 59:3577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, A. M., P. Weis, and G. Diamond. 1997. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 272:12008-12013. [DOI] [PubMed] [Google Scholar]
- 7.De Vuyst, L., and E. J. Vandamme. 1994. Nisin, a lantibiotic produced by Lactococcus lactis subsp. lactis: properties, biosynthesis, fermentation and applications, p. 151-221. In L. De Vuyst and E. J. Vandamme (ed.), Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. Blackie Academic and Professional, London, England.
- 8.Diep, D. B., and I. F. Nes. 2002. Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Curr. Drug Targets 3:107-122. [DOI] [PubMed] [Google Scholar]
- 9.Fimland, G., V. G. H. Eijsink, and J. Nissen-Meyer. 2002. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 41:9508-9515. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, J. T., A. L. Chopko, and P. D. van Wassenaar. 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 295:5-12. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, Jr., and R. A. N. B. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 13.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 14.Morgan, S. M., M. Galvin, J. Kelly, R. P. Ross, and C. Hill. 1999. Development of a lacticin 3147-enriched whey powder with inhibitory activity against foodborne pathogens. J. Food Prot. 62:1011-1016. [DOI] [PubMed] [Google Scholar]
- 15.Nes, I. F., H. Holo, G. Fimland, H. H. Hauge, and J. Nissen-Meyer. 2001. Unmodified peptide-bacteriocins (class II) produced by lactic acid bacteria. In C. J. Dutton, M. A. Haxell, H. A. I. McArthur, and R. G. Wax (ed.), Peptide antibiotics: discovery, modes of action, and applications. Dekker, New York, N.Y.
- 16.Nieto Lozano, J. C., J. Nissen-Meyer, K. Sletten, C. Pelaz, and I. F. Nes. 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985-1990. [DOI] [PubMed] [Google Scholar]
- 17.Nissen-Meyer, J., and I. F. Nes. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Biochem. Biophys. 167:67-77. [PubMed] [Google Scholar]
- 18.Oren, Z., and Y. Shai. 1998. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers (Pept. Sci.) 47:451-463. [DOI] [PubMed] [Google Scholar]
- 19.Patrzykat, A., C. L. Friedrich, L. Zhang, V. Mendoza, and R. E. Hancock. 2002. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 46:605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan, M. P., J. Flynn, C. Hill, R. P. Ross, and W. J. Meaney. 1999. The natural food grade inhibitor, lacticin 3147, reduced the incidence of mastitis after experimental challenge with Streptococcus dysgalactiae in nonlactating dairy cows. J. Dairy Sci. 82:2625-2631. [DOI] [PubMed] [Google Scholar]
- 21.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocin curvacin A from Lactobacillus curvatus LTH1174 and sakacin from L. sake LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 22.Uteng, M., H. H. Hauge, I. Brondz, J. Nissen-Meyer, and G. Fimland. 2002. Rapid two-step procedure for large-scale purification of pediocin-like bacteriocins and other cationic antimicrobial peptides from complex culture medium. Appl. Environ. Microbiol. 68:952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, M., E. Maier, R. Benz, and R. E. W. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 24.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]