Abstract
Phosphorylation of neural proteins in response to a diverse array of external stimuli is one of the main mechanisms underlying dynamic changes in neural circuitry. The NR2B subunit of the NMDA receptor is tyrosine-phosphorylated in the brain, with Tyr-1472 its major phosphorylation site. Here, we generate mice with a knockin mutation of the Tyr-1472 site to phenylalanine (Y1472F) and show that Tyr-1472 phosphorylation is essential for fear learning and amygdaloid synaptic plasticity. The knockin mice show impaired fear-related learning and reduced amygdaloid long-term potentiation. NMDA receptor-mediated CaMKII signaling is impaired in YF/YF mice. Electron microscopic analyses reveal that the Y1472F mutant of the NR2B subunit shows improper localization at synapses in the amygdala. We thus identify Tyr-1472 phosphorylation as a key mediator of fear learning and amygdaloid synaptic plasticity.
Keywords: amygdala, fear learning, NMDA receptor, synaptic plasticity, tyrosine phosphorylation
Introduction
The N-methyl-D-aspartate subtype of ionotropic glutamate receptor (NMDAR) is crucial for development, synaptic plasticity and neuronal excitotoxicity (Choi, 1988; McDonald and Johnston, 1990; Collingridge and Bliss, 1995). Long-term potentiation (LTP) of excitatory synaptic transmission is a candidate for the cellular mechanism underlying neural plasticity, such as learning and memory (Bliss and Collingridge, 1993). The NMDAR is composed of the NR1 (GluRζ) and NR2 (GluRɛ) subunits. The NR1 subunit is essential for the function of NMDAR channels, whereas NR2 subunits (NR2A (GluRɛ1), NR2B (GluRɛ2), NR2C (GluRɛ3) and NR2D (GluRɛ4)) determine the characteristics of NMDAR channels by forming different heteromeric configurations with the NR1 subunit (Monyer et al, 1994; Cull-Candy et al, 2001). The NMDAR complex is composed of NMDAR subunits and a number of associated regulatory proteins, including scaffolding proteins, cytoskeletal proteins, protein kinases and protein phosphatases (Kornau et al, 1997; Husi et al, 2000; Scannevin and Huganir, 2000; Sheng and Kim, 2002).
Recent studies have shown that NMDARs are subject to rapid activity-dependent redistribution at synapses (Carroll and Zukin, 2002; Wenthold et al, 2003). The NMDAR moves laterally between synaptic and extrasynaptic domains. LTP promotes rapid expression of NMDARs on the cell surface at hippocampal CA1 synapses. Conversely, long-term depression (LTD) correlates with a decrease in NMDAR protein levels in CA1 synaptoneurosomes (Carroll and Zukin, 2002; Wenthold et al, 2003). Moreover, the NMDAR is internalized by clathrin-mediated endocytosis from synaptic sites in a use-dependent manner (Roche et al, 2001; Vissel et al, 2001), which is thought to contribute to several forms of synaptic plasticity.
The amygdala is a group of spatially contiguous and anatomically interconnected nuclei located within the rostral pole of the temporal lobe of mammals. Accumulated evidence indicates that the amygdala is a crucial brain structure for the expression of fear memory (Davis, 1997; Fendt and Fanselow, 1999; Maren, 1999; LeDoux, 2000; Chapman et al, 2003; Sah et al, 2003). LTP is observed in both thalamo-amygdala and cortico-amygdala projections in vivo (Maren, 1999; LeDoux, 2000; Chapman et al, 2003). Given that LTP occurs at synapses receiving conditioned stimulus and that fear conditioning induces associative LTP-like changes (Maren, 1999; LeDoux, 2000; Chapman et al, 2003), the LTP is a candidate mechanism for memory storage during fear memory formation. Intra-amygdala blockade of NMDARs disrupts both the acquisition of fear memory and LTP, indicating that NMDARs in the amygdala are necessary for fear memory formation (Gewirtz and Davis, 1997; Lee and Kim, 1998; LeDoux, 2000; Fendt, 2001; Rodrigues et al, 2001; Bauer et al, 2002).
NR2 subunits have an unusually long C-terminal tail extended into the cytoplasm (Cull-Candy et al, 2001), which is likely to play essential roles in their regulation including receptor localization, receptor channel activity and downstream signaling via NMDAR-associated proteins (Kornau et al, 1997; Husi et al, 2000; Scannevin and Huganir, 2000; Sheng and Kim, 2002). Several proteins including scaffolding proteins, cytoskeletal proteins, signaling proteins, protein kinases and protein phosphatases interact directly with NR2B subunits (Kornau et al, 1997; Husi et al, 2000; Scannevin and Huganir, 2000; Sheng and Kim, 2002).
The NR2B subunit is a major tyrosine-phosphorylated protein (Moon et al, 1994). We have identified Tyr-1472 of the NR2B subunit as the main phosphorylation site in vitro and have shown that this tyrosine is phosphorylated by Fyn, a member of the Src family kinases (Nakazawa et al, 2001). The level of Tyr-1472 phosphorylation is increased after induction of LTP in the hippocampus, suggesting that Fyn-mediated phosphorylation of Tyr-1472 is involved in synaptic plasticity (Nakazawa et al, 2001). Tyr-1472 phosphorylation is also implicated in transient ischemia, lithium protection against excitotoxicity and ethanol inhibition of NMDAR-mediated responses (Salter and Kalia, 2004). Src and Fyn upregulate the current through recombinant NR1-NR2A channels, but not NR1-NR2B channels in HEK293 cells (Köhr and Seeburg, 1996). Thus, Tyr-1472 phosphorylation may not regulate NMDAR channel properties but rather regulate NMDAR localization or initiation of downstream signaling pathways. Such action may be triggered by providing binding sites for signaling proteins through SH2 or phospho-tyrosine-binding domains. However, the precise roles of Tyr-1472 phosphorylation in vivo remain unclear.
Here, we report analyses of knockin mice in which Tyr-1472 of the NR2B subunit is mutated to phenylalanine to prevent phosphorylation of this site in vivo. We find that the lack of Tyr-1472 phosphorylation results in poor fear-related learning, impaired amygdaloid synaptic plasticity, and improper NMDAR localization at synapses.
Results
Generation of NR2B Y1472F knockin mice
To examine the physiological significance of Tyr-1472 phosphorylation of the NR2B subunit, we generated mutant mice lacking the Tyr-1472 phosphorylation site by substituting Tyr-1472 (Y) with Phe-1472 (F) by a knockin technique (Figure 1). The success of these procedures was confirmed by Southern blot and PCR analysis (Figure 1A–C and data not shown). We confirmed the absence of Tyr-1472 phosphorylation in homozygous knockin mice (YF/YF mice) by immunoblot analysis using the anti-pY1472 antibody that specifically recognizes the NR2B subunit phosphorylated at Tyr-1472 (Nakazawa et al, 2001; Figure 1C, top panel). The expression level of the NR2B subunit in YF/YF mice was virtually identical to that in wild-type littermates (WT/WT mice), indicating that the targeted NR2B gene expressed the NR2B protein at normal levels (WT/WT, 100±7%, n=5 from five mice; YF/YF, 102±5%, n=5 from five mice; P>0.9, Student's t-test) (Figure 1C, bottom panel).
Figure 1.
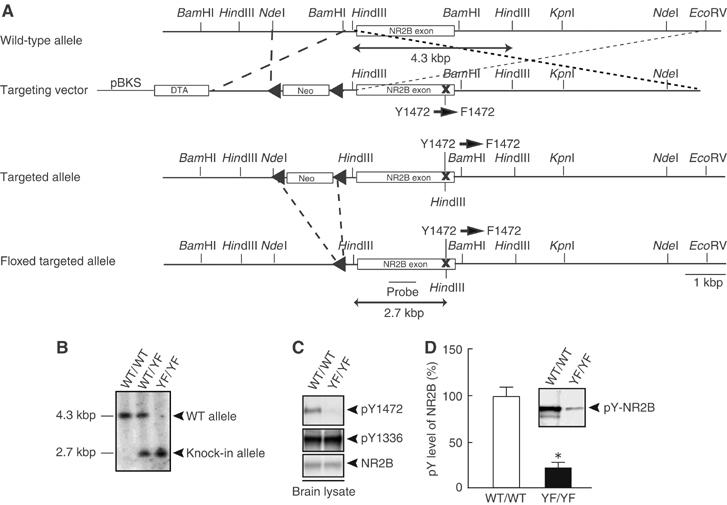
Generation of mice with a mutation of the NR2B tyrosine phosphorylation site. (A) Schematic representations of the structures of WT, targeting vector, and targeted and floxed-targeted NR2B alleles with relevant restriction sites. The probe for Southern blotting is indicated. pBKS, pBluescriptIIKS plasmid; DTA, diphtheria toxin gene; neo, neomycin-resistance gene. (B) The Southern blot analysis of HindIII-digested genomic DNAs from WT/WT, WT/YF and YF/YF mice. (C) The absence of Tyr-1472 phosphorylation in homozygous YF/YF mice (upper). Equal amounts of amygdaloid lysates from WT/WT and YF/YF mice were probed with the anti-pY1472 antibody. (D) A reduced level of NR2B tyrosine-phosphorylation in YF/YF mice. Equal amounts of NR2B immunoprecipitates from amygdaloid lysates of WT/WT and YF/YF mice were probed with the anti-pY (4G10) mAb. A representative blot is shown in the inset. All data are presented as the mean±s.e.m. (Figures 1, 2, 3, 4, 5, 6 and 7 and Supplementary Figures 1–7). *P<0.001, Student's t-test.
YF/YF mice appeared healthy with general behaviors (data not shown). Histological analysis with Nissl-stained coronal sections of CNS structures from YF/YF mice did not show any gross abnormalities in cytoarchitecture (data not shown). On the basis of the observed behavioral and electrophysiological phenotypes described below, we analyzed the amygdala in detail. In YF/YF mice, the levels of NR2B tyrosine phosphorylation in the amygdala (WT/WT, 100±11%, n=4 from four mice; YF/YF, 21±5%, n=4 from four mice; P<0.001, Student's t-test), cerebral cortex and hippocampus were significantly reduced in comparison with those of WT/WT mice, indicating that Tyr-1472 was the main phosphorylation site in vivo (Figure 1D). The phosphorylation level of Tyr-1336, a minor phosphorylation site of NR2B (Nakazawa et al, 2001), was unchanged in YF/YF mice (Figure 1C, middle panel). These findings indicate that the lack of Tyr-1472 phosphorylation does not alter gross anatomy of the brain or expression of the NR2B subunit.
Impaired fear-related learning in YF/YF mice
We performed the battery of behavioral tests (Morris water maze test, contextual fear conditioning test, passive avoidance test and auditory fear conditioning test) for examining the learning ability of YF/YF mice. While we did not detect abnormalities in hippocampus-dependent learning ability of YF/YF mice (data not shown), YF/YF mice showed impaired fear-related learning in auditory fear conditioning test. Mice were given a single pairing of a tone (CS) and a footshock (US) on the conditioning day (Figure 2A). At 24 h after the conditioning, the mice were placed in a novel chamber for 3 min before the tone (CS) was delivered for 3 min. Before the tone was presented, both WT/WT and YF/YF mice displayed only weak freezing behavior in the novel chamber (WT/WT, 3.49±1.10%, n=17; YF/YF, 5.54±2.01%, n=17; P>0.3, ANOVA) (Figure 2B and C). During the 1-min period immediately after delivery of the tone, WT/WT mice showed strong freezing responses (41.6±4.8%, n=17), whereas YF/YF mice showed significantly weaker responses (23.1±6.5%, n=17; F(1,32)=5.23, P<0.03, ANOVA) (Figure 2B and C). This deficit could be caused by failure to acquire the CS–US association or by failure to stabilize the fear-related memory. To distinguish between these alternatives, we performed the behavioral test 1 h after the conditioning to assess the learning. In this test, YF/YF mice also showed much weaker freezing responses (6.9±2.7%, n=10) in comparison to WT/WT mice (23.6±5.2%, n=20; F(1,28)=4.73, P<0.04, ANOVA) (Figure 2D), suggesting that YF/YF mice failed to acquire the CS–US association. Using visual conditioned stimuli, YF/YF mice also showed much weaker freezing responses in comparison to WT/WT mice (Supplementary Figure 1). Because pain sensitivity would affect freezing responses, we measured current thresholds for three reactions to the nociceptive shock; namely, flinch, vocalization and jump. There were no significant differences between WT/WT and YF/YF mice in pain thresholds for flinch (WT/WT, 0.07±0.01 mA; YF/YF, 0.08±0.01 mA; F(1,15)=0.294, P>0.5, ANOVA), vocalization (WT/WT, 0.12±0.01 mA; YF/YF, 0.12±0.01 mA; F(1,15)=0.340, P>0.5, ANOVA) and jump reactions (WT/WT, 0.29±0.02 mA; YF/YF, 0.31±0.03 mA; F(1,15)=0.406, P>0.5, ANOVA). Together with our observations that YF/YF mice normally respond to the tone in acoustic startle response test (Supplementary Figure 2), these data suggest that fear-related learning is severely impaired in YF/YF mice.
Figure 2.
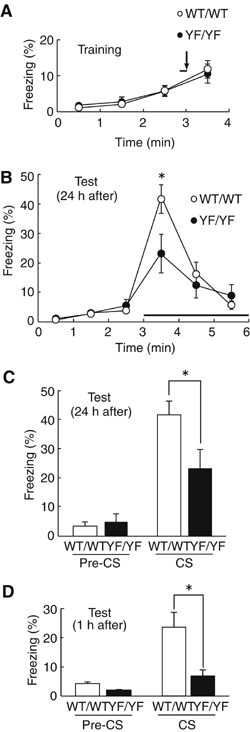
Impairment of auditory fear conditioning in YF/YF mice. (A) Freezing responses of WT/WT and YF/YF mice in auditory fear conditioning on the conditioning day (at 3 min; WT/WT, 5.88±1.43, n=17; YF/YF, 5.69±1.41, n=17; P>0.9, ANOVA). 170 s after mice were placed in the conditioning chamber, a tone was presented for 10 s (solid line); at the end of the tone mice were given a footshock (arrow). (B) Freezing responses 24 h after conditioning. At 3 min after mice were placed into a testing chamber with novel contexts, the tone was presented (solid line). WT/WT, n=17; YF/YF, n=17; *P< 0.03, ANOVA. (C, D) Summary of freezing responses 24 h after conditioning (C) and 1 h after conditioning (D). Freezing in the absence of the tone (Pre-CS) and in the presence of the tone (CS) was calculated as the mean freezing response from 2 to 3 min and from 3 to 4 min, respectively, after placement in the testing chamber. (24 h, *P< 0.03, ANOVA; 1 h, WT/WT, n=20; YF/YF, n=10; *P<0.04, ANOVA).
Impaired synaptic plasticity of YF/YF mice
In the lateral nucleus of amygdala (LA), LTP can be induced at thalamic input synapses with a pairing protocol in which weak presynaptic stimulation of thalamo-amygdala afferents that transmit CS information to the LA is presented concurrently with depolarization of the postsynaptic cell by current injection (Weisskopf et al, 1999). This form of LTP is essential for auditory fear conditioning in mice (LeDoux, 2000). We performed electrophysiological experiments in the LA of brain slices. Delivery of the conditioning stimulation to the thalamic inputs produced robust LTP in the LA of WT/WT mice (155±14% of baseline, n=17), whereas LTP in YF/YF slices (116±7% of baseline, n=16) was significantly smaller than that in WT/WT slices (P<0.02, Student's t-test) (Figure 3A and B). The LTP was almost completely blocked by D-APV (Supplementary Figure 3A) or D-APV plus the L-type voltage-gated calcium channel (VGCC) antagonist nitrendipine (Supplementary Figure 3B), suggesting that the LTP was mostly NMDAR dependent. We found no significant differences (P>0.8) between WT/WT (the ratio of integral of the synaptic current during the first burst to that of the single excitatory postsynaptic potential (EPSC), 4.24±0.24, n=14) and YF/YF (4.19±0.32, n=8) mice in the temporal summation of NMDAR-mediated EPSCs elicited by the same stimulation protocol used for the LTP induction (Figure 3C). Thus, not only single NMDAR-mediated synaptic responses but also the multiple NMDAR-mediated synaptic responses (and presumably, Ca2+ influx into the postsynaptic cell) during the conditioning were unaltered by the mutation.
Figure 3.
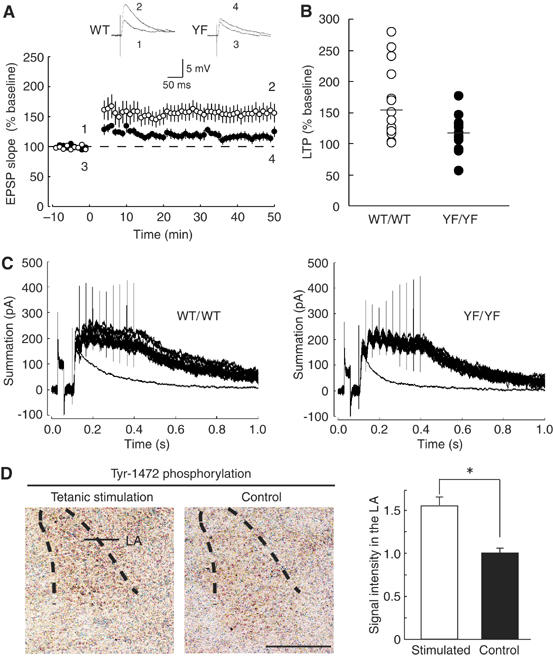
Impaired amygdaloid LTP in YF/YF mice. (A) The averaged time course of LTP in WT/WT (n=17) and YF/YF (n=16) mice. Maximal initial EPSP slopes were normalized in each experiment to the averaged maximal initial EPSP slope during the control period (−10 to 0 min). LTP was induced by ‘pairing' at time 0. Sample traces of EPSPs (average of 10 consecutive responses) in WT/WT and YF/YF mice recorded at the times indicated by the numbers are shown in the inset. (B) Summary of LTP (WT/WT, n=17; YF/YF, n=16; P<0.02, Student's t-test) expressed as percent of the mean EPSP slope from 40 to 50 min after the induction relative to the mean EPSP slope during the control period (−10 to 0 min). Horizontal bars indicate the means. (C) Sample traces showing the temporal summation of NMDAR-mediated EPSCs elicited by the same stimulation protocol used for the LTP induction. Fifteen traces evoked by 30-Hz stimulation at 10-s intervals and a single EPSC trace (average of 10 traces) are superimposed. (D) (left) The level of Tyr-1472 phosphorylation is increased after electrical stimulation in the LA. Coronal sections including the LA were stained with the anti-phospho-Tyr1472 antibody. La, lateral amygdala. (right) Quantification of Tyr-1472 phosphorylation in the LA. *P<0.004, Student's t-test, n=3. The calibration bar, 500 μm.
Increased phosphorylation of Tyr-1472 after tetanic stimulation in the amygdala
We then investigated whether the level of Tyr-1472 phosphorylation was enhanced after tetanic stimulation of thalamo-amygdala afferents (100 Hz for 1 s, repeated three times at 10-s intervals, three places). At 5 min after the stimulation, the level of Tyr-1472 phosphorylation was significantly enhanced in the amygdala (stimulated slices, 1.57±0.07; control slice, 1.00±0.05; P<0.004, Student's t-test) (Figure 3D). The data suggest that the lack of Tyr-1472 phosphorylation, rather than the Y1472F substitution, is responsible for the impaired amygdaloid synaptic plasticity in YF/YF mice.
Normal basic properties of synaptic transmission in YF/YF mice
The ratio of NMDAR-mediated EPSC amplitudes to α-amino-3-hydroxy-5-methylisoxazole-4-propionic-acid receptor (AMPAR)-mediated EPSC amplitudes did not differ between WT/WT and YF/YF mice (WT/WT, 68.9±2.2%, n=9; YF/YF, 69.3±2.9%, n=12; P>0.9, Student's t-test) (Figure 4A). Although the most straightforward explanation was that there was no change in NMDAR-mediated synaptic currents in YF/YF mice, one could argue that AMPAR-mediated synaptic responses were also decreased due to, for instance, a reduction of AMPAR surface expression at synapses. To examine this possibility, we recorded AMPAR-mediated miniature EPSCs (mEPSCs) in the whole-cell patch-clamp configuration from LA principal neurons. The averaged amplitudes of AMPAR-mediated mEPSCs were indistinguishable between WT/WT and YF/YF mice (WT/WT, 13.2±0.7 pA, n=23; YF/YF, 12.8±0.8 pA, n=20; P>0.8, Student's t-test) (Figure 4B). To obtain further support that the NMDAR-mediated response was unchanged, we examined the ratio of NMDAR-mediated mEPSC amplitudes to AMPAR-mediated mEPSC amplitudes (Figure 4C). No statistically significant difference was found in the averaged NMDAR-mediated mEPSC/AMPAR-mediated mEPSC ratio between WT/WT and YF/YF mice (WT/WT, 18.1±2.9%, n=9; YF/YF, 21.3±3.6%, n=7; P>0.7, Student's t-test). In addition, the I–V curves of WT/WT and YF/YF mice were virtually identical (Figure 4D), suggesting that Tyr-1472 phosphorylation did not affect the voltage-dependent Mg2+ block of NMDARs. Furthermore, the rise time (P>0.9, Student's t-test) and decay time constant (P>0.7, Student's t-test) of NMDAR-mediated EPSCs were not different between WT/WT (rise time, 8.40±0.33 ms; decay time constant, 134.4±9.7 ms; n=17) and YF/YF (rise time, 8.60±0.46 ms; decay time constant, 132.9±9.4 ms; n=12) mice, suggesting that the NMDAR subunit composition was not changed in YF/YF mice (Monyer et al, 1994). Together with our present results shown in Figure 3C, the data strongly suggest that basic properties of NMDARs are absolutely normal in YF/YF mice. Thus, the impaired amygdaloid LTP likely resulted from the abnormal signal transduction downstream of NMDAR activation.
Figure 4.
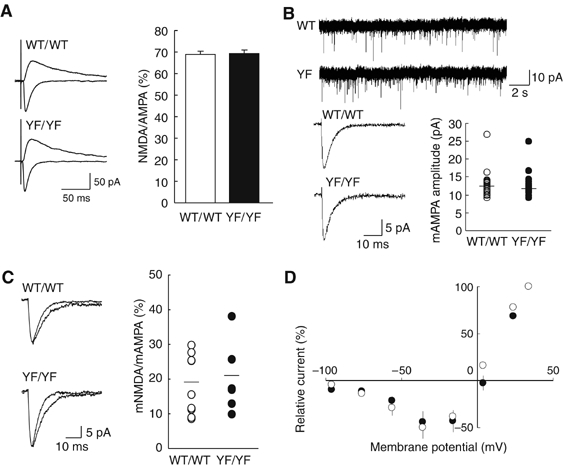
Normal basic properties of synaptic transmission in YF/YF mice. (A) Sample traces (average of 10 traces) of evoked AMPAR-mediated (downward traces) and NMDAR-mediated (upward traces) EPSCs in WT/WT and YF/YF mice (left). Ratio of amplitudes of NMDAR-mediated EPSCs to those of AMPAR-mediated EPSCs (right) (WT/WT mice, n=9; YF/YF mice, n=12). The ratio was calculated for each cell, and the values were then averaged for all cells. (B) Unaltered AMPAR-mediated mESPCs in YF/YF mice. Sample traces showing multiple events on a slower time scale (top). Averaged traces of mEPSCs recorded from single neurons (consecutive 100 events are averaged for WT/WT mice; consecutive 82 events for YF/YF mice) (bottom, left). Summary of the mean amplitude of AMPAR-mediated mEPSCs (bottom, right) in WT/WT (n=23) and YF/YF mice (n=20). Horizontal bars indicate the means. (C) Sample traces of AMPAR-mediated mEPSCs (faster traces) and AMPAR-plus NMDAR-mediated mEPSCs (slower traces) in WT/WT (average of 28 traces for AMPA and NMDA mEPSCs; average of 50 traces for AMPA mEPSCs) and YF/YF (average of 53 traces for both AMPA and NMDA mEPSCs and AMPA mEPSCs) mice (left). Ratio of the mean amplitude of NMDAR-mediated mEPSCs (AMPA mEPSCs were digitally subtracted from AMPA plus NMDA mEPSCs) to that of AMPAR-mediated mEPSCs (WT/WT, n=9; YF/YF, n=7). Horizontal bars indicate the means. (D) Current–voltage relationships of evoked NMDA synaptic currents recorded in the presence of 10 μM CNQX in WT/WT (n=5) and YF/YF (n=5) mice. The current values were normalized to the value at +34 mV in each cell, and the values were then averaged for all cells. The liquid junction potential was compensated.
Impaired NMDAR-mediated CaMKII signaling in YF/YF mice
We then examined the NMDAR-mediated downstream signaling in WT/WT and YF/YF mice. We immunoprecipitated NMDARs from the amygdala with an antibody against the NR2B subunit and probed for the levels of co-immunoprecipitated NMDAR-associated proteins. Among these molecules, we found that CaMKII, an essential molecule for the induction of NMDAR-dependent LTP in the amygdala (Bayer et al, 2001; Lisman et al, 2002; Rodrigues et al, 2004), was almost absent from the NMDAR complex in YF/YF mice (Figure 5A). Consistently, we found that glutamate-induced upregulation of CaMKII was severely impaired in YF/YF mice (WT/WT, 180±20%, n=3 from 3 mice; YF/YF, 115±7%, n=3 from 3 mice; P<0.01, Student's t-test) (Figure 5B and C). We found no differences between WT/WT and YF/YF mice in glutamate-induced upregulation of ERK and CREB (Figure 5B). Other NR2B-interacting signaling molecules such as PLCγ1 and RasGRF1 (Krapivinsky et al, 2003) interacted well with both the WT NR2B subunit and Y1472FNR2B (data not shown). These results suggest that the impaired LTP in YF/YF mice is caused by the impaired NMDAR-mediated CaMKII signaling in YF/YF mice.
Figure 5.
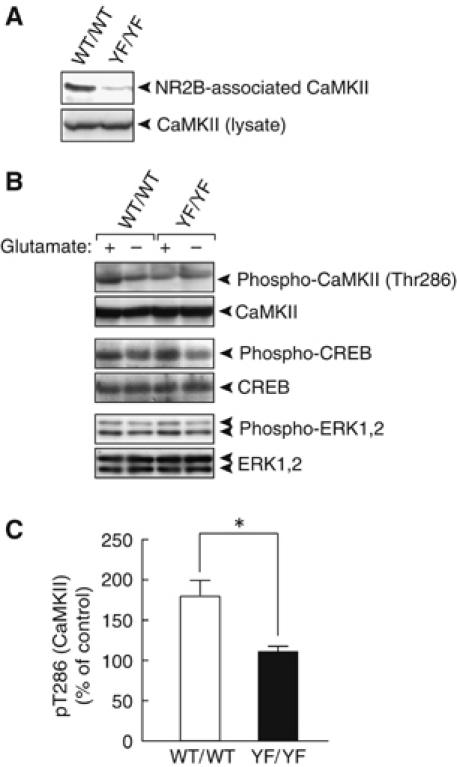
Impaired NMDAR-mediated CaMKII signaling in YF/YF mice. (A) Interaction of CaMKII with the WT NR2B, but very little interaction with Y1472FNR2B. NR2B immunoprecipitates (top) and lysates (bottom) were probed with the anti-CaMKII mAb. (B) Impaired glutamate-induced upregulation of CaMKII in YF/YF mice. Stimulation of coronal sections including the LA with glutamate was performed as described in Materials and methods. (C) Quantification of phospho-CaMKII in WT/WT (n=6 from 3 mice) and YF/YF mice (n=6 from 3 mice). *P<0.01, Student's t-test.
Improper localization of the NMDAR at synaptic and perisynaptic regions in YF/YF mice
Finally, we examined the structure of synapses and the distribution of NMDAR at synapses in detail by electron microscopy. While we found no obvious abnormalities in synaptic structure of the LA in YF/YF mice (data not shown), we found the improper localization of the NR2B and NR1 subunits at asymmetrical axo-spinous synapses in the LA by postembedding immunogold staining described below (Figures 6 and 7; Supplementary Figure 4). When analyzing cross-sections of LA synapses by transmission electron microscopy, we divided each synapse into central, peripheral, and perisynaptic regions and scored each region for immunogold particles. While the method did not quantify the absolute two-dimensional distribution of particles across the synaptic membrane surface, it provided one-dimensional values useful for comparison. In YF/YF mice, the distribution of NR2B was skewed toward the peripheral and perisynaptic regions, whereas, in WT/WT mice, NR2B was located more centrally at synapses, as reported previously (Racca et al, 2000; Figure 6A). The distribution ratios of central to peripheral NR2B signals were 1.85±0.22 for WT/WT mice and 0.99±0.25 for YF/YF mice (P<0.01, Student's t-test) (Figure 6B and C). The perisynaptic region, where NMDARs undergo endocytosis (Blanpied et al, 2002; Petralia et al, 2003; Racz et al, 2004), was similarly enriched for NR2B in YF/YF mice (Figure 6A). The proportions of the total signal in this region were 4.36±1.42% in WT/WT mice versus 11.72±4.19% in YF/YF mice (P<0.03, Student's t-test) (Figure 6B and D). As for NR2B signals detected below the synaptic membrane, their perpendicular distribution from the membrane did not differ between WT/WT and YF/YF mice (Figure 6E). Furthermore, WT/WT and YF/YF mice were indistinguishable with regard to the total number of NR2B signals present per profile of asymmetrical axo-spinous synapses (Figure 6F). The distribution of NR1 was also skewed toward the peripheral and perisynaptic regions in YF/YF mice (Figure 7A–D; Supplementary Figure 4). In contrast to NR2B and NR1 subunits, the distributions and total number of NR2A signals were unchanged in YF/YF mice (Figure 7E–H; Supplementary Figure 6). These data suggest that the Y1472F mutation interferes with proper localization of NMDARs across synaptic and perisynaptic regions.
Figure 6.
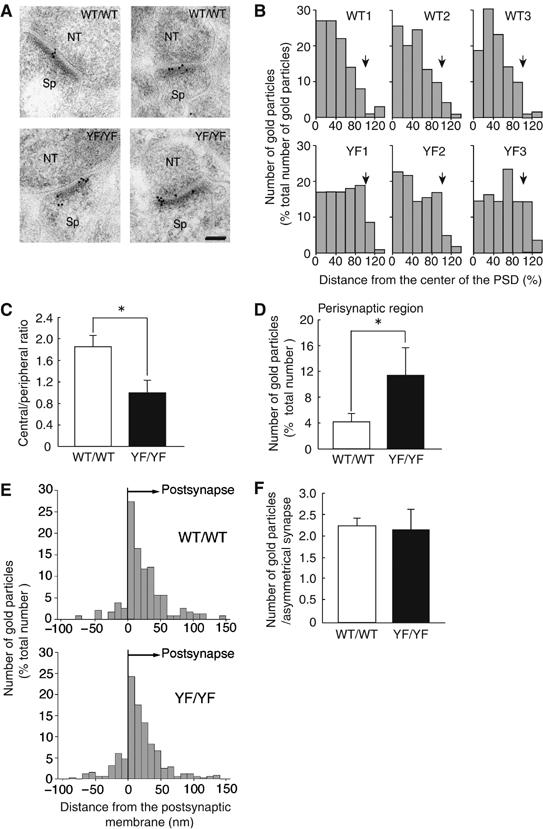
Improper localization of the NR2B subunit in synaptic and perisynaptic regions in YF/YF mice. (A) Postembedding immunogold staining of NR2B subunits at asymmetrical axo-spinous synapses in the LA of WT/WT (WT/WT1 in B) and YF/YF (YF/YF1 in B) mice. NT, nerve terminal; Sp, spine. The calibration bar, 50 nm. (B–D) Tangential distribution of the NR2B subunit (WT/WT, 88 synapses; YF/YF, 102 synapses). (B) Histograms for the tangential distribution of NR2B subunits at synapses of WT/WT and YF/YF mice. Arrows indicate a boundary of the PSD. (C) Ratio of the number of immunogold particles for the NR2B subunit in the central region to that in the peripheral region. *P<0.01, Student's t-test. (D) The number of immunogold particles for the NR2B subunit in the perisynaptic regions. Results are expressed as percent of the total number of immunogold particles at synapses. *P<0.03, Student's t-test. (E) Histograms for the perpendicular distribution of NR2B subunits at synapses of WT/WT (66 synapses) and YF/YF mice (72 synapses). (F) The number of immunogold particles for the NR2B subunit per profile of synapses in the LA of the WT/WT (447 synapses) and YF/YF (451 synapses) mice. P>0.7, Student's t-test.
Figure 7.
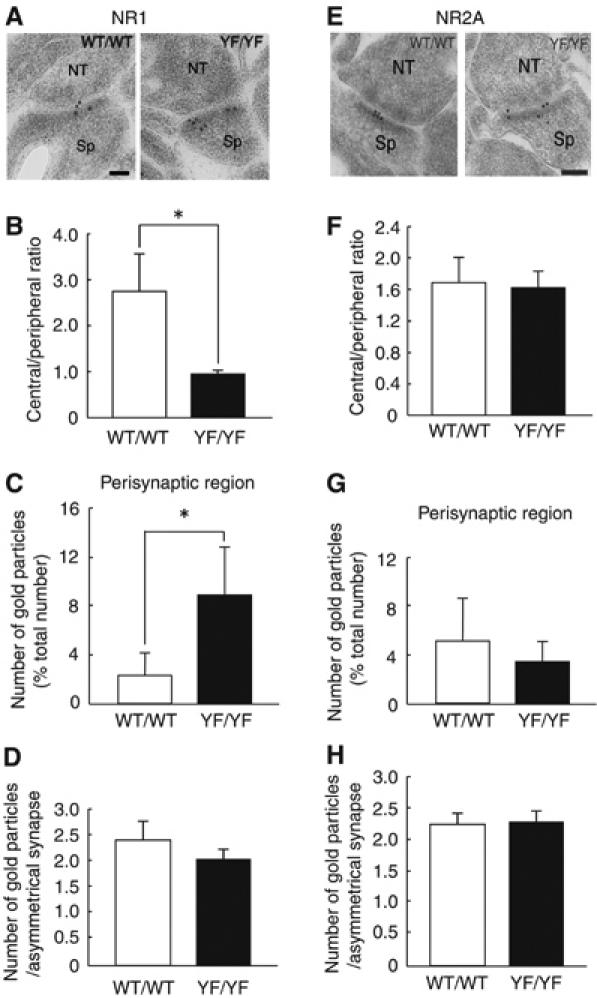
Improper localization of NR1 but normal localization of NR2A in synaptic and perisynaptic regions in YF/YF mice. (A) Postembedding immunogold staining of the NR1 subunit at asymmetrical axo-spinous synapses in the LA of WT/WT (WT/WT1 in Supplementary Figure 4A) and YF/YF (YF/YF1 in Supplementary Figure 4A) mice. NT, nerve terminal; Sp, spine. The calibration bar, 50 nm. (B, C) Tangential distribution of the NR1 subunit (WT/WT, 81 synapses; YF/YF, 84 synapses). (B) Ratio of the number of immunogold particles for the NR1 subunit in the central region to that in the peripheral region (WT/WT, 2.70±0.88; YF/YF, 0.79±0.14; *P<0.02, Student's t-test). (C) The number of immunogold particles for the NR1 subunit in the perisynaptic regions. Results are expressed as percent of the total number of immunogold particles at synapses (WT/WT, 2.27±1.63%; YF/YF, 8.47±3.89%; *P<0.03, Student's t-test). (D) The number of immunogold particles for the NR1 subunit per profile of synapses in the LA of WT/WT (2.45±0.43, 100 synapses) and YF/YF (2.07±0.20, 100 synapses; P>0.2, Student's t-test) mice. (E) Postembedding immunogold staining of the NR2A subunit at asymmetrical axo-spinous synapses in the LA of WT/WT (WT/WT1 in Supplementary Figure 4C) and YF/YF (YF/YF1 in Supplementary Figure 4C) mice. The calibration bar, 50 nm. (F, G) Tangential distribution of the NR2A subunit (WT/WT, 70 synapses; YF/YF, 55 synapses). (F) Ratio of the number of immunogold particles for the NR2A subunit in the central region to that in the peripheral region (WT/WT, 1.69±0.36; YF/YF, 1.59±0.25; P>0.7, Student's t-test). (G) The number of immunogold particles for the NR2A subunit in the perisynaptic regions (WT/WT, 5.38±3.15%; YF/YF, 3.55±1.51%; P>0.2, Student's t-test). (H) The number of immunogold particles for the NR2A subunit per profile of synapses in the LA of WT/WT (2.32±0.15, 70 synapses) and YF/YF (2.35±0.16, 55 synapses; P>0.7, Student's t-test) mice.
Discussion
We find that the mice carrying the mutation on the NR2B tyrosine phosphorylation site (YF/YF mice) show impaired fear learning and amygdaloid LTP. We also demonstrate that the YF/YF mice show improper localization of NMDARs at synapses. Our present findings strongly argue that proper localization of NMDARs at synapses depends on Tyr-1472 phosphorylation of NR2B, and that the proper localization may be essential for synaptic plasticity and fear learning.
We provide in vivo evidence that Tyr-1472 phosphorylation site is required for fear learning (Figure 2). YF/YF mice showed impaired fear learning 1 h after conditioning, suggesting that Tyr-1472 phosphorylation is essential to acquire the CS–US association during auditory fear conditioning. This observation is consistent with the report that intra-amygdala blockade of NR2B disrupts the acquisition of fear conditioning (Rodrigues et al, 2001). YF/YF mice have normal sensitivity to footshock and normal context-dependent freezing (data not shown), suggesting that the impaired fear-learning in YF/YF mice cannot be explained by impaired pain perception or impaired expression of freezing behavior. YF/YF mice normally respond to the tone in an acoustic startle response test (Supplementary Figure 2), suggesting that YF/YF mice have normal auditory sense. Furthermore, YF/YF mice show impaired visual cue/footshock association (Supplementary Figure 1), confirming that YF/YF mice show impaired fear learning. We find that the lack of Tyr-1472 phosphorylation leads to impaired LTP of the thalamo-amygdala pathway that transmits CS information to the LA (Figure 3). It is well accepted that the amygdaloid NMDAR is critical for the expression of conditioned fear responses and that the LTP of the thalamo-amygdala pathway is essential for auditory fear conditioning in mice (Gewirtz and Davis, 1997; Lee and Kim, 1998; for a review, see LeDoux, 2000; Fendt, 2001; Rodrigues et al, 2001; Bauer et al, 2002). Thus, we speculate amygdala is involved in the impaired fear learning of YF/YF mice but further study is needed to prove this.
Our present findings strongly suggest that basic properties of NMDAR channels are absolutely normal in YF/YF mice, indicating that the impaired LTP in YF/YF mice is due to the deficits in the signal downstream from NMDAR activation. Given that the components of the NMDAR complex show spatially restricted localization in the PSD (Kornau et al, 1997; Husi et al, 2000; Scannevin and Huganir, 2000; Sheng and Kim, 2002), it is possible that the improperly localized Y1472FNR2B in the peripheral and perisynaptic regions cannot organize the proper NMDAR signaling complex. We have found that NMDAR-associated CaMKII is dramatically reduced and that glutamate-induced upregulation of CaMKII was severely impaired in YF/YF mice (Figure 5). Because CaMKII is an essential protein for the induction of NMDAR-dependent LTP in the amygdala and the interaction between NR2B and CaMKII is required for synaptic plasticity (Lisman et al, 2002; Rodrigues et al, 2004; Barria and Malinow, 2005), the impaired LTP in YF/YF mice is likely to be caused by the impaired NMDAR-mediated CaMKII signaling in YF/YF mice.
We did not detect any major abnormalities in hippocampal synaptic plasticity and hippocampus-dependent learning using several standard protocols (data not shown). It is possible that NR2A, one of the members of NR2 family, has overlapping functions in the hippocampus, but not in the amygdala. Indeed, NR2A knockout mice show impairments in a hippocampus-dependent contextual fear-conditioning task, while these mice are normal in the performance of amygdala-dependent auditory fear conditioning (Kiyama et al, 1998). Alternatively, in the hippocampus, some compensatory event might have occurred to cover the hippocampal phenotypes in the YF/YF mice. Under specific conditions, we may detect abnormalities in hippocampal functions in YF/YF mice. In addition, Tyr-1472 phosphorylation may regulate synaptic functions in other brain areas such as nucleus accumbens and striatum.
Our data clearly demonstrate that localization of the NR1 subunit, an essential subunit for NMDARs, is determined by the NR2B subunit (Figure 7). In contrast to NR2B, the distribution and total number of NR2A at synapses is unaltered in YF/YF mice. These results indicate that the NR2B subunit is present at mature synapses and required for proper allocation of the heteromeric NMDAR complex at central synapses in vivo, although it has been reported that the NR2B protein is gradually replaced by the NR2A protein during LA development (Lopez de Armentia and Sah, 2003). In the adult amygdala, the NR1/NR2B complex, but not the NR1/NR2A or NR1/NR2A/NR2B complex, may be the major NMDAR complex and contribute to amygdaloid functions more closely than the other complex, and thus it is possible that Tyr-1472 phosphorylation of the NR2B subunit exerts more influence on synaptic plasticity in the amygdala than in the hippocampus.
We provide in vivo evidence suggesting that the Tyr-1472 phosphorylation site is relevant to proper localization of NMDARs at synapses and to regulation of the interaction of NMDARs with α-actinin2 and the AP-2 complex (Supplementary Figure 5). In WT/WT mice, NMDARs are located centrally at synapses (Figures 6 and 7) and a small portion of NR2B moves laterally at synapses in the basal state (Racca et al, 2000; Tovar and Westbrook, 2002). Our present results suggest that the Tyr-1472-phosphorylated NR2B is tightly associated with α-actinin2 to anchor NMDARs to the subsynaptic actin cytoskeleton. Such a link would be necessary for the central localization of NMDARs at synapses. On dephosphorylation at Tyr-1472, NR2B subunits would no longer be associated with postsynaptic actin filaments, allowing NMDARs to move laterally at synapses. Given that the surface NMDARs undergo endocytosis at the endocytotic zone (perisynaptic region) by interacting with the AP-2 complex in response to external stimuli (Roche et al, 2001; Vissel et al, 2001; Blanpied et al, 2002; Petralia et al, 2003; Racz et al, 2004), some portion of Tyr-1472-dephosphorylated NR2B can enter the perisynaptic region to be endocytosed. Replacing endocytosed NMDARs, newly assembled NMDARs are delivered to the synapses (Carroll and Zukin, 2002). In contrast, Y1472FNR2B in YF/YF mice interacts inefficiently with both α-actinin2 and the AP-2 complex (Supplementary Figure 5), leading to the scattered localization of Y1472FNR2B at synapses and to accumulation of Y1472FNR2B in the perisynaptic regions where WT NR2B would normally be endocytosed (Blanpied et al, 2002; Petralia et al, 2003; Racz et al, 2004; Figure 6). Although it remains unclear how Tyr-1472 phosphorylation regulates these interactions, Tyr-1472 phosphorylation has at least two modes of binding, one in which phosphorylation of Tyr-1472 allows binding to α-actinin2, and the other in which dephosphorylation of Tyr-1472 allows binding to the AP-2 complex. Because the AP-2 complex interacts directly with its substrate via tyrosine residues (Ohno et al, 1995), Tyr-1472 phosphorylation would negatively regulate direct interaction of the NR2B subunit with the AP-2 complex (Lavezzari et al, 2003). α-Actinin2 may also interact with Tyr-1472-phosphorylated NR2B directly. Alternatively, some scaffolding protein may exist to link NR2B and α-actinin2.
A recent study suggests that extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways (Hardingham et al, 2002; Vanhoutte and Bading, 2003). Although Y1472F NR2B containing NMDARs show improper localization at synapses (Figures 6 and 7), they are still associated with PSD as shown in the EM analysis. Furthermore, glutamate-induced CREB phosphorylation and cell death were normal in YF/YF mice (Figure 5; Supplementary Figure 7). Thus, these results strongly suggest that most Y1472FNR2B-containing NMDARs are in the synaptic and perisynaptic region, but not at the extrasynaptic region.
While a considerable number of NMDARs are in the perisynaptic regions in YF/YF mice (Figures 6 and 7), we observe no change in the NMDAR-mediated responses (Figure 4). Given that the NMDAR has a higher affinity for glutamate (Kullmann and Asztely, 1998), despite the relatively long distance from the presynaptic terminals to the perisynaptic Y1472FNR2B, improperly localized Y1472FNR2B may be able to sense almost the same amount of glutamate as WT NR2B. Thus, the basic properties of NMDAR may be unaltered in YF/YF mice.
Previously, we have found that the level of Tyr-1472 phosphorylation increases in parallel with brain development (Nakazawa et al, 2001). Recent reports have also shown that the level of tyrosine phosphorylation of the NR2B subunit increases after novel taste learning or the induction of hippocampal LTP (Salter and Kalia, 2004). Therefore, Tyr-1472 phosphorylation plays significant roles at relatively mature synapses. Indeed, YF/YF mice appear healthy in general behaviors, whereas mice lacking NR2B subunits or those expressing the C-terminal-truncated NR2B subunit die soon after their birth, suggesting that the tyrosine phosphorylation itself is not essential for early development (Mori et al, 1998; Sprengel et al, 1998). In addition, Tyr-1472 phosphorylation is implicated not only in learning and memory but also in ischemia and ethanol sensitivity (Salter and Kalia, 2004). Therefore, the knockin mice will also be useful for the analysis of the role of NMDARs on other diverse brain functions.
Recently, we have reported that the level of Tyr-1472 phosphorylation in fyn-deficient mice is much less than that in WT mice (Nakazawa et al, 2001). Consistent with our present data, fyn-deficient mice show impaired amygdala-dependent behaviors (Miyakawa et al, 1994). Interestingly, the transgenic mice, in which Fyn is overexpressed and the level of tyrosine phosphorylation of the NR2B subunit is elevated, also show impaired auditory fear learning (Kojima et al, 2005). Thus, proper regulation of Tyr-1472 phosphorylation may be critical for amygdala-dependent fear learning. We propose that the activity-dependent phosphorylation of the Tyr-1472 is a molecular switch that regulates fear-related memory formation.
Materials and methods
Generation of YF/YF mice
See Supplementary data for methods describing the generation YF/YF mice.
Antibodies
Polyclonal antibodies against phospho-Tyr-1472, NR1, NR2B, NR2A and PSD-95 were described previously (Watanabe et al, 1998; Nakazawa et al, 2001; Fukaya et al, 2003). The commercially available antibodies used in this study are described in Supplementary data.
Preparation of lysates, immunoprecipitation and immunoblotting
Preparation of lysates from amygdaloid slices and whole telencephalons, immunoprecipitation and immunoblotting were performed as described previously (Nakazawa et al, 2001, 2003). For quantification, the immunoreacted protein bands were analyzed with the NIH image software.
Auditory fear conditioning
Fear conditioning was conducted in a small conditioning chamber surrounded by a sound-attenuating chest (CL-M3, O'Hara & Co., Ltd, Tokyo, Japan). Mice were placed in the conditioning chamber for 170 s and then presented with a tone of 65 dB/10 kHz for 10 s through a speaker on the ceiling of the sound-attenuating chamber. At the end of the tone presentation, the mice were given a footshock (2 s/0.35 mA). Freezing responses were monitored for 1 min after the footshock, and the mice were then returned to their home cages. On the test day, the mice were placed in a novel chamber with contexts different from those of the conditioning chamber to minimize freezing caused by contextual fear conditioning. Freezing was scored for 3 min before delivery of the tone and then for 3 min in the presence of the tone. Freezing responses were analyzed on a Macintosh computer with Image FZC 2.22sr2 (O'Hara & Co., Ltd), the software based on the NIH Image program.
Electrophysiology
Whole-cell recordings were performed as described previously (Manabe et al, 2000). Coronal slices including the LA (400-μm thickness) were prepared from 4- to 6-week-old mice and placed in an interface-type holding chamber for at least 1 h. A slice was then transferred to the recording chamber and submerged beneath continuously perfusing artificial cerebrospinal fluid (ACSF) consisting of (in mM): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1.0 NaH2PO4, 26.2 NaH2CO3, 11 glucose, which had been saturated with 95% O2 and 5% CO2. All the perfusing solutions contained 100 μM picrotoxin (Wako Pure Chemical Industries, Osaka, Japan) to block GABAA receptor-mediated inhibitory synaptic responses. The dorsal region of the cortex was cut off to prevent the invasion of epileptiform activity. A whole-cell pipette was placed in the LA. For current-clamp recordings, the whole-cell pipette was filled with the internal solution containing (in mM): 135 potassium methanesulfonate, 8 NaCl, 10 HEPES, 2 Mg-ATP, and 0.3 Na3-GTP (pH 7.3; 290–300 mOsm). For voltage-clamp recordings, the internal solution contained (in mM): 122.5 cesium gluconate, 17.5 CsCl, 10 HEPES, 0.2 EGTA, 8 NaCl, 2 Mg-ATP and 0.3 Na3-GTP (pH 7.2; 290–300 mOsm). For current-clamp experiments, cells were clamped at −75 mV with DC current injection. To stimulate the presynaptic thalamic fibers, a tungsten bipolar electrode was placed in the ventral striatum just medial to the LA. The test stimuli were applied to the thalamic pathway at 0.1 Hz, and the stimulus strength was adjusted to evoke excitatory postsynaptic potentials (EPSPs) with amplitudes of 3–5 mV. To induce LTP, trains of 10 stimuli at 30 Hz were paired with depolarization (1 nA/8 ms) given 5 ms after the onset of each EPSP in the train. This pairing was given 15 times at 10-s intervals. For voltage-clamp experiments, AMPA receptor-mediated EPSCs were recorded at −80 mV, and NMDA receptor-mediated EPSCs were recorded at +40 mV with the same stimulus strength in the presence of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX: Tocris, Avonmouth, UK). MEPSCs were recorded in the presence of 1 μM tetrodotoxin (Sankyo, Tokyo, Japan) to block action potentials. Cells were clamped at −80 mV in the presence of Mg2+ (1.3 mM) to measure the AMPAR-mediated component, and then the external solution was switched to the nominally Mg2+-free solution. After washing out external Mg2+ (typically around 30 min), mEPSCs composed of both D-APV-sensitive and -insensitive components were observed. D-APV (50 μM: Tocris, Avonmouth, UK) was then applied to block the NMDAR-mediated component. MEPSCs were analyzed using the Mini Analysis (6.0.3) software. For recording AMPAR-mediated mEPSCs, the detection threshold was set at 10 pA, while it was increased to 21–25 pA for recording simultaneously both AMPAR-mediated and NMDAR-mediated mEPSCs partly because the background noise level was increased in the absence of Mg2+. Axoclamp 2B, Axopatch 1D or MultiClamp 700A amplifier, and Digidata 1320A or Digidata 1322A (Axon Instruments, Union City, CA) were used to record and store the data with the pClamp software (Axon Instruments, Union City, CA), respectively. The signal was filtered at 2–6 kHz and digitized at 10 kHz. All experiments were performed at 25–28°C. All data are presented as the mean±s.e.m.
Histology
Immunohistochemical analyses were performed as described previously (Fukaya et al, 2003).
Pharmacological treatment of brain slices
Coronal slices including the LA (400-μm thickness) were prepared from 4-to 6-week-old mice and placed in an interface-type holding chamber for at least 3 h. Slices were preincubated in ACSF with 1 μM TTX (Wako) and 1 μM okadaic acid (Calbiochem, SanDiego, CA) for 1 h and then stimulated with 10 μM glutamate and 10 μM glycine for 7 min. Preparations of cell lysates were described above.
Immunogold electron microscopy
Postembedding immunogold analysis was performed as described previously (Fukaya et al, 2003). To carry out a semiquantitative distribution analysis of the NR2A, NR2B or NR1 subunit across synapses, synapse cross-section lengths were measured, and any shorter than 150 nm were excluded to avoid cross-sections lying too far off-center. Immunogold particles at remaining axo-spinous asymmetrical synapses in the LA were counted by taking the central 50% of the total PSD cross-section length as the ‘central' region, and the two ends, each 25% of the total PSD cross-section length, as the ‘peripheral' region. An additional 20% length outside the PSD on each side was defined as the ‘perisynaptic' region.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank Dr I Saito for the recombinant adenovirus producing Cre recombinase. We also thank Drs J Fujimoto, S Kida, J Inoue, M Mishina, H Ohno, S Okabe, K Semba and Y Yamanashi for valuable discussion. TN was supported in part by Japan Society for the Promotion of Science fellowships for Japanese Junior Scientists. This research was supported in part by grants-in-aid for scientific research (TY, AMW and TM), by Center for Brain Medical Science, 21st Century COE Program (FA and TM), by Special Coordination Funds for Promoting Science and Technology (TM) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, by Uehara Memorial Foundation (TM), by Terumo Life Science Foundation (TM), by Sumitomo Foundation (TM), by Naito Foundation (TM), by Takeda Science Foundation (TM) and by RISTEX, Japan Science and Technology Agency (JST) (TM).
References
- Barria A, Malinow R (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301 [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE (2002) NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci 22: 5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Koninck PD, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805 [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36: 435–449 [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS (2002) NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci 25: 571–577 [DOI] [PubMed] [Google Scholar]
- Chapman PF, Ramsay MF, Krezel W, Knevett SG (2003) Synaptic plasticity in the amygdala: comparisons with hippocampus. Ann NY Acad Sci 985: 114–124 [DOI] [PubMed] [Google Scholar]
- Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11: 465–469 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TVP (1995) Memories of NMDA receptors and LTP. Trends Neurosci 18: 54–56 [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M (2001) NMDA receptor subunits: diversity, development, and disease. Curr Opin Neurobiol 11: 327–335 [DOI] [PubMed] [Google Scholar]
- Davis M (1997) Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci 9: 382–402 [DOI] [PubMed] [Google Scholar]
- Fendt M (2001) Injections of the NMDA receptor antagonist Aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. J. Neurosci 21: 4111–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23: 743–760 [DOI] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M (2003) Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci USA 100: 4855–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M (1997) Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature 388: 471–474 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDA receptors by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5: 405–414 [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SGN (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3: 661–669 [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M (1998) Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ɛ1 subunit. J Neurosci 18: 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhr G, Seeburg PH (1996) Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol 492: 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Sakamoto T, Endo S, Niki H (2005) Impairment of conditioned freezing to tone, but not to context, in Fyn-transgenic mice: relationship to NMDA receptor subunit 2B function. Eur J Neurosci 21: 1359–1369 [DOI] [PubMed] [Google Scholar]
- Kornau HC, Seeburg PH, Kennedy MB (1997) Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol 11: 327–335 [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40: 775–784 [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F (1998) Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci 21: 8–14 [DOI] [PubMed] [Google Scholar]
- Lavezzari G, Mccallum J, Lee R, Roche KW (2003) Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology 45: 729–737 [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184 [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ (1998) Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci 18: 8444–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Rev Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P (2003) Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci 23: 6876–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M (2000) Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci 20: 2504–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (1999) Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci 22: 561–567 [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV (1990) Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev 15: 41–70 [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Watanabe S, Niki H (1994) Increased fearfulness of Fyn tyrosine kinase deficient mice. Brain Res Mol Brain Res 27: 179–182 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540 [DOI] [PubMed] [Google Scholar]
- Moon IL, Apperson ML, Kennedy MB (1994) The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 91: 3954–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Manabe T, Watanabe M, Satoh Y, Suzuki N, Toki S, Nakamura K, Yagi T, Kushiya E, Takahashi T, Inoue Y, Sakimura K, Mishina M (1998) Role of the carboxy-terminal region of the GluRɛ2 subunit in synaptic localization of the NMDA receptor channel. Neuron 21: 571–580 [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRɛ2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276: 693–699 [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Watabe AM, Tezuka T, Yoshida Y, Yokoyama K, Umemori H, Inoue A, Okabe S, Manabe T, Yamamoto T (2003) p250GAP, a novel brain-enriched GTPase-activating protein for Rho family GTPases, is involved in the N-methyl-D-aspartate receptor signaling. Mol Biol Cell 14: 2921–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS (1995) Interaction of tyrosine-based sorting signal with clathrin-associated proteins. Science 269: 1872–1875 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Wenthold RJ (2003) Internalization at glutamatergic synapses during development. Eur J Neurosci 18: 3207–3217 [DOI] [PubMed] [Google Scholar]
- Racca C, Stephenson FN, Streit P, Roberts JDB, Somogyi P (2000) NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci 20: 2512–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ (2004) Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci 7: 917–918 [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Ly CD, Ehlers MD, Wenthold RJ (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802 [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE (2001) Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci 21: 6889–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE (2004) Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44: 75–91 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez DA, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834 [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5: 317–328 [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL (2000) Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci 1: 133–141 [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ (2002) Postsynaptic signaling and plasticity mechanisms. Science 298: 776–780 [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH (1998) Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92: 279–289 [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL (2002) Mobile NMDA receptors at hippocampal synapses. Neuron 34: 255–264 [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H (2003) Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signaling and BDNF gene regulation. Curr Opin Neurobiol 13: 366–371 [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL (2001) A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci 4: 587–596 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y (1998) Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fiber-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 10: 478–487 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE (1999) L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci 19: 10512–10519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS (2003) Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 43: 335–358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1


