Abstract
Recent studies have shown that phagosome maturation depends on the balance between pro-inflammatory and anti-inflammatory cytokines, indicating that cytokine modulates phagosome maturation. However, the mechanism of cytokine-mediated modulation of intracellular trafficking remains to be elucidated. Here, we have shown that treatment of macrophages with IL-6 specifically induce the expression of Rab5 through the activation of extracellular signal-regulated kinase, whereas IL-12 exclusively upregulate the expression of Rab7 through the activation of p38 MAPK. We have cloned the 5′-flanking regions of the rab5c or rab7 into the promoterless reporter vector. Our results have shown that cells transfected with rab5c chimera are transactivated by IL-6, and IL-12 specifically transactivates cells containing rab7 chimera. Moreover, our results also show that IL-12 induces lysosomal transport, whereas IL-6 stimulates the fusion between early compartments in macrophages and accordingly modulates Salmonella trafficking and survival in macrophages. This is the first demonstration showing that cytokine differentially regulates endocytic trafficking by controlling the expression of appropriate Rab GTPase, and provides insight into the mechanism of cytokine-mediated regulation of intracellular trafficking.
Keywords: cytokines, endocytosis, gene regulation, Rab GTPase, trafficking
Introduction
Several intracellular pathogens have developed strategies to interfere with normal cell signaling to disable the host defense mechanisms (Vieira et al, 2002; Rosenberger and Finlay, 2003; Alonso and Garcia-del Protillo, 2004). In addition, recent reports have shown that the phagosome maturation is modulated through signaling of Toll-like receptors (TLR) (Beutler, 2004; Underhill and Gantner, 2004). However, downstream effect of these signaling processes on phagosome maturation is not well characterized. The fact that TNF-α (Underhill et al, 1999) and IFN-γ significantly modify the phagosome maturation (Siren et al, 2005) suggests a link between regulations of membrane trafficking with cytokine. Moreover, survival of intracellular pathogens within macrophages depends on the balance between pro-inflammatory cytokines and anti-inflammatory cytokines (Hornef et al, 2002; Stuart and Ezekowitz, 2005). For instance, infection with Mycobacterium tuberculosis, Salmonella typhimurium, Legionella pneumophilia and Yersinia pestis has been shown to induce IL-10 production and suppress the production of IL-12 and TNF-α (Redpath et al, 2001), whereas intracellular pathogens like Leishmania major, Toxoplasma gondii and Listeria monocytogenes directly impair IL-12 production (Mosser and Karp, 1999; Hornef et al, 2002) in the infected cells. Consequent studies have shown that treatment with IL-12 and IFN-γ kills intracellular pathogens (Greenberger et al, 1996; de Jong et al, 1998), whereas IL-6 promotes the growth of similar pathogens (Dube et al, 2004) in the host. These results suggest that these cytokines possibly modulate distinct steps in the endocytic pathway and thereby regulate phagosome maturation. However, how cytokine modulates intracellular trafficking is not yet known.
Interestingly, our recent studies have shown that intracellular delivery of muramyl di-peptide to macrophages predominantly enhances the secretion of IL-6 and IL-12 (Srividya et al, 2000) and modulates the intracellular content of endocytic Rab GTPase (Mukherjee et al, 2002). In addition, treatment of macrophages with IFN-γ is shown to increase the cellular content of Rab5 (Alvarez-Dominguez and Stahl, 1998), but the mechanism of induction is not known. Taken together, it is tempting to speculate a correlation between cytokine-mediated modulations of Rab GTPases, which regulate intracellular trafficking (Zerial and McBride, 2001). Here, we have shown that IL-6 and IL-12 specifically induce the expression of endocytic Rab GTPases like Rab5 and Rab7, respectively, via the activation of distinct signaling pathways and thereby modulate intracellular trafficking.
Results
IL-6 and IL-12 regulate the expression of Rab5 and Rab7
To determine the role of IL-6 and IL-12 in the expression of endocytic Rab GTPases like Rab5 and Rab7, J774E cells were treated with different concentrations of IL-6 or IL-12, and the cellular contents of Rab5 and Rab7 in treated and untreated cells were determined by Western blot analysis using specific antibodies (Figure 1A and B). About three-fold increase in the cellular content of Rab5 or Rab7 was observed at 0.5 μg/ml of IL-6 or IL-12 treatment, respectively (lower panels of Figure 1A and B). Quantification of the results demonstrated that IL-6 and IL-12 upregulated the expression of Rab5 and Rab7, respectively, in a concentration-dependent manner (lower panels of Figure 1A and B) and maximum increase of the expression of specific Rab was observed at 0.5 μg/ml. Therefore, this concentration was used for subsequent experiments.
Figure 1.
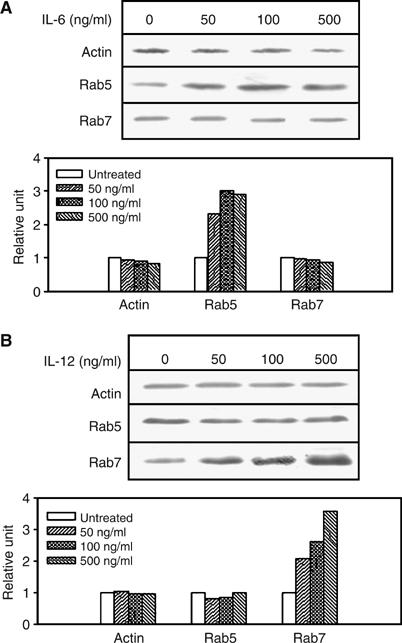
IL-6 and IL-12 induce the expression of Rab5c and Rab7. To determine cytokine-induced expression of Rab GTPase, J774E cells (5 × 106) were treated with the indicated concentrations of IL-6 (A) or IL-12 (B) for 6 h at 37°C. Western blot analyses were carried out to determine the levels of actin, Rab7 and Rab5 in the treated cell lysates (40 μg) using specific antibodies. Proteins were visualized using appropriate HRP-labeled second antibody by ECL. The 0-h time point indicates the level of protein in untreated control. Results from Western blots are representative of three independent preparations. Lower panel of each figure indicates the quantitation of the respective data. Results are represented as the mean of three observations and normalized to untreated control, arbitrarily chosen as one unit.
Specificity of the cytokine-mediated regulation of the expression of Rab GTPase
To determine the specificity of cytokine-mediated regulation of the Rab expression, cellular contents of several Rab proteins were analyzed in the cells treated with the respective cytokines. The Western blot analyses presented in Figure 2A show that IL-6 treatment specifically increased the level of Rab5, but no significant change in the expression of other Rab GTPases, for example, Rab6, Rab4, Rab11 and Rab7, was observed. Quantitation of Rab5 by densitometry showed (lower panel of Figure 2A) that about two-fold more Rab5 is present in IL-6-treated cells after 6 h of treatment (115±3.2 arbitrary units) than in untreated control cells (59±5.4 arbitrary units). In contrast, about 2.5-fold more (123±5.2 arbitrary units) Rab7 was detected in IL-12-treated cells (Figure 2B) than in untreated cells (46±7.2 arbitrary units), whereas the content of Rab6, Rab4, Rab11 and Rab5 levels were unaltered (lower panel of Figure 2B).
Figure 2.

Determination of the specificity of IL-6- and IL-12-mediated expression of Rab5 and Rab7. To determine the kinetics and specificity of cytokine in the regulation of Rab expression, cells were treated with 0.5 μg/ml of IL-6 (A) or IL-12 (B) for the indicated periods of time and Western blot analyses were carried out to determine the levels of actin, Rab4, Rab6, Rab11, Rab7 and Rab5 in the treated cell as described previously. The 0-h time point indicates the level of protein in untreated control. Results from Western blots are representative of three independent preparations. Similarly, to detect the mRNA levels of different Rabs in IL-6- (C) or IL-12- (D) treated cells, total RNA was isolated from the treated macrophages and limiting dilution RT–PCR was performed as described in Materials and methods. The 0-h time point indicated the level of mRNA in untreated control. Lower panel of each figure indicates the quantitation of the respective data. Results are represented as the mean of three observations and normalized to untreated control, arbitrarily chosen as one unit.
Subsequently, mRNA levels of different Rabs were compared in cytokine-treated and untreated cells at the indicated times by limiting dilution RT–PCR using specific primers. The data presented in Figure 2C show that all isoforms of Rab5 mRNA were significantly induced after 1 h treatment with IL-6 and remained higher in the treated cells for up to 6 h. Quantitation of Rab5 mRNAs revealed that 6 h after treatment with IL-6, about three-folds more Rab5c mRNA and less than two-folds more of Rab5a or Rab5b mRNAs were detected than in untreated control (lower panel of Figure 2C). In contrast, IL-12 specifically induced about two-fold more Rab7 mRNA in 6 h post-treatment (Figure 2D). However, the levels of β-actin mRNA as well as other indicated Rabs remained unaltered by either IL-6 or IL-12 treatment (lower panels of Figure 2C and D). Moreover, our results also showed that induced expression of specific Rab by cytokines is not due to the altered stability of the transcript (Supplementary Figure 1A and B).
Role of different kinases in the regulation of Rab expression by cytokines
To understand the mechanism of the activation of Rabs, cells were treated with specific pharmacological inhibitors of various kinases in the signaling pathways (Giri et al, 2003), for example, tyrosine kinase, PI3 kinase, MEK1/2 and p38 MAP kinase, along with the respective cytokines, and the level of Rab-specific mRNA was monitored by RT–PCR. Our results showed that IL-6-induced Rab5c expression is inhibited by PD98059 and U0126 (Figure 3A), inhibitors of MEK1/2, demonstrating that increased expression of Rab5 is regulated by extracellular signal-regulated kinase (ERK). Moreover, IL-6 treatment induced the phosphorylation of ERK1/2 in comparison to untreated cells in a time-dependent manner (Figure 3B). In contrast, SB203580, a selective p38 MAPK inhibitor, abrogated the IL-12-induced Rab7 expression (Figure 3C). In addition, IL-12 induced the phosphorylation of p38 MAPK (Figure 3D) in the treated cells demonstrating the role of p38 MAPK in the induction of Rab7.
Figure 3.

Regulation of induced expression of Rab 5c and Rab7 by the respective cytokine through specific signaling pathways. J774E cells (5 × 106) cells were preincubated for 30 min with inhibitors of tyrosine kinase (Genistein, 25 μg/ml), PI3 kinase (Wortmanin, 200 nM and LY294002, 10 μM), MEK1/2 (PD98059, 10 μM and U0126, 150 nM), P38 MAP kinase (SB203580, 1 μM) or JNK (SP600125, 100 nM) and subsequently treated with 0.5 μg/ml of either IL-6 (A) or IL-12 (C) for 6 h. Subsequently, RT–PCR was performed to determine levels of the respective mRNA as described previously using specific primers of Rab5c and Rab7. β-Actin was used as an internal control. Results are representative of three independent preparations. Lower panels of (A) and (C) indicate the quantitation of the respective data. Results are represented as the mean of three observations and normalized to untreated control, arbitrarily chosen as one unit. To determine the levels of indicated kinases by cytokine treatment, cells were treated with 0.5 μg/ml of IL-6 (B) or IL-12 (D) for the indicated periods of time and Western blot analyses were carried out using specific antibodies. Results from Western blots are representative of three independent preparations. Level of respective protein in untreated control is represented by 0 h treatment.
IL-6 or IL-12 regulates the expression of specific rab by activation of the 5′-flanking regulatory regions of the gene
To investigate if IL-6 or IL-12 regulates the expression of rab genes by activation of the respective promoter, we prepared chimeric constructs by ligating 5′-flanking regulatory regions of the rab5c or rab7 genes with luciferase as a heterologous reporter (Seshadri et al, 2002). First, mouse rab5c (+51 to −999) and rab7 (+23 to −886) 5′-flanking regulatory regions containing promoter sequence were identified from mouse genome database and amplified using appropriate primers (Supplementary Figure 2A and B), which were subsequently cloned into the promoterless reporter vector (pGL3-basic). The chimeras were transiently transfected to J774E cells and incubated with the indicated concentrations of IL-6 or IL-12 to determine the expression of the heterologous reporter. The results presented in Figure 4A show that transactivation of the rab5 construct (rab5 +51 to −999-Luc) was increased about 3.8-fold by 0.5 μg/ml of IL-6 over the control, whereas no significant activation was detected with IL-12 treatment. In contrast, 2.7-fold increase of luciferase activity of the rab7 construct (rab5 +23 to −886-Luc) was detected by IL-12 over the control, whereas IL-6 failed to show any activation (Figure 4A). Significant inductions of rab5c and rab7 promoter by specific cytokines were also obtained even at low concentration (0.05 μg/ml) of respective cytokines. Moreover, IL-6-mediated increase in Rab5c promoter activity was specifically blocked by ERK inhibitor (PD 98059) but not by p38 MAPK inhibitor, whereas activation of Rab7 promoter by IL-12 was specifically inhibited by p38 MAPK inhibitor, SB 203587 but not by PD 98059 (Figure 4B). These results demonstrated that IL-6 and IL-12 specifically activate a precise signal transduction pathway to increase the expression of specific rab genes.
Figure 4.
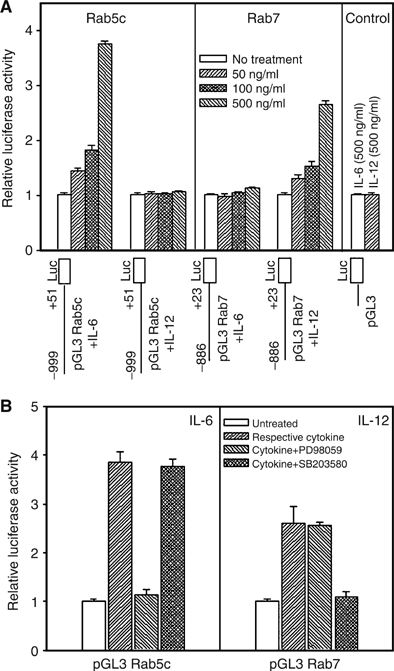
Determination of the molecular mechanisms of the cytokine-mediated activation of specific Rabs. To determine the cytokine responsive element present in the 5′-flanking region of Rab5c and Rab7, chimeric pGL3-basic vectors containing Rab5c or Rab7 5′-flanking regulatory sequence (A) were transiently transfected into semiconfluent J774E cells along with a reporter gene construct containing β-galactosidase. Subsequently, transfected cells were treated with the indicated concentrations of IL-6 or IL-12 and luciferase activity in cell extracts was measured and normalized for β-galactosidase activity as described in Materials and methods. Results are expressed as an average of three independent experiments±s.d. To determine the role of kinases in the transactivation of rab genes (B), transfected cells containing Rab5c or Rab7 chimeras were treated with different kinase inhibitors like PD 98059 (20 μM) and SB203580 (5 μM), 30 min before addition of 0.5 μg/ml of IL-6 or IL-12 and processed as described previously. Results are expressed as an average of three independent experiments±s.d.
Role of IL-6 and IL-12 in intracellular trafficking
Finally, studies were carried out to determine the modulation of phagosome maturation by IL-6- and IL-12-mediated increased expression of Rab5 and Rab7. The results presented in Figure 5A showed that cytosol prepared from IL-6-treated cells enhances the fusion between early endosomes and early phagosomes in comparison to cytosol isolated from untreated cells. Moreover, this enhanced fusion between the early compartments was abrogated in the presence of cytosol prepared from the cells treated with IL-6 along with ERK inhibitor (Figure 5A) as well as immunodepletion of Rab5 from IL-6-treated cytosol (Figure 5B). Furthermore, addition of Rab5 in the immunodepleted cytosol significantly recovered the fusion almost to the fusion observed with IL-6-treated cytosol (Figure 5B), demonstrating that enhanced fusion between the early compartments is due to increased content of the Rab5 in IL-6-treated cells.
Figure 5.
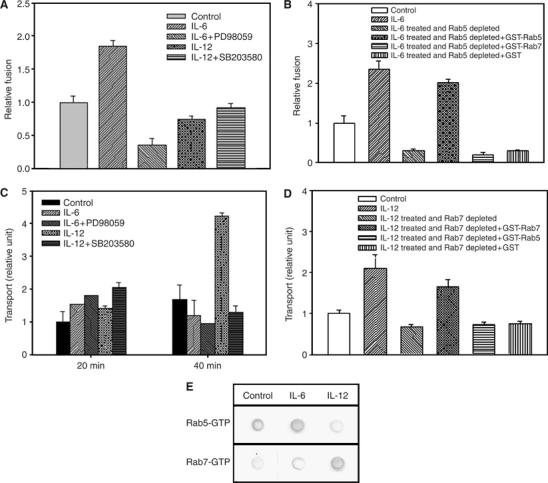
Role of IL-6 and IL-12 on endocytic and phagocytic pathways. (A) To determine the role of cytokine-induced Rab expression, in vitro fusion between early endosomes containing avidin-HRP with early phagosome containing biotinylated-latex beads was carried out either in the presence of normal cytosol or cytosol prepared from IL-6- or IL-12-treated cells as described in Materials and methods. Fusion observed between endosomes and phagosomes in the presence of cytosol isolated from untreated cells was normalized to one unit and results are expressed as relative fusion of three independent experiments±s.d. One unit corresponds to 9.2 ng of HRP activity/mg of protein. (B) To determine the role of Rab5 in IL-6-treated cytosol, similar fusion assay was carried out in the presence of Rab5 immunodepleted from IL-6-treated cytosol as well as cytosol supplemented with the indicated Rab proteins. Fusion observed between endosomes and phagosomes in the presence of cytosol isolated from untreated cells was normalized to one unit and results are expressed as relative fusion of three independent experiments±s.d. One unit corresponds to 10.5 ng of HRP activity/mg of protein. (C) To determine the role of the indicated cytokine on phagosome maturation, biotinylated-latex beads containing phagosome transport to avidin-HRP preloaded lysosome was carried out in cytokine-treated and untreated cells as described in Materials and methods. At the indicated times, the formation of bead–biotin–avidin–HRP complex was measured to determine the transport of phagosomes to lysosomes. Transport observed at 20 min in the untreated control cells was taken as one unit and results are expressed as relative unit of transport to lysosome from three independent experiments±s.d. One unit corresponds to 12.7 ng of HRP activity/mg of protein. (D) To determine the role of Rab7 in the phagosome–lysosome transport, reconstitution of phagosome–lysosome transport was carried out under indicated conditions as described in Materials and methods. Formation of bead–biotin–avidin–HRP complex was measured after 40 min of chase to determine the lysosomal transport. Transport observed in the untreated control cells was taken as one unit and results are expressed as relative unit of transport to lysosome from three independent experiments±s.d. One unit corresponds to 19.9 ng of HRP activity/mg of protein. (E) To determine the activation of Rab5 and Rab7 by respective cytokines, Rab5 and Rab7 were immunoprecipitated from the respective cytokine-treated cytosol and GTP binding assay was carried out using [α-32P]GTP as described in Materials and methods. Results are representative of three independent preparations.
Furthermore, when lysosomal targeting of paramagnetic-bead-containing phagosomes was measured, enhanced transport of these phagosomes to the avidin-horseradish peroxidase (avidin-HRP)-loaded lysosomes was observed within 40 min in IL-12-treated cells in comparison to untreated cells (Figure 5C). Moreover, IL-12-mediated enhanced transport of beads to the lysosomes was specifically blocked when the cells were treated with IL-12 along with SB 203587. Furthermore, immunodepletion of Rab7 from IL-12-treated cytosol completely abrogated the IL-12-induced lysosomal transport of the phagocytic probe which was significantly recovered by the supplementation of the Rab7 in the same cytosol, demonstrating that enhanced transport to the lysosomes in the IL-12-treated cells is due to increased amounts of Rab7 in the treated cells (Figure 5D). Interestingly, GTP blot analysis of the immunoprecipitated Rabs from IL-6- and IL-12-treated cell showed that IL-6-treated cytosol has increased amount of Rab5 in GTP form, whereas IL-12-treated cytosol show significantly more GTP form of Rab7 (Figure 5E) in comparison to untreated control. Similarly, our results also showed that treatment of J774E cells with IL-6 and IL-12 appropriately modulates the uptake and degradation of maleylated bovine serum albumin (Supplementary Figure 3), a ligand recognized by macrophage scavenger receptor. Taken together, these results demonstrated that IL-6 and IL-12 modulate phagocytic and endocytic pathways through the overexpression of appropriate endocytic Rab proteins.
Effect of IL-6 and IL-12 in intracellular trafficking of Salmonella in macrophages
To determine the effect of IL-6 and IL-12 on the trafficking of Salmonella in macrophages, J774E cells were treated with the respective cytokines and infected with GFP-S. typhimurium at a ratio of 1:10 (macrophage: Salmonella). Cells were washed and chased for 90 min at 37°C and their localization with lysosomes labeled with lysotracker Red was determined. The results presented in the Figure 6A demonstrated that live GFP-Salmonella colocalized with Lysotracker Red-labeled lysosomal compartment in IL-12-treated cells, whereas live GFP-Salmonella inhibited their transport to the lysotracker-labeled lysosomal compartment in IL-6-treated cells as observed in untreated control cells. It is pertinent to mention that more than 90% of the GFP-Salmonella colocalized with Lysotracker Red-labeled lysosomal compartment in IL-12-treated cells, whereas less than 5% of the GFP-Salmonella colocalized with Lysotracker Red-labeled compartment in IL-6-treated cells. Subsequently, studies were carried out to determine the ability of Salmonella to survive in IL-6- or IL-12-treated J774E cells for different periods of time. Data presented in Figure 6B show that about 49.7% Salmonella were present in IL-12-treated cells in comparison to untreated control (100%) after 15 h of cytokine treatment. Whereas about 20% more intracellular Salmonella were detected in IL-6-treated than in control cells.
Figure 6.

Effect of IL-6 and IL-12 on Salmonella trafficking in J774E macrophages. (A) Confocal images showing the GFP-Salmonella localization with Lysotracker Red-labeled lysosomes in untreated and indicated cytokine-treated J774E cells. Lysotracker Red-labeled lysosomes appear in red channel, green channel shows the GFP-Salmonella and yellow indicates the colocalization of GFP-Salmonella with lysotracker-labeled lysosomes in merged images. Infection of J774E cells with Dead GFP-Salmonella was used as control. Results are representative of three independent observations. (B) Cytokine-treated or untreated J774E cells were infected with S. typhimurium as described in Materials and methods. At the indicated times, cells were lysed and an aliquot of the cell lysates was used to determine the number of viable bacteria under each condition. Results are expressed as an average of three determinations±s.d.
Discussion
Endocytosis is a fundamental process that mediates a wide range of cellular functions, including nutrient uptake, downregulation of cell surface receptor, maintenance of homeostasis and pathogen defense (Conner and Schmid, 2003; Maxfield and McGraw, 2004). Moreover, recent studies have shown that binding of a ligand with cell surface receptor triggers large number of signaling pathways, both in endocytic and phagocytic pathways (Di Fiore and De Camilli, 2001; McPherson et al, 2001; Janeway and Medzhitov, 2002; Underhill and Ozinsky, 2002; Gonzalez-Gaitan, 2003; Pelkmans et al, 2005). However, how these downstream signaling pathways modulate intracellular trafficking is not clearly established. Our finding that IL-6- and IL-12-mediated activation of specific kinase of the signal transduction pathway regulates the expression of particular endocytic Rab GTPase demonstrates a novel mechanism of regulation of intracellular trafficking through signaling pathway.
Subsequent analyses of the mechanisms of activation of Rab5 and Rab7 by IL-6 and IL-12 have shown that increased expression of Rab5 is regulated via the activation of ERK by IL-6, whereas IL-12 induces Rab7 expression through p38 MAPK. Recent studies have shown that activation of p38 MAKP regulates endocytosis by regulating the activity of Rab5 accessory proteins like Rab5-GDI (Cavalli et al, 2001), EEA1 and rabenosyn-5 (Mace et al, 2005), which are known to regulate membrane transport during endocytosis (Simonsen et al, 1998; Nielson et al, 2000). Similarly, several independent studies have also demonstrated that activation of ERK regulates endocytic traffic of multiple receptor systems, for example, 5-HT1A receptor, m1 muscarinic receptor, μ and δ opiod receptors (Della Rocca et al, 1999; Volger et al, 1999; Whistler and von Zastrow, 1999; McPherson et al, 2001). Thus, these results further support our finding that activation of different kinases regulates intracellular trafficking. In addition, our results provide additional link that activation of specific kinase could regulate the expression of appropriate Rab GTPase. Moreover, a recent study has shown that TLR signaling activates p38 MAPK and modulates phagosome maturation towards lysosomes (Blander and Medzhitov, 2004), however, the mechanism of which is not clearly addressed. Our results indicate an interesting possibility that TLR-mediated activation of p38 MAPK might regulate phagosome maturation by enhanced expression of Rab7, which regulates the transport to the lysosomes (Feng et al, 1995; Mukhopadhyay et al, 1997b). This is supported by the fact that IL-6 promotes the fusion between early compartments and IL-12 induces transport of the internalized cargo to the lysosomes and these induced transport steps by particular cytokine are also inhibited in the presence of appropriate inhibitor of specific kinase activated by that cytokine. Our results have also shown that induction of specific transport step by IL-6 or IL-12 is due to the increased content of Rab5 or Rab7, respectively, as immnodepletion of the specific Rab from the respective cytokine-treated cytosol completely abrogated the cytokine-induced relevant transport processes. These results are in correlation with previous finding that Rab5 is involved in the fusion between early compartments (Grovel et al, 1991; Mukhopadhyay et al, 1997a) and Rab7 regulates the transport to the late/lysosomal compartment (Feng et al, 1995; Mukhopadhyay et al, 1997b). Furthermore, our results also show the presence of increased amount of GTP form of the Rab5 or Rab7 in IL-6- or IL-12-treated cells, respectively, which could be due to the consequence of enhanced content of that Rab in particular cytokine-treated cells. However, it could also be possible that cytokine-mediated activation of the particular kinase might modulate some molecules of the Rab cycle and thereby activate respective Rab protein. Interestingly, recent studies have shown that stress-induced activation p38 MAPK regulates the activity of some effector molecules of Rab5 (Cavalli et al, 2001; Mace et al, 2005) and thereby modulates endocytosis. In contrast, our results have shown that IL-12-mediated activation of the p38 MAKP induces the expression of Rab7, which is possibly due to induction of specific transcription factor for Rab7 by p38 MAKP. These apparent discrepancies could be due to the complex signaling pathways activated by p38 MAPK, as it has been shown that p38 MAPK induces a large variety of biological effects in response to a wide range of stimuli resulting in a bewildering array of possibilities (Zhang and Kaplan, 2000; Kaminska, 2005). Thus, our results demonstrated a new mechanism of cytokine-mediated regulation of intracellular trafficking by modulating the expression of Rab GTPases through the activation of definite signal transduction pathway.
Subsequently, attempts have been made to understand how IL-6 or IL-12 regulates the expression of specific rab genes. Accordingly, we have cloned the 5′-flanking regulatory regions containing promoter sequence of the rab5c or rab7 genes to determine the presence of any cytokine response element in the 5′-flanking regulatory regions of the respective genes. Interestingly, our results have shown that cells transfected with the promoterless reporter vector containing 5′-flanking regulatory region of rab5c specifically expresses enhanced level of heterologous reporter when treated with IL-6. Whereas, cells transfected with similar construct containing 5′-flanking regulatory region of rab7 specifically induce the expression of reporter gene by IL-12. Moreover, in correlation with our previous finding, activation of ERK is required for the expression of the reporter gene containing 5′-flanking regulatory region of rab5c by IL-6 treatment. In contrast, expression of the reporter gene through 5′-flanking regulatory region of rab7 by IL-12 requires p38 MAPK activity. Thus, our results demonstrated that cytokine-mediated specific activation of ERK and p38 MAPK plays pivotal role in the regulation of the expression of Rab genes. It is tempting to speculate that cytokine-mediated activation of a specific kinase of signal transduction pathway induced some transcription factors; those in turn bind with the particular promoter present in the 5′-flanking regulatory region of a rab gene and regulate the expression of the particular rab gene. Interestingly, previous studies have shown that IL-6 induces Ets and AP-1 transcription factors through the activation of ERK MAP kinase pathway (Jenkins et al, 2004); those are also present in the 5′-flanking regulatory region of rab 5c. Similarly, IL-12-activated p38 MAP kinase is shown to induce several trans-regulatory factors including ATF, ELK, CHOP, MEF2C, SAP1 or CREB (Zhang and Kaplan, 2000). Among them, ATF-, MEF 2C- and CREB-binding regions are present in the 5′-flanking regulatory region of rab7. Whether any of these transcription factors are activated by specific cytokines and contribute to the induced expression of particular rab needs further investigations.
Finally, our results have shown that cells infected with live Salmonella is targeted to the lysosomes in IL-12-treated cells, whereas transport of Salmonella to the lysosomes is inhibited in IL-6-treated cells. In correlation with this finding, survival of intracellular Salmonella in macrophages is significantly inhibited in IL-12-treated cells in comparison to untreated control cells. In contrast, our results have shown that a relatively large number of intracellular bacteria are present in IL-6-treated cells. Taken together, these results have shown that increased expression of Rab7 by IL-12 treatment accounts for the enhanced killing of Salmonella by targeting them to the lysosomes, whereas overexpression of Rab5 by IL-6 treatment possibly promotes the fusion of Salmonella containing phagosomes with early endosomes and thereby inhibits their transport to the lysosomes. However, IL-12 treatment failed to eliminate all intracellular Salmonella from the treated macrophages, probably owing to the Salmonella-mediated inhibition of their transport to the lysosomes by the recruitment of Rab5 from the host cells (Hashim et al, 2000; Mukherjee et al, 2000). Thus, our results provide the mechanistic explanation for why different intracellular pathogens are killed by IL-12 treatment (Greenberger et al, 1996; de Jong et al, 1998), but not by IL-6 treatment (Dube et al, 2004) of the host cells.
In conclusion, our results reveal the existence of a unique regulation of membrane trafficking directly dependent on different cytokines. This is the first demonstration that cytokines like IL-6 and IL-12 specifically regulate the expression of endocytic Rab proteins through the activation of a definite signal transduction pathway, resulting in specific modulation of intracellular trafficking pathways. Moreover, cytokine-mediated regulation of Rab expression might also suggest the plausible mechanisms of modulation of antigen presentation by cytokines. Our results showing that endocytosis and intracellular trafficking can be modulated by external stimuli like cytokines raise the intriguing possibility of regulating the progression of infection, aging and number of degenerative diseases by appropriate interventions.
Materials and methods
Unless otherwise stated, all reagents were obtained from Sigma Chemical Co. Tissue culture supplies were obtained from the Grand Island Biological Co. Recombinant mouse IL-6 and IL-12 were obtained from R&D systems (Minneapolis, MN). Affinity-purified rabbit polyclonal anti-Rab4, Rab6, Rab7 and Rab11 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-Rab5 antibody was generous gift by Dr A Wandinger-Ness (University of New Mexico, Albuquerque, NM). Rab5 and Rab7 constructs were provided by Dr Philip Stahl (Washington University School of Medicine, St Louis, MO). Pharmacological inhibitors, different kinases and kinase-specific antibodies were purchased from Cell Signaling (Beverly, MA). Mouse anti-Actin antibody and all the secondary antibodies labeled with HRP were purchased from Calbiochem (La Jolla, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Avidin-HRP and avidin were purchased from Vector laboratories (Burlingame, CA). Other reagents used were of analytical grade. Dr V Bal (National Institute of Immunology, New Delhi) kindly provided the virulent strain of Salmonella typhimurium and GFP-Salmonella was obtained as a gift from Dr A Aballay (Massachusetts General Hospital, Boston). These bacteria were routinely grown in Luria broth (LB) at 37°C with constant shaking (300 rpm) and used in infection experiments as described previously (Mukherjee et al, 2002).
Detection of levels of different Rab proteins in cytokine-treated cells
To determine the effect of cytokine treatment on Rab expression, J774E cells (5 × 106) were treated with the indicated concentrations of IL-6 or IL-12 for 6 h at 37°C in FCS-free RPMI-1640 medium. Cells were washed and further incubated for 3 h at 37°C in RPMI-1640 medium with 10% FCS. Subsequently, cells were washed with PBS and lysed by sonication and 40 μg of the respective cell lysates were analyzed by 12% SDS–PAGE. The proteins were transferred onto nitrocellulose membranes and checked for the presence of actin, Rab4, Rab6, Rab11, Rab7 and Rab5 by Western blot analysis using specific antibodies. Subsequently, membranes were probed with appropriate HRP-labeled second antibodies and visualized by ECL.
Detection of mRNA levels of different Rabs in cytokine-treated cells
J774E cells (5 × 106 cells) were treated with the indicated concentrations of IL-6 or IL-12 at 37°C in FCS-free RPMI-1640 medium for different periods of time. At the indicated time point, total RNA was isolated from the control and treated cells, using TRIZOL reagent (Invitrogen). Subsequently, complementary DNA was synthesized using 2 μg of total RNA by Thermo Script RT–PCR kit according to the manufacturer's (Invitrogen) protocol.
Then, PCRs were performed with specific sets of primers for the indicated Rabs using 1 μl of cDNA as template. The primers for PCR were synthesized at ‘Microsynth AG (Switzerland)'.
A. β-actin: forward 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and reverse 5′-TAAAACGCAGCTCAGTAACAGTCC-3′; B. Rab4: forward 5′-ATGGCCGAGACCTACGACTTC-3′ and reverse 5′-TCAGCAGCCACAGGGCTGAG-3′; C. Rab5a: forward 5′-ATGGCTAGTCGAGGCGCA-3′ and reverse 5′-TTAGTTACTACAACACTG-3′; D. Rab5b: forward 5′-ATGACTAGCAGAACAGCTAGGCCC-3′ and reverse 5′-TCAGTTGCTACAACACTGGCTCTTGTTCTGCTGGG-3′; E. Rab5c: forward 5′-ATGGCGGGTCGGGGAGGCGCACG-3′ and reverse 5′-TCAGTTGCTGCACTGGCTCCGGC-3′; F. Rab6: forward 5′-ATGTCCGCGGGCGGAGAC-3′ and reverse 5′-TCAGCAGGAACAGCCGCCTTC-3′; G. Rab7: forward 5′-ATGACCTCTAGGAAGAAAGTG-3′ and reverse 5′-CCTTCAACAACTGCAGCTTTCTGCGG-3′.
PCRs for Rab4, Rab5b, Rab5c, Rab6, Rab7 and β-actin were performed in a Perkin-Elmer thermocycler for 30 cycles: denaturation for 30 s at 94°C; annealing for 30 s at 57°C and extension for 1 min at 68°C. However, to amplify Rab5a, a pre-PCR of five cycles was carried out with lower annealing temperature (45°C), followed by 25 cycles under similar conditions using Rab5a-specific primers.
Cloning and construction of vectors containing Rab5c- or Rab7 5′-flanking regulatory region
The mouse Rab5c (NM_024456) and Rab7 (NM_009005) 5′-flanking regulatory regions corresponding to nucleotides −999 to +51 and −886 to +23 (relative to the transcription start site), respectively, were PCR amplified from mouse macrophage (J774E) genomic DNA using specific primers:
A. Rab5c: forward 5′-ATACATCTCGAGCACTCCAGAAGTAGAG-3′ and reverse 5′-ATACATAAGCTTCCCACCCCCGGCGGTGTC-3′; B. Rab7: forward 5′-ATATTGGAGCTCGGACCTACAAACCTTCTC-3′ and reverse 5′-ATATTGCTCGAGATGGTCAAAGCTCCG-3′.
PCRs were performed in a Perkin–Elmer thermocycler using Pfu polymerase (Stratagene) with initial denaturation for 5 min at 94°C followed by 30 cycles of denaturation for 1 min at 94°C; annealing for 1 min at 55°C and extension for 1.5 min at 72°C with a final extension for 7 min at 72°C. The amplified product of Rab5c was digested with XhoI and HindIII, whereas Rab7 fragment was digested with SacI and XhoI; then inserted into the respective pGL3-basic vector (Promega, Madison, WI). All the constructs were sequenced and verified with the reported genome sequences.
Transient transfection of cells and reporter gene assays
To measure the transcriptional efficiency of chimeric constructs of Rab5c and Rab7 5′-flanking regulatory region, individual constructs (2 μg) were transfected into semiconfluent J774E cells in 35 mm dishes using Lipofectamin 2000 (Invitrogen). To monitor transfection efficiency, a reporter gene construct (0.5 μg) containing β-galactosidase behind SV40 promoter was cotransfected. Transfected cells were allowed to recover for 6 h in RPMI medium with 10% FCS at 37°C and treated with the indicated concentrations of IL-6 or IL-12 in serum-free medium for 8 h. Luciferase and β-galactosidase (both are from Promega) activities in cell extracts were determined by chemiluminescence and spectrophotometric assay, respectively (according to the company's protocol). Cells containing appropriate constructs were treated with different kinase inhibitors like PD 98059 (20 μM) and SB203580 (5 μM), 30 min before addition of IL-6 or IL-12 to determine the signaling pathway involved in the cytokine-mediated induction of gene expression.
Assay for in vitro fusion between early endosomes and early phagosomes
To determine the role of cytokine-induced Rab5 and Rab7 expression, we have reconstituted the fusion between early endosomes containing avidin-HRP with early phagosome containing biotinylated-latex beads, using the procedure described previously (Mukherjee et al, 2000). Briefly, J774E cells were allowed to internalize biotinylated-latex beads for 5 min at 37°C and early phagosomes were purified. Similarly, early endosomes containing avidin-HRP were prepared as described previously (Mukherjee et al, 2000). Subsequently, phagosomes were mixed with early endosomes in fusion buffer (250 mM sucrose, 0.5 mM EGTA, 20 mM HEPES–KOH, pH 7.2, 1 mM dithiothreitol, 1.5 mM MgCl2, 100 mM KCl, including an ATP regenerating system, 1 mM ATP, 8 mM creatine phosphate, 31 Units/ml creatine phosphokinase and 0.25 mg/ml avidin as scavenger) supplemented with 1.5 mg/ml of gel-filtered cytosol prepared from untreated or indicated cytokine-treated cells. Fusion was carried out for 10 min at 37°C and the reaction was stopped by chilling on ice. The HRP–avidin–biotin–bead complex was recovered by centrifugation (10 000 g for 5 min) after solubilization of the membrane in solubilization buffer (SB, PBS containing 0.5% Triton X-100 with 0.25 mg/ml avidin as scavenger). The HRP activity associated with the biotinylated bead was measured as fusion unit.
To directly demonstrate the role of Rab5 in the enhanced fusion by IL-6-treated cytosol, Rab5 was immunodepleted from the respective cytosol as described previously (Mukherjee et al, 2001). Subsequently, fusion was carried out with Rab5-immunodepleted cytosol supplemented with indicated in vitro prenylated Rab (1 μg) proteins using the similar assay. Rab5 and Rab7 were preincubated with cytosol in the fusion buffer at room temperature for 30 min for in vitro prenylation (Lombardi et al, 1993) before addition to the fusion assay.
Assay for transport to lysosomes
To determine the role of cytokine-induced Rab expression on phagosome maturation, we have used a ligand mixing assay to measure the transport of biotinylated-paramagnetic-beads containing early phagosome to avidin-HRP-loaded late/lysosomal compartments (Mukherjee et al, 2002). J774E cells (1 × 106 cells) were treated with the respective cytokines as described previously. Briefly, J774E-treated or -untreated cells were incubated in the presence of avidin-HRP (200 μg/ml) at 4°C to allow binding. Subsequently, avidin-HRP was chased for 90 min at 37°C to label the lysosomes. After washing, uptake of biotinylated beads (1 × 107) was carried out for 5 min at 37°C to level the early compartment. Cells were washed to remove unbound beads and uninternalized surface-bound biotinylated beads were quenched by free avidin (0.25 mg/ml). Cells were washed twice and chased for the indicated times at 37°C. Finally, cells were solubilized in SB. The HRP–avidin–biotin–bead complexes were washed four times with SB by using a magnet and the HRP activity associated with the biotinylated beads was measured as relative transport unit.
Reconstitution of phagosome–lysosome transport in permeabilized cells
To determine the role of Rab7 content in IL-12-treated cells in lysosomal targeting, reconstitution of phagosome–lysosome transport assay was carried out in permeabilized cells by using a similar assay as described previously (Mukherjee et al, 2002). Briefly, lysosomes of J774E cells (1 × 106 cells) were loaded with avidin-HRP and biotinylated beads were internalizated to the early compartment as mentioned in the previous section. Subsequently, cells were resuspended in ice-cold permeabilzation buffer (10 mM K-phosphate, 120 mM KCl, 0.5 mM EGTA, pH 7.2 containing 50 μg/ml avidin) and quickly frozen in liquid nitrogen. Cells were kept at −80°C for 12 h and thawed by warming the tubes at room temperature. Under these conditions, more than 80% of the cells were permeabilized, as measured by the release of lactate dehydrogenase. After permeabilization, cells were incubated for 30 min at 4°C to deplete cytosol and subsequently loaded with indicated cytosol (4 mg/ml) supplemented with in vitro prenylated Rab protein (500 ng) as described previously (Mukherjee et al, 2002). Rab7 was immunodepleted from the IL-12-treated cytosol as described previously (Mukherjee et al, 2001). Finally, cells were incubated for 40 min at 37°C to allow transport to the lysosomes. Cells were solubilized in SB and HRP–avidin–biotin–bead complexes were washed four times with SB by using a magnet. The HRP activity associated with the biotinylated beads was measured as relative transport unit.
GTP binding assay
In order to determine the level of GTP form of Rab5 or Rab7 in IL-6 or IL-12 cells, respectively, an in vitro GTP binding assay was carried out (Stenmark et al, 1994). Briefly, Rab5 and Rab7 were immunoprecipitated from the IL-6- and IL-12-treated cytosols as described previously. The beads were washed thrice in PBSTM (50 mM phosphate buffer, pH 7.5, containing 5 mM MgCl2 and 0.1% Tween-20) and incubated with 1 μCi/ml of [α-32P]GTP (3000 Ci/mM, NEN) in the same buffer for 1 h at 37°C. Then, the beads were washed extensively in PBSTM and bound nucleotides were eluted in 25 μl of sample buffer (phosphate buffer, 50 mM, pH 7.5, containing 0.2% SDS, 2 mM EDTA and 10 mM GTP) at 70°C for 2 min. Beads were separated by centrifugation and the supernatant was spotted onto a TLC plate. GTP binding status of the indicated Rabs by the respective cytokine treatment was visualized by autoradiography.
Determination of the effect of IL-6 and IL-12 on the maturation of Salmonella in macrophages by confocal microscopy
To determine the modulation of the intracellular transport of Salmonella in macrophages by IL-6 or IL-12 treatment, J774E cells (1 × 106) were plated on sterile glass coverslip placed in six-well tissue culture plate and treated with 0.5 μg/ml of cytokines as described previously. Subsequently, treated or untreated J774E cells (1 × 106 cells) were incubated with live GFP-S. typhimurium (1 × 107 cells) for 10 min at 37°C to restrict their entry to the early compartment. Cells were washed three times to remove unbound bacteria and chased for another 90 min at 37°C. During the last 30 min of the chase, Lysotracker Red (100 nM) was added to GFP-Salmonella-infected macrophages to label the lysosomes. Cells were washed three times with cold PBS and confocal microscopy was carried out using a Zeiss, LSM 510 confocal microscope using oil immersion objective.
Effect of cytokines on the survival of Salmonella in macrophages
To determine the effect of IL-6 or IL-12 on the survival of Salmonella in macrophages, J774E cells (1 × 106 cells/well) were cultured in RPMI-1640 medium containing 10% FCS in the presence of 0.5 μg/ml of the respective cytokine for 6 h at 37°C. Cells were washed twice with PBS and incubated with S. typhimurium (1 × 107) for 20 min at 37°C for infection. Uninternalized bacteria were removed by washing with PBS and followed by gentamycin treatment as described earlier (Mukherjee et al, 2002). The infected macrophages were further incubated in the respective cytokine-containing medium at 37°C for the indicated periods of time. Finally, the cells were lysed in SB and an aliquot of the cell lysates was plated on Salmonella–Shigella agar plates to determine the number of viable Salmonella present in the lysate in terms of colony-forming units.
Supplementary Material
Supplementary Figures
Acknowledgments
This study was supported by grants from the Department of Biotechnology, Government of India to National Institute of Immunology and to CKM. MB and SS were supported by fellowships from Council of Scientific & Industrial Research, Government of India.
References
- Alonso A, Garcia-del Protillo F (2004) Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int Microbiol 7: 181–191 [PubMed] [Google Scholar]
- Alvarez-Dominguez C, Stahl PD (1998) Interferon-γ selectively induced rab5a synthesis and processing in mononuclear cells. J Biol Chem 273: 33901–33904 [DOI] [PubMed] [Google Scholar]
- Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signaling. Nature 430: 257–263 [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R (2004) Regulation of phagosome maturation by signals from toll-like receptors. Science 304: 1014–1018 [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberge J (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell 7: 421–432 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH (1998) Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280: 1435–1438 [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Raymond JR (1999) Serotinin 5-HT1A receptor mediated ERK activation requires calcium/calmodulin dependent receptor endocytosis. J Biol Chem 274: 4749–4753 [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P (2001) Endocytosis and signaling. An inseparable partnership. Cell 106: 1–4 [DOI] [PubMed] [Google Scholar]
- Dube PH, Handley SA, Lewis J, Miller VL (2004) Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect Immun 72: 3561–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A (1995) Rab7: an important regulator of late endocytic membrane traffic. J Cell Biol 131: 1435–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri RK, Selvaraj SK, Kalra VK (2003) Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immonol 170: 5281–5294 [DOI] [PubMed] [Google Scholar]
- Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, Graham FL, Hitt M, Danforth JM, Standiford TJ (1996) IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol 157: 3006–3012 [PubMed] [Google Scholar]
- Gonzalez-Gaitan M (2003) Signal dispersal and transduction through endocytic pathway. Nat Rev Mol Cell Biol 4: 213–224 [DOI] [PubMed] [Google Scholar]
- Grovel JP, Chavrier P, Zerial M, Gruenberg J (1991) Rab5 controls early endosome fusion in vitro. Cell 64: 915–925 [DOI] [PubMed] [Google Scholar]
- Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A (2000) Live Salmonella modulate the expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J Biol Chem 275: 16281–16288 [DOI] [PubMed] [Google Scholar]
- Hornef MW, Wick MJ, Rhen M, Normark S (2002) Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3: 1033–1040 [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216 [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Grail D, Inglese M, Quilici C, Bozinovski S, Wong P, Ernst M (2004) Imbalanced gp130-dependent signaling in macrophages alters macrophage colony-stimulating factor responsiveness via regulation of c-fms expression. Mol Cell Biol 24: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy from molecular mechanisms to therapeutic benefits. Biochem Biophys Acta 1754: 253–262 [DOI] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Reiderer MA, Goda Y, Zerial M, Pfeffer SR (1993) Rab9 functions in transport between late endosome to trans Golgi network. EMBO J 12: 677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace G, Miaczynska M, Zerial M, Nebreda AR (2005) Phosphorylation of EEA1 by p38 MAP kinase regulates μ opioid receptor endocytosis. EMBO J 24: 3235–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132 [DOI] [PubMed] [Google Scholar]
- McPherson PS, Kay BK, Hussain NK (2001) Signaling on the endocytic pathway. Traffic 2: 375–384 [DOI] [PubMed] [Google Scholar]
- Mosser DM, Karp CL (1999) Receptor mediated subversion of macrophage cytokine production by intracellular pathogens. Curr Opin Immunol 11: 406–411 [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Parashuraman S, Krishnamurthy G, Majumdar J, Yadav A, Kumar R, Basu SK, Mukhopadhyay A (2002) Diverting intracellular trafficking of Salmonella to the lysosome through activation of the late endocytic Rab7 by intracellular delivery of muramyl dipeptide. J Cell Sci 115: 3693–3701 [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A (2001) SopE acts as an Rab5 specific exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J Biol Chem 276: 23607–23615 [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Siddiqi SA, Hashim S, Raje M, Basu SK, Mukhopadhyay A (2000) Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome. J Cell Biol 148: 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Barbieri AM, Funato K, Roberts R, Stahl PD (1997a) Sequential actions of rab5 and rab7 regulate endocytosis in the Xenopus oocyte. J Cell Biol 136: 1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Funato F, Stahl PD (1997b) Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem 272: 13055–13059 [DOI] [PubMed] [Google Scholar]
- Nielson E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M (2000) Rabenosyn-5, a novel Rab 5 effector, is complexed with hVPS45 and recruited to endosomes through FYVE finger domain. J Cell Biol 151: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M (2005) Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86 [DOI] [PubMed] [Google Scholar]
- Redpath S, Ghazal P, Gascoigne NR (2001) Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol 9: 86–92 [DOI] [PubMed] [Google Scholar]
- Rosenberger CM, Finlay BB (2003) Phagocyte sabotage: disruption of macrophage signaling by bacterial pathogens. Nat Rev Mol Cell Biol 4: 385–396 [DOI] [PubMed] [Google Scholar]
- Seshadri V, Fox PL, Mukhopadhyay CM (2002) Dual role of insulin in transcriptional regulation of the acute phase reactant ceruloplasmin. J Biol Chem 277: 27903–27911 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerail M, Stenmark H (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome function. Nature 394: 494–498 [DOI] [PubMed] [Google Scholar]
- Siren J, Pirhonenm J, Julkunenm I, Matikainen S (2005) IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol 174: 1932–1937 [DOI] [PubMed] [Google Scholar]
- Srividya S, Roy RP, Basu SK, Mukhopadhyay A (2000) Scavenger receptor-mediated delivery of muramyl dipeptide activates antitumor efficacy of macrophages by enhanced secretion of tumor-suppressive cytokines. J Leuk Biol 67: 683–690 [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13: 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA (2005) Phagocytosis: elegant complexity. Immunity 22: 539–550 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Gantner B (2004) Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect 6: 1368–1373 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A (2002) Toll-like receptors: key mediators of microb detection. Curr Opin Immunol 14: 103–110 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401: 811–815 [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Grinstein S (2002) Phagosome maturation: aging gracefully. Biochem J 366: 689–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volger O, Nolte B, Voss M, Schmidt M, Jakobs KH, van Koppen CJ (1999) Regulation of muscarininc receptor sequestration and function by beta-arrestin. J Biol Chem 274: 12333–12338 [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M (1999) Dissociation of functional roles of dyanamin in receptor-mediated endocytosis and mitogenic signal transduction. J Biol Chem 274: 24575–24578 [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zhang S, Kaplan MH (2000) The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-γ expression. J Immunol 165: 1374–1380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


