Abstract
Soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptors (SNAREs) are core machinery for membrane fusion during intracellular vesicular transport. Synaptosome-associated protein of 23 kDa (SNAP23) is a target SNARE previously identified at the plasma membrane, where it is involved in exocytotic membrane fusion. Here we show that SNAP23 associates with vimentin filaments in a Triton X-100 insoluble fraction in fibroblasts in primary culture and HeLa cells. Upon treatment of human fibroblasts with N-ethyl-maleimide, SNAP23 dissociates from vimentin filaments and forms a protein complex with syntaxin 4, a plasma membrane SNARE. The vimentin-associated pool of SNAP23 can therefore be a reservoir, which would supply the plasma membrane fusion machinery, in fibroblasts. Our observation points to a yet unexplored role of intermediate filaments.
INTRODUCTION
SNAP23 is a ubiquitously expressed isoform of synaptosomal-associated protein of 25 kDa (SNAP25) (Ravichandran et al., 1996), a component of the whole neuronal plasma membrane (Oyler et al., 1989; Galli et al., 1995). SNAP25 plays a role as a target soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein (SNAP) receptor (t-SNARE), through the formation of a ternary complex with syntaxin 1, another neuronal plasma membrane protein, and synaptobrevin 2, a synaptic vesicle SNARE (Sollner et al., 1993). Formation of trans-SNARE complexes between adjacent membranes mediates lipid bilayer fusion (Weber et al., 1998; Bock and Scheller, 1999; Nickel et al., 1999). SNAP23 localizes to the apical and lateral plasma membranes in epithelial cells and it is involved in exocytosis at both domains (Galli et al., 1998; Leung et al., 1998; Low et al., 1998a; Lafont et al., 1999). SNAP23 is also involved in granule exocytosis in nonpolarized cell types, including mast cells (Guo et al., 1998), adipocytes (Foster et al., 1999), and platelets (Chen et al., 2000).
The mechanism and regulation of targeting of SNAP25 and SNAP23 to the plasma membrane is not fully understood. Palmytoylation of cysteine residues located in the center of these proteins is required (Gonzalo and Linder, 1998; Gonzalo et al., 1999) but the interaction with a plasma membrane syntaxin (Veit, 2000) and phosphorylation by SNAP kinase (Cabaniols et al., 1999) are also important in this process. It was recently reported that SNAP23 partially sediments with cytoskeletal elements in a Triton X-100 insoluble fraction (Guo et al., 1998; Foster et al., 1999). In this study we show that the cytoskeleton-associated pool of SNAP23 is bound to vimentin filaments in fibroblasts in primary culture and may be recruited to form SNARE complexes at the plasma membrane.
MATERIALS AND METHODS
Cells
HeLa cells, human fibroblasts, and embryonic mouse fibroblasts were used. Human fibroblasts were obtained from Dr. G. de Saint Basile (Hôpital Necker Enfants Malades, Paris, France) and cultured in RPMI 1640, 10% fetal calf serum, 5 mM glutamine. Embryonic mouse fibroblasts from wild-type and vimentin knockout mice were prepared from E13.5 embryo as described (Gillard et al., 1998) and cultured in Dulbecco's modified Eagle's medium, 10% fetal calf serum, 5 mM glutamine, 1 mM sodium pyruvate.
Antibodies
Primary antibodies included rabbit affinity-purified anti-SNAP23 recombinant protein (TG7); anti-Nter peptide (Nter, 1–17 residues; generous gift of Dr. P. Roche, National Institutes of Health, Bethesda, MD; Low et al., 1998b); anti-peptide (residues 196–211; Synaptic Systems, Göttingen, FRG); and mouse antibodies directed against β-tubulin (clone tub2.1; Sigma, St. Louis, MO), syntaxin 4 (clone 49; Transduction Laboratories, San Diego, CA), vimentin (clone7A3), and keratin (anti pan-keratin, clone PCK-26; Sigma) in phosphate-buffered saline (PBS). Secondary antibodies included Texas Red-coupled anti-rabbit F(ab′)2 (Jackson Laboratories, West Grove, PA) or rhodamine-coupled phalloidin (Sigma), and Alexa 488-coupled donkey anti-mouse secondary antibodies (Molecular Probes, Leiden, The Netherlands). Horseradish peroxidase-coupled antibodies against mouse and rabbit was purchased from Jackson Laboratories.
Immunofluorescence
Cells were grown on glass coverslips and fixed in methanol at −20°C for 3–5 min, incubated in PBS/bovine serum albumin/saponin (0.2%/0.05%) for 10 min, and subsequently with the different antibodies for 20–30 min at each step. The coverslips were mounted in Mowiol. Analysis of the samples was performed on a TCS confocal microscope (Leica, Heidelberg, FRG). In certain experiments, cells were incubated during 16 h in RPMI containing 4 mM acrylamide before fixation (Eckert, 1985; Olink-Coux et al., 1992).
N-ethyl Maleimide (NEM) Treatment
Human fibroblasts were treated with 1 mM NEM in PBS for 15 min on ice and then with 2 mM dithiothreitol (DTT) in PBS for another 15 min on ice, or with 1 mM NEM + 2 mM DTT in PBS for 30 min on ice, washed extensively with ice-cold PBS, and incubated in culture medium at 37°C for 30 min. They were lysed with 1% Triton X-100 in 50 mM Tris, 150 mM NaCl, 5 mM EDTA, and a cocktail of protease inhibitors (Sigma). The extract was centrifuged at 100,000 × g for 30 min, resulting in Triton X-100 insoluble and soluble fractions. Immunoprecipitation was carried out from the Triton X-100 soluble fraction with antibody-coated magnetic beads (Dynal, Oslo, Norway)
Electron Microscopy
The protocol was previously published by Maison et al. (1993). Briefly, cells were incubated for 45 min at 37°C in 2 ml of culture medium containing 20 ng/ml nocodazole and 20 μM cytochalasin B. They were washed twice in PBS at 4°C and once in KHM buffer (78 mM KCl, 50 mM HEPES KOH, pH 7.0, 4 mM MgCl2, 10 mM EGTA, 8.37 mM CaCl2, 1 mM DTT, 20 μM cytochalasin B). Cells were resuspended in KHM buffer and homogenized in a tight-fitting Dounce homogenizer. The homogenate was put onto grids and incubated for 3 min. The grids were fixed in 3.7% paraformaldehyde for 10 min and quenched in 50 mM PBS/glycine for 10 min.
Immunoisolation
The cells were broken as described for electron microscopy. Subsequently, anti-vimentin-coated Dynabeads were incubated with the homogenate. The beads were washed three times in PBS and proteins were solubilized by incubating in SDS-Laemmli buffer. The samples were separated on a SDS-PAGE, transferred onto Immobilon-membrane. Vimentin and SNAP23 were revealed by chemiluminiscence and quantified by densitometry by using Bio1D software (Vilbert-Lourmat, Marne-La-Vallée, France).
RESULTS
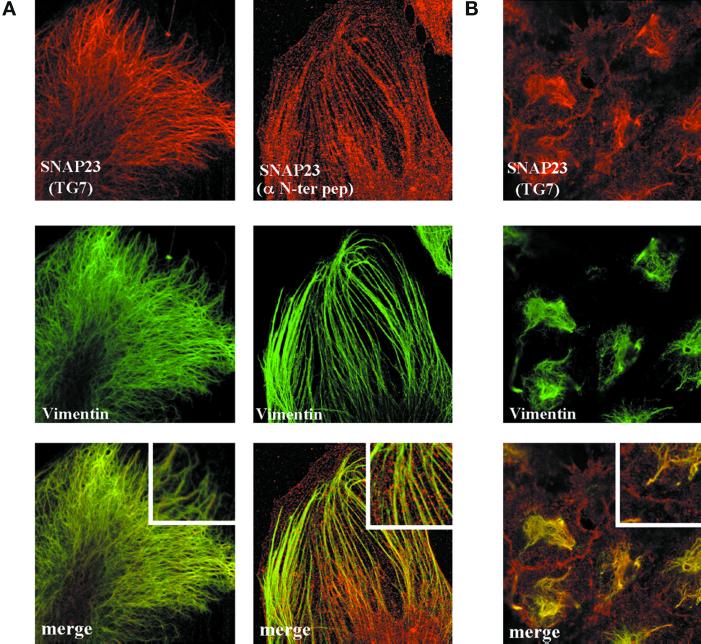
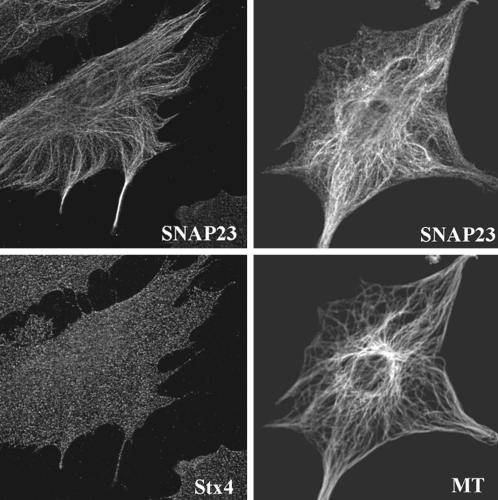
In primary culture of human fibroblasts, SNAP23 immunoreactivity recognized by a rabbit antibody raised against the recombinant human protein produced in Escherichia coli (TG7) appeared as spots associated with filamentous structures in confocal microscopy. These structures were also vimentin-positive (Figure 1A) but not tubulin-, keratin-, or actin-positive (Figure 2 and our unpublished data). The same structures were observed using two anti-peptide antibodies directed against SNAP23, the anti-Nter peptide (1–17) (Figure 1A) and the anti-peptide [196–211] (our unpublished data). SNAP23 was the only SNARE found on vimentin filaments among the tested ones (cellubrevin, TI-VAMP/VAMP7, endobrevin/VAMP8, syntaxin 3,4). In particular, syntaxin 4, a main t-SNARE partner of SNAP23, localized to punctate structures not aligned along cytoskeletal structures (Figure 1A). Vimentin association of SNAP23 was intriguing because SNAP23 immunoreactivity was restricted to the apical and lateral plasma membrane in CaCo-2 cells (Galli et al., 1998). However, it should be noted that these cells are free of vimentin intermediate filaments. Interestingly, in HeLa cells, which produce vimentin and keratin intermediate filament networks, SNAP23 localized to vimentin filaments as well as to the plasma membrane (Figure 1B) but not to keratin filaments (our unpublished data). We wondered whether the vimentin-bound pool of SNAP23 could correspond to vesicular structures anchored to intermediate filaments or to a direct protein–protein association.
Figure 1.
SNAP23 is targeted to vimentin filaments in fibroblasts. (A) Confocal sections of human and murine fibroblasts in primary culture double-stained for vimentin and SNAP23 by using two different anti-SNAP23 antibodies: TG7, directed against the full recombinant human protein, and α-Nter, directed against the first 17 N-terminal amino acids of human SNAP23. In both cases, SNAP23 immunoreactivity appears as discontinuous spots along vimentin filaments. (B) Confocal section of HeLa Cells double-stained for SNAP23 (with TG7) and vimentin. SNAP23 immunoreactivity is present both at the plasma membrane and along vimentin filaments.
Figure 2.
Confocal sections of human and murine fibroblasts in primary culture double-stained for SNAP23 and α-tubulin (MT), or syntaxin 4 (Stx4). Note that SNAP23 does not significantly colocalize with α-tubulin or syntaxin 4.
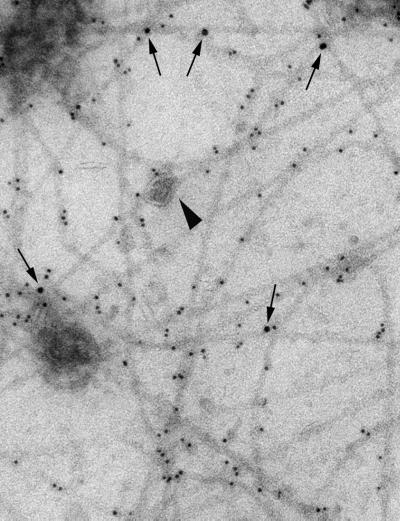
To investigate this question, we prepared extracts from human fibroblasts by passing the cells through a cell cracker followed by combined cytochalasin B and nocodazole treatments to disrupt microfilaments and microtubules. This preparation was then processed for immunogold labeling of SNAP23 and vimentin followed by electron microscopy observation (Maison et al., 1993). All of the intermediate filaments remaining in the preparation were positive for vimentin. SNAP23 immunoreactivity associated directly with vimentin filaments and not with membranes attached to them (Figure 3).
Figure 3.
Direct association of SNAP23 on vimentin filaments. Human fibroblast were homogenized in a cell cracker, treated with cytochalasin B and nocodazole, and deposited on electron microscopy grids. The sample was double-stained for SNAP23 (10-nm gold particle, arrows) and vimentin (5-nm gold particle). The arrowhead points to a vimentin-bound vesicle, which is SNAP23-negative.
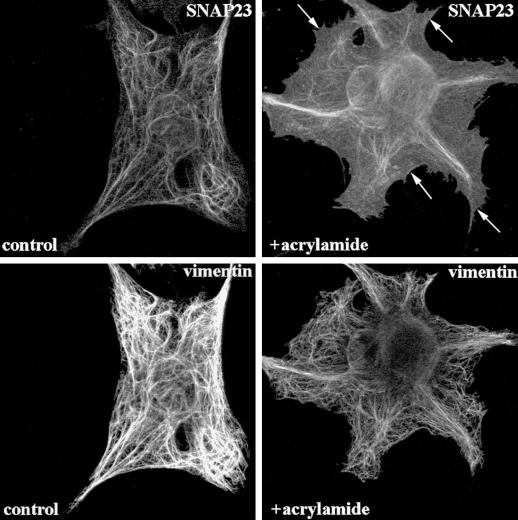
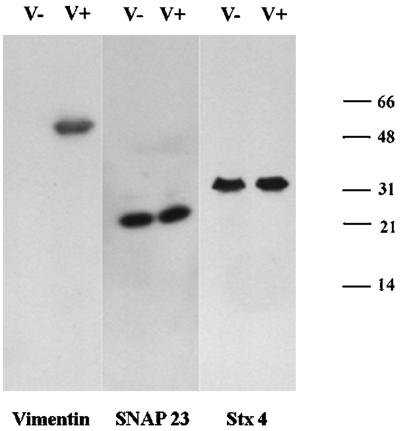
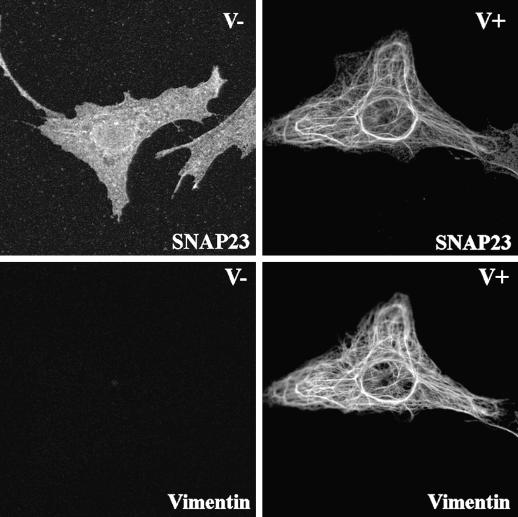
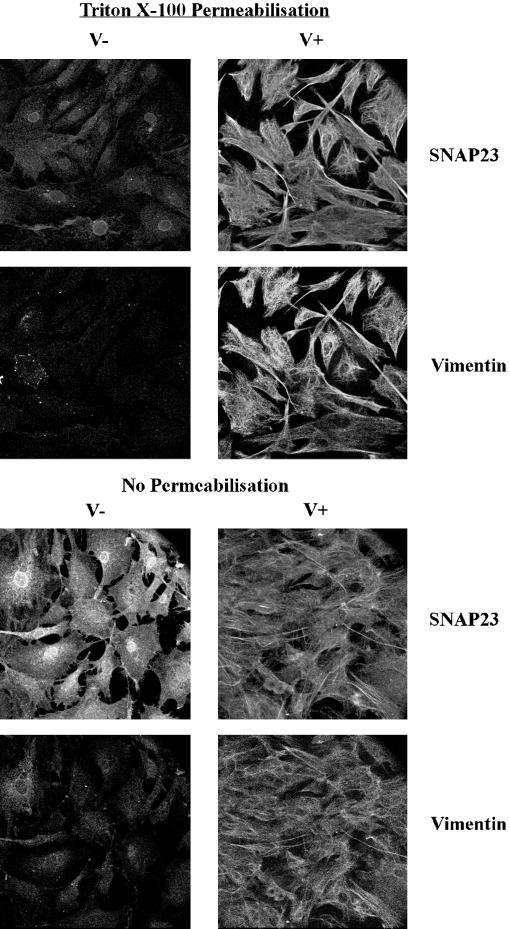
This prompted us to investigate the effect of vimentin filament disruption on SNAP23 intracellular distribution. First, we treated human fibroblasts in primary culture with acrylamide, a poison of intermediate filament organization (Eckert, 1985; Olink-Coux et al., 1992). Acrylamide induced massive, but incomplete, aggregation of vimentin filaments and a significant redistribution of SNAP23 to the plasma membrane (Figure 4). Second, we studied the subcellular localization of syntaxin 4 and SNAP23 in primary culture fibroblasts derived from wild-type (V+) and vimentin knockout embryonic mice (V−) (Colucci-Guyon et al., 1994; Gillard et al., 1998). Both cell types expressed equal amounts of syntaxin 4 and SNAP23, whereas vimentin expression was totally obliterated in V− cells (Figure 5A). The anti-SNAP23 antibody recognized a single band corresponding to the expected molecular weight for SNAP23 in Western blot of cell extracts. We did not observe any cross-reactivity with any other antigen, including vimentin, a major protein in V+ cell extracts (Figure 5A). Confocal sections of V+ fibroblast cells in primary culture showed that SNAP23 immunoreactivity appeared as spots associated with filamentous vimentin (Figure 5B). Strikingly, SNAP23 immunoreactivity was concentrated in spots partially associated or in proximity with the plasma membrane in V− cells (Figure 5B). This distribution was similar to that of syntaxin 4 in human (Figure 1), V− and V+ mouse (our unpublished data) fibroblasts in primary culture. Permeabilization of cells with Triton X-100 before fixation (Kreis, 1987) induced the loss of SNAP23 immunoreactivity in V−, but not in V+ cells (Figure 6). We conclude the following: 1) SNAP23 association with vimentin filaments was dependent upon their integrity; 2) in cells producing vimentin filaments, intracellular pools of SNAP23 are observed; and 3) the vimentin-associated pool of SNAP23 is Triton X-100 insoluble in contrast to the plasma membrane pool.
Figure 4.
Vimentin alone targets SNAP23. Confocal sections of human fibroblasts showing redistribution of SNAP23 following acrylamide-induced vimentin disruption. SNAP23 immunoreactivity redistributes in large vimentin-positive structures and is found at the plasma membrane. Arrows point to plasma membrane localization of SNAP23.
Figure 5.
(A) Western blot analysis of extracts of fibroblasts from wild-type (V+) and vimentin knockout (V−) mice. Vimentin is expressed only by V+ fibroblasts, whereas SNAP23 and syntaxin 4 (Stx4) equally expressed by V+ and V− cells. Note the high specificity of the anti-SNAP23 (TG7) antibody. (B) SNAP23 localizes at the plasma membrane in V− fibroblasts. V+ and V− fibroblasts were double-stained for SNAP23 and vimentin. SNAP23 colocalizes with vimentin in V+ cells but is found in punctate structures mostly at the plasma membrane in V− cells.
Figure 6.
Vimentin-associated SNAP23 is Triton X-100 insoluble. V+ and V− fibroblasts were permeabilized with Triton X-100 (Triton X-100 prepermeabilization) or not (no prepermabilization) before methanol fixation, double-stained for SNAP23 and vimentin, and observed on the confocal microscope by using the same parameters in all cases. The images were then treated equally. SNAP23 immunoreactivity is extracted by Triton X-100 prepermeabilization in V− cells but not in V+ cells.
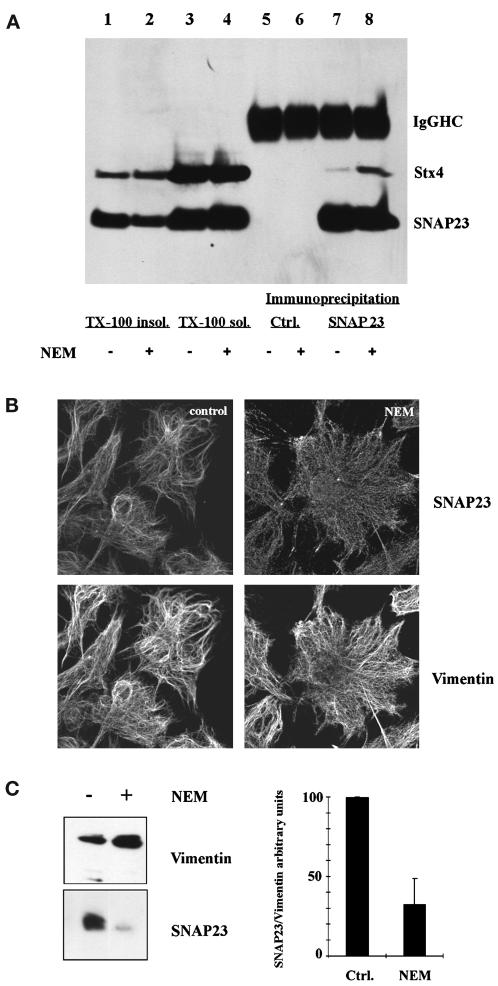
Altogether, these results suggested that the vimentin-associated pool of SNAP23 could constitute a reservoir of the plasma membrane pool of SNAP23. To test this hypothesis, we treated human fibroblasts with NEM to inactivate NSF (Beckers et al., 1989). As expected, the SNAP23–syntaxin 4 complex immunoprecipitated from the Triton X-100 soluble fraction was more abundant in NEM-treated cells than in control (NEM + DTT-treated) cells (Figure 7A, lanes 5–8). Hence, inactivation of NSF leads to the stabilization of plasma membrane SNARE complexes as shown previously (Sogaard et al., 1994; Banerjee et al., 1996; Galli et al., 1998). If the pool of SNAP23 assembling into SNARE complexes was mobilized from the vimentin-associated pool, then the latter should decrease upon NEM treatment. Indeed, NEM treatment decreased the concentration of SNAP23 in the Triton X-100 insoluble fraction (Figure 7A, compare lane 1 and 2). No difference was observed in the case of syntaxin 4, a minor pool of this SNARE being found in the Triton X-100 insoluble fraction in both cases (Figure 7A). Accordingly, NEM treatment of human fibroblasts in primary culture decreased the vimentin-associated pool of SNAP23, as observed by confocal microscopy (Figure 7B). Next, we immunoisolated vimentin filaments, in the absence of Triton X-100, from cell homogenates treated with nocodazole and cytochalasin B (Maison et al., 1993), as in our study by electron microscopy shown in Figure 3. We used magnetic beads coated with anti-vimentin antibodies and the amount of SNAP23 in vimentin immunoisolates was measured by Western blot. Treatment with NEM induced a 70% decrease in the amount of SNAP23 associated with vimentin (Figure 7C). Syntaxin 4 was not found in vimentin immunoisolates (our unpublished data). Altogether, these results show that stabilization of SNARE complexes by inactivation of NSF decrease the vimentin-associated pool of SNAP23, but increase the syntaxin 4- and most likely plasma membrane-associated pool of SNAP23.
Figure 7.
NEM induces the redistribution of SNAP23. (A) NEM treatment of human fibroblasts leads to a decrease of the Triton X-100 insoluble (TX-100 insol.) pool of SNAP23 and an increased recovery of syntaxin 4 (Stx4) in SNAP23 immunoprecipitates. Immunoprecipitation was carried out from the Triton X-100 soluble (TX-100 sol.) fraction with sheep anti-rabbit magnetic beads coated with control rabbit immunoglobulins (Ctrl.) or anti-SNAP23 antibodies. The amounts loaded in each condition correspond to an equivalent cell number. Control rabbit immunoglobulins did not immunoprecipitate SNAP23 or Stx4. (Immunoglobulin heavy chains, IgG HC.) (B) SNAP23 localizes to the plasma membrane following NEM treatment. Human fibroblasts were treated (NEM) or not (Ctrl.) with NEM and double-stained for SNAP23 and vimentin. (C) NEM treatment decreases the vimentin-associated pool of SNAP23. Human fibroblasts were homogenized with a cell cracker and vimentin filaments were immunoisolated. Bars represent the average of three independent experiments. In each experiment, the intensity of the vimentin and SNAP23 bands was quantified. The amount of SNAP23 was expressed as a ratio of the amount of vimentin recovered in each sample. In each experiment, the SNAP23/vimentin ratio was set to 100 arbitrary units for control cells.
DISCUSSION
Our results showing mobilization of SNAP23 from a reservoir pool on vimentin filaments to functional SNARE complexes at the plasma membrane suggests a new role of vimentin in the regulation of SNAP23 function and maybe other membrane proteins in fibroblasts. Vimentin has been implicated in the formation of aggresomes, a pericentriolar structure accumulating misfolded proteins, including membrane proteins such as cystic fibrosis transmembrane conductance regulator, which have not been degraded by the proteasome (Johnston et al., 1998), and ubiquitin-related proteins have been found to regulate the interaction of vimentin filaments with the plasma membrane (Wu et al., 1999)
Thus, vimentin could be involved in sequestering proteins, such as SNAP23, along their biosynthesis or degradation pathways. How could SNAP23 move from the vimentin-associated pool to the plasma membrane? Targeting of SNAP23 to vimentin filaments could be direct or it could involve partners such as a recently identified SNAP25-interacting protein (SNIP) that partitions entirely in Triton X-100 insoluble fractions (Chin et al., 2000). Targeting to the plasma membrane likely involves syntaxins (Cabaniols et al., 1999; Veit, 2000). If vimentin-associated SNAP23 were palmytoylated, then its targeting to the plasma membrane could depend on proteins similar to GDP dissociation inhibitor, which solubilize and target small GTPases to membranes (Soldati et al., 1994; Ullrich et al., 1994). If vimentin-associated SNAP23 were not palmytoylated, depalmytoylation/palmytoylation cycles, as for the α subunits of heterotrimeric G proteins (Levis and Bourne, 1992), would occur in the course of SNAP23 cycling between vimentin filaments and the plasma membrane. Phosphorylation of SNAP23 by SNAP23 kinase decreases its degradation and increases the kinetics of t-SNARE assembly (Cabaniols et al., 1999) so it could regulate SNAP23 targeting to the plasma membrane.
In this study, we propose that transfer of SNAP23 from the vimentin-associated reservoir to the functional plasma membrane pool may modulate availability of SNAP23 to form SNARE complexes in fibroblasts. How could the vimentin-associated reservoir of SNAP23 play a role in membrane traffic? Although the role of microtubules and the actin cytoskeleton in membrane traffic has been well documented (Goodson et al., 1997), only few reports examine the role of intermediate filaments in membrane traffic. Recently, the group of Marcus has found a decreased synthesis of glycosphingolipids (GSL) in fibroblast cells derived from vimentin knockout mice (Gillard et al., 1998). This result led the authors to postulate a possible implication of vimentin in intracellular membrane trafficking, in particular in the recycling of GSL between the Golgi apparatus and the endosomes, a pathway responsible for the incorporation of sugars into GSL. Intermediate filaments reorganization is coupled to granule secretion activation in neutrophils (Pryzwansky and Merricks, 1998) but a direct involvement of vimentin in exocytosis has not been demonstrated. SNAP23 is involved in transferring receptor recycling in Madin-Darby canine kidney cells (Leung et al., 1998). Thus, we have measured the rate of endocytosis and recycling to the plasma membrane of iodinated mouse transferrin in primary cultures of V+ and V− fibroblasts. We found that endocytosis of holo-transferrin and exocytosis of recycled apo-transferrin were identical in both cell types (our unpublished data). This may indicate that a low amount of SNAP23 at the plasma membrane is enough to sustain normal exocytosis of transferrin receptor in V+ cells. Nevertheless, our observations could suggest that targeting of SNAP23 to vimentin may affect other membrane transport pathways. The latter could include release of Golgi secretory vesicles, including secretory granules that depend on compound exocytosis (Guo et al., 1998; Chen et al., 2000). Alternatively, the vimentin-associated reservoir of SNAP23 may regulate membrane traffic only in a yet uncovered signal transduction or cell-specific functional context. Indeed, vimentin together with actin are present in podosomes, a specialized type of focal adhesions found in macrophages (Correia et al., 1999). Interestingly, dynamin and endophilin, two proteins involved in endocytosis, are also found in podosomes (Ochoa et al., 2000).
The association of a pool of t-SNARE to cytoskeletal structures is not specific to SNAP23. Indeed, SNAP25 is also partially Triton X-100 insoluble in neuronal cells (Chin et al., 2000). In these cells, SNAP25 and SNIP, partially colocalize with cortical actin but Chin et al. (2000) have not compared the localization of SNAP25 with that of intermediate filaments and have not demonstrated direct association of SNAP25 to actin. Thus, it is not yet clear whether the insoluble pool of SNAP25, like that of SNAP23, is associated with intermediate filaments. Future studies should clarify this point and address the functional relevance of the cytoskeletal pools of SNAP23 and SNAP25.
ACKNOWLEDGMENTS
We thank Paul Roche for the anti-SNAP23 antibody, Christèle Maison for helping with the preparation of vimentin filaments, Graça Raposo for electron microscopy, and Daniel Meur and Dominique Morineau for photographic documentation. This work was supported in part by Fritz Thyssen Foundation and Vaincre les Maladies Lysosomiales fellowships to W.F., and Action Concertée Incitative-Jeunes Chercheurs (N°5254) from the Ministère de la Recherche et des Technologies to T.G.
REFERENCES
- Banerjee A, Barry VA, DasGupta BR, Martin TFJ. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- Beckers CJ, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989;339:397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. SNARE proteins mediate lipid bilayer fusion. Proc Natl Acad Sci USA. 1999;96:12227–12229. doi: 10.1073/pnas.96.22.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaniols J, Ravichandran V, Roche PA. Phosphorylation of SNAP-23 by the novel kinase SNAK regulates t-SNARE complex assembly [In Process Citation] Mol Biol Cell. 1999;10:4033–4041. doi: 10.1091/mbc.10.12.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–929. [PubMed] [Google Scholar]
- Chin LS, Nugent RD, Raynor MC, Vavalle JP, Li L. SNIP, a novel SNAP-25-interacting protein implicated in regulated exocytosis. J Biol Chem. 2000;275:1191–1200. doi: 10.1074/jbc.275.2.1191. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Eckert BS. Alteration of intermediate filament distribution in PtK1 cells by acrylamide. Eur J Cell Biol. 1985;37:169–174. [PubMed] [Google Scholar]
- Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. Integrating the actin and vimentin cytoskeletons. Adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J Cell Biol. 1999;146:831–842. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, Yaworsky K, Trimble WS, Klip A. SNAP23 promotes insulin-dependent glucose uptake in 3T3–L1 adipocytes: possible interaction with cytoskeleton. Am J Physiol. 1999;276:C1108–C1114. doi: 10.1152/ajpcell.1999.276.5.C1108. [DOI] [PubMed] [Google Scholar]
- Galli T, Garcia EP, Mundigl O, Chilcote TJ, De Camilli P. v- and t-SNAREs in neuronal exocytosis: a need for additional components to define sites of release. Neuropharmacology. 1995;34:1351–1360. doi: 10.1016/0028-3908(95)00113-k. [DOI] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard BK, Clement R, Colucci-Guyon E, Babinet C, Schwarzmann G, Taki T, Kasama T, Marcus DM. Decreased synthesis of glycosphingolipids in cells lacking vimentin intermediate filaments. Exp. Cell Res. 1998;242:561–572. doi: 10.1006/excr.1998.4126. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Greentree WK, Linder ME. SNAP-25 is targeted to the plasma membrane through a novel membrane-binding domain. J Biol Chem. 1999;274:21313–21318. doi: 10.1074/jbc.274.30.21313. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SM, Chen D, DasGupta BR, Whiteheart SW, Apodaca G. SNAP-23 requirement for transferrin recycling in Streptolysin-O-permeabilized Madin-Darby canine kidney cells. J Biol Chem. 1998;273:17732–17741. doi: 10.1074/jbc.273.28.17732. [DOI] [PubMed] [Google Scholar]
- Levis MJ, Bourne HR. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998a;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Roche PA, Anderson HA, van Ijzendoorn SCD, Zhang M, Mostov KE, Weimbs T. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J Biol Chem. 1998b;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- Maison C, Horstmann H, Georgatos SD. Regulated docking of nuclear membrane vesicles to vimentin filaments during mitosis. J Cell Biol. 1993;123:1491–1505. doi: 10.1083/jcb.123.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Weber T, McNew JA, Parlati F, Sollner TH, Rothman JE. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, DeCamilli P. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olink-Coux M, Huesca M, Scherrer K. Specific types of prosomes are associated to subnetworks of the intermediate filaments in PtK1 cells. Eur J Cell Biol. 1992;59:148–159. [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky KB, Merricks EP. Chemotactic peptide-induced changes of intermediate filament organization in neutrophils during granule secretion: role of cyclic guanosine monophosphate. Mol Biol Cell. 1998;9:2933–2947. doi: 10.1091/mbc.9.10.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature. 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Veit M. Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem J. 2000;345:145–151. [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wu AL, Wang J, Zheleznyak A, Brown EJ. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane. Mol Cell. 1999;4:619–625. doi: 10.1016/s1097-2765(00)80212-9. [DOI] [PubMed] [Google Scholar]