Abstract
Some of the important open questions concerning the physiology of the secretory pathway relate to its homeostasis. Secretion involves a number of separate compartments for which their transport activities should be precisely cross-coordinated to avoid gross imbalances in the trafficking system. Moreover, the membrane fluxes across these compartments should be able to adapt to environmental ‘requests' and to respond to extracellular signals. How is this regulation effected? Here, we consider evidence that endomembrane-based signalling cascades that are similar in organization to those used at the plasma membrane coordinate membrane traffic. If this is the case, this would also represent a model for a more general inter-organelle signalling network for functionally interconnecting different intracellular activities, a necessity for the maintenance of cellular homeostasis and to express harmonic global cellular responses.
Keywords: Golgi, membrane transport, signalling
In vivo physiology of the secretory pathway
The genetics and biochemistry of intracellular membrane trafficking have been progressively elucidated over the last few decades, and some of the molecular machineries underlying elementary transport events, such as membrane bending, budding, fusion and fission, are now understood in great detail (Lee et al, 2004). In contrast, there has been much less progress towards a true understanding of supramolecular, or ‘global', aspects of the trafficking system that underlie the physiology of membrane transport in vivo. Among these are the issues concerning the micro-anatomy and micro-dynamics of the trafficking compartments and the organizational principles of transport in vivo (Trucco et al, 2004; Luini et al, 2005), some aspects of which have seen significant advances only recently.
The reasons for this knowledge gap are multiple, and although these include historical/cultural aspects, the main cause has been the lack of suitable technologies. An understanding of the physiology of trafficking requires the visualization of traffic events in vivo with high resolution in time and space—a possibility that has only become (partially) available in the last few years owing to the advent of increasingly powerful video-microscopy and electron tomography approaches, as well as of effective ancillary techniques (e.g. traffic synchronization and correlative electron microscopy) (Polishchuk et al, 2000). These advances have spurred a series of studies that have led to new models of the organization of transport in vivo—models that, incidentally, have not always been easy to reconcile with previous schemes that were based mostly on genetic and in vitro biochemical data (Mellman and Warren, 2000; Pelham and Rothman, 2000; Lee et al, 2004; Behnia and Munro, 2005). Moreover, as a natural consequence of these studies, new fundamental issues about the physiology of traffic have come to the fore. One in particular is the issue of the identity of the mechanism underlying the internal coordination and homeostasis of these compartments as well as the regulation of transport organelles by exogenous cues. Here, we will focus on the signalling circuits that might support the coordination of the compartments of the secretory pathway.
Homeostasis of the secretory compartments
Intracellular trafficking pathways comprise a number of anatomically separate compartments that are constantly exchanging their membranes and cargo proteins in an organized sequence of trafficking segments (e.g. endoplasmic reticulum (ER) to Golgi complex, Golgi complex to plasma membrane (PM), etc.). The membrane fluxes along each of these segments should be precisely coordinated under physiological conditions to avoid gross imbalances in the system; moreover, the overall trafficking fluxes (including those regulated and constitutive) should be able to adapt to environmental ‘requests' and to react to extracellular signals as part of the global cellular response.
How is this regulation effected? Several mechanisms are in principle conceivable. For instance, it is possible to explain the ability of the secretory system to control traffic fluxes across different compartments and to maintain the size and composition of these compartments simply on the basis of a few parameters, such as the properties and amounts of the coat and SNARE proteins in the system (Bonifacino and Glick, 2004; Heinrich and Rapoport, 2005). Moreover, it is possible that simple constitutive phosphorylation and dephosphorylation events, such as those proposed to participate in the clathrin-dependent endocytic cycle, might also have roles in complex trafficking responses (Flett et al, 2005). Other considerations, however, suggest that more sophisticated control mechanisms might exist (see below), superimposed onto these basic ‘automatic' processes, acting perhaps in the same way in which computerized electronic circuits regulate the performance of mechanical parts of modern cars. Here, we will consider one specific hypothesis; namely, that these regulatory circuits involve endomembrane-based signalling cascades that are similar in their organization and components to those used by the cell at the PM. Moreover, these signalling cascades should be initiated by traffic itself and affect the activity of other organelles (this type of signalling organization could therefore be defined as ‘inter-organelle signalling'). This hypothesis is supported by two main arguments. The first is that a variety of ‘classical' signalling molecules, including G proteins, kinases and phospholipases, are physically associated with secretory endomembranes, and in particular with the Golgi complex (Stow and de Almeida, 1993; Denker et al, 1996; Donaldson and Lippincott-Schwartz, 2000; Bard et al, 2002; Nagahama et al, 2002; Bivona and Philips, 2003; Wylie et al, 2003; Larocca et al, 2004; Preisinger et al, 2004; Diaz Anel and Malhotra, 2005; Ghanekar and Lowe, 2005; Le Niculescu et al, 2005). They are therefore well placed to participate in traffic-initiated signalling (Helms et al, 1998; Cabrera et al, 2003). The second, more speculative argument is that given the sophisticated spatio-temporal control that has evolved for PM-initiated signal transduction pathways (Pierce et al, 2002), it would be uneconomical for cells not to use these mechanisms to generate signals operating across intracellular organelles to mediate inter-compartment coordination of membrane trafficking.
In the following, we will review some of the available evidence pertaining to the presence of signalling molecules on endomembranes and to the role of these molecules in the regulation/coordination of the trafficking pathway. We do not plan to be exhaustive in this survey. Potentially relevant aspects of the relationship between signalling and traffic have been reviewed recently and will not be treated here. These include the well-established links between signalling and endocytosis, whereby signalling complexes generated at the PM can modulate endocytic activity and can themselves be modulated while they reside on endocytic organelles (Di Fiore and De Camilli, 2001; Sorkin and Von Zastrow, 2002; Conner and Schmid, 2003; Miaczynska et al, 2004). They also include the links between signalling and autophagy (Codogno and Meijer, 2005). Neither will we discuss the significance and the mechanisms of regulation of overall secretory traffic rates by surface receptors, aspects that have been previously reviewed by us and others (Luini and De Matteis, 1993; Bannykh et al, 1995). Rather, we will focus mainly on the secretory pathway and on a selected group of ‘traditional' signalling protein families and pathways that have been more extensively characterized in the context of secretory traffic and that provide relevant examples of the issues we are dealing with here. We will also summarize the literature data on a larger number of molecules for which only limited information is at present available, but which might turn out to be relevant to the issue being examined (Table I).
Table 1.
Signalling proteins and their functions on intracellular organelles
| Signalling protein | Organelle localization | Molecular function | Role on endomembranes | References |
|---|---|---|---|---|
| AKAP350 | Centrosome, ERGIC | A-kinase-anchoring protein | Unknown | Shanks et al (2002) |
| ATF6 | ER | Transcription factor | Transduces effects of UPR | Rutkowski and Kaufman (2004) |
| BIG2 | Golgi | Nucleotide exchange factor and AKAP | Unknown | Li et al (2003) |
| C3G | Golgi | Rap1 exchange factor | MAP kinase pathway | Radha et al (2004) |
| Cbl | Golgi | E3 ubiquitin ligase | Unknown | Bard et al (2002) |
| Cdc42 | Golgi | Small GTP-binding protein | Involved in ER-to-Golgi, TGN-to-PM and Golgi-to-ER transport | Luna et al (2002), Matas et al (2004), Stamnes (2002) |
| ERK | Golgi | Serine/threonine kinase | Involved in Golgi disassembly during mitosis | Cha and Shapiro (2001), Acharya et al (1998) |
| Fps | Golgi | Tyrosine kinase | Unknown | Zirngibl et al (2001) |
| GIV | COPI vesicles | Regulator of G-protein signalling | Unknown | Le Niculescu et al (2005) |
| GRP1 | Golgi | Ras exchange factor | Necessary for Ras activation on the Golgi | Bivona et al (2003) |
| Gαi | Golgi | Heterotrimeric GTP-binding protein | Regulates TGN-to-PM transport | Pimplikar and Simons (1993) |
| Gαi3 | Cis-Golgi | Heterotrimeric GTP-binding protein | Regulates transport of constitutively secreted proteins | Wilson et al (1994), Stow and de Almeida (1993) |
| Gαq | Golgi, mitochondria | Heterotrimeric GTP-binding protein | Unknown | Wilson et al (1994), Denker et al (1996) |
| Gαs | TGN | Heterotrimeric GTP-binding protein | TGN-to-PM transport | Pimplikar and Simons (1993) |
| Gαz | Golgi | Heterotrimeric GTP-binding protein | Maintenance of Golgi structure | Nagahama et al (2002) Yamaguchi et al (2000) |
| IRE1 | ER | Serine/threonine kinase | Transduces effects of UPR | Rutkowski and Kaufman (2004) |
| JAK-2 | Transitional ER | Tyrosine kinase | Coordinates ERES assembly | Lavoie et al (2000) |
| KDELr | ER, Golgi | Receptor protein | Transduces effects of UPR | Yamamoto et al (2003) |
| Lck | Golgi | Tyrosine kinase | Unknown | Bijlmakers et al (1997) |
| LimK | Golgi | Serine/threonine kinase | Kinase-dead mutant produces tubular processes emerging from the Golgi stack | Rosso et al (2004) |
| MEK-1 | Golgi | Serine/threonine kinase | Involved in Golgi disassembly during mitosis | Colanzi et al (2003) |
| MINK | Golgi | Serine/threonine kinase | Unknown | Hu et al (2004) |
| MST4 | Golgi | Serine/threonine kinase | Depletion by siRNA causes Golgi dispersion | Preisinger et al (2004) |
| MTG | Cis-Golgi | Myeloid translocation gene | Unknown | Asirvatham et al (2004) |
| Myomegalin | Golgi, centrosome | Scaffold protein | Unknown | Verde et al (2001) |
| PCTAIRE | ER | Cycin-dependent kinase | Regulator of ER-to-Golgi transport | Palmer et al (2005) |
| PDE4D3 | Golgi, centrosome | Phosphodiesterase | Unknown | Jin et al (1998) |
| PDE7A | Cis-Golgi | Phosphodiesterase | Unknown | Asirvatham et al (2004) |
| PERK | ER | Serine/threonine kinase | Transduces effects of UPR | Rutkowski and Kaufman (2004) |
| PI3K | Golgi | Lipid kinase | Involved in Golgi disassembly during mitosis | Domin et al (2000) |
| PKA | Golgi | Serine/threonine kinase | Regulates endosome-to-TGN, Golgi-to-ER, and TGN-to-PM transport | Birkeli et al (2003), Muniz et al (1997), Cabrera et al (2003) |
| PKC | Golgi | Serine/threonine kinase | Regulates TGN-to-PM transport and apoptosis | Buccione et al (1996) Kajimoto et al (2004) |
| PKD | Golgi | Serine/threonine kinase | TGN-to-PM transport | Diaz Anel and Malhotra (2005), Ghanekar and Lowe (2005) |
| PLK3 | Golgi | Serine/threonine kinase | Involved in Golgi disassembly during mitosis | Xie et al (2004) |
| PSKH1 | Cis-Golgi, centrosome, nuclei | Serine/threonine kinase | Mutant kinase causes Golgi disassembly | Brede et al (2003) |
| PTPH1 | ERES | Protein tyrosine phosphatase | Coordinates ERES assembly | Lavoie et al (2000) |
| Rac | TGN | Small GTP-binding protein | TGN sorting | Faucherre et al (2003) |
| Rap1 | Golgi | Small GTP-binding protein | Unknown | Wienecke et al (1996) |
| Ras | Golgi, ER | Small GTP-binding protein | Supports cell transformation via MAPK | Bivona and Philips (2003) |
| RGS2 | Golgi | Regulator of G-protein signalling | Unknown | Sullivan et al (2000) |
| RGS4 | Golgi | Regulator of G-protein signalling | Regulates Golgi-to-PM transport via interaction with β-COP | Sullivan et al (2000) |
| RGS-GAIP | TGN | Regulator of G-protein signalling | Unknown | De Vries et al (1998) |
| RGSZ | Golgi | Regulator of G-protein signalling | Regulates ER-to-PM transport and Golgi disruption | Chatterjee and Fisher (2000), Nagahama et al (2002) |
| Sef | Golgi | Scaffold protein | Spatial regulator of ERK signalling | Torii et al (2004) |
| sGαi2 | Golgi | Heterotrimeric GTP-binding protein | Maintenance of Golgi structure | Montmayeur and Borrelli (1994) |
| Src | Golgi | Tyrosine kinase | Regulates retrograde transport of KDELr | Bard et al (2003), Bard et al (2002) |
| TrkA | Golgi | Tyrosine kinase receptor | Neuronal survival | Rajagopal et al (2004) |
| XLGαs | Golgi | Heterotrimeric GTP-binding protein | Unknown | Pasolli et al (2000) Ugur and Jones (2000) |
| YSK1 | Golgi | Serine/threonine kinase | Kinase-dead and siRNA causes Golgi dispersion | Preisinger et al (2004) |
| βγ | Unknown | Heterotrimeric GTP-binding protein | TGN-to-PM transport and regulation of Golgi complex organization | Jamora et al (1999), Diaz Anel and Malhotra (2005) |
| AKAP, protein kinase A-anchoring scaffold protein; GTP, guanine 5′-triphosphate; MAP, mitogen-activated protein; PM, plasma membrane; ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment; TGN, trans-Golgi network; ERES, ER exit sites; UPR, unfolded protein response; KDELr, KDEL receptor. | The membranes of the secretory system host several signalling proteins, ranging from the heterotrimeric G-proteins to small G-proteins, scaffold proteins, kinases, phosphodiesterases, GTPase-activating proteins and guanine nucleotide exchange factors. For many of these, the only information available relates to the effects of their perturbation on Golgi morphology and function, with their physiological roles remaining, as yet, unknown. |
While this evidence will be discussed in the light of an inter-organelle signalling hypothesis for traffic coordination (Figure 1A), it is also necessary to consider other schemes that can explain the presence of signalling molecules on endomembranes. An alternative possibility, for instance, is that Golgi-based transduction proteins might serve to mediate inputs that initiate at the PM but regulate Golgi function (Figure 1C), as shown previously by us and others (De Matteis et al, 1993; Luton et al, 1999) (a similar regulatory mechanism has been shown to exist also in the case of endocytosis (Sorkin and Von Zastrow, 2002; Miaczynska et al, 2004)). Furthermore, it is possible that transduction molecules present on the Golgi complex act as relay devices in signalling networks initiated at the PM that control cell growth and other functions (Figure 1D). This has been shown to be the case for Golgi- and endosome-based signalling proteins (Di Fiore and De Camilli, 2001; Sorkin and Von Zastrow, 2002; Bivona et al, 2003; Miaczynska et al, 2004). Finally, signalling proteins might simply be in transit through the Golgi to the PM following their synthesis (Michaelson et al, 2002) (Figure 1E). It should also to be borne in mind that these models (schematized in Figure 1) are not mutually exclusive.
Figure 1.
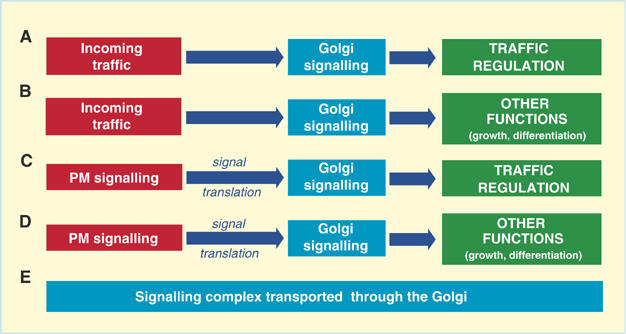
Generic models for the roles of signalling proteins at the Golgi complex. (A, B). Direct activation of Golgi signalling by endogenous events (e.g. incoming traffic)—self-regulation of the trafficking pathway (A) and/or regulation of other cellular functions (B); (C) activation of PM signals—signal translation—activation of Golgi signaling—regulation of trafficking; (D) activation of PM signals—signal translation—activation of Golgi signaling—regulation of other cellular functions (e.g. cell proliferation, motility); (E) passive residence of signalling molecules in transit to the PM.
The cAMP-PKA pathway
The centrepiece of the cAMP-PKA pathway is protein kinase A (PKA), a tetrameric serine/threonine kinase that consists of two regulatory and two catalytic subunits. The kinase becomes active when the catalytic and regulatory subunits dissociate in the presence of cAMP. In addition to PKA, the pathway includes the enzyme adenylyl cylase (AC, which synthesizes cAMP) and AC activators (such as Gαs), pathway inactivating phosphodiesterases (PDEs, which hydrolyze cAMP) and the PKA-anchoring scaffold proteins(s) (AKAPs, see below). Remarkably, all of these components have been shown to be located on endomembranes, and particularly on the Golgi complex (Cheng and Farquhar, 1976a, 1976b; Maier et al, 1995; Denker et al, 1996; Pooley et al, 1997; Martin et al, 1999; Birkeli et al, 2003; Li et al, 2003; Asirvatham et al, 2004; Larocca et al, 2004); our unpublished observations).
The cAMP-PKA pathway can exert potent effects on many trafficking steps. Transport from the ER to the Golgi complex can be accelerated through stimulation of cAMP synthesis and impaired by the PKA inhibitor H89 (Muniz et al, 1996). However, this transport step is not affected by other PKA blockers (i.e. KT5720 and PKI) (Aridor and Balch, 2000; Lee and Linstedt, 2000), suggesting that H89 acts through other kinase(s) and indicating that while the cAMP-PKA cascade can accelerate this step, it is not required for its execution at steady state. PKA also regulates intra-Golgi transport and transport from the trans Golgi network (TGN) to the PM (Muniz et al, 1996). Velasco and co-workers showed that PKA catalytic activity is required for the budding of constitutive transport vesicles from the TGN in vitro (Muniz et al, 1997) and that the stimulation of cAMP synthesis in vivo accelerates transport from the Golgi to the PM. An increase in cAMP also modifies the structure of the Golgi complex, with induction of the formation of numerous tubules interconnecting nonequivalent Golgi cisternae (Muniz et al, 1996). Finally, the activity of PKA appears also to be required for retrograde transport from the endosomal compartment to the Golgi complex and from the Golgi to the ER (Birkeli et al, 2003) (Cabrera et al, 2003).
The mechanisms of action through which PKA exerts these effects have been identified only in part. One mechanism relates to the ability of PKA to recruit ADP ribosylation factor-1 (ARF1) to the Golgi complex (Martin et al, 2000). ARF1 belongs to the Ras superfamily of small GTP-binding proteins and is the master regulator of many fundamental steps along the secretory pathway, including the assembly of coat proteins, such as COPI and clathrin (Donaldson et al, 2005). The recruitment of ARF1 from the cytosol to Golgi membranes has been shown to be increased by the catalytic portion of PKA and by cAMP (Martin et al, 2000). Conversely, ARF1 recruitment is impaired by PKI (a selective PKA inhibitor) and by PKA depletion (Martin et al, 2000). This has been confirmed in vivo by direct stimulation of cAMP formation using forskolin, which led to an increase in the recruitment of ARF1 to the Golgi complex (Martin et al, 2000).
Another target of PKA that might contribute to the regulation of membrane transport is the KDEL receptor (KDELr). The KDELr is a seven-transmembrane-domain protein that cycles between the ER and the Golgi complex (Lewis and Pelham, 1992). In doing so, it returns to the ER the ER-resident chaperones that have leaked through to post-ER compartments during membrane transport. PKA phosphorylates a COPI-binding motif on the C-tail of the KDELr, which is necessary for the retrograde transport of the KDELr itself (Cabrera et al, 2003). PKA also phosphorylates certain SNAREs (soluble N-ethyl-maleimide-sensitive fusion protein attachment protein receptor) and regulates their ability to support membrane fusion in endocytic and exocytic steps in mammals (Hong, 2005), as well as in ER-to-Golgi and intra-Golgi transport in yeast (Hong, 2005; Weinberger et al, 2005). Furthermore, PKA is involved in the ER quality control process for a class of proteins that bear the RXR transient-ER-retention motif through which they bind to COPI (Michelsen et al, 2005). These include G-protein-coupled receptors (GPCRs) and multimeric ion channels, the transport of which is delayed in the ER through a COPI-mediated retrieval mechanism. When these protein complexes are properly assembled, their RXR motif is masked by an interaction with the partner subunit(s) or by PKA or protein kinase C (PKC)-mediated phosphorylation of a nearby serine or threonine, which itself generates a binding site for the adaptor protein 14-3-3 that prevents the RXR motif from interacting with COPI (Kuwana et al, 1998; Michelsen et al, 2005).
The presence of the components of the cAMP-PKA pathway (including those responsible for the formation of cAMP) on the Golgi and their regulation of traffic through this organelle is consistent with a scheme (Figure 1A) in which PKA-based Golgi-initiated signalling coordinates the activities of different Golgi subcompartments. However, the complete circuit in which this pathway might be involved, and the circumstances in which this circuit might be activated, remains to be defined, and alternative explanations for the presence of the pathway components on the Golgi cannot be excluded at this time (e.g. schemes in Figure 1B and C).
The DAG-PKC pathway
The protein kinase C (PKC) are a family of at least 10 serine/threonine kinases that can be structurally and functionally subclassified into three main groups: those Ca2+- and diacylglycerol (DAG)-dependent, those calcium-independent and those atypical, calcium- and DAG-independent. Most of these can localize to the Golgi complex via their C1 lipid-binding domains (Schultz et al, 2004), which can bind to DAG and/or to ceramides, retinoic acid and archidonic acid. This binding can have important functional consequences. In neuroblastoma and HeLa cells treated with ceramides, PKCδ and PKCɛ show a C1-domain-dependent translocation to the Golgi complex, and this translocation is necessary for the apoptotic effects promoted by ceramides (Kajimoto et al, 2001; Schultz et al, 2003; Kajimoto et al, 2004). Similar effects are seen with IFN-γ treatment: this acts via ceramide production and the consequent translocation of PKCδ to the Golgi complex, which results in cell apoptosis. PKCδ is activated only after its Golgi translocation, via direct phosphorylation on tyrosines 311 and 332 by a Golgi-located Src, and this phosphorylation is necessary for the apoptotic effect promoted by PKCδ (Kajimoto et al, 2004). Another Golgi targeting mechanism is via the RACK (receptor for activated PKC)-anchoring proteins. PKCɛ is targeted to the Golgi complex via a direct interaction with the β′ subunit of the coatomer β′-COP, which acts as a RACK through its characteristic WD-40 repeat that is found in other RACKs (Csukai et al, 1997).
The recruitment of the PKCs to endomembranes also has regulatory effects on traffic. The intermediate compartment that operates between the ER and the Golgi is an important sorting station where anterograde-directed proteins are segregated from recycling proteins. Here, Rab2, a small GTPase and a key player in the intermediate compartment function, recruits PKCι/λ (Tisdale and Artalejo, 2006), which promotes the binding of COPI to intermediate compartment membranes (Tisdale, 2000, 2003), and phosphorylates glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH then ultimately influences microtubule dynamics and transport in the early secretory pathway (Tisdale, 2002). ER-to-Golgi transport is impaired by the specific PKC inhibitor calphostin C, whereas it is activated by DAG analogues (e.g. phorbol 12-myristate 13-acetate (PMA)) (Fabbri et al, 1994). However, several other PKC inhibitors that act as ATP competitors (e.g. H7, H8, staurosporine) and the downregulation of PKC have no effects on ER-to-Golgi transport, thus casting doubts as to the role of PKC here, and suggesting instead the involvement of another (as yet unidentified) protein that contains a DAG-binding domain (Fabbri et al, 1994).
Constitutive intra-Golgi and TGN-to-PM transport can be modulated by PKC (De Matteis et al, 1993; Fabbri et al, 1994). De Matteis et al (1993) have demonstrated that the transport of glucosaminoglycans (GAGs) and the temperature-sensitive variant of the G protein of vesicular stomatitis virus (VSVG) from the Golgi to the PM requires PKC activity (Buccione et al, 1996), and that the direct activation of PKC by PMA or via activation of the IgE receptor stimulates GAG secretion. In this context, the action of PKC is likely to involve more than one mechanism, including the regulation of the GTP-dependent binding of ARF1 to Golgi membranes (De Matteis et al, 1993). As the IgE receptor has been shown to stimulate traffic from the ER to the Golgi in addition to Golgi-to-PM traffic (Bannykh et al, 1995; Buccione et al, 1996), these data identify a case of stimulation of the entire constitutive secretory pathway by an extracellular ligand. The significance of these observations has been discussed previously (Luini and De Matteis, 1993). In an analogous study, Fabbri et al (1994) showed that the PKC inhibitor calphostin C blocks the transport of VSVG from the TGN to the PM. Using an in vitro system to generate post-Golgi vesicles, Sabatini and co-workers showed that PKC is involved in membrane carrier fission from the TGN (however, they proposed that in this case PKC does not act via its kinase activity) (Simon et al, 1996). Finally, in a series of studies that is further discussed below, the Malhotra laboratory (see above) showed that PKCη acts as a kinase that activates protein kinase D (PKD) and that this is required for the fission of TGN carriers (Diaz Anel and Malhotra, 2005).
Therefore, PKC on the Golgi complex appears to be involved in at least two main functions: apoptosis-related signalling and the regulation of traffic. One of the PKC-dependent pathways that controls traffic initiates at the cell surface with the activation of IgE receptors, and so this fits well with scheme C in Figure 1. Whether PKC is also activated on the Golgi by traffic itself (scheme A in Figure 1) is unknown, but it is possible that traffic can induce changes in the lipid composition (DAG, ceramides) in specific Golgi domains, which could then result in the regulation of PKC activity (Baron and Malhotra, 2002; Diaz Anel and Malhotra, 2005).
Heterotrimeric G proteins
Heterotrimeric G proteins are a family of GTPases that are primarily involved in the transduction of signals initiated at the PM by seven-transmembrane-domain receptors. However, they have also been proposed to have direct roles in membrane transport based on the evidence that a number of different Gα subunits are located on internal membranes, including the Golgi complex (Denker et al, 1996; Helms et al, 1998; Qian et al, 2002), and that manipulation of the levels or activities of these G proteins markedly affects the functioning of transport pathways.
The distribution of the different Gα subunits on endomembranes has been the target of many studies in different laboratories (Stow et al, 1991b; Wilson et al, 1994). Stow and co-workers demonstrated that Gαi3 is present on Golgi membranes in epithelial cells (Stow et al, 1991a; Stow and de Almeida, 1993) and Farquhar and co-workers (Wilson et al, 1994) showed that Gαi3 is mostly on the cis side of the Golgi in pituitary cells. A partial Golgi distribution was also described for Gαq (Denker et al, 1996). Both a short and a long form of Gαs have been identified in the Golgi in liver and adrenal medulla cells (Maier et al, 1995), and Huttner and co-workers showed that Gαs colocalizes with the trans-Golgi marker protein TGN38 in PC12 cells (Leyte et al, 1992). Gαz has also been shown to localize to the Golgi complex in BHK cells, where it overlaps with the Golgi marker mannosidase II and follows the redistribution of Golgi proteins after treatment with brefeldin A (BFA, a fungal toxin that disassembles and redistributes the Golgi into the ER) (Nagahama et al, 2002).
From a functional standpoint, several secretory transport steps have been proposed to be regulated by heterotrimeric G proteins on the basis of a variety of lines of evidence. Transport from the ER to the Golgi complex was suggested to be controlled by these G proteins, since mastoparan (an activator of Gαi) causes a block in the exit of VSVG from the ER (Schwaninger et al, 1992). A similar result was also seen in permeabilized cells treated with purified βγ subunits (which deplete the Gα pool by associating with it), suggesting that both activatory and inhibitory heterotrimeric G proteins are involved in the export of proteins from the ER.
Heterotrimeric G proteins have also been implicated in intra-Golgi and post-Golgi trafficking. Manipulation (overexpression or treatment with pertussis toxin) of Gαi3 (which is located in the Golgi; see above) indicated that this protein has an overall inhibitory role on anterograde secretory traffic (Stow et al, 1991a). COPI (a protein complex crucially involved in intra-Golgi traffic (Rothman, 1994)) has been shown to interact with the hetrotrimeric G protein GTPase-activating protein RGS4 (regulator of G protein signalling-4) (Sullivan et al, 2000). This interaction impairs COPI binding to Golgi membranes (although ARF1 localization and Golgi integrity are not affected), and apparently results in inhibition of the transport of acquaporin 1 to the PM and the secretion of placental alkaline phosphatase. Thus, it was hypothesized that RGS4 regulates transport through the sequestration of β-COP from the cytosol, with this sequestration inhibiting β-COP recruitment to Golgi membranes. Potentially linked to these observations, Velasco and co-workers showed that the in vitro interaction of PKA with Golgi membranes is sensitive to modulators of heterotrimeric G proteins, and that myristoylated Gαi3 can stimulate this interaction, suggesting a role for G proteins in the recruitment of PKA to Golgi membranes (Martin et al, 1999). PKA participates in COPI-dependent retrograde transport of the KDELr by the phosphorylation of the C-tail of the KDELr, which is mandatory for its interaction with β-COP and its inclusion in COPI-coated vesicles (see also below). Collectively, the above data suggest that G-protein-dependent signalling is involved in the regulation of the COPI trafficking machinery, although the precise mechanism of this involvement remains unclear.
Heterotrimeric G proteins also appear to be involved in the regulation of TGN-to-PM transport. In an early study, Pimplikar and Simons (1993) showed that treatment with reagents that influence Gαs specifically impair apical, and not basolateral, transport. On the other hand, a selectivity towards basolateral transport was seen with treatments influencing Gαi, leading to the conclusion that Gαs and Gαi could be involved in the regulation of polarized TGN-to-PM transport. In addition to Gα subunits, the βγ subunits appear to have a role in transport from the TGN. In what is the most complete series of studies in this area to date, Malhotra and co-workers showed that PKD, a key regulator of membrane fission at the TGN, is activated by a signalling pathway that involves βγ subunits (β1γ2/β3γ2), and that this activation passes through PKCη, in this way regulating transport to the cell surface and organization of the Golgi complex (Diaz Anel and Malhotra, 2005) (Liljedahl et al, 2001; Yeaman et al, 2004). RGS proteins are also present on the TGN, and it has been shown that RGS-Gα-interacting protein (RGS-GAIP) is localized to clathrin-coated buds and vesicles in the Golgi region. However, at this stage, the function of RGS-GAIP remains to be defined (De Vries et al, 1998; Wylie et al, 1999, 2003; Chatterjee and Fisher, 2000; Gleeson et al, 2004).
Gαz and Gαi2 also appear to have key roles in the maintenance of the overall structure of the Golgi complex (Yamaguchi et al, 2000). Their overexpression results in inhibition of nordihydroguaiaretic acid (NDGA)-induced disassembly of the Golgi complex. Conversely, overexpression of the regulator (repressor) of Gαz signalling (RGSz) stimulated disruption of the Golgi complex, and delayed ER-to-PM transport of VSVG (Nagahama et al, 2002).
Thus, there is abundant (albeit heterogeneous) evidence for a role for heterotrimeric G proteins and of some of their accessory proteins in secretory transport, as well as in the control of the structure of the Golgi. The fact that they are both present and able to exert effects on this organelle suggests that they might operate within local regulatory circuits, which would be consistent with scheme A in Figure 1. However, a coherent picture of the pathway(s) in which they are involved remains to be defined.
The Ras signalling pathway
Ras is the prototype of a large family of small GTPases that are involved in multiple cellular functions, including the transduction of the effects of many extracellular ligands, such as growth factors, via PM receptors. Recently, Ras has been shown to be present on the Golgi and to be activated there as a consequence of PM-based signalling. Different Ras family proteins localize to distinct subdomains of the PM (Prior et al, 2001) and/or to various intracellular compartments, including the Golgi, in a palmitoylation-dependent fashion (Choy et al, 1999; Michaelson et al, 2001; Rocks et al, 2005). Moreover, downstream elements of the Ras pathway have also been demonstrated to be located on the Golgi complex (Philips, 2004). Over the last few years, this has prompted a number of studies to address the question of whether these proteins can actually signal from endomembranes. In particular, Philips and co-workers (Chiu et al, 2002) investigated the pool of Ras localized on endomembranes using an in vivo probe for activated Ras. They concluded that stimulation with mitogens results in the activation of Ras both on the PM and on the Golgi complex, but with different kinetics and through different mechanisms (some of these findings have, however, been disputed by others (Augsten et al, 2006). Philips and colleagues have also shown that activation of H-Ras on the Golgi complex, but not on the PM, is dependent on Src-family kinases (Chiu et al, 2002). Thus, following Src-dependent activation of phospholipase Cγ (PLCγ), Ras guanine nucleotide releasing protein 1 (RasGRP1) translocates to the Golgi complex, where it activates Ras (Bivona and Philips, 2003). This pathway differs from that at the PM, indicating that distinct modes of activation and deactivation can regulate Ras in different subcellular compartments. This strategy might allow the cell to differentially regulate the activation of Ras in different cellular locations (Bivona and Philips, 2003).
As noted, not only Ras but also other components of the Ras pathway appear to be located on the Golgi complex. Recently, Nishida and co-workers (Torii et al, 2004) demonstrated the ‘spatial' modulatory activity of a novel scaffold protein, Sef, which is localized to the Golgi. Sef had been previously identified as a negative regulator of FGF signalling, and here it was shown to bind the active MEK/ERK complex, anchoring it to the Golgi complex (Sorkin, 2005). When ERK is trapped within this heterotrimeric complex, it can still phosphorylate one of its cytosolic substrates (RSK2), although it cannot dissociate and translocate to the nucleus to activate the array of nuclear ERK effectors (e.g. Elk-1). Reinforcing the concept that Sef works as a spatial modulator, it was shown that Golgi-entrapped ERK can still phosphorylate the nuclear target Elk-1 if the latter is made to move into the cytosol (Torii et al, 2004). What is still unknown is which proteins control the function of Sef and when they promote the switch from nuclear to cytosolic signalling of the ERK pathway. To complete the picture, Sef activation switches off the nuclear ERK pathway while activating the TAK1/JNK apoptotic pathway (Yang et al, 2004).
Finally, it has been shown that the nerve growth factor (NGF) receptor (TrkA) can localize to the Golgi complex, where it can be functionally activated, and thus impinge on the Ras pathway (Sorkin, 2005). Stimulation of the G protein-coupled receptors for adenosine or pituitary adenylate cyclase-activating polypeptide (PACAP) in neuronal cells has been shown to support cell survival via trans-activation of the pool of Golgi-based TrkA receptor. TrkA transactivation requires new protein synthesis and engages a population of immature receptors that are localized exclusively at the Golgi complex (Rajagopal et al, 2004). A BFA treatment, which results in Golgi disassembly and redistribution into the ER, completely prevented TrkA transactivation, while not affecting its stimulation via NGF from the PM, indicating the requirement of a preserved Golgi structure for this signalling pathway. Moreover, trans-activation of TrkA on the Golgi complex depends on Src and calcium ions (Rajagopal et al, 2004).
To date, these observations have been interpreted as indicating a role of the Ras signalling pool on the Golgi complex as a relay device for signalling cascades that are initiated at the PM and are involved in growth and proliferation (Figure 1D). There is no evidence so far for a link between Ras and the regulation of traffic. If this is so, the case of Ras might be different from that of the signalling pathways discussed above, which appeared to be involved in traffic regulation.
Physiological significance of endomembrane-based signalling and concluding remarks
In addition to the signalling pathways discussed above, a myriad of other signalling molecules of various kinds have been found to reside on secretory compartments, and in particular in the Golgi complex (Table 1). Most of these are only partially (or not yet) characterized in terms of their function at the Golgi, or of their precise subcompartment locations. This collective body of knowledge strongly suggests the existence of a hugely complex and rich endomembrane-based signalling network, the function and connectivity of which are only beginning to be glimpsed at.
To begin to bring order to this complex body of information, we propose the five generic model pathways illustrated in Figure 1. Evidence is so far available for the sequence in Figure 1C (activation of PM signals—signal translation— activation of Golgi signaling—regulation of traffic (De Matteis et al, 1993; Fabbri et al, 1994; Buccione et al, 1996) and Figure 1D (activation of PM signals—signal translation—activation of Golgi signaling—regulation of other functions (e.g. cell proliferation and motility) (Bivona and Philips, 2003). Moreover, for some G proteins, evidence has been produced that their Golgi location reflects their transit or cycling through the secretory pathway (Michaelson et al, 2002; Takida and Wedegaertner, 2004; Rocks et al, 2005) (Figure 1E). The formal demonstration of direct activation of a Golgi-based signalling pathway by a traffic event (Figure 1A and B) is instead still lacking, despite a large number of tantalizing observations that have indicated that such pathways exist. However, an example of initiation of signalling cascades on a secretory organelle by endogenous events is provided by the case of the ER stress responses (Patil and Walter, 2001; Zhang and Kaufman, 2004), whereby ER stresses can activate signalling pathways on the cytosolic surface of the ER, which then regulate survival-related and other cellular functions.
The overall picture discussed above defines almost by itself the tasks being called for. Much is known about the endomembrane localization and the effects on traffic of signalling molecules. What is largely lacking, however, is an understanding of how information flows through this maze of signalling molecules that would allow us to define complete specific pathways, along with their physiological significance and context. In particular, the question of the activation mechanisms of signalling complexes at trafficking endomembranes remains open. Let us take, for instance, the case of the heterotrimeric G proteins. How might they be activated at their intracellular sites? GPCR-like molecules that can bind G proteins on their cytosolic tail have been reported to reside on internal endomembranes (Boivin et al, 2003; Konger et al, 2005). Moreover, more than one type of GPCR-unrelated molecule has been reported that might in principle participate in the activation of G proteins at the Golgi complex (Blumer and Lanier, 2003). However, no information is available so far on possible functional links between any of these molecules and the Golgi heterotrimeric G proteins, nor is it known whether the initial activating stimuli are driven by extracellular signals or by endogenous (e.g. traffic initiated) events. Also, the identity of the G protein effectors on endomembranes remains unclear, although there is some evidence for the existence of traffic regulation by ‘classical' G-protein-effector pathways (as discussed above, and see Figure 2). Similar questions apply to other signalling pathways. The discovery of endogenous activation mechanisms that can initiate inter-organelle signalling would greatly advance our understanding of the coordination of the many intracellular activities involved in the maintenance of harmonic global cellular behaviour, a critical, yet largely unexplored, aspect of the physiology of traffic and of other cell functions.
Figure 2.
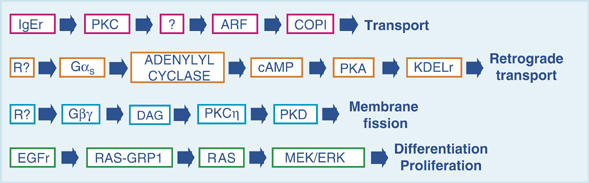
Representative Golgi-based signalling pathways. Several signalling complexes have been shown to be present on intracellular organelles. Here we depict the main elements of four representative pathways: the IgE receptor (IgEr) on the PM activates PKC, which promotes the recruitment of ARF1 and COPI to the Golgi complex via an unknown mediator. This pathway regulates the transport of cargo toward the PM. A hypothetical receptor (R?) with a potential intracellular location activates Gαs that, in turn, activates adenylyl cyclase, to generate the cAMP that is necessary for activation of PKA at the Golgi complex. This active PKA phosphorylates the KDELr, uncovering the COPI-binding motif that is necessary for its retrograde transport. A hypothetical receptor (R?) activates a heterotrimeric G protein to release its βγ subunit. This generates diacylglycerol (DAG) through an unknown mechanism, which in turn activates PKCη. The subsequent phosphorylation and activation of PKD is required for membrane fission at the TGN. Stimulation of the EGF receptor (EGFr) produces via PLCγ the Ca2+ and DAG that are necessary for translocation of the Ras exchange factor Ras-GRP1 to the Golgi complex. Ras-GRP1 activates Ras on the Golgi, and the resulting activation of the MAP kinase (MEK/ERK) pathway supports basic cellular functions, such as cell growth and differentiation.
Acknowledgments
We thank CP Berrie and C Wilson for editorial assistance, and E Fontana for artwork preparation. We also like to apologise to all of the authors of original studies whom we have not been able to reference and for the general use of review referencing, both of which arise from space limitations. We also acknowledge and thank the Italian Association for Cancer Research (AIRC, Milan, Italy), Telethon Italia and the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, Italy) for financial support. TP is the recipient of a fellowship of the Italian Foundation for Cancer Research (FIRC, Milan, Italy) and a fellowship from Fondazioni Bancarie Abruzzesi and Fondazione Negri Sud ONLUS (Progetto Sviluppo Sud).
References
- Acharya U, Mallabiabarrena A, Acharya JK, Malhotra V (1998) Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92: 183–192 [DOI] [PubMed] [Google Scholar]
- Aridor M, Balch WE (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem 275: 35673–35676 [DOI] [PubMed] [Google Scholar]
- Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, Carr DW (2004) A-kinase anchoring proteins interact with phosphodiesterases in T lymphocyte cell lines. J Immunol 173: 4806–4814 [DOI] [PubMed] [Google Scholar]
- Augsten M, Pusch R, Biskup C, Rennert K, Wittig U, Beyer K, Blume A, Wetzker R, Friedrich K, Rubio I (2006) Live-cell imaging of endogenous Ras-GTP illustrates predominant Ras activation at the plasma membrane. EMBO Rep 7: 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh S, Aridor M, Plutner H, Rowe T, Balch WE (1995) Regulated export of cargo from the endoplasmic reticulum of mammalian cells. Cold Spring Harb Symp Quant Biol 60: 127–137 [DOI] [PubMed] [Google Scholar]
- Bard F, Mazelin L, Pechoux-Longin C, Malhotra V, Jurdic P (2003) Src Regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum. J Biol Chem 278: 46601–46606 [DOI] [PubMed] [Google Scholar]
- Bard F, Patel U, Levy JB, Jurdic P, Horne WC, Baron R (2002) Molecular complexes that contain both c-Cbl and c-Src associate with Golgi membranes. Eur J Cell Biol 81: 26–35 [DOI] [PubMed] [Google Scholar]
- Baron CL, Malhotra V (2002) Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295: 325–328 [DOI] [PubMed] [Google Scholar]
- Behnia R, Munro S (2005) Organelle identity and the signposts for membrane traffic. Nature 438: 597–604 [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Isobe-Nakamura M, Ruddock LJ, Marsh M (1997) Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol 137: 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeli KA, Llorente A, Torgersen ML, Keryer G, Tasken K, Sandvig K (2003) Endosome-to-Golgi transport is regulated by protein kinase A type II alpha. J Biol Chem 278: 1991–1997 [DOI] [PubMed] [Google Scholar]
- Bivona TG, Perez DC, I Ahearn IM, Grana TM, Chiu VK, Lockyer PJ, Cullen PJ, Pellicer A, Cox AD, Philips MR (2003) Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424: 694–698 [DOI] [PubMed] [Google Scholar]
- Bivona TG, Philips MR (2003) Ras pathway signaling on endomembranes. Curr Opin Cell Biol 15: 136–142 [DOI] [PubMed] [Google Scholar]
- Blumer JB, Lanier SM (2003) Accessory proteins for G protein-signaling systems: activators of G protein signaling and other nonreceptor proteins influencing the activation state of G proteins. Receptors Channels 9: 195–204 [PubMed] [Google Scholar]
- Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG (2003) Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem 278: 29153–29163 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116: 153–166 [DOI] [PubMed] [Google Scholar]
- Brede G, Solheim J, Stang E, Prydz H (2003) Mutants of the protein serine kinase PSKH1 disassemble the Golgi apparatus. Exp Cell Res 291: 299–312 [DOI] [PubMed] [Google Scholar]
- Buccione R, Bannykh S, Santone I, Baldassarre M, Facchiano F, Bozzi Y, Di Tullio G, Mironov A, Luini A, De Matteis MA (1996) Regulation of constitutive exocytic transport by membrane receptors. A biochemical and morphometric study. J Biol Chem 271: 3523–3533 [DOI] [PubMed] [Google Scholar]
- Cabrera M, Muniz M, Hidalgo J, Vega L, Martin ME, Velasco A (2003) The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol Biol Cell 14: 4114–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H, Shapiro P (2001) Tyrosine-phosphorylated extracellular signal-regulated kinase associates with the Golgi complex during G2/M phase of the cell cycle: evidence for regulation of Golgi structure. J Cell Biol 153: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Fisher RA (2000) Cytoplasmic, nuclear, and golgi localization of RGS proteins. Evidence for N-terminal and RGS domain sequences as intracellular targeting motifs. J Biol Chem 275: 24013–24021 [DOI] [PubMed] [Google Scholar]
- Cheng H, Farquhar MG (1976a) Presence of adenylate cyclase activity in Golgi and other fractions from rat liver. I. Biochemical determination. J Cell Biol 70: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Farquhar MG (1976b) Presence of adenylate cyclase activity in Golgi and other fractions from rat liver. II. Cytochemical localization within Golgi and ER membranes. J Cell Biol 70: 671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL II, Cox AD, Philips MR (2002) Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol 4: 343–350 [DOI] [PubMed] [Google Scholar]
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR (1999) Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98: 69–80 [DOI] [PubMed] [Google Scholar]
- Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12 (Suppl 2): 1509–1518 [DOI] [PubMed] [Google Scholar]
- Colanzi A, Sutterlin C, Malhotra V (2003) RAF1-activated MEK1 is found on the Golgi apparatus in late prophase and is required for Golgi complex fragmentation in mitosis. J Cell Biol 161: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Csukai M, Chen CH, De Matteis MA, Mochly-Rosen D (1997) The coatomer protein beta'-COP, a selective binding protein (RACK) for protein kinase Cepsilon. J Biol Chem 272: 29200–29206 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Santini G, Kahn RA, Di Tullio G, Luini A (1993) Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature 364: 818–821 [DOI] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG (1996) Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol 133: 1027–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries L, Elenko E, McCaffery JM, Fischer T, Hubler L, McQuistan T, Watson N, Farquhar MG (1998) RGS-GAIP, a GTPase-activating protein for Galphai heterotrimeric G proteins, is located on clathrin-coated vesicles. Mol Biol Cell 9: 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Anel AM, Malhotra V (2005) PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol 169: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P (2001) Endocytosis and signaling an inseparable partnership. Cell 106: 1–4 [DOI] [PubMed] [Google Scholar]
- Domin J, Gaidarov I, Smith ME, Keen JH, Waterfield MD (2000) The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J Biol Chem 275: 11943–11950 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Honda A, Weigert R (2005) Multiple activities for Arf1 at the Golgi complex. Biochim Biophys Acta 1744: 364–373 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J (2000) Sorting and signaling at the Golgi complex. Cell 101: 693–696 [DOI] [PubMed] [Google Scholar]
- Fabbri M, Bannykh S, Balch WE (1994) Export of protein from the endoplasmic reticulum is regulated by a diacylglycerol/phorbol ester binding protein. J Biol Chem 269: 26848–26857 [PubMed] [Google Scholar]
- Faucherre A, Desbois P, Satre V, Lunardi J, Dorseuil O, Gacon G (2003) Lowe syndrome protein OCRL1 interacts with Rac GTPase in the trans-Golgi network. Hum Mol Genet 12: 2449–2456 [DOI] [PubMed] [Google Scholar]
- Flett A, Semerdjieva S, Jackson AP, Smythe E (2005) Regulation of the clathrin-coated vesicle cycle by reversible phosphorylation. Biochem Soc Symp 72: 65–70 [DOI] [PubMed] [Google Scholar]
- Ghanekar Y, Lowe M (2005) Protein kinase D: activation for Golgi carrier formation. Trends Cell Biol 15: 511–514 [DOI] [PubMed] [Google Scholar]
- Gleeson PA, Lock JG, Luke MR, Stow JL (2004) Domains of the TGN: coats, tethers and G proteins. Traffic 5: 315–326 [DOI] [PubMed] [Google Scholar]
- Heinrich R, Rapoport TA (2005) Generation of nonidentical compartments in vesicular transport systems. J Cell Biol 168: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Helms-Brons D, Brugger B, Gkantiragas I, Eberle H, Nickel W, Nurnberg B, Gerdes HH, Wieland FT (1998) A putative heterotrimeric G protein inhibits the fusion of COPI-coated vesicles. Segregation of heterotrimeric G proteins from COPI-coated vesicles. J Biol Chem 273: 15203–15208 [DOI] [PubMed] [Google Scholar]
- Hong W (2005) SNAREs and traffic. Biochim Biophys Acta 1744: 493–517 [PubMed] [Google Scholar]
- Hu Y, Leo C, Yu S, Huang BC, Wang H, Shen M, Luo Y, Daniel-Issakani S, Payan DG, Xu X (2004) Identification and functional characterization of a novel human misshapen/Nck interacting kinase-related kinase, hMINK beta. J Biol Chem 279: 54387–54397 [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V (1999) Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98: 59–68 [DOI] [PubMed] [Google Scholar]
- Jin SL, Bushnik T, Lan L, Conti M (1998) Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. Differential targeting and activation of the splicing variants derived from the PDE4D gene. J Biol Chem 273: 19672–19678 [DOI] [PubMed] [Google Scholar]
- Kajimoto T, Ohmori S, Shirai Y, Sakai N, Saito N (2001) Subtype-specific translocation of the delta subtype of protein kinase C and its activation by tyrosine phosphorylation induced by ceramide in HeLa cells. Mol Cell Biol 21: 1769–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, Saito N (2004) Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem 279: 12668–12676 [DOI] [PubMed] [Google Scholar]
- Konger RL, Billings SD, Thompson AB, Morimiya A, Ladenson JH, Landt Y, Pentland AP, Badve S (2005) Immunolocalization of low-affinity prostaglandin E receptors, EP and EP, in adult human epidermis. J Invest Dermatol 124: 965–970 [DOI] [PubMed] [Google Scholar]
- Kuwana T, Peterson PA, Karlsson L (1998) Exit of major histocompatibility complex class II-invariant chain p35 complexes from the endoplasmic reticulum is modulated by phosphorylation. Proc Natl Acad Sci USA 95: 1056–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca MC, Shanks RA, Tian L, Nelson DL, Stewart DM, Goldenring JR (2004) AKAP350 interaction with cdc42 interacting protein 4 at the Golgi apparatus. Mol Biol Cell 15: 2771–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie C, Chevet E, Roy L, Tonks NK, Fazel A, Posner BI, Paiement J, Bergeron JJ (2000) Tyrosine phosphorylation of p97 regulates transitional endoplasmic reticulum assembly in vitro. Proc Natl Acad Sci USA 97: 13637–13642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20: 87–123 [DOI] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD (2000) Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol Biol Cell 11: 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG (2005) Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum–Golgi transport vesicles. J Biol Chem 280: 22012–22020 [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR (1992) Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68: 353–364 [DOI] [PubMed] [Google Scholar]
- Leyte A, Barr FA, Kehlenbach RH, Huttner WB (1992) Multiple trimeric G-proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO J 11: 4795–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M (2003) Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2). Proc Natl Acad Sci USA 100: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V (2001) Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104: 409–420 [DOI] [PubMed] [Google Scholar]
- Luini A, De Matteis MA (1993) Receptor-mediated regulation of constitutive secretion. Trends Cell Biol 3: 290–292 [DOI] [PubMed] [Google Scholar]
- Luini A, Ragnini-Wilson A, Polishchuck RS, De Matteis MA (2005) Large pleiomorphic traffic intermediates in the secretory pathway. Curr Opin Cell Biol 17: 353–361 [DOI] [PubMed] [Google Scholar]
- Luna A, Matas OB, Martinez-Menarguez JA, Mato E, Duran JM, Ballesta J, Way M, Egea G (2002) Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP. Mol Biol Cell 13: 866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Verges M, Vaerman JP, Sudol M, Mostov KE (1999) The SRC family protein tyrosine kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell 4: 627–632 [DOI] [PubMed] [Google Scholar]
- Maier O, Ehmsen E, Westermann P (1995) Trimeric G protein alpha subunits of the Gs and Gi families localized at the Golgi membrane. Biochem Biophys Res Commun 208: 135–143 [DOI] [PubMed] [Google Scholar]
- Martin ME, Hidalgo J, Rosa JL, Crottet P, Velasco A (2000) Effect of protein kinase A activity on the association of ADP-ribosylation factor 1 to Golgi membranes. J Biol Chem 275: 19050–19059 [DOI] [PubMed] [Google Scholar]
- Martin ME, Hidalgo J, Vega FM, Velasco A (1999) Trimeric G proteins modulate the dynamic interaction of PKAII with the Golgi complex. J Cell Sci 112 (Part 22): 3869–3878 [DOI] [PubMed] [Google Scholar]
- Matas OB, Martinez-Menarguez JA, Egea G (2004) Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic 5: 838–846 [DOI] [PubMed] [Google Scholar]
- Mellman I, Warren G (2000) The road taken: past and future foundations of membrane traffic. Cell 100: 99–112 [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Pelkmans L, Zerial M (2004) Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol 16: 400–406 [DOI] [PubMed] [Google Scholar]
- Michaelson D, Ahearn I, Bergo M, Young S, Philips M (2002) Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell 13: 3294–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR (2001) Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 152: 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K, Yuan H, Schwappach B (2005) Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep 6: 717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Borrelli E (1994) Targeting of G alpha i2 to the Golgi by alternative spliced carboxyl-terminal region. Science 263: 95–98 [DOI] [PubMed] [Google Scholar]
- Muniz M, Alonso M, Hidalgo J, Velasco A (1996) A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem 271: 30935–30941 [DOI] [PubMed] [Google Scholar]
- Muniz M, Martin ME, Hidalgo J, Velasco A (1997) Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA 94: 14461–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M, Usui S, Shinohara T, Yamaguchi T, Tani K, Tagaya M (2002) Inactivation of Galpha(z) causes disassembly of the Golgi apparatus. J Cell Sci 115: 4483–4493 [DOI] [PubMed] [Google Scholar]
- Palmer KJ, Konkel JE, Stephens DJ (2005) PCTAIRE protein kinases interact directly with the COPII complex and modulate secretory cargo transport. J Cell Sci 118: 3839–3847 [DOI] [PubMed] [Google Scholar]
- Pasolli HA, Klemke M, Kehlenbach RH, Wang Y, Huttner WB (2000) Characterization of the extra-large G protein alpha-subunit XLalphas. I. Tissue distribution and subcellular localization. J Biol Chem 275: 33622–33632 [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13: 349–355 [DOI] [PubMed] [Google Scholar]
- Pelham HR, Rothman JE (2000) The debate about transport in the Golgi—two sides of the same coin? Cell 102: 713–719 [DOI] [PubMed] [Google Scholar]
- Philips MR (2004) Sef: a MEK/ERK catcher on the Golgi. Mol Cell 15: 168–169 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, Simons K (1993) Role of heterotrimeric G proteins in polarized membrane transport. J Cell Sci Suppl 17: 27–32 [DOI] [PubMed] [Google Scholar]
- Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA (2000) Correlative light-electron microscopy reveals the tubular–saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol 148: 45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley L, Shakur Y, Rena G, Houslay MD (1997) Intracellular localization of the PDE4A cAMP-specific phosphodiesterase splice variant RD1 (RNPDE4A1A) in stably transfected human thyroid carcinoma FTC cell lines. Biochem J 321 (Part 1): 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C, Short B, De CV, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA (2004) YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol 164: 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF (2001) GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol 3: 368–375 [DOI] [PubMed] [Google Scholar]
- Qian L, Yang T, Chen H, Xie J, Zeng H, Warren DW, MacVeigh M, Meneray MA, Hamm-Alvarez SF, Mircheff AK (2002) Heterotrimeric GTP-binding proteins in the lacrimal acinar cell endomembrane system. Exp Eye Res 74: 7–22 [DOI] [PubMed] [Google Scholar]
- Radha V, Rajanna A, Swarup G (2004) Phosphorylated guanine nucleotide exchange factor C3G, induced by pervanadate and Src family kinases localizes to the Golgi and subcortical actin cytoskeleton. BMC Cell Biol 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV (2004) Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci 24: 6650–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI (2005) An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307: 1746–1752 [DOI] [PubMed] [Google Scholar]
- Rosso S, Bollati F, Bisbal M, Peretti D, Sumi T, Nakamura T, Quiroga S, Ferreira A, Caceres A (2004) LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol Biol Cell 15: 3433–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE (1994) Mechanisms of intracellular protein transport. Nature 372: 55–63 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28 [DOI] [PubMed] [Google Scholar]
- Schultz A, Jonsson JI, Larsson C (2003) The regulatory domain of protein kinase Ctheta localises to the Golgi complex and induces apoptosis in neuroblastoma and Jurkat cells. Cell Death Differ 10: 662–675 [DOI] [PubMed] [Google Scholar]
- Schultz A, Ling M, Larsson C (2004) Identification of an amino acid residue in the protein kinase C C1b domain crucial for its localization to the Golgi network. J Biol Chem 279: 31750–31760 [DOI] [PubMed] [Google Scholar]
- Schwaninger R, Plutner H, Bokoch GM, Balch WE (1992) Multiple GTP-binding proteins regulate vesicular transport from the ER to Golgi membranes. J Cell Biol 119: 1077–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RA, Steadman BT, Schmidt PH, Goldenring JR (2002) AKAP350 at the Golgi apparatus. I. Identification of a distinct Golgi apparatus targeting motif in AKAP350. J Biol Chem 277: 40967–40972 [DOI] [PubMed] [Google Scholar]
- Simon JP, Ivanov IE, Adesnik M, Sabatini DD (1996) The production of post-Golgi vesicles requires a protein kinase C-like molecules, but not its phosphorylating activity. J Cell Biol 135: 355–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A (2005) TRKing signals through the Golgi Sci. STKE 2005: e1. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Von Zastrow M (2002) Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 3: 600–614 [DOI] [PubMed] [Google Scholar]
- Stamnes M (2002) Regulating the actin cytoskeleton during vesicular transport. Curr Opin Cell Biol 14: 428–433 [DOI] [PubMed] [Google Scholar]
- Stow JL, de Almeida JB (1993) Distribution and role of heterotrimeric G proteins in the secretory pathway of polarized epithelial cells. J Cell Sci Suppl 17: 33–39 [DOI] [PubMed] [Google Scholar]
- Stow JL, de Almeida JB, Narula N, Holtzman EJ, Ercolani L, Ausiello DA (1991a) A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol 114: 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow JL, Sabolic I, Brown D (1991b) Heterogeneous localization of G protein alpha-subunits in rat kidney. Am J Physiol 261: F831–F840 [DOI] [PubMed] [Google Scholar]
- Sullivan BM, Harrison-Lavoie KJ, Marshansky V, Lin HY, Kehrl JH, Ausiello DA, Brown D, Druey KM (2000) RGS4 and RGS2 bind coatomer and inhibit COPI association with Golgi membranes and intracellular transport. Mol Biol Cell 11: 3155–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takida S, Wedegaertner PB (2004) Exocytic pathway-independent plasma membrane targeting of heterotrimeric G proteins. FEBS Lett 567: 209–213 [DOI] [PubMed] [Google Scholar]
- Tisdale EJ (2000) Rab2 requires PKC iota/lambda to recruit beta-COP for vesicle formation. Traffic 1: 702–712 [DOI] [PubMed] [Google Scholar]
- Tisdale EJ (2002) Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Ciota/lambda and plays a role in microtubule dynamics in the early secretory pathway. J Biol Chem 277: 3334–3341 [DOI] [PubMed] [Google Scholar]
- Tisdale EJ (2003) Rab2 interacts directly with atypical protein kinase C (aPKC) iota/lambda and inhibits aPKCiota/lambda-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J Biol Chem 278: 52524–52530 [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Artalejo CR (2006) Src-dependent aPKCiota/lambda tyrosine phosphorylation is required for aPKCiota/lambda association with Rab2 and glyceraldehyde-3-phosphate dehydrogenase on Pre-golgi intermediates. J Biol Chem 281: 8436–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E (2004) Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell 7: 33–44 [DOI] [PubMed] [Google Scholar]
- Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts WJ, Koster AJ, Burger KN, Mironov AA, Luini A (2004) Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol 6: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Ugur O, Jones TL (2000) A proline-rich region and nearby cysteine residues target XLalphas to the Golgi complex region. Mol Biol Cell 11: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M (2001) Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem 276: 11189–11198 [DOI] [PubMed] [Google Scholar]
- Weinberger A, Kamena F, Kama R, Spang A, Gerst JE (2005) Control of Golgi morphology and function by Sed5 t-SNARE phosphorylation. Mol Biol Cell 16: 4918–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke R, Maize JC Jr, Shoarinejad F, Vass WC, Reed J, Bonifacino JS, Resau JH, de Gunzburg J, Yeung RS, DeClue JE (1996) Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Jr Oncogene 13: 913–923 [PubMed] [Google Scholar]
- Wilson BS, Komuro M, Farquhar MG (1994) Cellular variations in heterotrimeric G protein localization and expression in rat pituitary. J Endocrinol 134: 233–244 [DOI] [PubMed] [Google Scholar]
- Wylie F, Heimann K, Le TL, Brown D, Rabnott G, Stow JL (1999) GAIP, a Galphai-3-binding protein, is associated with Golgi-derived vesicles and protein trafficking. J Am J Physiol 276: C497–C506 [DOI] [PubMed] [Google Scholar]
- Wylie FG, Lock JG, Jamriska L, Khromykh T, Brown DL, Stow JL (2003) GAIP participates in budding of membrane carriers at the trans-Golgi network. Traffic 4: 175–189 [DOI] [PubMed] [Google Scholar]
- Xie S, Wang Q, Ruan Q, Liu T, Jhanwar-Uniyal M, Guan K, Dai W (2004) MEK1-induced Golgi dynamics during cell cycle progression is partly mediated by Polo-like kinase-3. Oncogene 23: 3822–3829 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagahama M, Itoh H, Hatsuzawa K, Tani K, Tagaya M (2000) Regulation of the Golgi structure by the alpha subunits of heterotrimeric G proteins. FEBS Lett 470: 25–28 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hamada H, Shinkai H, Kohno Y, Koseki H, Aoe T (2003) The KDEL receptor modulates the endoplasmic reticulum stress response through mitogen-activated protein kinase signaling cascades. J Biol Chem 278: 34525–34532 [DOI] [PubMed] [Google Scholar]
- Yang X, Kovalenko D, Nadeau RJ, Harkins LK, Mitchell J, Zubanova O, Chen PY, Friesel R (2004) Sef interacts with TAK1 and mediates JNK activation and apoptosis. J Biol Chem 279: 38099–38102 [DOI] [PubMed] [Google Scholar]
- Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V (2004) Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol 6: 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ (2004) Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279: 25935–25938 [DOI] [PubMed] [Google Scholar]
- Zirngibl R, Schulze D, Mirski SE, Cole SP, Greer PA (2001) Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp Cell Res 266: 87–94 [DOI] [PubMed] [Google Scholar]


