Abstract
The infection of maize by Fusarium verticillioides can result in highly variable disease symptoms ranging from asymptomatic plants to severe rotting and wilting. We produced F. verticillioides green fluorescent protein-expressing transgenic isolates and used them to characterize early events in the F. verticillioides-maize interaction that may affect later symptom appearance. Plants grown in F. verticillioides-infested soil were smaller and chlorotic. The fungus colonized all of the underground parts of a plant but was found primarily in lateral roots and mesocotyl tissue. In some mesocotyl cells, conidia were produced within 14 to 21 days after infection. Intercellular mycelium was detected, but additional cells were not infected until 21 days after planting. At 25 to 30 days after planting, the mesocotyl and main roots were heavily infected, and rotting developed in these tissues. Other tissues, including the adventitious roots and the stem, appeared to be healthy and contained only a small number of hyphae. These results imply that asymptomatic systemic infection is characterized by a mode of fungal development that includes infection of certain tissues, intercellular growth of a limited number of fungal hyphae, and reproduction of the fungus in a few cells without invasion of other cells. Development of visibly rotted tissue is associated with massive production of fungal mycelium and much less organized growth.
Fusarium verticillioides (synonym, Fusarium moniliforme Sheldon; teleomorph, Gibberella moniliformis [synonym, Gibberella fujikuroi mating population A]) is the most commonly reported fungal species infecting maize (26, 36, 37). F. verticillioides can be found in plant residues in almost every maize field at harvest, yet the disease symptoms vary widely and range from asymptomatic infection to severe rotting of all plant parts. In many cases diseased and asymptomatic plants occur in the same field planted with a genetically uniform host. Environmental conditions, water availability (8, 9, 28, 29, 35), and the genetic background of the plant and the pathogen (2, 19, 25, 45) may all be important factors in disease development, but it is not known why the disease does not occur during asymptomatic infections or what causes rotting and wilting in the diseased tissues.
Infection of maize by F. verticillioides can occur via several routes. The most commonly reported method of kernel infection is through airborne conidia that infect the silks (15, 31, 32). After invasion through the silks, the fungus infects the kernels, but usually only a small percentage of the infected kernels become symptomatic (33). Another proposed infection pathway is systemically through the seed (12). Systemic infection can start from fungal conidia or mycelia that are either carried inside the seeds or on the seed surface. The fungus develops inside the young plant, moving from the roots to the stalk and finally to the cob and kernels. Movement of the fungus from infected seeds to the stalk and kernels has been established in a number of studies. Kedera et al. (20) used vegetative compatibility (vegetative compatibility groups) as a marker to track specific strains and obtained evidence that the fungus moves from the seeds to kernels by recovering the isolate inoculated into the seeds from approximately 10% of the kernels. Similar findings were reported by Munkvold and coworkers, who also used vegetative compatibility groups to track fungal movement in the plant (32, 33). These researchers also showed that although infected seeds may contribute to kernel infection, local infection via silks is the main pathway of kernel infection. Systemic infection also may result from inoculum that survives in crop residues in the soil; however, the relative importance of soilborne and seed-borne inocula as the cause of systemic infections is not known (25, 39).
F. verticillioides produces several toxins that have potential toxicity for humans and domesticated animals. The most significant of these toxins produced by F. verticillioides are the fumonisins (7, 37). Since fumonisins can be detected in symptomatic and asymptomatic maize kernels, control of fumonisin contamination in maize has become a priority area in food safety research (3), and some guidelines for maximum fumonisin levels in human food and animal feeds have been issued (13). The presence of fumonisins in asymptomatic grain means that a better understanding of the asymptomatic endophytic portion of the life cycle of F. verticillioides is badly needed. Work with genetically tractable strains has suggested that the limiting step in the movement of the fungus from the seeds to the upper parts of the plant is the transition of the fungus from the seedling crown to the stalk (19, 33). However, the data generated by such analyses are limited to isolation of the fungus from different tissues and do not provide a clear picture of the colonization of the plant by the fungus. Moreover, not all experiments have produced the same results (45).
Light microscopy and electron microscopy have been used to obtain more detailed information on the infection of maize by F. verticillioides (1, 34, 45). These methods provide information on events such as seed infection and root penetration, but the data are limited to particular time points. More continuous information on in planta fungal development may be obtained by tracing transgenic isolates that express reporter genes. Work with β-glucuronidase-expressing isolates has provided some insight into the F. verticillioides-maize interaction (3, 46); however, the tissue sectioning and other experimental manipulations required to monitor β-glucuronidase activity limit its utility in such analyses. Recently, the green fluorescent protein (GFP) has become a commonly used tool in the analysis of fungus-plant interactions (27). Spores and hyphae of GFP-expressing fungal isolates can be identified by fluorescence microscopy in intact tissues or tissue sections without extensive manipulation and provide highly informative data on processes of plant colonization (10, 11, 16, 23, 30).
In the present study we generated F. verticillioides GFP-expressing transgenic isolates and used them to characterize early stages of the F. verticillioides-maize interaction. By using GFP-expressing transgenic isolates we directly monitored development of the fungus in planta during early stages of the interaction and determined the spread of the fungus to upper parts of the plant, including leaves and cobs, at different stages of plant growth. These analyses provided new information on the disease, which can help explain the differences between aggressive and asymptomatic interactions.
MATERIALS AND METHODS
Fungi, plants, and growth conditions.
F. verticillioides strain A-00149 (= FGSC 7600) was provided by J. F. Leslie. This strain was first isolated from maize in California. It has been used as a standard mating type tester strain for this species, and it is one of the progenitors of the mapping population of F. verticillioides (21, 22). Wild-type strain A-00149 and its derivative transgenic strains were used in all experiments. The fungus was maintained at 28°C on Czapek-Dox medium (40) or was stored as a conidial suspension in 20% glycerol at −70°C (21). For mycelium and spore production the fungus was grown in shake cultures at 28°C, and the mycelium was collected by filtration.
Sweet maize cultivar Jubilee was used for plant inoculation experiments. This commercial line is widely grown and is highly susceptible to F. verticillioides (17). Plants were grown in pots of various sizes. Greenhouse experiments were conducted in 3- or 10-liter pots containing a mixture of 40% peat, 40% loam, 10% vermiculite, and 10% compost. The greenhouse temperature was 25 to 27°C, and daylight was supplemented with light from fluorescent tubes to provide 14 h of continuous light. The light intensities in the greenhouse during the day were between 120 and 200 microeinsteins m−2. The plants used in greenhouse experiments were irrigated daily with a hand sprinkler. Experiments in the nursery were conducted in 10-liter pots containing soil from fields in which wilted plants were observed. The plants were drip irrigated, and the soil moisture content was maintained at 70% of the soil water capacity to provide optimal water conditions. Soil disinfestation was performed with Basamid G (BASF, Munich, Germany) used according to the manufacturer's instructions. After disinfestation the soil was washed for 24 h with water (drip irrigation, 2 liters h−1) to remove traces of Basamid. An absence of Basamid residual activity was verified by the development of healthy, asymptomatic seedlings after maize seeds were planted.
Seed inoculation.
Before seeds were inoculated or planted, all the seeds were soaked for 30 min in a 0.2% solution of the fungicide Octav (AgrEvo UK, Cambridge, England) to prevent infection from a seed-borne inoculum. No colonies developed when Octav-treated seeds were plated on the isolation medium. Octav-treated seeds were rinsed with water and then placed in a 0.2% Tween 20 solution containing 5 × 109 conidia ml−1. After 30 min, the seeds were removed from the spore suspension and dried in a laminar flow hood overnight.
Soil inoculation.
The soil inoculum consisted of 3-day-old mycelium that was inoculated onto dry maize leaves. Fresh mycelium (25 g) was homogenized with a blender and suspended in 200 ml of water. Chopped maize leaves (10 g) were mixed with 50 ml of the original or diluted mycelial suspension, depending on the treatment. The top 12 cm of soil was removed from six pots (either 3- or 10-liter pots) and mixed with the inoculum, and then the inoculated soil was distributed among the pots so that the upper 12 cm in each pot contained inoculated soil. Seeds were planted immediately after inoculation of the soil.
Low-light experiment.
Experiments to test the effect of low light on disease development were conducted in 50-liter containers in a growth room. Cool white fluorescent tubes supplied a light intensity of 20 microeinsteins m−2. The temperature was maintained at 25 ± 2°C.
Fungal isolation.
We used the methods described by Burgess et al. (4) for fungal isolation. Plant parts were surface sterilized by submerging and shaking them in 1% sodium hypochlorite-10% ethanol for 30 s. The tissues were then washed with sterile water and dried in a laminar flow hood. Three pieces of sterilized tissue were sectioned with a razor (0.5-cm-thick stalk disks, 5-cm-long roots) and placed on peptone-pentachloronitrobenzene (PCNB) medium (4).
Plasmids and fungal transformation.
A DNA fragment was amplified by PCR from F. verticillioides genomic DNA by using the decamer primer 3′-GGGTGCAGTT-5′. A 500-bp PCR amplification product was cloned into plasmid pUC57 (MBI-Fermentas, Hanover, Md.) and sequenced. No putative open reading frame was found. The fragment was cloned into the vector gGFP (30) at the HindIII and XbaI restriction sites to produce the transformation vector fgGFP.
Protoplast preparation and transformation were performed as described by Tudzynski et al. (44), with some modifications. A mycelial suspension (1 ml) was added to 100 ml of complete medium (44) and cultured for 16 to 18 h at 200 rpm on an orbital shaker at 28°C. The mycelium was filtered and washed with 0.8 N NaCl, and 0.5 g of the mycelium was suspended in an enzyme solution (0.5% Novozyme [InterSpex Products, Inc., Foster City, Calif.], 0.1% driselase [Sigma, Rehovot, Israel], 0.01% chitinase [Sigma]) for 2 to 3 h with shaking at 100 rpm until protoplasts were released. The protoplasts were washed with cold 0.8 N NaCl, and the final concentration was adjusted to 5 × 107 protoplasts ml−1 in STC. Linear plasmid (10 μg) was added to 50 μl of the protoplast solution together with 50 μl of polyethylene glycol 6000 (Sigma). The mixture was incubated on ice for 25 min, an additional 2 ml of polyethylene glycol 6000 was added to each of the tubes, and the preparations were incubated for another 10 min at room temperature before dilution with 4 ml of STC. The protoplasts were incorporated into 100 ml of regeneration medium containing (per liter of water) 145.7 g of mannitol (Merck), 4 g of yeast extract (Difco, Detroit, Mich.), 1 g of soluble starch (Merck), and 16 g of agar; hygromycin B was added to a final concentration of 100 μg ml−1, and the medium was then distributed into six petri dishes. The plates were incubated at 28°C, and colonies were subcultured 4 to 8 days later.
Microscopy.
Fluorescence microscopy was performed with a Leica DMRBE epifluorescence microscope (Leica, Deerfield, Ill.). Each of the tissues examined was placed on a microscope slide, submerged in a water droplet, and covered with a glass coverslip. The K3/L4 filter cube with peak transmission for excitation at 470 to 490 nm and peak transmission for suppression at 515 to 560 nm was used for GFP detection. Light microscopy was performed with the same microscope without filters. Confocal microscopy was performed with an inverted laser scanning confocal microscope (CLSM 510; Zeiss, Cberkochen, Germany) with excitation and emission at 488 and 510 nm, respectively. GFP expression in entire colonies was visualized with an Olympus SZx12 fluorescence stereoscope by using an eGFP filter.
Experimental procedures.
The nursery and greenhouse inoculation experiments were conducted in six replications, with six plants per pot. The growth chamber experiment at a low light intensity was conducted in three replications, with 20 plants per container. To determine plant weight or root colonization, one plant from each replication was sampled at every time point; the fresh weight of the plants was determined, and tissue samples were used for colony isolation.
RESULTS
GFP-expressing strains.
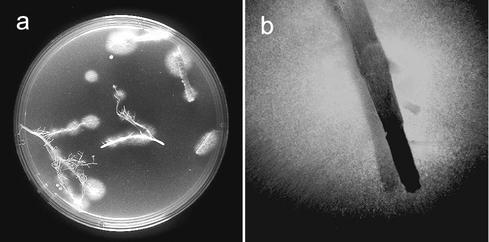
Transgenic isolates were obtained at a rate of two transformants per microgram of plasmid DNA. Only 60% of the hygromycin B-resistant isolates expressed GFP, a value which is similar to values reported for other fungi (23, 41). GFP expression was generally strong and uniform in all fungal tissues and remained high and stable after successive transfers on Czapek-Dox medium with and without hygromycin B. Southern analysis showed that in all cases the plasmid integrated into chromosomal DNA in one or more locations and that tandem integration of two or more copies was common (data not shown). We analyzed 20 isolates and selected one, gf12, to study in planta fungal development. This isolate expressed the highest level of GFP, produced abundant conidia, and had wild-type colony morphology. Inoculation of plants with gf12 and recovery on semiselective medium showed that gf12 was as infective as the wild type and that it could be easily detected by observation with the fluorescence stereoscope (Fig. 1).
FIG. 1.
Isolation of GFP-expressing F. verticillioides from infected maize roots. Seeds were inoculated with the transgenic isolate gf12 and planted in disinfested soil. Roots of 14-day-old plants were excised, rinsed in water, and plated on peptone-PCNB semiselective medium. Colonies emerged after 4 to 6 days (a), and GFP expression was detected with a fluorescence stereoscope (b) (the white halo fluoresces in green).
Plant infection by using seed and soil inocula.
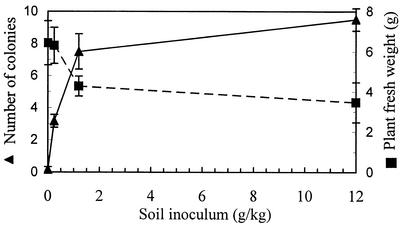
Nursery experiments were conducted with the wild-type isolate to compare the effects of soil inoculation and seed inoculation on infection of maize plants. Seed inoculation had no effect on the weight of the plants grown in untreated soil compared to the weight of control plants that developed under similar conditions (Table 1). Growth enhancement was observed in the disinfested soil compared to the growth in untreated soil. Although seed inoculation did not suppress seedling growth, high rates of root colonization were detected even at the first sampling time after 20 days (Table 1). Soil inoculation reduced plant growth, with greater reductions occurring at higher inoculum levels, and the number of colonies isolated from the roots was proportional to the amount of the soil inoculum (Fig. 2).
TABLE 1.
Effect of seed inoculation with F. verticillioides on seedling fresh weight and root colonization
| Soil treatment | Seed treatment | No. of coloniesa | Plant fresh wt (g) |
|---|---|---|---|
| Sterilized | Inoculated | 8.3 ± 2.6 a | 11 ± 6.2 a |
| Sterilized | Uninoculated | 0.17 ± 0.41 b | 6.43 ± 2.6 ab |
| Untreated | Inoculated | 8.2 ± 2.4 a | 2.53 ± 1.6 b |
| Untreated | Uninoculated | 7.3 ± 3.9 a | 2.83 ± 0.53 b |
The number of colonies and plant fresh weight were determined 20 days after planting. Data are averages ± standard deviations for six replications. Values followed by different letters are statistically significantly different (P < 0.05) according to the Tukey multiple-comparison test.
FIG. 2.
Effect of soil inoculation with F. verticillioides on seedling fresh weight and root colonization. Disinfested soil was inoculated with an F. verticillioides mycelium homogenate. Plant fresh weight and root colonization were determined 20 days after planting. The error bars indicate the standard errors.
Early events in plant colonization.
We detected only trace amounts of fluorescent mycelium during microscopic examination of plants that developed from inoculated seeds, although the fungus was readily recovered from the roots of these plants by plating on peptone-PCNB medium (Fig. 1). Soil inoculation resulted in more pronounced infection (Fig. 3). The transgenic fungus was easily detected on the surfaces of underground organs, such as seeds, roots, and hypocotyl (Fig. 3a and b). During the first few days after planting, the fungus grew both on the surface and inside lateral roots (Fig. 3c and d). Only single hyphae were detected growing along the root, and there was no branching or change in the direction of growth. Thickened organelles were observed in hyphae that developed on the surfaces of lateral roots. These organelles resembled conidia; however, the true nature of these organs and their role are unclear. Such organs were not observed in the hyphae that developed inside the roots.
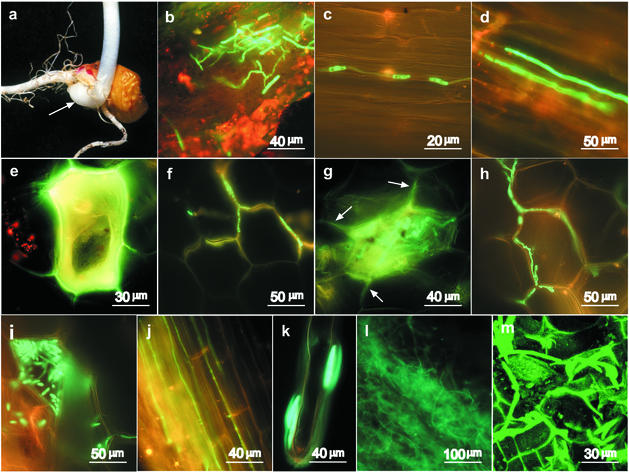
FIG. 3.
Early events during plant colonization by F. verticillioides. Seeds were planted in soil that was inoculated with a transgenic isolate gf12 mycelium homogenate. The photographs were obtained with a fluorescence microscope unless otherwise indicated. (a) The organs and tissues examined included the root, lateral roots and root hairs, mesocotyl (arrow), seed pericarp, and lower part of the shoot. (b) Adherence of mycelium and direct penetration of the mesocotyl 3 days after planting. (c and d) Hyphae growing on the surface of a lateral root (c) and inside a lateral root (d) 7 days after planting. (e to i) Colonization and reproduction of the fungus in mesocotyl cells. Single cells accumulated fluorescing material after 7 days (e), but fungal growth was restricted to the cell wall spaces (f). After 14 days, conidia started to differentiate (g), and more developed mycelium was detected in the intercellular wall spaces (h). Hyphae grew out of the cells in which conidia were formed, but through specific points of contact with other cells and without rupturing the cells (g, arrows). After 21 days conidia matured (i) but were still retained in the cells in which they were formed. (j) After 21 days, the development of the mycelium on the root intensified, with hyphae growing in the intercellular spaces along the root. (k) Macroconidia attached to the mucilage layer on a root hair 21 days after planting. (l and m) Large amounts of mycelium developed after 30 days and infected the entire mesocotyl tissue (l) and the main root (m) (confocal microscopy).
After 7 days, the fungus also was detected in the mesocotyl tissue but not in the tissues above the mesocotyl. The mesocotyl is defined as the intermediate tissue between the root and the hypocotyl (Fig. 3a). A very organized pattern of development was observed: most cells in the mesocotyl were unaffected, but a few cells developed yellow-green fluorescence (Fig. 3e). The emission spectrum of this fluorescence was different from the GFP emission spectrum, indicating that the cells accumulated autofluorescing material. Very little mycelium could be found in the mesocotyl at this stage, and the mycelia that we did detect were retained at the cell boundaries without cell penetration (Fig. 3f).
Green fluorescing fungal material composed of undefined rounded organelles that filled single cells in the mesocotyl was detected after 14 days (Fig. 3g). The adjacent cells were unaffected, but mycelium could be detected in wall connections between an infected cell and the surrounding cells (Fig. 3g). More mycelium was observed in the cell wall spaces at this stage (Fig. 3h). This mycelium contained thick organelles that looked like conidiophores or aerial branching.
After 21 days, the infected cells were filled with mature conidia (Fig. 3i). Conidia were found only inside a cell in which an infection was established, while the surrounding cells appeared to be normal. A large number of hyphae that developed along the root axis (Fig. 3j) and green-fluorescing macroconidia (Fig. 3k) were detected on root hairs. Disease symptoms were not observed at this stage.
The initial phase of infection and establishment in the roots and mesocotyl was followed by a second phase 25 to 35 days after inoculation, in which the fungus proliferated in the mesocotyl and in the main root (Fig. 3l and m). Large amounts of mycelium were produced in these organs and caused rotting of the mesocotyl and the main root. No fluorescing hyphae were observed in the stem by fluorescence or confocal microscopy; however, a small number of green fluorescent, transgenic colonies could be isolated from the stems and even leaves by plating on selective medium (data not shown).
The amount of mycelium that developed in the mesocotyl was larger at high soil inoculum rates. More mycelium was detected in the mesocotyl tissue of plants that developed in soil containing 0.6 g of inoculum kg of soil−1 than in plants growing in soil containing 0.1 g of inoculum kg of soil−1 (Fig. 4a and b). These results are consistent with the results of the nursery experiments performed with the wild-type strain, in which the number of colonies that were isolated from roots increased as the size of the soil inoculum increased (Fig. 2).
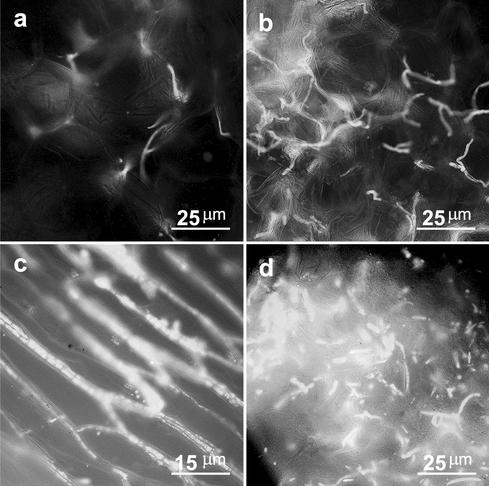
FIG. 4.
Effects of inoculum levels and light conditions on inoculation of maize plants by F. verticillioides. (a and b) Effect of two levels of inoculum on the amount of mycelium that developed in the mesocotyl 30 days after seeds were planted in inoculated soil. The inocula used were 0.1 g (fresh weight) per kg of soil (a) and 0.6 g (fresh weight) per kg of soil (b). (c and d) Development of mycelium in roots (c) and mesocotyls (d) of plants grown under low-light conditions (20 microeinsteins m−2) 14 days after inoculated seeds were planted in disinfested soil.
Infection of plants grown under low-light conditions.
Two-week-old plants that were grown under low-light conditions (20 microeinsteins m−2) had intense rotting, and large amounts of fungal mycelium were detected on and inside the plants. The mycelia grew without any special order, infecting the entire root system, the mesocotyl, and the stem (Fig. 4a and d). The massive infection resulted in rapid rotting and seedling mortality 2 weeks after planting (Table 2). The aggressive fungal development under low-light conditions was in sharp contrast to development of mycelium in tissues of plants that were grown with higher light intensities; under these conditions the myclelium did not invade the cells and developed mainly along the cell walls.
TABLE 2.
Disease development in F. verticillioides-infected maize seedlings grown under low-light conditions
| Treatmenta | % of diseased plantsb | No. of plants |
|---|---|---|
| Control | 15 ± 0.41c | 53 |
| Seed inoculation | 36 ± 2.0 | 59 |
| Soil (1.2 g/kg)d | 62 ± 0.77 | 60 |
| Soil (12 g/kg)d | 80 ± 1.6 | 57 |
Disinfested soil was used in all treatments.
The number of dead and rotted plants was determined 14 days after planting.
The values are means ± standard errors for the total number of plants.
Soil was inoculated with 1.2 or 12 g of fresh mycelium homogenate per kg of soil.
DISCUSSION
Systemic movement of F. verticillioides from infected seeds to roots, crowns, stalks, and ears has been documented by using various methods (3, 31, 33, 34, 38, 45). Although systemic infection can cause rotting in all plant organs, it is considered a major cause of stalk rot, while the significance of this pathway in kernel infection appears to be low (9, 31, 33, 38). Munkvold et al. (33) reported that transmission of the fungus from seedling crown tissue to stalk tissue appeared to be the rate-limiting factor in fungal movement from infected seeds to the upper parts of the plants. These results are consistent with previous findings of Nelson (36) and Lawrence et al. (24), who suggested that seed-transmitted strains do not advance to above-ground parts of a plant until after pollination. However, various reports have indicated that the fungus has been isolated from all parts of young (7- to 14-day-old) seedlings (2, 45). The different results may be explained by different genetic backgrounds of the plant and the fungus (5, 7, 15, 18, 19, 35), by differences in the inoculation methods and the experimental conditions (9, 28, 29, 33, 38), or by differences in the sensitivities of the detection methods (3, 31, 34, 45). In the present study, we isolated the transgenic fungus from stems of 14-day-old seedlings by plating stem sections on semiselective medium (Fig. 1). However, the fungus was not detectable in planta by fluorescence microscopy at this stage, which indicates that there were no more than trace amounts of fungal mycelium in the above-ground tissues at this time. These findings confirm that the fungus is capable of moving to above-ground parts early in the interaction, but relatively little fungal biomass which does not cause rotting or other disease symptoms develops during these early infection stages. This growth pattern may explain why the fungus was detected in the upper parts in some studies but not in other studies (2, 31, 36, 45).
The levels of soil inoculum and root colonization correlated with reduced seedling growth, which was more pronounced at the early growth stages, while seed inoculation did not suppress plant growth and may even have enhanced plant development (Fig. 2 and 3). Enhanced growth of Fusarium-infected plants has been reported previously and was attributed to the endophytic nature of the fungus (25, 45). Our results suggest that the same strain may cause growth enhancement and growth retardation, depending on the level of plant colonization, which is influenced by the amount of fungal inoculum in the soil and seed. Although F. verticillioides can survive in crop residue in the soil, this inoculum source has not been considered a major source of plant infection (25, 31). Our results suggest that soil inoculum, in addition to infected seeds, may be important in initiation of F. verticillioides systemic infection. Furthermore, in our study soil inoculum resulted in more diseased plants than seed inoculation, suggesting that problems associated with stand establishment attributed to Fusarium are more likely to result from soilborne inoculum than from seed-borne inoculum.
GFP-expressing transgenic isolates can be visualized in living tissues without any processing or manipulation of the samples. This property makes GFP extremely useful for analysis of in planta fungal development (27). In the present study, we used GFP-expressing transgenic isolates to obtain detailed information on the interaction between F. verticillioides and maize plants. The main sites of plant penetration were the lateral roots and the mesocotyl, which is the tissue connecting the emerging root with the emerging shoot. The fungus was detected in these tissues as early as 72 h after the seeds were planted in infested soil. These findings support previous findings of Murillo et al. (34), who showed that there was direct penetration of the seed pericarp and root epidermal cells by F. verticillioides 3 days after inoculated maize seeds were planted. These authors also found that fungal growth at this stage was mainly intercellular. Lagopodi et al. (23) used GFP-expressing transgenic Fusarium oxysporum to study tomato root colonization and infection. They found that the contact between the fungus and the host was initiated predominantly at the root hairs, while colonization of the crown was predominant after the initial contact. These findings are similar to our results and suggest that there is a common mode of plant colonization at the early stages, in which the pathogens first attach to the lateral roots and root hairs and then penetrate directly through the cuticle and epidermis of these tissues.
After penetration the fungus develops in the intercellular spaces for various periods of time (23, 34, 45). In the F. oxysporum-tomato interaction, the mycelium continued to grow along the junctions of the plant epidermal cells and covered large areas of the roots after 4 days. Necrosis started to appear 4 to 5 days after planting and was associated with a dense hyphal network that covered the necrotic tissues (23). In the F. verticillioides-maize interaction only single hyphae growing along the roots were observed after 7 to 14 days (Fig. 4c and d). A larger number of hyphae were detected after 21 days, but even these hyphae did not cover the entire root but rather retained the preexisting hyphal network architecture (Fig. 3j). Rotting associated with the massive growth of mycelium in these tissues was observed 3 to 4 weeks after planting and was restricted to the mesocotyl and the root (Fig. 3l and m).
These differences in fungal development in maize and tomato may reflect differences in the growth rates and aggressiveness of the highly virulent pathogen F. oxysporum and the less virulent pathogen F. verticillioides. These two fungi appear to use similar routes to penetrate and colonize their hosts, and the differences in disease appearance result from differences in the rates of fungal growth within the plant. In the more virulent pathogen F. oxysporum, the whole process proceeds much faster, and the transition from the initial symptomless phase to the necrotrophic phase occurs within a few days. The less aggressive pathogen F. verticillioides develops more slowly, leaving the plant more time to respond and restrict fungal growth. Indeed, activation of defense responses, such as deposition of lignins (45) and accumulation of PR proteins (34), by F. verticillioides-infected maize has been reported. Moreover, development of the fungus in physiologically weakened plants (e.g., plants grown with low light intensities) was much more aggressive, and large amounts of mycelium that caused severe rotting and seedling mortality were observed on and in the roots, mesocotyls, crowns, and stems of 2-week-old plants (Fig. 4 and 2).
Our data suggest that the well-orchestrated development of F. verticillioides inside maize tissues may represent a delicate balance between the fungus and the plant. Under conditions that favor symptomless infection, fungal growth may be restricted to specific tissues, where it penetrates only specific cells within which it reproduces without damaging the surrounding cells (Fig. 3e to i). The only rotting observed at this stage, in the mesocotyl and the primary root, was compensated for by the development of adventitious roots above the mesocotyl tissue, which were unaffected by the fungus (data not shown). This response may explain the initial slower growth of seedlings in the soil inoculation experiments (Table 1) and their later recovery and resumption of normal growth.
Under conditions that favor pathogenic development more mycelium develops, and the fungus switches to a more aggressive phase that probably involves secretion of hydrolytic enzymes and toxins (6, 7, 14, 18, 37, 42, 43). The magnitude and time of appearance of symptoms may vary, from no symptoms during the entire life cycle of the plant to seedling blight and severe rotting of the entire plant, depending in part on the physiological conditions of the plant during the early stages of colonization and later developmental stages that may favor disease outbreak.
Acknowledgments
We thank Shimon Sharon for his assistance in figure preparation.
This work was supported in part by Israeli Ministry of Agriculture grant 891-0148-00.
REFERENCES
- 1.Bacon, C., R. Bennett, D. Hinton, and K. Voss. 1992. Scanning electron microscopy of Fusarium moniliforme within asymptomatic corn kernels and kernels associated with equine leukoencephalomalacia. Plant Dis. 76:144-148. [Google Scholar]
- 2.Bacon, C. W., and D. M. Hinton. 1996. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 74:1195-1202. [Google Scholar]
- 3.Brown, R., T. Cleveland, C. Woloshuk, G. A. Payne, and D. Bhatnagar. 2001. Growth inhibition of a Fusarium verticillioides GUS strain in corn kernels of aflatoxin-resistant genotypes. Appl. Biotechnol. Microbiol. 57:708-711. [DOI] [PubMed]
- 4.Burgess, L. W., B. A. Summerell, S. Bullock, K. P. Gott, and D. Backhouse. 1994. Laboratory manual for Fusarium research, 3rd ed. University of Sydney, Sydney, Australia.
- 5.Danielsen, S., and D. Jensen. 1998. Relationships between seed germination, fumonisin content, and Fusarium verticillioides infection in selected maize samples from different regions of Costa Rica. Plant Pathol. 47:609-614. [Google Scholar]
- 6.Daroda, L., K. Hahn, D. Pashkoulov, and E. Benvenuto. 2001. Molecular characterization and in planta detection of Fusarium moniliforme endopolygalacturonase isoforms. Physiol. Mol. Plant Pathol. 59:317-325. [Google Scholar]
- 7.Desjardins, A. E., R. D. Plattner, T. C. Nelsen, and J. F. Leslie. 1995. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 61:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd, J. 1980. The role of plant stresses in development of corn stalk rots. Plant Dis. 64:533-537. [Google Scholar]
- 9.Drepper, W., and B. Renfro. 1990. Comparison of methods for inoculation of ears and stalks of maize with Fusarium moniliforme. Plant Dis. 74:952-956. [Google Scholar]
- 10.Du, W., Z. Huang, J. E. Flaherty, K. Wells, and G. A. Payne. 1999. Green fluorescent protein as a reporter to monitor gene expression and food colonization by Aspergillus flavus. Appl. Environ. Microbiol. 65:834-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumas, B., N. Centis, N. Sarrazin, and M. Esquere-Tugaye. 1999. Use of green fluorescent protein to detect expression of an endopolygalacturonase gene of Colletotrichum lindemuthianum during bean infection. Appl. Environ. Microbiol. 65:1769-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley, D. C. 1962. Systemic infection of corn by Fusarium moniliforme. Phytopathology 52:870-872. [Google Scholar]
- 13.Food and Drug Administration. 2000. Guidance for industry: fumonisin levels in human foods and animal feeds—draft guidance. Food and Drug Administration, Washington, D.C.
- 14.Garcia-Maceira, F., A. Di Pietro, M. Huertas-Gonzalez, M. Ruiz-Roldan, and M. Roncero. 2001. Molecular characterization of an endopolygalacturonase from Fusarium oxysporum expressed during early stages of infection Appl. Environ. Microbiol. 67:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Headrick, J., and J. Pataky. 1991. Maternal influence on the resistance of sweet corn lines to kernel infection by Fusarium moniliforme. Phytopathology 81:268-274. [Google Scholar]
- 16.Horowitz, S., S. Freeman, and A. Sharon. 2002. Use of green fluorescent protein-transgenic strains to study pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum. Phytopathology 92:743-749. [DOI] [PubMed] [Google Scholar]
- 17.Huang, R., M. Galperin, Y. Levy, and R. Perl-Treves. 1997. Genetic diversity of Fusarium moniliforme detected by vegetative compatibility groups and random amplified polymorphic DNA markers. Plant Pathol. 46:871-881. [Google Scholar]
- 18.Jardine, D. J., and J. F. Leslie. 1999. Aggressiveness to mature maize plants of Fusarium strains differing in ability to produce fumonisin. Plant Dis. 83:690-693. [DOI] [PubMed] [Google Scholar]
- 19.Kedera, C. J., J. F. Leslie, and L. E. Claflin. 1994. Genetic diversity of Fusarium section Liseola (Gibberella fujikuroi) in individual maize stalks. Phytopathology 84:603-607. [Google Scholar]
- 20.Kedera, C. J., J. F. Leslie, and L. E. Claflin. 1992. Systemic infection of corn by Fusarium moniliforme. Phytopathology 82:1138. [Google Scholar]
- 21.Kerenyi, Z., K. Zeller, L. Hornok, and J. F. Leslie. 1999. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 65:4071-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keying, Y., and M. B. Dickman. 1993. Sensitivity of field strains of Gibberella fujikuroi (Fusarium section Liseola) to benomyl and hygromycin B. Mycologia 85:206-213.
- 23.Lagopodi, A., A. Ram, G. Lamers, P. Punt, C. A. M. J. J. van den Hondel, B. Lugtenberg, and G. Bloemberg. 2002. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant-Microbe Interact. 15:172-179. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence, E., P. Nelson, and J. Ayers. 1981. Histopathology of sweet corn seeds and plants infected with Fusarium moniliforme and F. oxysporum. Phytopathology 71:379-386. [Google Scholar]
- 25.Leslie, J. F., C. Pearson, P. Nelson, and T. Toussoun. 1990. Fusarium spp. from corn, sorghum, and soybean fields in the central and eastern United States. Phytopathology 80:343-350. [Google Scholar]
- 26.Leslie, J. F. 1996. Introductory biology of Fusarium moniliforme, p. 153-164. In L. S. Jackson, J. W. De Vries, and L. B. Bullerman (ed.), Fumonisins in food. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 27.Lorang, J. M., R. P. Tuori, J. P. Martinez, T. L. Sawyer, R. S. Redman, J. A. Rollins, T. J. Wolpert, K. B. Johnson, R. J. Rodriguez, M. B. Dickman, and L. M. Ciuffetti. 2001. Green fluorescent protein is lighting up fungal biology. Appl. Environ. Microbiol. 67:1987-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magan, N., and J. Lacey. 1984. Effect of water activity, temperature and substrate on interactions between field and storage fungi. Trans. Br. Mycol. Soc. 82:83-93. [Google Scholar]
- 29.Magan, N., S. Marin, A. Ramos, and V. Sanchis. 1997. The impact of ecological factors on germination, growth, fumonisin production of F. moniliforme and F. proliferatum and their interactions with other common maize fungi. Cereal Res. Commun. 25:643-645. [Google Scholar]
- 30.Maor, R., M. Puyesky, B. A. Horowitz, and A. Sharon. 1998. Use of the green fluorescent protein (GFP) for studying development and fungal-plant interaction in Cochliobolus heterostrophus. Mycol. Res. 102:491-496. [Google Scholar]
- 31.Munkvold, G. P., and W. M. Carlton. 1997. Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds. Plant Dis. 81:211-216. [DOI] [PubMed] [Google Scholar]
- 32.Munkvold, G. P., R. L. Helmich, and W. B. Showers. 1997. Reduced Fusarium ear rot and symptomless infection in kernels of maize genetically engineered for European corn borer resistance. Phytopathology 87:1071-1077. [DOI] [PubMed] [Google Scholar]
- 33.Munkvold, G. P., D. C. McGee, and W. M. Carlton. 1997. Importance of different pathways for maize kernel infection by Fusarium verticillioides. Phytopathology 97:209-217. [DOI] [PubMed] [Google Scholar]
- 34.Murillo, I., L. Cavalarin, and S. San Segundo. 1999. Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRms protein. Phytopathology 89:737-747. [DOI] [PubMed] [Google Scholar]
- 35.Nagy, E., I. Cabulea, and I. Has. 1997. The role of genotype in maize × Fusarium pathosystem. Cereal Res. Commun. 25:789-790. [Google Scholar]
- 36.Nelson, P. E. 1992. Taxonomy and biology of Fusarium moniliforme. Mycopathologia 117:29-36. [DOI] [PubMed] [Google Scholar]
- 37.Nelson, P. E., A. E. Desjardins, and R. D. Plattner. 1993. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry and significance. Annu. Rev. Phytopathology 31:233-252. [DOI] [PubMed] [Google Scholar]
- 38.Reid, L. M., T. Woldemariam, X. Zhu, D. Stewart, and A. Schaafsma. 2002. Effect of inoculation time and point of entry on disease severity in Fusarium graminearum, Fusarium verticillioides, or Fusarium subglutinans inoculated maize ears. Can. J. Plant Pathol. 24:162-167. [Google Scholar]
- 39.Rheeder, J. P., and W. F. O. Marasas. 1998. Fusarium species from plant debris associated with soils from maize production areas in the Transkei region of South Africa. Mycopathologia 143:113-119. [DOI] [PubMed] [Google Scholar]
- 40.Robinson, M., J. Riov, and A. Sharon. 1998. Indole-3-acetic acid biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl. Environ. Microbiol. 64:5030-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson, M., and A. Sharon. 1999. Transformation of the bioherbicide Colletotrichum gloeosporioides f. sp. aeschynomene by electroporation of germinated conidia. Curr. Genet. 36:98-104. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Roldan, M., A. Di Pietro, M. Huertas-Gonzalez, and M. Roncero. 1999. Two xylanase genes of the vascular wilt pathogen Fusarium oxysporum are differentially expressed during infection of tomato plants. Mol. Plant-Microbe Interact. 261:530-536. [DOI] [PubMed] [Google Scholar]
- 43.Seo, J., R. Proctor, and R. Plattner. 2001. Characterization of four clustered and coregulated genes associated with fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 34:155-165. [DOI] [PubMed] [Google Scholar]
- 44.Tudzynski, B., K. Mende, K. M. Weltring, S. E. Unkles, and J. R. Kinghorn. 1996. The Gibberella fujikuroi niaD gene encoding nitrate reductase: isolation, sequence, homologous transformation and electrophoretic karyotype location. Microbiology 142:533-539. [DOI] [PubMed] [Google Scholar]
- 45.Yates, I. E., C. W. Bacon, and D. M. Hinton. 1997. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 81:723-728. [DOI] [PubMed] [Google Scholar]
- 46.Yates, I. E., K. Hiett, D. Kapczynski, W. Smart, A. Glenn, D. M. Hinton, C. W. Bacon, R. Meinersmann, S. Liu, and A. Jaworski. 1999. GUS transformation of the maize fungal endophyte Fusarium moniliforme. Mycol. Res. 103:129-136. [Google Scholar]