Abstract
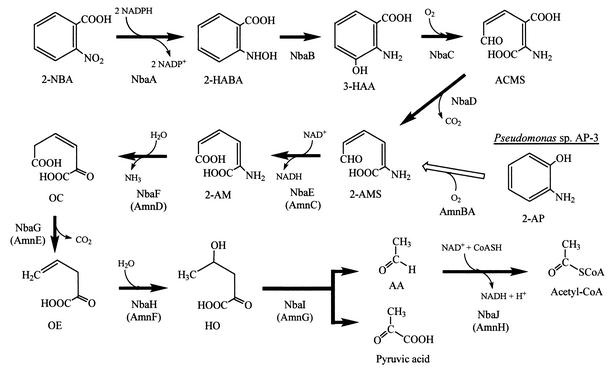
The 2-nitrobenzoic acid degradation pathway of Pseudomonas fluorescens strain KU-7 proceeds via a novel 3-hydroxyanthranilate intermediate. In this study, we cloned and sequenced a 19-kb DNA locus of strain KU-7 that encompasses the 3-hydroxyanthranilate meta-cleavage pathway genes. The gene cluster, designated nbaEXHJIGFCDR, is organized tightly and in the same direction. The nbaC and nbaD gene products were found to be novel homologs of the eukaryotic 3-hydroxyanthranilate 3,4-dioxygenase and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase, respectively. The NbaC enzyme carries out the oxidation of 3-hydroxyanthranilate to 2-amino-3-carboxymuconate-6-semialdehyde, while the NbaD enzyme catalyzes the decarboxylation of the latter compound to 2-aminomuconate-6-semialdehyde. The NbaC and NbaD proteins were overexpressed in Escherichia coli and characterized. The substrate specificity of the 23.8-kDa NbaC protein was found to be restricted to 3-hydroxyanthranilate. In E. coli, this enzyme oxidizes 3-hydroxyanthranilate with a specific activity of 8 U/mg of protein. Site-directed mutagenesis experiments revealed the essential role of two conserved histidine residues (His52 and His96) in the NbaC sequence. The NbaC activity is also dependent on the presence of Fe2+ but is inhibited by other metal ions, such as Zn2+, Cu2+, and Cd2+. The NbaD protein was overproduced as a 38.7-kDa protein, and its specific activity towards 2-amino-3-carboxymuconate-6-semialdehyde was 195 U/mg of protein. Further processing of 2-aminomuconate-6-semialdehyde to pyruvic acid and acetyl coenzyme A was predicted to proceed via the activities of NbaE, NbaF, NbaG, NbaH, NbaI, and NbaJ. The predicted amino acid sequences of these proteins are highly homologous to those of the corresponding proteins involved in the metabolism of 2-aminophenol (e.g., AmnCDEFGH in Pseudomonas sp. strain AP-3). The NbaR-encoding gene is predicted to have a regulatory function of the LysR family type. The function of the product of the small open reading frame, NbaX, like the homologous sequences in the nitrobenzene or 2-aminophenol metabolic pathway, remains elusive.
There is a continuing need to isolate new microorganisms from the environment, not only because we want to enrich the little that we know about microbial diversity or community structure (41) but also because new microorganisms may have interesting new metabolic or biocatalytic properties. As this study illustrated, a soil pseudomonad can continue to amaze us because of its metabolic potential and can be a source for discovering new biocatalysts. Furthermore, the strain described here provides new insight into the possible evolutionary process of a catabolic pathway.
Pseudomonas fluorescens strain KU-7 was isolated from a petrochemical-contaminated site and is able to utilize 2-nitrobenzoic acid (2-NBA) as a sole source of carbon, nitrogen, and energy (17). A novel feature of the 2-NBA degradation pathway of strain KU-7 is the formation of 3-hydroxyanthranilate (3-HAA) as an intermediate (Fig. 1). Until now, 3-HAA was only known to be an intermediate in the kynurenine pathway for the degradation of tryptophan and the biosynthesis of nicotinic acid in yeast and mammalian systems (23). In Arthrobacter protophormiae strain RKJ100, there is an alternative route of 2-NBA metabolism (9). Although the initial reaction is also reductive, the conversion of 2-NBA to 2-hydroxylaminobenzoate was found to proceed via the formation of 2-aminobenzoate, which is also known as anthranilate. Subsequently, anthranilic acid is believed to go through deamination and β-ketoadipic acid formation before entering the Krebs cycle (9).
FIG. 1.
Proposed pathway for degradation of 2-NBA by P. fluorescens strain KU-7. The responsible Nba enzymes are as follows: NbaA, 2-NBA nitroreductase; NbaB, 2-hydroxylaminobenzoate mutase; NbaC, HAO; NbaD, ACMSD; NbaE, 2-AMS dehydrogenase; NbaF, 2-aminomuconate deaminase; NbaG, 4-oxalocrotonate decarboxylase; NbaH, 2-oxopent-4-dienoate hydratase; NbaI, 4-hydroxy-2-oxovalerate aldolase; and NbaJ, acylating aldehyde dehydrogenase. For comparison, the 2-AP degradation pathway and enzymes of Pseudomonas sp. strain AP-3 are shown (37). AmnBA, 2-aminophenol 1,6-dioxygenase. Abbreviations: 2-HABA, 2-hydroxylaminobenzoate; 2-AM, 2-aminomuconate; OC, 4-oxalocrotonate; OE, 2-oxopent-4-dienoate; HO, 4-hydroxy-2-oxovalerate; AA, acetaldehyde; CoA, coenzyme A.
To gain a better understanding of the metabolism of 2-NBA in bacteria, in this study we performed the first analysis of a genetic locus of strain KU-7 that is responsible for the conversion of 3-HAA to Krebs cycle intermediates. We concentrated on the characterization of two novel encoding genes, nbaC and nbaD, and the evolution of the new pathway is discussed below in relation to convergence or divergence of the 2-aminophenol (2-AP) and nitrobenzene metabolic pathways in other bacteria.
(A portion of this work was presented at the American Society for Microbiology Conference on Biodegradation, Biotransformation, and Biocatalysis (B3), San Juan, Puerto Rico, 2 to 4 October, 2001 [for a review see reference 29].)
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Pseudomonas strains were grown in Luria-Bertani (LB) broth, MY medium (17), or MS medium (MY medium without the yeast extract). Escherichia coli strains were routinely cultured in LB media. Cultures were incubated at 30°C for the Pseudomonas strains and at 37°C for the E. coli strains. Antibiotics were added at the following final concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and trimethoprim 200 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| BL21 | F−ompT hsdSB (rB− mB−) gal dcm | Novagen |
| DH5α | supE44 thi-1 recA1 hsdR17 endA1 gyrA (Nalr) Δ(lacIZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | 32 |
| S17-1 | recA pro thi hsdR RP4-2-Tc::Mu-Km::Tn7 Tra+ Tpr Smr | 34 |
| XL1-blue | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 [F′ lacIq ZM15 Tn10 (Tetr)] | 6 |
| P. fluorescens strains | ||
| KU-7 | Wild type, grows on 2-NBA and 4-hydroxybenzoate | 17 |
| KUM-1 | KU-7, nbaJ::Tn5-31Tp | This study |
| KUM-2 | KU-7, nbaR::Tn5-31Tp | This study |
| KUM-3 | KU-7, nbaD::Tn5-31Tp | This study |
| KUM-4 | KU-7, nbaF::Tn5-31Tp | This study |
| Plasmids | ||
| pHAO | EcoRI*-PstI* fragment containing nbaC in pSD80 | This study |
| pHAO-H52A | His52 (NbaC) is replaced by Ala by site-directed mutagenesis in pHAO | This study |
| pHAO-H93A | His93 (NbaC) is replaced by Ala by site-directed mutagenesis in pHAO | This study |
| pHAO-H96A | His96 (NbaC) is replaced by Ala by site-directed mutagenesis in pHAO | This study |
| pACMS1 | EcoRI*-PstI* fragment containing nbaD1 (the first potential ATG codon) in pSD80 | This study |
| pACMS2 | EcoRI*-PstI* fragment containing nbaD2 (the second potential ATG codon) in pSD80 | This study |
| pKN31 | Kmr Tpr; mob RP4 delivery plasmid for Tn5-31Tp | 1 |
| pNBA1 | Apr Tpr; pUC19 with 6.1-kb EcoRI fragment from P. fluorescens strain KUM-1 | This study |
| pNBA2 | Apr Kmr; pUC19 with 6.1-kb BamH1 fragment from P. fluorescens strain KUM-1 | This study |
| pNBA3 | Apr; pUC19 with 9.3-kb HindIII fragment (1-9312) from P. fluorescens strain KU-7 | This study |
| pNBA4 | Apr; pUC19 with 8.4-kb HindIII fragment (10705-19065) from P. fluorescens strain KU-7 | This study |
| pSD80 | Apr; expression vector with tac promoter | 35 |
| pUC19 | Apr; high-copy-number cloning vector | 44 |
The numbers in parentheses are the positions in the sequence deposited in the databases (DDBJ/GenBank/EMBL accession no. AB088043). EcoRI* and PstI* are restriction endonucleases introduced by PCR design.
Transposon mutagenesis and screening for 2-NBA-negative mutant strains.
E. coli strain S17-1 carrying the Tn5-31Tp transposon on pKN31 (Table 1) was grown overnight with shaking at 37°C in LB medium containing kanamycin. Recipient strain KU-7 was grown at 30°C in LB medium. Donor and recipient cultures were mixed at different ratios and filtered onto a 0.45-μm-pore-size membrane (Nihon, Millipore Ltd., Tokyo, Japan). The membrane was placed with the bacterium side up on an LB medium plate. After overnight incubation at 30°C, the bacteria were resuspended in 10 ml of 0.85% NaCl and plated (200 μl/plate) on MS medium containing 0.2% 4-hydroxybenzoate, a growth substrate utilized by strain KU-7 but not by E. coli S17-1. Kanamycin was used as a selective marker for the transposon. Transconjugants of strain KU-7 were identified following incubation at 30°C for 2 days. Mutants that were not able to grow on 2-NBA were identified by replica plating on MS medium containing 0.2% 2-NBA and kanamycin. The production of ammonia, detected colorimetrically by the method of Fawcett and Scott (14), was used as a marker for the deamination step after ring cleavage of 3-HAA (Fig. 1).
DNA manipulations.
Unless otherwise stated, standard methods were used for DNA manipulations (32). Total DNA was prepared by the method of Wilson (43), and plasmid DNA was isolated by the method of Birnboim and Doly (4). Restriction endonucleases and T4 DNA ligase were obtained from New England Biolabs (Beverly, Mass). Purified DNA fragments were labeled with digoxigenin (DIG)-11-dUTP by using a DIG DNA labeling kit (Roche Diagnostics K.K., Tokyo, Japan). For Southern blot experiments, DNA was transferred onto a positively charged nylon membrane (Hybond-N; Amersham Biosciences Corp., Piscataway, N.J.), and the DIG labels were visualized by using a DIG luminescent detection kit (Roche Diagnostics K.K.) according to the supplier's instructions.
DNA sequencing and sequence analysis methods.
The nucleotide sequence was determined with an Applied Biosystems automated sequencer (model 310) by using a BigDye terminator cycle sequencing FS Ready Reaction kit (Applied Biosystems Japan Ltd., Tokyo, Japan). Synthetic primers were used for sequencing reactions. DNA sequences were analyzed with GENETYX-Mac software (Software Development Co., Ltd., Tokyo, Japan). Nucleotide and protein sequence similarity searches were done with the BLAST program (2) via the National Center for Biotechnology Information server. Amino acid sequences were analyzed with the Proteomics and Sequence Analysis Tools available at the Swiss Institute of Bioinformatics ExPASy web site. Multiple-protein-sequence alignments were constructed with the CLUSTAL W program (40) at the DNA Data Bank of Japan server.
Expression of nbaC and nbaD in E. coli.
The nbaC gene, including its potential Shine-Dalgarno (SD) sequence, was amplified by PCR with the following pairs of primers having built-in EcoRI and PstI restriction sites (underlined sequences): 5′-CGGAATTCAAGCAGTTGCCTACAAACC and 5′-AAAACTGCAGAAGTGCGAGTGCATATC. PCR amplifications were carried out in 50-μl reaction mixtures containing 0.1 μg of template DNA, 50 pmol of each primer, each deoxynucleoside triphosphate at a concentration of 200 μM, 5 μl of Pfu DNA polymerase 10× buffer with MgSO4, and 1.25 U of Pfu DNA polymerase (Promega K.K., Tokyo, Japan). After a 3-min hot start at 94°C, each reaction mixture was subjected to 30 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C. The PCR product was purified from a 1.2% agarose gel, digested with EcoRI and PstI, and ligated into the equivalent sites of pSD80, placing the genes of interest in frame with the tac promoter to form plasmid pHAO (Table 1). The insert was sequenced to ensure that mutations had not been incorporated during the PCR. A 100-ml culture of E. coli DH5α(pHAO) in LB broth containing 100 μg of ampicillin per ml was grown at 30°C to an A660 of 0.4 to 0.5, and then 1.0 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added and growth was continued for a further 3 h prior to harvesting.
For expression of nbaD1 (the first possible ATG start codon of nbaD) in E. coli, the gene was amplified by PCR with forward primer 5′-CGGAATTCATGAAAAAACCGCGGATTGATA and reverse primer 5′-AAAACTGCAGACCATTAAACATTGATATTG (the built-in EcoRI and PstI sites are underlined). The EcoRI site was introduced into the forward primer sequence to optimize the space between the vector SD sequence and the possible ATG codon of nbaD1. For expression of nbaD2 (the second possible ATG start codon of nbaD), the gene was amplified by using forward primer 5′-CGGAATTCATGCACTCGCACTTCTTCC and reverse primer 5′-AAAACTGCAGACCATTAAACATTGATATTG. Again, the EcoRI site was introduced to optimize the space between the vector SD sequence and the possible ATG codon of nbaD2. PCR amplification was carried out as described above for nbaC. The resulting plasmids containing nbaD1 and nbaD2 were designated pACMS1 and pACMS2, respectively. Both inserts were sequenced to ensure that mutations had not been incorporated during the PCR. E. coli BL21 cells harboring pACMS1 or pACMS2 were grown and induced by using the same procedure that was used for E. coli DH5α(pHAO).
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by using the method of Laemmli (24) and a Mini-PROTEAN II electrophoresis cell (Nippon Bio-Rad Laboratories, Tokyo, Japan).
Determination of NH2-terminal amino acid sequences.
The cell extracts derived from E. coli DH5α(pHAO) were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane (MiniProBlott; Applied Biosystems Japan Ltd.) with a Mini Trans-Blot electrophoretic transfer cell (Nippon Bio-Rad Laboratories) as described by the manufacturer. The area on the membrane containing NbaC was cut out and subjected to N-terminal amino acid sequencing with a model PPSQ-21 protein sequencer (Shimadzu Co., Kyoto, Japan).
Preparation of cell extracts.
Cells were harvested by centrifugation and washed twice with 50 mM MOPS (3-morpholinopropanesulfonic acid)-NaOH buffer (pH 6.5) for preparation of the NbaC protein or with 50 mM potassium phosphate buffer (pH 7.0) for preparation of the NbaD protein. They were resuspended in the same buffer at a concentration of approximately 0.2 g (wet weight)/ml. Cells were disrupted by sonication with a model 250 Sonifier (Branson, Danbury, Conn.) by using 40-s bursts on ice, and particulates were removed by centrifugation at 18,000 × g and 4°C for 30 min.
Enzyme activities. (i) HAO activity.
3-Hydroxyanthranilate 3,4-dioxygenase (HAO) activity was measured spectrophotometrically by monitoring the formation of 2-amino-3-carboxymuconate-6-semialdehyde (ACMS) at 360 nm by the method of Walsh et al. (42). The assay mixture (final volume, 1 ml) contained 3-HAA (0.1 μmol), Fe(NH4)2(SO4)2 · 6H2O (0.1 μmol), MOPS buffer (50 μmol, pH 6.5), and an aliquot of cell extract. The assay mixture was preincubated without substrate for 1 min at 25°C, and the reaction was started by adding 3-HAA and monitoring the increase in A360 for 20 s at 25°C. One unit of activity was defined as the amount of enzyme that produced 1 μmol of ACMS per min under the assay conditions. A molar extinction coefficient of 47,500 M−1 cm−1 for ACMS at 360 nm was used (22).
Relative rates for alternate substrates of NbaC were determined by using a Clarke-type oxygen electrode (YSI model 5300 biological oxygen monitor; YSI Inc., Yellow Springs, Ohio). The test compounds used were 3-amino-4-hydroxybenzoic acid, 4-aminoresorcinol, and 2-amino-m-cresol (purchased from Sigma-Aldrich Japan K.K., Tokyo, Japan); 4-amino-3-hydroxybenzoic acid, 3-aminosalicylic acid, 6-amino-m-cresol, 3-methylcatechol, 4-methylcatechol, 1,2,4-trihydroxybenzene, 2,3-dihydroxybenzoic acid, 4-amino-m-cresol, 5-aminosalicylic acid, gentisic acid, and homogentisic acid (obtained from Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan); and 2-AP, 2-amino-4-chlorophenol, 2-amino-p-cresol, catechol, 1,2,3-trihydroxybenzene, protocatechuic acid, and hydroquinone (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Each reaction mixture (3.0 ml) contained MOPS buffer (50 mM; pH 6.5), Fe(NH4)2(SO4)2 · 6H2O (0.1 mM), substrate (0.1 mM), and the appropriate amount of cell extract. The assay was started by adding the substrate, and the reaction mixture was incubated at 30°C. One unit of enzyme activity was defined as the amount of enzyme which catalyzed utilization of 1 μmol of O2 per min under the conditions specified above. The rate of O2 consumption caused by NbaC was calculated by subtracting the value for the control reaction mixture without NbaC.
(ii) ACMSD activity.
2-Amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) activity was determined as described by Nishizuka et al. (28) by measuring the conversion of ACMS to 2-aminomuconate-6-semialdehyde (2-AMS) by using a preassay mixture that consisted of 25 μM 3-HAA, 0.1 mM Fe(NH4)2(SO4)2 · 6H2O, and a solution of NbaC (39 mU/ml) prepared as described above in 50 mM MOPS buffer (pH 6.5). The reaction mixture was incubated at 25°C, and the increase in A360 due to the formation of ACMS from 3-HAA was monitored. After the reaction was completed, a solution of NbaD extract was added, and the decrease in A360 was monitored at 20-s intervals. One unit of ACMSD activity was defined as the amount of enzyme that converted 1 μmol of ACMS per min under the assay conditions. For calculation of activity, a molar extinction coefficient of 47,500 M−1 cm−1 for ACMS at 360 nm was used (22). The rate of the decrease in absorbance caused by NbaD was calculated by subtracting the value for the control reaction mixture without NbaD.
Protein concentrations were determined by the Bradford assay (5) with bovine serum albumin (Pierce, Rockford, Ill.) as the protein standard.
Site-directed mutagenesis.
PCR overlap extension mutagenesis (19) was used to generate nbaC mutants (Table 1). The following mutagenic primers were used (the base changes are underlined): for H52A, 5′-TCGCACAGATTTTGCGGATGATCCGATGGA and 5′-TCCATCGGATCATCCGCAAAATCTGTGCGA; for H93A, 5′-TTCTTCTGCCACCGGCGTTGCGCCACTCGC and 5′-GCGAGTGGCGCAACGCCGGTGGCAGAAGAA; and for H96A, 5′-CACCGCATTTGCGCGCGTCGCCACAACGAC and 5′-GTCGTTGTGGCGACGCGCGCAAATGCGGTG. The insert in pSD80 was sequenced to ensure that base changes had been introduced correctly.
Nucleotide sequence accession number.
The DNA sequence encompassing the nba EXHJIGFCDR gene cluster (8.7 kb) and additional open reading frames (ORFs) (data not shown) on either side of the nba cluster (total, 19,065 bp) has been deposited in the DDBJ database under accession number AB088043.
RESULTS AND DISCUSSION
Isolation of 2-NBA-negative mutant strains.
To provide a handle on the 2-NBA catabolic pathway gene locus, transposon mutagenesis was carried out to generate mutant derivatives of wild-type strain KU-7 that are unable to grow on 2-NBA as a sole carbon source. As a result, screening of approximately 3,000 transconjugants produced four candidates, designated strains KUM-1, -2, -3, and -4 (Table 1). Strain KUM-1 was analyzed first and was found to accumulate ammonia in the culture fluid (about two-thirds of the available nitrogen), indicating that the insertion of the transposon had inactivated the gene or genes involved after the deamination step. Subsequent DNA sequencing indicated that the nbaJ ORF had been disrupted (Fig. 1). The remaining three mutations were located in the predicted nbaD, nbaF, and nbaR ORFs as described below.
Cloning of the Tn5-31Tp disrupted regions.
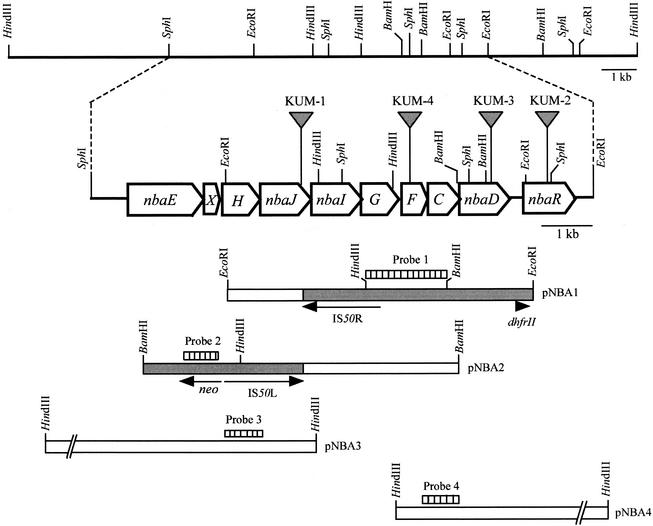
Insertion of the Tn5-31Tp transposon into strain KUM-1 was verified by Southern blot analysis of the total DNA digested with EcoRI and BamHI. As a result, only one hybridization band (a 6.1-kb EcoRI fragment and a 6.1-kb BamHI fragment), which was probed with a DIG-11-dUTP-labeled DNA probe (1.8-kb HindIII-BamHI probe 1 or PCR-amplified probe 2 [Fig. 2 ]), was observed in each digest, indicating that a single insertion event had occurred (data not shown).
FIG. 2.
Physical map and genetic organization of the P. fluorescens strain KU-7 nba gene cluster. The large open arrows indicate the unidirectional arrangement of the genes or ORFs described in Table 2. The following gene pairs have overlapping stop and potential start codons: nbaJ and nbaI (8-bp overlap); nbaI and nbaG (4-bp overlap); nbaF and nbaC (1-bp overlap); and nbaC and nbaD (4-bp overlap). Otherwise, there is a short intergenic space, as follows: nbaE and nbaX (11-bp space); nbaX and nbaH (10-bp space); nbaH and nbaJ (6-bp space); nbaG and nbaF (21-bp space); and nbaD and nbaR (284-bp space). A potential promoter consensus sequence (−10 sequence, TAAAAT; −35 sequence, TTGAAT or TTTGCT) is predicted immediately upstream (35 bp) of the start codon of nbaE. Following nbaD, two inverted repeat sequences may act as transcriptional terminators of a potential operon. The gray triangles indicate the sites of transposon insertion (nbaJ codon 286, nbaR codons 181 and 182, nbaD codon 228, and between codons 80 and 81 of nbaF in mutant strains KUM-1, KUM-2, KUM-3, and KUM-4, respectively). The open boxes in pNBA1 and pNBA2 were derived from KUM-1 DNA; pNBA3 and pNBA4 were derived from KU-7 DNA. The grey region indicates the Tn5-31Tp DNA. Probes 1, 2, 3, and 4 were used in the hybridization experiments. dhfrII and neo are trimethoprim resistance- and kanamycin resistance-encoding genes, respectively.
Cloning of the 6.1-kb EcoRI fragment or the 6.1-kb BamHI fragment was carried out by using a pUC19 plasmid transformed in E. coli XL1-blue. In the former case, transformants were selected on trimethoprim- and ampicillin-containing plates. In the latter case, kanamycin and ampicillin were used as the selection markers. The resulting plasmids were designated pNBA1 and pNBA2, respectively. The pNBA1 insert contained a 4.5-kb Tn5-31Tp segment and 1.6 kb of KUM-1 DNA. Plasmid pNBA2 was found to contain a 3-kb Tn5-31Tp insert and 3.1 kb of KUM-1 DNA (Fig. 2). Further cloning of the DNA upstream of the pNBA1 insert resulted in plasmid pNBA3, which contained a 9.3-kb HindIII fragment. Cloning of DNA downstream of the pNBA2 insert resulted in plasmid pNBA4, which contained a 8.4-kb HindIII fragment (Fig. 2).
Identification of the gene disrupted by the transposon and structural analysis of the nba genes.
We sequenced the Tn5-31Tp flanking regions in pNBA1 and pNBA2 with two oligonucleotides derived from IS50R and IS50L of the transposon, respectively (Fig. 2). Data analysis of the initial sequences revealed that the point of transposon disruption was in a locus, subsequently designated nbaJ, that had a high level of sequence similarity to the loci encoding the acylating acetaldehyde dehydrogenases in the database.
Further nucleotide sequencing and analyses of the four overlapping clones revealed 10 complete ORFs, which we designated nbaEXHJIGFCDR, all of which are oriented in the same direction and are organized in a tight manner. Either the intergenic spaces are short or the stop and potential start codons overlap, which is indicative of translational coupling (16) and an operon structure (Fig. 2).
Table 2 shows the location of each ORF or gene and the predicted molecular masses of the products, along with details for proteins in the National Center for Biotechnology Information nonredundant databases exhibiting significant similarity to the predicted gene products. Below we describe the novel characteristics of the nba gene cluster in the order of the predicted biochemical reaction (Fig. 1).
TABLE 2.
Predicted functions of the proteins encoded by the genes located in the nba gene cluster
| Gene | Nucleotide positionsa | Predicted function | Deduced molecular mass (Da) | Homology
|
|||
|---|---|---|---|---|---|---|---|
| % Amino acid identity | Protein(s) | Source | Accession no. | ||||
| nbaE | 5394-6896 | 2-Aminomuconate 6-semi- aldehyde dehydrogenase | 53,678 (498)b | 59 | DmpC | Pseudomonas sp. strain CF600 | X52805 |
| 57 | AmnC | Pseudomonas sp. strain AP-3 | AB020521 | ||||
| nbaX | 6908-7300 | Unknown | 13,839 (130) | 50 | ORF1 (YjgF-like protein) | P. pseudoalcaligenes JS45 | AF036343 |
| 50 | NbzI (putative ferredoxin) | P. putida HS12 | AF319593 | ||||
| nbaH | 7311-8102 | 2-Oxopent-4-dienoate hydratase | 27,928 (263) | 61 | AmnF | Pseudomonas sp. strain AP-3 | AB020521 |
| 60 | NbzG | P. putida HS12 | AF319593 | ||||
| nbaJ | 8109-9071 | Acetaldehyde dehydrog- enase | 33,394 (320) | 71 | NbzI | P. putida HS12 | AF319593 |
| 68 | AmnH | Pseudomonas sp. strain AP-3 | AB020521 | ||||
| 68 | CarF | Pseudomonas sp. strain CA10 | AB047548 | ||||
| nbaI | 9064-10092 | 4-Hydroxy-2-oxovalerate aldolase | 36,715 (342) | 82 | MhpE | Comamonas testosteroni TA441 | AB024335 |
| 81 | TobH | P. putida PB4071 | AF180147 | ||||
| nbaG | 10089-10844 | 4-Oxalocrotonate decarboxylase | 27,011 (251) | 50 | NbzF | P. putida HS12 | AF319593 |
| 49 | XylI | Sphingomona aromaticivorans F199 | AF079317 | ||||
| nbaF | 10866-11297 | 2-Aminomuconate deaminase | 15,549 (143) | 72 | AmnD | Pseudomonas sp. strain AP-3 | AB020521 |
| 70 | NbzE | P. putida HS12 | AF319593 | ||||
| 70 | AmnD | P. pseudoalcallgenes JS45 | P81593 | ||||
| nbaC | 11297-11854 | HAO | 21,245 (185) | 37 | Possible HAO | Caenorhabditis elegans | Z70751 and Z70755 |
| 35 | HADI | Saccharomyces cerevisiae | Z49525 | ||||
| 33 | HAO | Rattus norvegicus | D44494 | ||||
| nbaD | 11851-12855 | ACMSD | 37,140 (334) | 40 | ACMSD | Rattus norvegicus | AB069781 |
| 40 | ACMSD | Homo sapiens | AB071418 | ||||
| nbaR | 13140-14099 | Regulatory protein | 35,117 (319) | 31 | Probable transcriptional regulatory protein | P. aeruginosa PAO1 | AE004647 |
| 31 | FldY | Xanthomonas campestris ATCC 33913 | AE012524 | ||||
The numbers are the positions in sequence deposited in the databases (DDBI/GenBank/EMBL accession no. AB088043).
The values in parentheses are the numbers of amino acid residues.
Identification of NbaC as HAO and its overexpression in E. coli.
The deduced amino acid sequence of NbaC does not show any similarity to the amino acid sequences of the widely known extradiol dioxygenases and aminophenol dioxygenases, but instead it exhibits 33 to 37% identity to the amino acid sequences of the HAOs (EC 1.13.11.6), all of which have eukaryotic origins (Table 2). Among the human liver, rat, mouse, sponge, worm, and yeast proteins (20, 23, 26, 27, 39), only the human and yeast proteins have been characterized genetically and biochemically. The human HAO is a 286-amino-acid enzyme that is involved in the synthesis of quinolinic acid from 3-HAA, an intermediate in the kynurenine pathway of tryptophan metabolism (26). On the other hand, the yeast HAO (Had1) is shorter; it is 177 amino acids long, which is similar to the length of the 185-amino-acid NbaC protein. A multiple-sequence alignment of these sequences revealed that the N terminus for the first 120 amino acids was more conserved than the C terminus, although the sequences of the mouse (accession no. AK002608), rat (D44494), human (Z29481), sponge (AJ298053), and worm (Z70751 and Z70755) proteins are more closely related to each other (86 to 93% identity among the mouse, rat, and human sequences; 38 to 48% identity between the sponge and worm sequences).
The DNA sequence predicts that there are two consecutive ATG codons that are possible nbaC start codons. As a result of overexpression of the nbaC gene in pHAO, we established that the first 20 amino acids of NbaC are MFTFGKPLNFQRWLDDHSDL, a sequence that confirms the DNA prediction and the notion that nbaC is translated from the first ATG. By using SDS-PAGE, the molecular mass of NbaC was estimated to be 23.8 kDa, which is in good agreement with the predicted molecular mass (21,245 Da) (Fig. 3, lane 2).
FIG. 3.
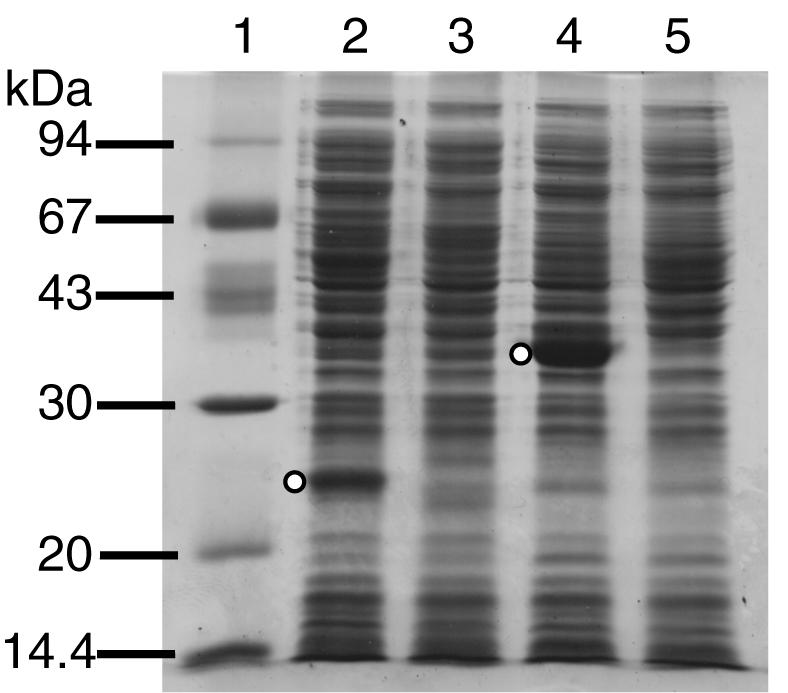
Expression of the nbaC and nbaD gene products of P. fluorescens strain KU-7 by recombinant E. coli strains by using Ptac induction. An SDS-12% PAGE gel was used and stained with Brilliant blue R (Sigma). The open circles in lanes 2 and 4 indicate the positions of the overexpressed NbaC and NbaD proteins, which had estimated molecular masses of 23.8 and 38.7 kDa, respectively. Lane 1, LMW marker (Amersham Biosciences); lane 2, cell extracts of E. coli DH5α(pHAO); lane 4, cell extracts of E. coli BL21(pACMS1); lanes 3 and 5, controls consisting of E. coli DH5α and BL21 cells containing only the pSD80 vector.
Substrate specificity of NbaC.
The specific activity of NbaC toward 3-HAA was found to be 7.9 U/mg of protein. No activity was observed with the extract from E. coli DH5α carrying pSD80. We next examined the substrate specificity of NbaC using a variety of aminophenol and catechol analogs as described in Materials and Methods. Briefly, NbaC was found to be specific for the substrate 3-HAA, which gave an oxygen consumption rate of 10.9 U/mg, whereas all other activities were not detectable.
Requirement of histidine residues 52 and 96 and ferrous iron for NbaC activity.
A large number of meta-ring cleavage dioxygenases have been characterized, and these enzymes have conserved histidine residues that function as ferrous iron ligands for catalytic activity (12, 36). An alignment of the NbaC sequence with the sequences of eukaryotic HAOs revealed that two histidine residues (His52 and His96 in NbaC) are conserved in all sequences (data not shown). To test whether these residues are required for activity, we generated nbaC mutants that resulted in His-to-Ala substitutions (H52A and H96A). In addition, we carried out mutagenesis of His93 since this residue is adjacent to His96. As a result, even though the amounts of the desired proteins produced were equivalent to the amount of unmodified NbaC produced (data not shown), both mutations resulted in no detectable NbaC activity. On the other hand, HAO with the H93A substitution was found to exhibit 24.8% of the activity of the native HAO, indicating that His93 is not as critical as His96 or His52.
We also tested the effects of chelating agents, chemical modification reagents, and metal ions on the HAO activity (Table 3). The activities of NbaC were completely eliminated by the presence of 2,2′-dipyridyl and 1,10-phenanthroline, which are Fe2+ chelators. This indicated that NbaC is dependent upon Fe2+ for catalytic activity. Diethyl pyrocarbonate, which has previously been shown to modify histidine residues of catechol dioxygenases (36), also inhibited the HAO activity (Table 3). Complete inactivation by dithionitrobenzoic acid, a cysteine-directed reagent, indicated that there is a cysteine residue at or near the active site(s) of the enzyme.
TABLE 3.
Effects of chelating agents, chemical modification reagents, and metal ions on NbaCa
| Reagent added | Concn (mM) | Remaining activity (%) |
|---|---|---|
| None | 100 | |
| Fe2+ chelators | ||
| 2,2′-Dipyridyl | 1.0 | 0 |
| 1,10-Phenanthroline | 1.0 | 0 |
| Nonspecific chelator EDTA | 1.0 | 1 |
| Cysteine-directed reagents | ||
| DTNBb | 1.0 | 0 |
| Sodium iodoacetate | 1.0 | 58 |
| Histidine-directed reagent diethyl pyrocarbonate | 1.0 | 30 |
| Carboxyl-directed reagent EDCc | 1.0 | 74 |
| Metal ions | ||
| Mo2+ | 1.0 | 85 |
| Mg2+ | 1.0 | 98 |
| Ca2+ | 1.0 | 105 |
| Fe3+ | 0.1 | 18 |
| Ni2+ | 1.0 | 18 |
| Co2+ | 1.0 | 8 |
| Mn2+ | 1.0 | 54 |
| Cu2+ | 0.1 | 0 |
| Zn2+ | 0.1 | 1 |
| Cd2+ | 0.1 | <1 |
| Pb2+ | 1.0 | 76 |
Each reagent was added to the assay mixture after catalytic activity was initiated by the introduction of Fe2+, and it was preincubated for 30 min at 25°C prior to the assay.
DTNB, dithionitrobenzoic acid.
EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide methiodide.
The effects of metal ions on HAO activity were investigated by introducing the metals into the assay mixture following addition of the divalent iron cofactor, which initiated catalytic activity. Table 3 shows that complete or nearly complete inhibition of HAO activity was caused by the presence of 0.1 mM Cu2+, 0.1 mM Zn2+, 0.1 mM Cd2+, or 0.1 mM Fe3+. At a concentration of 1 mM, Ni2+ and Co2+ were also quite inhibitory, although Ca2+, Mg2+, Mo2+, and Pb2+ had little or no effect. Presumably, some of these metals can bind to the active site or block the access of iron to the active site.
Inhibition of human HAO by Zn2+ and Cd2+ has been reported recently, and the former effect was interpreted to indicate the possible physiological relevance of zinc in the metabolism of human cerebral cells (8).
Identification of NbaD as ACMSD and its expression in E. coli.
Like the NbaC sequence, the sequence of NbaD has no prokaryotic counterpart in the available databases except for a few eukaryotic homologs. The deduced amino acid sequence of NbaD exhibits 31 to 40% overall identity to the rat, mouse, and human ACMSD (EC 4.1.1.45) sequences (15, 38). Caenorhabditis elegans has two ACMSD sequences; one (Y71D11A.3b; accession no. AC006816) consists of 401 amino acids, while the other (Y71D11A.3a; accession no. AC006816) has 352 amino acid residues, a size that is more consistent with the sizes of the other sequences (304 to 336 amino acids). We confirmed the finding of Fukuoka et al. (15) that no typical consensus sequences for nucleotide, metal, or any other cofactor binding sites were detectable with motif search programs. However, a multiple-sequence alignment of these sequences with the NbaD sequence revealed the following two conserved peptides that are at least 6 amino acids long: VHPWDM (amino acids 176 to 181 [NbaD numbering]) and LG(S/T)DYPFPLGE (amino acids 291 to 301). These peptides may be regarded as signature peptides for this group of proteins.
There are two potential ATG start codons for nbaD, both of which are preceded by a consensus ribosome binding sequence (GGAGA for nbaD1 and GGA for nbaD2). The two methionines are 6 amino acids apart. To examine which of the two methionines could be translated into protein, PCR-amplified nbaD was expressed in E. coli in two possible constructs, pACMS1 and pACMS2, as described in Materials and Methods. The cellular protein extracts were analyzed by SDS-PAGE. As a result, only pACMS1 yielded a polypeptide (Mr, 38,700) of the expected size (Fig. 3, lane 4), indicating that the first methionine is the start codon.
The cellular extract of E. coli BL21(pACMS1) gave an ACMSD activity of 194.9 U/mg of protein. For comparison, the specific activities of ACMSD prepared from crude rat liver and kidney homogenate were 1.8 and 4.6 mU/mg, respectively (38).
In the course of transposon tagging experiments, nbaD::Tn5-31Tp (strain KUM-3) was obtained. Sequencing revealed that the transposon was inserted at codon 228 of nbaD (data not shown). This indicated that ACMS was not utilized because of the absence of a functional NbaD in the KUM-3 cells.
2-AMS is a convergent point in nitrobenzene metabolism, 2-AP metabolism, and 3-HAA metabolism.
2-AMS is emerging as a common intermediate compound in the nitrobenzene pathway in Pseudomonas putida HS12 (30) and Pseudomonas pseudoalcaligenes JS45 (18), the 2-AP pathway in Pseudomonas sp. strain AP-3 (37), and the 2-NBA pathway in P. fluorescens strain KU-7 (17) (Fig. 1). This is reminiscent of the formation of 2-hydroxymuconate semialdehyde as a result of meta-cleavage of catechols (e.g., from the metabolism of xylenes, phenol, and related aromatic hydrocarbons) (21).
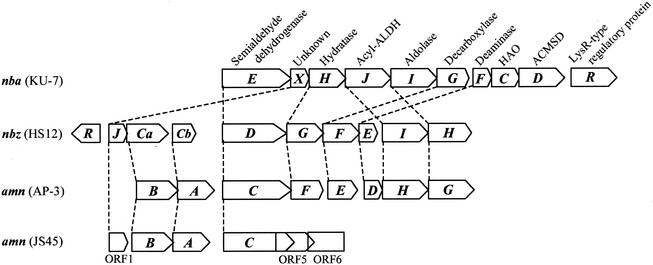
In a previous study a particular route for the 2-NBA degradation pathway in strain KU-7 beyond the ACMS formation step was not established (17). However, based on the extensive homology between the predicted gene products of nbaEFGHIJ and a familiar set of homologs in the databases (Table 2) and the inherent sequence motifs (data not shown), one could predict that further metabolism of 2-AMS in strain KU-7 proceeds in the same manner as the metabolism encoded by the nitrobenzene catabolic genes (nbz) and the 2-AP meta-cleavage genes (nbzDEFGHI) in P. putida HS12 (30). In Pseudomonas sp. strain AP-3, the corresponding genes of the 2-AP pathway are amnCDEFGH (37).
Elusive gene X and a potential regulatory gene, nbaR.
The small ORF between nbaE and nbaH was designated nbaX. The predicted amino acid sequence has 50% identity with the amino acid sequence of a YjgF-like protein (ORF1) from Pseudomonas pseudoalcaligenes JS45 and with the amino acid sequence of NbzJ from P. putida HS12 (Table 2 and Fig. 4). Kim et al. (30) proposed that the latter compound is a possible ferredoxin. We were unable to confirm this by motif searches. Instead, the sequence of NbaX (residues 103 to 121) was found to match the consensus sequence pattern of the YjgF-like family protein (Prosite accession no. PS01094; pfam01042) (3, 13). Hence, the possible function of NbaX and its counterparts in the nitrobenzene or aminophenol pathways remains unclear.
FIG. 4.
Comparison of the nba gene cluster of strain KU-7 with the nitrobenzene degradative genes (nbz) of P. putida HS12 (30) and the 2-AP degradative genes (amn) of Pseudomonas sp. strain AP-3 (37) and P. pseudoalcaligenes JS45 (11). Homologous genes are connected by dashed lines. Gene designations: nbaE, nbzD, and amnC, 2-aminomuconate 6-semialdehyde dehydrogenase genes; nbaX and ORF1, YjgF-like protein genes; nbzJ, putative ferredoxin gene; nbaH, nbzG, and amnF, 2-oxopent-4-dienoate hydratase genes; nbaJ, nbzI, and amnH, acetaldehyde dehydrogenase genes; nbaI, nbzH, and amnG, 4-hydroxy-2-oxovalerate aldolase genes; nbaG, nbzF, and amnE, 4-oxalocrotonate decarboxylase genes; nbaF, nbzE, and amnD, 2-aminomuconate deaminase genes; nbaC, HAO gene; nbaD, ACMSD gene; nbaR, LysR-type transcriptional regulatory gene; nbzR, MarR-type transcriptional regulatory gene; nbzCa and amnB, genes encoding the β-subunit of 2-aminophenol 1,6-dioxygenase; nbzCb, and amnA, genes encoding the α-subunit of 2-aminophenol 1,6-dioxygenase; ORF5 and ORF6 (incomplete ORF), genes similar to the genes encoding the ATP-dependent RNA helicases.
One of the transposon mutant strains generated in this study was KUM-2 (Table 1). Like the other mutant strains, KUM-2 was deficient in terms of utilizing 2-NBA as a sole source of carbon and energy. By using DNA sequencing, the site of transposon insertion was found to be located between codons 181 and 182 of an ORF designated nbaR (Fig. 2). The predicted amino acid sequence of NbaR indicates that the molecular mass is 35,117 Da (319 amino acids), which is similar to the molecular masses of the LysR-type transcriptional regulators (33). Moreover, the N-terminal NbaR sequence (residues 31 to 61) possesses sequence features of the LysR-type transcriptional regulator helix-turn-helix motif (Prosite accession no. PS00044). In addition, it is possible to locate the consensus binding motif of LysR-type regulators (viz., the T-N11-A sequence) upstream of the regulated promoter. Specifically, this sequence (TCTGGTATT-N9-AATAACTGA [inverted repeat sequences are underlined]) is located 78 bp upstream of the possible ATG start of nbaE, presumably the first gene of the nba operon (Fig. 2). Because of the numerous characteristics that have been determined and the transposon mutagenesis data, NbaR is expected to be a bona fide positive regulator of the nba pathway. A detailed study of NbaR regulation was outside the scope of this study.
Concluding remarks.
A molecular view of 2-NBA metabolism in bacteria is emerging for the first time. Although the initial reductase- and 2-hydroxylaminobenzoate mutase-encoding genes in the 2-NBA pathway of strain KU-7 have not been characterized yet (they were not found in the 19-kb region sequenced), this study nonetheless filled a void in our knowledge of microbial degradation of mononitrobenzoates or nitroaromatics in general (for a review see reference 45). Besides the two known routes of 2-NBA metabolism, one via the formation of anthranilic acid in A. protophormiae (9) and the other via the formation of 3-HAA (this study), it has been suggested that 2-NBA degradation in Nocardia could occur through an oxidative route via catechol formation, following an initial release of nitrite from 2-NBA (7). However, there has been no confirmatory data for this putative Cain pathway. Nevertheless, it is clear that bacteria can evolve multiple ways of degrading 2-NBA, just as there are at least five ways in which aerobic bacteria can metabolize toluene. Importantly, different gene families may be involved in the regulation of these pathways (25). In this study, we propose a LysR-type transcriptional regulation for 2-NBA metabolism in strain KU-7. The lack of molecular data for the anthranilate pathway of A. protophormiae prevents a comparative analysis at this time. However, given the convergence of 2-AP metabolism as shown by the formation of the common 2-AMS intermediate (Fig. 1), it is worth noting that it has been proposed that the regulatory unit for 2-AP metabolism in P. putida HS12 (nbzR) was derived from the MarR family of regulators (30).
The genes of the nitrobenzene and 2-AP pathways provide valuable insight into both convergence and divergence pertaining to the intermediate steps (Fig. 1). The steps in the AP-3 and HS12 strains are rather similar (Fig. 4). In terms of pathway convergence, one step is at the level of 2-AMS formation, the result of decarboxylation of ACMS by ACMSD (NbaD) in strain KU-7 and dioxygenation of 2-AP by 2-aminophenol 1,6-dioxygenase in strain AP-3 (37). In the case of nitrobenzene degradation in strain JS45, 2-AP is formed after the action of a nitrobenzene nitroreductase and a hydroxylaminobenzene mutase(s). Although the genes encoding the mutases in strain JS45 have been cloned, other genes are not yet available (10). A second level of convergence is at the formation of 2-oxopent-4-dienoic acid or its isomer, 2-hydroxypenta-2,4-dienoate. Indeed, degradation of these isomers is known to proceed by means of a rather universal and common set of enzymes, including a hydratase, an aldolase, and an acylating dehydrogenase that are present in the pathways for degradation of aromatic hydrocarbons in general (31).
It has been proposed that the 2-AP operon of P. putida HS12 has evolved in a modular fashion involving transfer and fusion of regulatory and structural units of meta-cleavage genes (30). A similar mode of gene acquisition may be responsible for the nature of the nba gene organization in strain KU-7.
Once again, a soil pseudomonad has been found to display diversity in its catabolic potential and to provide a unique link to tryptophan metabolism in eukaryotes insofar as HAO- and ACMSD-encoding genes are concerned. These enzymes have been studied because they may have relevance to the progression of Huntington's disease and related diseases according to the so-called quinolinate hypothesis (15). Briefly, the connection is as follows. In mammalian cells, quinolinate is a potent endogenous excitotoxin of neuronal cells, and it can be nonenzymatically derived from ACMS. As in bacterial 2-NBA metabolism, ACMS is generated from 3-HAA via the action of HAO. The HAO activity in the brains of Huntington's disease patients has been found to be elevated and to be 3.5-fold higher than the activity in normal controls, suggesting that there is an association between the formation of quinolinate and the disease. Furthermore, ACMSD has been postulated to play an important role in preventing progression of the disease by diverting ACMS to a nonbenign metabolite, which is 2-AMS (15). The possible contribution of NbaD as a prokaryotic enzyme supplement is exciting.
Acknowledgments
We thank A. Nakazawa for providing pKN31 and A. Keane for carefully reading the manuscript.
This research was financially supported by the Kansai University Grant-in-Aid for the Faculty Joint Research Program, 2002.
Footnotes
This paper is issued as NRCC number 45897.
REFERENCES
- 1.Abe, M., M. Tsuda, M. Kimito, S. Inouye, A. Nakazawa, and T. Nakazawa. 1996. A genetic analysis system of Burkholderia cepacia: construction of mobilizable transposons and a cloning vector. Gene 174:191-194. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Bullock, W. O., J. M. Fernandez, and J. M. Stuart. 1987. XL-1 Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 7.Cain, R. B., and N. J. Cartwright. 1960. Intermediary metabolism of nitrobenzoic acids by bacteria. Nature 185:868-869. [Google Scholar]
- 8.Calderone, V., M. Trabucco, V. Menin, A. Negro, and G. Zanotti. 2002. Cloning of human 3-hydroxyanthranilic acid dioxygenase in Escherichia coli: characterisation of the purified enzyme and its in vitro inhibition by Zn2+. Biochim. Biophys. Acta 1596:283-292. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan, A., and R. K. Jain. 2000. Degradation of o-nitrobenzoate via anthranilic acid (o-aminobenzoate) by Arthrobacter protophormiae: a plasmid-encoded new pathway. Biochem. Biophys. Res. Commun. 267:236-244. [DOI] [PubMed] [Google Scholar]
- 10.Davis, J. K., G. C. Paoli, Z. He, L. J. Nadeau, C. C. Somerville, and J. C. Spain. 2000. Sequence analysis and initial characterization of two isozymes of hydroxylaminobenzene mutase from Pseudomonas pseudoalcaligenes JS45. Appl. Environ. Microbiol. 66:2965-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, J. K., Z. He, C. C. Somerville, and J. C. Spain. 1999. Genetic and biochemical comparison of 2-aminophenol 1,6-dioxygenase of Pseudomonas pseudoalcaligenes JS45 to meta-cleavage dioxygenases: divergent evolution of 2-aminophenol meta-cleavage pathway. Arch. Microbiol. 172:330-339. [DOI] [PubMed] [Google Scholar]
- 12.Eltis, L. D., and J. T. Bolin. 1996. Evolutionary relationships among extradiol dioxygenases. J. Bacteriol. 178:5930-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falquet, L., M. Pagni, P. Bucher, N. Hulo, C. J. A. Sigrist, K. Hofmann, and A. Bairoch. 2002. The PROSITE database, its status in 2002. Nucleic Acids Res. 30:235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawcett, J. K., and J. E. Scott. 1960. A rapid and precise method for the determination of urea. J. Clin. Pathol. 13:156-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuoka, S., K. Ishiguro, K. Yanagihara, A. Tanabe, Y. Egashira, H. Sanada, and K. Shibata. 2002. Identification and expression of a cDNA encoding human α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase (ACMSD). J. Biol. Chem. 277:35162-35167. [DOI] [PubMed] [Google Scholar]
- 16.Gold, L. 1988. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu. Rev. Biochem. 57:199-233. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa, Y., T. Muraki, T. Tokuyama, H. Iwaki, M. Tatsuno, and P. C. K. Lau. 2000. A novel degradative pathway of 2-nitrobenzoate via 3-hydroxyanthranilate in Pseudomonas fluorescens strain KU-7. FEMS Microbiol. Lett. 190:185-190. [DOI] [PubMed] [Google Scholar]
- 18.He, Z., J. K. Davis, and J. C. Spain. 1998. Purification, characterization, and sequence analysis of 2-aminomuconic 6-semialdehyde dehydrogenase from Pseudomonas pseudoalcaligenes JS45. J. Bacteriol. 180:4591-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton, R. M. 1995. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol. Biotechnol. 3:93-99. [DOI] [PubMed] [Google Scholar]
- 20.Kawai, J., A. Shinagawa, K. Shibata, M. Yoshino, M. Itoh, Y. Ishii, T. Arakawa, A. Hara, Y. Fukunishi, H. Konno, J. Adachi, S. Fukuda, K. Aizawa, M. Izawa, K. Nishi, H. Kiyosawa, S. Kondo, I. Yamanaka, T. Saito, Y. Okazaki, T. Gojobori, H. Bono, T. Kasukawa, R. Saito, K. Kadota, H. Matsuda, M. Ashburner, S. Batalov, T. Casavant, W. Fleischmann, T. Gaasterland, C. Gissi, B. King, H. Kochiwa, P. Kuehl, S. Lewis, Y. Matsuo, I. Nikaido, G. Pesole, J. Quackenbush, L. M. Schriml, F. Staubli, R. Suzuki, M. Tomita, L. Wagner, T. Washio, K. Sakai, T. Okido, M. Furuno, H. Aono, R. Baldarelli, G. Barsh, J. Blake, D. Boffelli, N. Bojunga, P. Carninci, M. F. de Bonaldo, M. J. Brownstein, C. Bult, C. Fletcher, M. Fujita, M. Gariboldi, S. Gustincich, D. Hill, M. Hofmann, D. A. Hume, M. Kamiya, N. H. Lee, P. Lyons, L. Marchionni, J. Mashima, J. Mazzarelli, P. Mombaerts, P. Nordone, B. Ring, M. Ringwald, I. Rodriguez, N. Sakamoto, H. Sasaki, K. Sato, C. Schönbach, T. Seya, Y. Shibata, K.-F. Storch, H. Suzuki, K. Toyo-oka, K. H. Wang, C. Weitz, C. Whittaker, L. Wilming, A. Wynshaw-Boris, K. Yoshida, Y. Hasegawa, H. Kawaji, S. Kohtsuki, and Y. Hayashizaki. 2001. Functional annotation of a full-length mouse cDNA collection. Nature 409:685-690. [DOI] [PubMed]
- 21.Kim, S., H.-J. Shin, Y. Kim, S. J. Kim, and Y.-C. Kim. 1997. Nucleotide sequence of the Pseudomonas sp. DJ77 phnG gene encoding 2-hydroxymuconic semialdehyde dehydrogenase. Biochem. Biophys. Res. Commun. 240:41-45. [DOI] [PubMed] [Google Scholar]
- 22.Koontz, W. A., and R. Shiman. 1976. Beef kidney 3-hydroxyanthranilic acid oxygenase. J. Biol. Chem. 251:368-377. [PubMed] [Google Scholar]
- 23.Kucharczyk, R., M. Zagulski, J. Rytka, and C. J. Herbert. 1998. The yeast gene YJR025c encodes a 3-hydroxyanthranilic acid dioxygenase and is involved in nicotinic acid biosynthesis. FEBS Lett. 424:127-130. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lau, P. C. K., Y. Wang, A. Patel, D. Labbé, H. Bergeron, R. Brousseau, Y. Konishi, and M. Rawlings. 1997. A bacterial basic region leucine zipper histidine kinase regulating toluene degradation. Proc. Natl. Acad. Sci. USA 94:1453-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malherbe, P., C. Köhler, M. D. Prada, G. Lang, V. Kiefer, R. Schwarcz, H.-W. Lahm, and A. M. Cesura. 1994. Molecular cloning and functional expression of human 3-hydroxyanthranilic-acid dioxygenase. J. Biol. Chem. 269:13792-13797. [PubMed] [Google Scholar]
- 27.Nakagawa, Y., H. Asai, H. Mori, J. Kitoh, and K. Nakano. 1995. Increase in the level of mRNA for 3-hydroxyanthranilate 3,4-dioxygenase in brain of epilepsy-prone El mice. Biosci. Biotechnol. Biochem. 59:2191-2192. [DOI] [PubMed] [Google Scholar]
- 28.Nishizuka, Y., A. Ichiyama, and O. Hayaishi. 1970. Metabolism of the benzene ring of tryptophan. II. Picolinic carboxylase (cat liver) (α-amino-β-carboxymuconic-ɛ-semialdehyde β-decarboxylase). Methods Enzymol. 17:471-476. [Google Scholar]
- 29.Parales, R. E., N. C. Bruce, A. Schmid, and L. P. Wackett. 2002. Biodegradation, biotransformation, and biocatalysis (B3). Appl. Environ. Microbiol. 68:4699-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, H.-S., and H.-S. Kim. 2001. Genetic and structural organization of the aminophenol catabolic operon and its implication for evolutionary process. J. Bacteriol. 183:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt, A., V. Shingler, S. C. Taylor, and P. A. Williams. 1995. The 4-hydroxy-2-oxovalerate aldolase and acetaldehyde dehydrogenase (acylating) encoded by the nahM and nahO genes of the naphthalene catabolic plasmid pWW60-22 provide further evidence of conservation of meta-cleavage pathway gene sequences. Microbiology 141:2223-2233. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 34.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Smith, S. P., K. R. Barber, S. D. Dunn, and G. S. Shaw. 1996. Structural influence of cation binding to recombinant human brain S100b: evidence for calcium-induced exposure of a hydrophobic surface. Biochemistry 35:8805-8814. [DOI] [PubMed] [Google Scholar]
- 36.Spence, E. L., M. Kawamukai, J. Sanvoisin, H. Braven, and T. D. H. Bugg. 1996. Catechol dioxygenases from Escherichia coli (MhpB) and Alcaligenes eutrophus (MpcI): sequence analysis and biochemical properties of a third family of extradiol dioxygenases. J. Bacteriol. 178:5249-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takenaka, S., S. Murakami, Y.-J. Kim, and K. Aoki. 2000. Complete nucleotide sequence and functional analysis of the genes for 2-aminophenol metabolism from Pseudomonas sp. AP-3. Arch. Microbiol. 174:265-272. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe, A., Y. Egashira, S. Fukuoka, K. Shibata, and H. Sanada. 2002. Purification and molecular cloning of rat 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase. Biochem. J. 361:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The C. elegans sequencing consortium. 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282:2012-2018. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torsvik, V., L. Ovreas, and T. F. Thingstad. 2002. Prokaryotic diversity—magnitude, dynamics, and controlling factors. Science 296:1064-1066. [DOI] [PubMed] [Google Scholar]
- 42.Walsh, J. L., W. P. Todd, B. K. Carpenter, and R. Schwarcz. 1991. 4-Halo-3-hydroxyanthranilic acids: potent competitive inhibitors of 3-hydroxyanthranilic acid oxygenase in vitro. Biochem. Pharmacol. 42:985-990. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. A. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 44.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 45.Zylstra, G. J., S.-W. Bang, L. M. Newman, and L. L. Perry. 2000. Microbial degradation of mononitrophenols and mononitrobenzoates, p. 145-160. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. Lewis Publishers, Boca Raton, Fla.