Abstract
Sixty-one strains of alkane-oxidizing bacteria were tested for their ability to oxidize N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide to imidazol-2-yl amino acids applicable for pharmaceutical purposes. After growth with n-alkane, 15 strains formed different imidazol-2-yl amino acids identified by chemical structure analysis (mass and nuclear magnetic resonance spectrometry). High yields of imidazol-2-yl amino acids were produced by the strains Gordonia rubropertincta SBUG 105, Gordonia terrae SBUG 253, Nocardia asteroides SBUG 175, Rhodococcus erythropolis SBUG 251, and Rhodococcus erythropolis SBUG 254. Biotransformation occurred via oxidation of the alkyl side chain and produced 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid and the butanoic acid derivative. In addition, the acetylamino group of these products and of the substrate was transformed to an amino group. The product pattern as well as the transformation pathway of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide differed in the various strains used.
Substitued imidazoles have been extensively used in medicine and agriculture. The recognition early in the last century that histamine, 2-(4-imidazolyl)ethylamine, acts as a messenger molecule in cell-to-cell communication (3, 10, 11) led to the development and use of antihistamine drugs, defined as H1, H2, and H3 receptor antagonists with structures related to histamine (4, 46). Burimamid and cimetidin were described as selective H2 receptor antagonists (4, 5), and phenoxyalkyl imidazoles have been developed as potent H3 receptor antagonists (19). Some substituted imidazoles have antifungal activities (14, 15, 39, 44, 49, 53, 54), while various 1-substituted imidazoles have anticonvulsant properties (9, 21, 22, 24-26, 38, 43, 44). Furthermore, the biologically active angiotensin II receptor antagonists have an imidazole moiety (1). Apart from N-benzylimidazoles (8), eprosartan (36), and related compounds (6, 32), imidazolylalkylamino acids are also potent angiotensin II receptor antagonists (28).
Because of the manifold biological activities of imidazole derivatives, we investigated whether novel imidazol-2-yl amino acids, applicable for pharmaceutical purposes, might be synthesized from N-(2-alkylamino-4-phenylimidazol-1-yl)-acetamides by using microbial biotransformation reactions. Alkane-oxidizing bacteria should be able to oxidize the terminal methyl group of the alkylamino substituent of the N-(2-alkylamino-4-phenylimidazol-1-yl)-acetamides to yield the desired imidazol-2-yl amino acids, because the most common pathway of oxidation of alkyl chains is the monoterminal oxidation via an alcohol and an aldehyde intermediate (37, 41) to the corresponding carboxylic acid. Such microbial transformation has also been found for alkyl substituents of aromatics and heteroaromatics such as phenyl alkanes (51), alkyl benzene sulfonates (55), 2,5-alkyl thiophenes (17), or 6-alkyl-2-pyrones (40) and offers an attractive route for organic synthesis to produce novel aromatic and heteroaromatic substituted aliphatic acids.
The aim of this study was (i) to investigate whether the alkane-oxidizing ability of bacteria can be used for the synthesis of imidazol-2-yl amino acids from an alkylated parent compound, (ii) to select the most efficient strains and to characterize the product pattern, and (iii) to adapt the incubation conditions for high product yields.
MATERIALS AND METHODS
Chemicals.
N-(2-Hexylamino-4-phenylimidazol-1-yl)-acetamide was synthesized from 2-amino-5-methyl-3-phenacyl-1,3,4-oxadiazolium bromide and hexylamine according to procedures published in the literature (27).
1-Amino-2-hexylamino-4-phenylimidazole was synthesized from N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide. N-(2-Hexylamino-4-phenylimidazol-1-yl)-acetamide (0.50 g) was treated with 2 ml of 48% solution of HBr. After addition of water, the precipitate was filtered off and recrystallized from ethanol to yield a yellow-brown substance; the yield was 0.30 g (70%).
Organisms and growth conditions.
For the experiments, 61 strains of n-alkane-utilizing bacteria were used. Some bacteria were obtained from the strain collection. Most of the strains were isolated from hydrocarbon-contaminated soils in the north of Germany by enrichment cultivation performed in 500-ml flasks containing 100 ml of mineral salts medium (5 g of NH4H2PO4, 2.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, 0.5 g of NaCl, 0.46 g of K2SO4, 0.07 g of CaCl2, 2 mg of FeCl3 · 6H2O, 0.5 mg of H3BO3, 0.1 mg of CuSO4 · 5H2O, 0.1 mg of KI, 0.4 mg of MnSO4 · 5H2O, 0.4 mg of ZnSO4 · 7H2O, 0.2 mg of Na2MoO4, and 0.1 mg of CoCl3 per liter of deionized water) and 1% hexadecane as the only source of carbon and energy. After appropriate times (3 to 7 days) of shaking cultures at 30°C and 180 rpm, 5 ml was transferred to 90 ml of fresh medium and incubated under the same conditions. Identical transfers were performed two more times. Afterwards, microorganisms were obtained by plating 0.1 ml of the cultures on nutrient broth plates. Pure cultures were maintained on nutrient broth slants and deposited at the strain collection of the Department of Biology of the University of Greifswald (SBUG).
The efficient strains belong to the genera Gordonia, Nocardia, and Rhodococcus, and isolates were analyzed by morphological and physiological testing, mainly by methods of Groth et al. (23). Further identification to the species level by using physiological characteristics (Table 1) was performed by the methods of Tsukamura (48), Goodfellow (20), and Holt et al. (29).
TABLE 1.
Differentiating characteristics of strains identified
| Characteristic | Results fora:
|
|
|---|---|---|
| G. rubropertincta SBUG 105 | G. terrae SBUG 253 | |
| Color of colonies | Red | Red |
| Morphology | Rod-shaped | Rod-shaped |
| Growth on sole carbon source | ||
| d-Galactose (1.0%) | + | + |
| Mesoinositol (1.0%) | − | + |
| Sorbitol (0.5%) | + | + |
| l-Rhamnose (1.0%) | − | + |
| Citric acid (Na salt, 0.1%) | + | + |
| Butan-2,3-diol (1.0%) | − | + |
| Propan-1-ol (0.1%) | − | + |
| l-Serine (0.1%) | − | + |
| d-Alanine (0.1%) | − | − |
| l-Alanine (0.1%) | − | + |
| Mycolic acid | One, long chain | One, long chain |
| Fatty acid composition | S, U, T | S, U, T |
| Major menaquinone | MK-9 (H2) | MK-9 (H2) |
| 16:1 fatty acid present | + | + |
+, positive; −, negative; S, saturated; U, monounsaturated; T, tuberculostearic acid.
For more detailed studies, we used Gordonia rubropertincta SBUG 105 (isolated strain), Gordonia terrae SBUG 253 (isolated strain), Nocardia asteroides SBUG 175 (ATCC 19247), Rhodococcus erythropolis SBUG 251, and R. erythropolis SBUG 254 (ATCC 4277).
Biotransformation experiments.
For incubation experiments, bacteria grown on agar plates with n-alkanes were used. The agar medium contained mineral salts medium (31, 45), trace element solution (18), and agar-agar (18 g liter−1). The agar plates were supplemented with gases of n-alkanes (C10 to C20) (mostly n-tetradecane) evaporated from a sterile filter sheet in the plate cover (33) as carbon source for 2 to 7 days at 30°C to allow growth of cells. In case of use of n-alkanes with shorter chain lengths (C6 to C9), the inoculated plates were put in a larger glass with evaporating n-alkane (closed by a lid) according to method F 10 of Kreisel and Schauer (33).
Bacterial biomass from five agar plates was transferred to 500-ml flasks with 100 ml of sterilized mineral salts medium supplemented with trace elements (see above), and N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (0.1 g liter−1) was added. For controls we used (i) cells in mineral salts medium without the compound that was to be transformed and (ii) N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide in mineral salts medium without cells. After incubation for 3 days at 30°C and 220 rpm on a rotary shaker, formation of metabolites was followed by analyzing 0.1 ml of the culture supernatant by high-performance liquid chromatography (HPLC). For kinetic studies the formation of metabolites was analyzed by HPLC from time to time during the course of the incubation period.
To avoid a decrease of inducable alkane hydroxylase during the biotransformation experiments and to increase the product yields, alkane-oxidizing enzymes were partially induced again during incubation with N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by supplementation with gaseous n-alkane from a reservoir inside the incubation flask. For this purpose the incubation flasks with cells of N. asteroides SBUG 175 were supplemented one time with n-tetradecane (5 ml in the reservoir) and with cells of G. rubropertincta SBUG 105, G. terrae SBUG 253, and R. erythropolis SBUG 251 two times with n-octane (2 ml in the reservoir) to maintain alkane vapor phases in the flasks.
All experiments described were carried out in duplicate. The deviation was no more than 5%.
Chemical analysis, isolation, and identification of products.
For the detection and quantification of metabolites in the aqueous supernatant, culture samples were centrifuged (6,000 × g for 5 min) to remove cells and were analyzed by an HPLC system (1050 M; Hewlett-Packard, Bad Homburg, Germany) equipped with a quaternary pump system, an HP 1040 M series I diode array detector, and an HP HPLC Chemstation. The separation was carried out on an end-capped, 5-μm, LiChroCart 125-4 RP 18 column (Merck, Darmstadt, Germany). The initial solvent composition was 20% methanol-80% phosphate buffer (20 mM), pH 4.8, reaching 100% methanol within 14 min at a flow rate of 1 ml min−1.
For isolation of products, the cells were removed from the incubation medium by centrifugation at 6,000 × g for 5 min at the end of the biotransformation experiments. An RP 18 silicagel solid-phase extraction column (polypropylene, 3 ml; and 200 mg of absorbent material; Baker, Gross-Gerau, Germany) was charged with 10 ml of cell-free culture media. The substrate and several products were eluted together with 1 ml of methanol. The separation of products from the methanolic fraction obtained by solid-phase extraction was performed on an HPLC module system (Merck) equipped with a model L 6200 A Intelligent Pump, a Rheodyne 7161 injection valve with a 100-μl loop, and a model L 4250 absorbance detector operating at 220 nm. High purity of products was achieved on an end-capped, 5-μm, LiChroCart 125-4 RP 18 column (Merck) at a flow rate of 1 ml min−1. For sufficient separation, the solvent system, consisting of methanol (eluent A) and ammonium acetate buffer (20 mM, pH 4.8) (eluent B), was started in a ratio of 20% A and 80% B and reached 70% A and 30% B within 12.5 min, and then it was changed to 100% A within 30 s and held constant for another 5 min.
Analysis of purified products by mass spectrometry was carried out by electron impact analysis at 70 eV with sample introduction via a direct insertion probe on an M-40 mass spectrometer (AMD Intectra).
The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker (Karlsruhe, Germany) ARX 300 instrument at 300 MHz in (CD3)2SO. Tetramethylsilane was used as an internal standard.
RESULTS
Oxidation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide.
We compared 61 alkane-oxidizing bacterial strains regarding their potential for the oxidation of alkylamino imidazoles. None of the strains tested used N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide that was added to mineral salts medium as the only source for carbon and energy, and no oxidation products were detectable. However, 15 strains transformed the substrate to one to four different products after growth on n-tetradecane (Table 2).
TABLE 2.
Bacterial isolates and transformation products formed during incubation with N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (10 mg) after growth with n-tetradecane
| Strain | Result for producta:
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Acinetobacter calcoaceticus SBUG 39 | ++ | 0 | 0 | 0 | 0 |
| G. rubropertincta SBUG 105 | + | 0 | 0 | 0 | ++ |
| G. terrae SBUG 253 | ++ | + | 0 | 0 | ++ |
| Mycobacterium phlei SBUG 119 | +++ | 0 | 0 | 0 | +++ |
| Mycobacterium vaccae SBUG 109 | ++ | 0 | 0 | 0 | +++ |
| N. asteroides SBUG 175 | 0 | +++ | 0 | +++ | +++ |
| Nocardia opaca SBUG 177 | ++ | 0 | 0 | 0 | 0 |
| R. erythropolis SBUG 251 | ++ | 0 | 0 | 0 | 0 |
| R. erythropolis SBUG 254 | ++ | 0 | 0 | 0 | 0 |
| Rhodococcus sp. strain SBUG 82 | + | 0 | 0 | 0 | 0 |
| Strain SBUG 108 | ++ | 0 | 0 | 0 | 0 |
| Strain SBUG 143 | ++ | 0 | 0 | 0 | 0 |
| Strain SBUG 303 | + | 0 | 0 | 0 | 0 |
| Strain SBUG 304 | + | 0 | 0 | 0 | 0 |
| Strain SBUG 259 | ++ | + | + | 0 | +++ |
Amount of metabolites accumulated in 100-ml culture medium is shown. 0, not detectable; +, 10 to 100 μg; ++, 100 to 400 μg; and +++, >400 μg.
Because of the short amino alkyl side chain in the parent compound (C6) and the high water insolubility of tetradecane, a variety of shorter n-alkanes (C6 to C12) were used in further experiments as growth substrates for the selected strains in order to get better induction and higher product yields.
A higher product level was found only for some strains, such as R. erythropolis SBUG 251 (up to 10-fold), R. erythropolis SBUG 254 (up to 10-fold), and G. terrae (up to twofold). The highest yield of products was recovered after growth and induction of oxidizing enzymes with n-octane. The strain G. rubropertincta SBUG 105 transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide not only in greater amounts (up to sevenfold) but also produced more metabolites (four instead of two) after growth with octane than with tetradecane.
Induction with alkanes ranging from hexane to dodecane had no positive influence on the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by the other strains listed in Table 2. In most cases the strains tested showed limited growth on the shorter-chain n-alkanes.
Detection of products by HPLC.
Transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide was demonstrated by metabolites, which were detected by HPLC analysis of the cell-free supernatant.
Most strains accumulated only one oxidation product, designated product A, which had a retention time of 7.2 min.
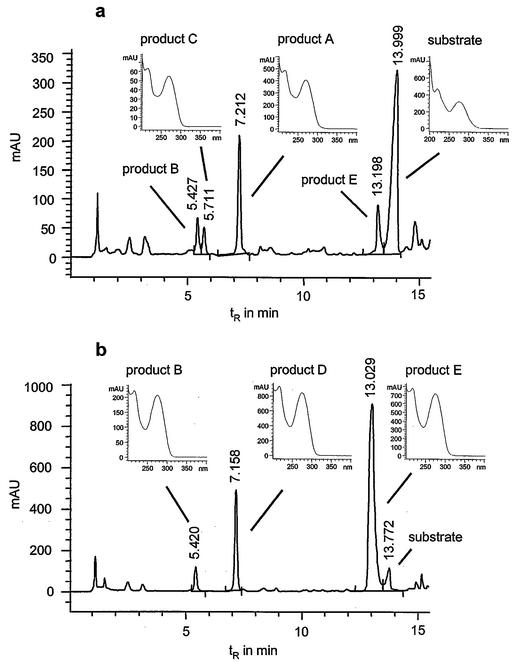
The elution profiles of other strains, including G. rubropertincta SBUG 105, G. terrae SBUG 253, and N. asteroides SBUG 175, showed more than one oxidation product. These products were designated B to E and differ clearly in their HPLC retention times (Fig. 1).
FIG. 1.
HPLC elution profiles of the aqueous culture supernatant after incubation of G. rubropertincta SBUG 105 (a) and N. asteroides SBUG 175 (b) with N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (0.1 g liter−1; 72 h) and UV absorption spectra of products A through E. AU, absorbance units.
Identification of products A to E. (i) Product A.
The short HPLC retention time of product A (7.2 min) suggested that this compound is more hydrophilic than the parent compound, which would be expected for the formation of an imidazol-2-yl amino acid derivative. Product A was separated by HPLC from the cell-free culture medium of the strain R. erythropolis SBUG 251 and was characterized by mass spectrometry and NMR analysis. The mass spectral data showed a molecular ion peak at m/z 330. The peak at m/z 272 resulted from the loss of the acetylamino group (CH3CONH) from the molecular ion. Further fragment ions at m/z 257 (M−CO2/−C2H4/−H), 229 (M−CH3CONH2/−CO2/+2H), 228 (M−CO2/−2xC2H4/−H), 187 (M−CH3CONH2/−CO2/−C3H6/+2H), 186 (M−CO2/−2xC2H4/−H/−COCH3/+H), 173 (M−CH3CONH2/−CO2/−C4H8/+2H), 159 (M−CH3CONH2/−CO2/−C3H6/−C2H4/+2H), 104 (C6H5—CH=CH2), 103 (C6H5—C☰N), 77 (C6H5), and 43 (COCH3) correspond with a structure containing one carboxyl function, one acetylamino group, an n-pentyl chain, and a phenyl ring, indicating that the structure of product A is acetylamino-phenylimidazolyl-aminohexanoic acid.
Comparison of proton-proton spin systems and couplings of the 1H NMR spectrum and the 13C NMR data of the product A with those of the substrate showed structural similarities (Tables 3 and 4). All resonance signals of product A were identical with those of the substrate with two exceptions: the spectra failed in the signals for the methyl group of the substrate but contained signals specific for a carboxyl group, and the signals of the neighboring group changed. The absence of the aliphatic methyl protons (showing a triplet at δ = 0.87 ppm) in the 1H NMR spectrum of the product and the absence of the carbon signal of the methyl group indicated the transformation of this group. The carbon signal at the low field value of δ = 174.5 ppm in the 13C NMR spectrum of the product demonstrates the presence of a carbonyl structure not detectable in the substrate spectrum. The lower field value of δ = 2.2 ppm of one methylene group in the 1H NMR spectrum of the product was in accordance with that of a neighboring carboxyl group and together with the remaining resonance signals confirmed the structure of 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid for product A.
TABLE 3.
1H NMR data of the substrate N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide and the products A, B, C, and E
| Data for:
|
Proton assignmenta | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate
|
Product A
|
Product B
|
Product C
|
Product E
|
||||||
| δ (ppm) | 3J (Hz) | δ (ppm) | 3J (Hz) | δ (ppm) | 3J (Hz) | δ (ppm) | 3J (Hz) | δ (ppm) | 3J (Hz) | |
| 10.65 | 10.66 | 10.69 | s, 1H, NH, NH—COCH3 | |||||||
| 7.65 | 7.8 | 7.65 | 8.2 | 7.62 | 8.2 | 7.64 | 7.8 | 7.70 | 7.8 | d, 2H, H0, C6H5 |
| 7.30 | 7.8, 7.2 | 7.29 | 8.2, 7.3 | 7.3 | 8.2, 7.3 | 7.29 | 7.8, 7.3 | 7.46 | 7.8, 7.3 | dd, 2H, Hm, C6H5 |
| 7.13 | 7.2 | 7.11 | 7.3 | 6.9 | 7.3 | 7.11 | 7.3 | 7.36 | 7.3 | t, 1H, Hp, C6H5 |
| 7.08 | 7.07 | 7.1 | 7.09 | 7.49 | s, 1H, CH, imidazol | |||||
| 5.5 | 6.01 | s, 2H, NH2 | ||||||||
| 5.83 | 5.7 | 5.84 | 5.8 | 7.88 | 5.98 | 5.8 | 7.85 | 6.1 | t, 1H, NH, NH—CH2 | |
| 3.21 | 3.21 | 3.31 | 3.24 | 3.38 | m, 2H, CH2, CH2—NH | |||||
| 2.22 | 7.3 | 2.28 | 8.2 | 2.29 | 7.4 | t, 2H, CH2, CH2—COOH | ||||
| 1.97 | 1.97 | 1.97 | s, 3H, CH3 | |||||||
| 1.83 | 1.8 | m, 2H, CH2, NH—CH2—CH2—CH2COOH | ||||||||
| 1.54 | 1.59 | m, 2H, CH2, CH2—CH3 | ||||||||
| 1.29 | 1.5, 1.3 | 1.31 | m, 6H, 3xCH2 | |||||||
| 0.87 | 6.6 | 0.88 | 6.7 | t, 3H, CH3, CH3(CH2)5NH | ||||||
Underlining reflects assignment of the single NMR signal.
TABLE 4.
13C NMR data of the substrate N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide and the products A, B, C, and E
| Substrate δ (ppm) | Product A δ (ppm) | Product C δ (ppm) | Product E δ (ppm) | Proton assignmenta |
|---|---|---|---|---|
| 174.5 | 174.5 | C=O, COOH | ||
| 168.9 | 168.8 | 168.8 | C=O, NH—COCH3 | |
| 149.6 | 149.4 | 149.3 | 147.1 | C2, imidazol |
| 135.2 | 135.0 | 134.9 | 128.1 | C1, C6H5 |
| 133.6 | 132.1 | 133.3 | 123.5 | C4, imidazol |
| 128.4 | 128.2 | 128.2 | 128.7 | CO, C6H5 |
| 125.7 | 125.5 | 125.6 | 127.5 | Cp, C6H5 |
| 123.9 | 123.7 | 123.7 | 124.7 | Cm, C6H5 |
| 112.0 | 111.9 | 111.9 | 115.3 | C5, imidazol |
| 42.7 | 42.3 | 41.8 | 42.5 | CH2, NH—CH2(CH2)2COOH |
| 33.6 | 31.2 | CH2, CH2—COOH | ||
| 31.3, 29.5, 26.3, 22.3 | 30.8, 28.9, 25.6, 22.1 | (CH2)4, CH3(CH2)4CH2NH | ||
| 29.0, 25.9, 24.3 | (CH2)3, NH—CH2(CH2)3CH2—COOH | |||
| 24.8 | CH2, NH—CH2—CH2—CH2—COOH | |||
| 21.0 | 20.8 | 20.8 | CH3, CH3—CONH | |
| 14.2 | 13.8 | CH3, CH3(CH2)5NH |
Underlining reflects assingment of the single NMR signal.
(ii) Product B.
Product B was formed by cells of G. rubropertincta SBUG 105 and N. asteroides SBUG 175. The mass spectrum of this compound showed a molecular ion peak at m/z 260. The main fragment ions at m/z 217 (M−CO2/+H), 204 (M−CO2/−CH4/+4H), 203 (M−CO2/−NH3/+4H), 189 (M−CO2/−C2H4/+H), 175 (M−CO2/−C3H6/+H), 173 (M−CO2/-NH3/−C2H4/+2H), 160 (M−CO2/−C2H4/−HCN/-H), 158 (M−CO2/−C3H6/−NH3/+H), 142 (M−CO2/−C3H6/−2xNH3/+2xH), 104 (C6H5—CH=CH2), 103 (C6H5—C☰N), and 77 (C6H5) correspond with a structure containing one carboxyl function, a primary amino group, an n-propyl chain, and a phenyl residue. In comparison with the mass data of the substrate (data not shown) and product A, no fragment ions indicating the acetylamino and the pentyl group were detectable in the mass spectrum of product B.
The three aromatic resonance signals in the 1H NMR spectrum (δ = 7.62, 7.3, and 6.9 ppm) of product B were identical with those of the substrate and product A (Table 3). Additionally, the proton of the imidazol ring gave the same signal in all three compounds, indicating identical structural elements characteristic for the phenyl and imidazol ring. Three signals in the 1H NMR spectrum of product B (a multiplet, a triplet, and a multiplet at δ = 3.31, 2.28, and 1.83 ppm) confirmed the existence of six aliphatic protons corresponding to three methylene groups in contrast to five methylene groups in the substrate and product A. The missing signal of the acetyl group, the new signal at 5.5 ppm (singlet) belonging to a primary amino group, and the downfield shift of the proton signal of the secondary amino group of the alkylamino chain all indicate the loss of the acetyl group. Thus, an oxidation of the methyl group of the alkylamino chain, a loss of two methylene groups, and a loss of the acetyl group result in the structure of 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid for product B.
(iii) Product C.
Product C was separated by HPLC from the cell-free culture medium of the species G. rubropertincta SBUG 105 only. Mass spectral analysis revealed a molecular weight of 302. The difference of 42 in comparison with the molecular weight of product B (M+ 260) is consistent with an intact acetylamino group in this molecule. Besides the molecular ion peak at m/z 302 (M+, C15H18N4O3), the mass spectrum contains the characteristic fragment ions m/z 285 (M−OH), 256 (M−HCOOH), 244 (M−CH3CONH2/+H), 229 (M−CO2/−C2H4/−H), 199 (M−CH3CONH2/−CO2), 172 (M−CH3CONH2/−CO2/−C2H4/+H), 158 (M−CH3CONH2/−CO2/−C3H6/+H), 104 (C6H5—CH=CH2), 103 (C6H5—C☰N), 77 (C6H5), and 43 (COCH3).
The structure of product C was also analyzed by NMR (Tables 3 and 4). In accordance with the structures of the substrate and products A and B, the spectra of product C show three aromatic resonance signals in the 1H NMR (δ = 7.64, 7.29, and 7.11 ppm) and four in the 13C NMR (δ = 134.9, 128.2, 125.6, and 123.7 ppm). Furthermore, the signal of the proton of the imidazol ring was registered. In contrast to that of product B, the signal pattern of the oxidized propylamino chain of product C remains unchanged apart from the upfield shift of the proton signal of the secondary amino group that confirmed the intact acetylamino group. The singlet at 1.97 ppm in the 1H NMR spectrum and the corresponding signal at 20.8 ppm in the 13C NMR spectrum are the expected signals of an acetyl group, as in product A and in the substrate, showing that product C is 1-acetylamino-4-phenylimidazol-2-yl-4-aminobutanoic acid.
(iv) Product D.
Product D resulted from the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by N. asteroides SBUG 175 (Fig. 1b). In comparison with products A through C, product D has an HPLC retention value and a UV spectrum, suggesting that this compound could be an imidazol-2-yl amino acid.
The mass spectrum showed a molecular ion peak at m/z 288 (M+, C15H20N4O2). The peak at m/z 229 (M-CO2/-CH4/+H) resulted from the loss of the carboxyl and methylene group from the molecular ion. Further main fragment ions at m/z 214 (M−CO2/−CH4/−NH3/+3H), 186 (M−CO2/−CH4/−C3H6), 172 (M−CO2/−CH4/−NH3/−C3H6/+3H), 159 (M−CO2/−CH4/−C3H6/−HCN), 158 (M−CO2/−CH4/−NH3/−2xC2H4/+3H), 104 (C6H5—CH=CH2), 103 (C6H5—C☰N), and 77 (C6H5) correspond with a structure containing one carboxyl function, a primary amino group, an n-pentyl chain, and a phenyl residue, suggesting the structure 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid for product D.
Compound D was an intermediate that accumulated for only a short period during the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (see kinetic data). Therefore, the amount of product D was not sufficient for NMR analysis.
(v) Product E.
In contrast to products A to D, product E showed an HPLC retention value very similar to that of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide, indicating few changes in the hydrophobic parts of the parent compound. The mass spectrum of product E, formed by G. rubropertincta SBUG 105, N. asteroides SBUG 175, and four other strains, showed a molecular ion peak at m/z 258, which was consistent with the calculated molecular mass of 1-amino-2-hexylamino-4-phenylimidazole. Therefore, this compound was chemically synthesized from N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide according to the method described in Materials and Methods. The spectroscopic data of the biotransformation product E were identical with those of the synthesized 1-amino-2-hexylamino-4-phenylimidazole. Mass data are as follows: m/z 258 (molecular mass, M+, C15H22N4), 243 (M−CH3), 242 (M−CH4), 228 (M−C2H6), 172 (M−C2H6/−2xC2H4), 159 (M−3xC2H4/−NH3/+2H), 158 (M−3xC2H4/−NH3/+H), 104 (C6H5—CH=CH2), 103 (C6H5—C☰N), 77 (C6H5); 1H NMR and 13C NMR (Tables 3 and 4).
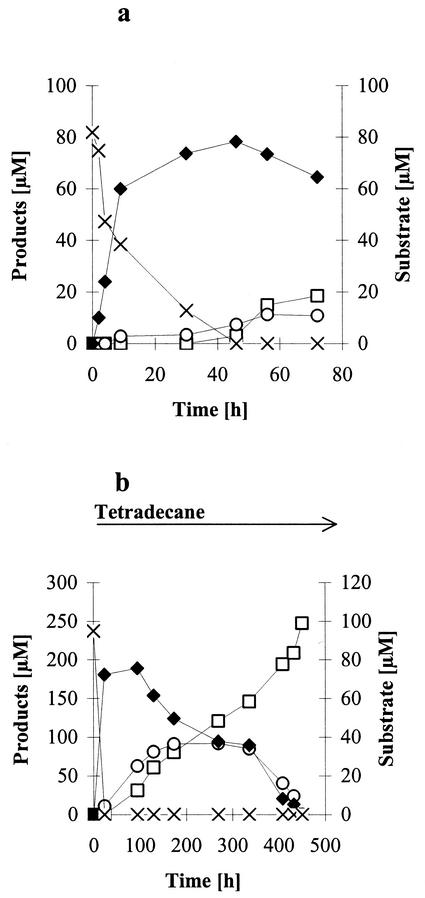
Kinetics of the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide.
n-Tetradecane-grown cells of N. asteroides SBUG 175 transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide completely within 48 h. The main product, which formed rapidly, was 1-amino-2-hexylamino-4-phenylimidazole, demonstrating an active deacetylation system in cells of N. asteroides SBUG 175 (Fig. 2a). The deacetylated imidazol-2-yl-aminohexanoic acids accumulated at a low rate. The amount of this imidazol-2-yl-amino acid decreased with accumulation of the 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid. At the end of incubation, 1-amino-2-hexylamino-4-phenylimidazole was still present in high quantity. The yield of the 1-amino-4-phenylimidazol-2-yl-aminoalkanoic acids was between 3 and 5%.
FIG. 2.
Kinetics of the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (0.1%) by N. asteroides SBUG 175 as determined by HPLC. (a) Incubation with n-tetradecane-grown cells; (b) incubation with n-tetradecane-grown cells and continuous induction by n-tetradecane. ×, N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide; ♦, 1-amino-2-hexylamino-4-phenylimidazole; ○, 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid; and □, 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid.
To increase the amount of the desired aminoalkanoic acid derivatives, we chose a continuous induction of alkane-oxidizing enzymes by n-tetradecane during the biotransformation process. The deacetylation of the parent compound remained the first step of transformation (Fig. 2b). However, the resulting product 1-amino-2-hexylamino-4-phenylimidazole was transformed to the desired 1-amino-4-phenylimidazol-2-yl-aminoalkanoic acids continuously. The production of the aminohexanoic acid derivative was enhanced fivefold within 180 h, after which it was increasingly metabolized and the amount of 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid increased continuously during the incubation period. At the end (after 450 h), it was harvested as the only product (yield, 70%).
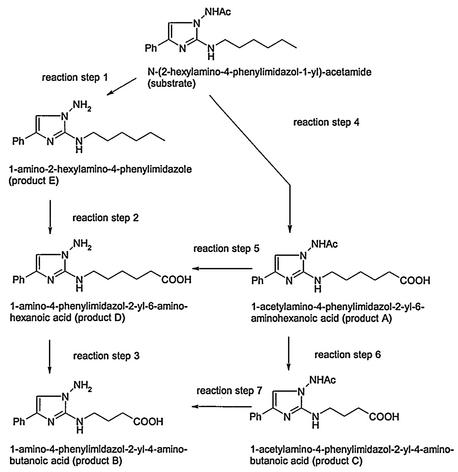
This kinetic study of the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by N. asteroides SBUG 175 showed clearly that the first product formed, 1-amino-2-hexylamino-4-phenylimidazole (product E, reaction step 1, Fig. 4), is converted to 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid (product D, reaction step 2), which can be further transformed to 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid (product B, reaction step 3).
FIG. 4.
Products detected during biotransformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by G. rubropertincta SBUG 105, G. terrae SBUG 253, N. asteroides SBUG 175, and R. erythropolis SBUG 251 and SBUG 254. For G. rubropertincta SBUG 105, there was one biotransformation pathway via reaction steps 4, 6, and 7 to produce end product B and a second biotransformation pathway via reaction steps 1 through 3 to produce end product B; for G. terrae SBUG 253, there was one biotransformation pathway via reaction steps 4, 5, and 3 to produce end product B and a second biotransformation pathway via reaction steps 1 through 3 to produce end product B; for N. asteroides SBUG 175, there was one biotransformation pathway via reaction steps 1, 2, and 3 to produce end product B; and for R. erythropolis SBUG 251 and SBUG 254, there was one biotransformation pathway via the reaction step 4 to produce end product A.
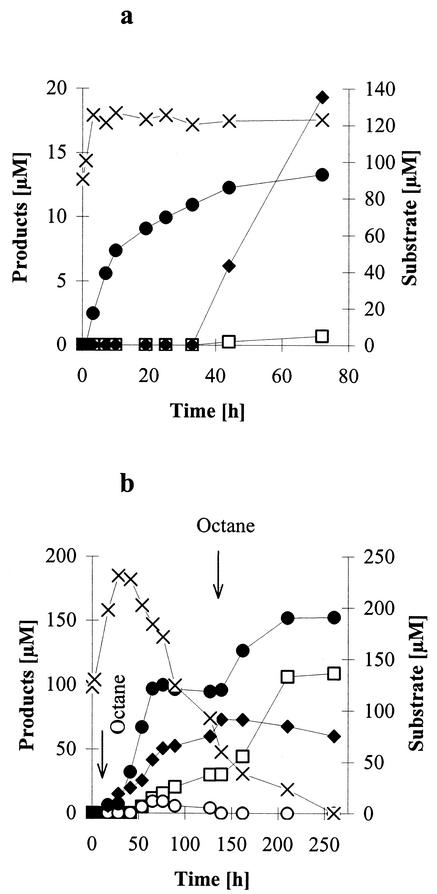
G. terrae SBUG 253 grown with n-octane oxidized the N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide to the 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid from the beginning of incubation (Fig. 3a). The formation rate decreased after about 12 h, perhaps due to a decrease of active alkane-oxidizing enzymes in the cells. The imidazole-2-yl-4-aminobutanoic acid derivative formation occurred only weakly and started after 40 h together with the deacetylation of the substrate to produce 1-amino-2-hexylamino-4-phenylimidazole. The yield of the 4-phenylimidazol-2-yl-aminoalkanoic acids was not higher than 5%. To increase the yield of 4-phenylimidazol-2-yl-aminoalkanoic acids, octane was supplemented as cosubstrate during the biotransformation process in two steps (Fig. 3b).
FIG. 3.
Kinetics of the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide (0.1%) by G. terrae SBUG 253 as determined by HPLC. (a) Incubation with n-octane-grown cells; (b) incubation with n-octane-grown cells and addition of n-octane in two steps.×, N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide; ♦, 1-amino-2-hexylamino-4-phenylimidazole; •, 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid; ○, 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid; and □, 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid.
Alkane supplementation of incubation assays led to growth of cells followed by a decrease in pH and an increase of dissolved substrate, which is more water soluble at low pH. Because of this and because of an increase of alkane hydroxylase during the incubation period, higher concentrations of both substrate and products were detected by HPLC analysis. However, every addition of n-octane led to a drastic increase of the concentration of the oxidation product 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid, indicating an active monooxygenase system involved in the oxidation of the alkylamino group. The second addition of n-octane, necessary because of the rapid evaporation of octane, caused a strong enrich-ment of the 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid, too. In this way high yields of the 4-phenylimidazol-2-yl-aminoalkanoic acids (1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid, 50%; and 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid, 30%) could be achieved.
The amount of the deacetylated product 1-amino-2-hexylamino-4-phenylimidazole was more or less independent from the supplementation with n-octane and decreased at the end of incubation. The deacetylated terminal oxidized product 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid was detected as an intermediate in low concentration over a period of 70 h.
The kinetic data demonstrate that N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide was transformed via two different pathways by G. terrae SBUG 253 (Fig. 4). One pathway starts with the oxidation of the hexylamino group (reaction step 4, Fig. 4), followed by a deacetylation of the product (reaction step 5, Fig. 4). The initial reaction of the second pathway is the deacetylation of the substrate (reaction step 1, Fig. 4), and the resulting 1-amino-2-hexylamino-4-phenylimidazole is oxidized (reaction step 2, Fig. 4). In both cases 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid (product D) is produced and is converted to 1-amino-4-phenylimidazol-2-yl-6-aminobutanoic acid (product B, reaction step 3).
Transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by n-octane-grown cells of R. erythropolis SBUG 251 revealed the accumulation of 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid as a single product (data not shown). This product was enriched within the first 15 h (yield, 15%) and did not further increase thereafter, although the substrate was detectable in large amounts. Addition of an inducing n-alkane to the incubation mixtures did not lead to any increase of product yield.
Cells of G. rubropertincta SBUG 105 grown with n-octane transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide via two pathways similar to that shown for G. terrae (Fig. 4). However, transformation occurred faster and all products and intermediates (also 1-acetylamino-4-phenylimidazol-2-yl-4-aminobutanoic acid) were formed within less than 20 h. The low yield of the imidazol-2-yl amino acids of around 4% could not be increased by addition of n-octane as described for G. terrae.
The highest yields (50 to 70%) of the desired imidazol-2-yl amino acids were obtained from the strains G. terrae SBUG 253 and N. asteroides SBUG 175 by continuous induction of alkane-oxidizing enzymes during incubation with N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide.
DISCUSSION
Fifteen of 61 tested strains of alkane-oxidizing bacteria transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamides to imidazol-2-yl amino acids after growth on n-alkane. High yields of imidazol-2-yl amino acids were obtained from the transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by the strains G. rubropertincta SBUG 105, G. terrae SBUG 253, N. asteroides SBUG 175, R. erythropolis SBUG 251, and R. erythropolis SBUG 254. Species of the genera Nocardia (30, 34, 50) and Rhodococcus (2, 42, 50, 56) are well known for their ability to degrade aliphatic hydrocarbons, especially shorter-chain alkanes to carboxylic acids and further oxidation products. Malachowsky et al. (35) described isolates of Rhodococcus, which utilized n-alkanes ranging in chain length from propane to hexadecane as a carbon and energy source. Probably this is the reason for better oxidation of the hexyl group of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by species of Gordonia, Nocardia, and Rhodococcus than by other alkane-oxidizing bacteria.
Only alkane-induced cells could oxidize the substrate N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide to the imidazol-2-yl amino acids. The capacity to oxidize short-chain alkyl substituents has already been shown for other bacteria of this group. For instance, strains of Rhodococcus, after growth on n-alkanes, are able to oxidize substituted phenoxypropane to phenoxy propanoic acids (47). Most strains selected transformed the N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide better to the imidazol-2-yl amino acids after growth on shorter-chain n-alkanes (C6, C8) than after growth on n-tetradecane. The induction with shorter-chain n-alkanes is more effective than with longer-chain n-alkanes, because the shorter alkanes, despite leading to lower growth rates, induce unadapted cells much better (52). The yields of imidazol-2-yl amino acids were further increased by n-alkane supplementation during the biotransformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide. This result can be caused by various reasons. On the one hand, the n-alkanes induce alkane hydroxylase, which is necessary for oxidation of the hexyl group of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide, during the whole incubation period. However, the hydroxylase seems to be quickly inactivated by biotransformation without alkanes. On the other hand, the terminal oxidation of the hexyl group of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide is an energy-consuming process, and the bacteria take the necessary energy from the cometabolism with n-alkane. The alkyl chains in phenylalkanes or alkylcyclohexanes with lesser chain length (C2 to C4) were also oxidized cometabolically by a Nocardia species with n-alkanes only (12). In contrast to these results, phenylalkanes with greater chain lengths (C9 to C18) were transformed by nocardiae at the alkyl chain and served as inductor and as a source of carbon and energy without the necessity of an additional alkane induction (12, 51).
Many other alkane-utilizing bacteria can grow on alkanes of greater chain length (≥ C12) only. Nevertheless, most can also oxidize alkanes of lesser chain length (C6 to C11) after growth with alkanes of >C12. The hydrophilic aminoimidazol unit at the alkyl chain of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide increases the hydrophilic properties of the whole molecule and may be also a steric hindrance of the alkyl configuration to enzyme activation and may in this way increase the problems of many strains during the oxidation of the hexyl chain of this molecule.
The occurrence of 1-(acetyl)amino-4-phenylimidazol-2-yl-6-aminohexanoic acid in all bacterial cultures, which transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide, and the fact that only alkane-induced cells could oxidize the substrate point to active monooxygenase systems involved not only in alkane oxidation but also in the oxidation of alkyl substituents of more hydrophilic molecules. The initial step of oxidation of alkanes in the investigated gram-positive bacteria is catalyzed by a cytochrome P450 monooxygenase (7, 47). Obviously, the hexylamino group of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide was oxidized via an alcohol and aldehyde intermediate to the corresponding carboxylic acid.
Both the transformation pathway and the product pattern of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide differed in the various strains. R. erythropolis SBUG 251 and R. erythropolis SBUG 254 oxidized the terminal methyl group of the hexylamino substituent to a carboxylic group (reaction step 4, Fig. 4). The resulting 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid was the only product of the transformation and seemed to be a dead-end product. Although the oxidation of phenyl alkanes to phenyl acetic acid was described for rhodococci (34), R. erythropolis SBUG 251 and SBUG 254 were not able to metabolize the 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid intermediate further by β-oxidation.
G. rubropertincta SBUG 105, G. terrae SBUG 253, and N. asteroides SBUG 175 transformed N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide via different intermediates to 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid (product B).
There exist several examples for the oxidation of alkyl residues in more hydrophilic compounds than n-alkanes, e.g., the oxidation of alkyl groups of phenyl alkanes (12, 16, 51), alkyl benzene sulfonates (55), 2,5-alkyl thiophenes (17), and 6-alkyl-2-pyrones (40). The transformation of decyl- and hexadecyltriammonium bromides was described by Dean-Raymond and Alexander (13) and the degradation of long-chain n-alkylcyclohexanes by Dutta and Harayama (16). All these compounds were oxidized to the corresponding carboxylic acids, which undergo a shortening of the chain by β-oxidation (mostly to C2 or C3). 1-Amino-4-phenylimidazol-2-yl-4-aminobutanoic acid also represents such a product arising from β-oxidation of 1-amino-4-phenylimidazol-2-yl-6-aminohexanoic acid (reaction step 3, Fig. 4), though further β-oxidation to the level of an acetic acid derivative was not detected. Webley et al. (51) reported the rapid conversion of 2-phenylpropionic acid (oxidation product of phenyl alkanes) via cinnamic to benzoic acid by two strains of Nocardia and the failure of such a chain shortening with 2-α-naphthylpropionic acid (oxidation product of 2-α-naphthyl alkanes). The lack of β-oxidation was attributed by these authors (51) to an electronic or steric effect of the larger nucleus of 2-α-naphthylpropionic acid. In the same way, the heteroaromatic ring of 1-(acetyl)amino-4-phenylimidazol-2-yl aminoalkanoic acids seems to prevent the undesirable biodegradation of the products formed.
This hindrance of further oxidation and therefore the selective production of definite imidazol-2-yl amino acids by bacterial strains represent a considerable advantage for applications in organic synthesis. The transformation of N-(2-hexylamino-4-phenylimidazol-1-yl)-acetamide by bacteria provides an attractive and practicable route to imidazol-2-yl amino acids. As reported here, R. erythropolis SBUG 251 produces only 1-acetylamino-4-phenylimidazol-2-yl-6-aminohexanoic acid, while N. asteroides SBUG 175 produces only 1-amino-4-phenylimidazol-2-yl-4-aminobutanoic acid. The pharmaceutical activity of these derivatives will be investigated in further studies.
Acknowledgments
This study was supported by a grant from Cusanuswerk.
We thank Renate Schulze for help with screening bacterial strains, M. Kindermann, S. Siegert, and B. Witt (Institute of Chemistry and Biochemistry, University of Greifswald) for performing NMR spectroscopy, and R. Jack (Institute of Immunology, University of Greifswald) for help in preparing the manuscript. We are grateful to P. Schumann (Deutsche Sammlung von Mikroorganismen und Zellkulturen) for help with identification of isolated strains.
REFERENCES
- 1.Alexander, J. C., J. R. Schuh, and R. J. Gorczynski. December1996. Epoxy-steroidal aldosterone antagonist and angiotensin II antagonist combination therapy for treatment of cardiovascular disorders, including congestive heart failure. PCT Int. Appl. WO 96 40 257.
- 2.Andreoni, V., S. Bernasconi, M. Colombo, J. B. van Beilen, and L. Cavalca. 2000. Characterization of Rhodococcus sp. strain 1BN bearing alkane (alk) and naphthalene (nar) catabolic pathways. Environ. Microbiol. 2:572-577. [DOI] [PubMed] [Google Scholar]
- 3.Barger, G., and H. H. Dale. 1910. 4-β-Aminoethylglyoxaline (β-iminazolyl-ethylamine) and the other active principles of ergot. J. Chem. Soc. 97:2592-2595. [Google Scholar]
- 4.Black, J. W., W. A. M. Duncan, G. J. Durant, C. R. Ganellin, and M. E. Parsons. 1972. Definition and antagonism of histamine H2-receptors. Nature (London) 236:385-390. [DOI] [PubMed] [Google Scholar]
- 5.Brimblecombe, R. W., W. A. M. Duncan, G. J. Durant, J. C. Emmett, C. R. Ganellin, G. B. Leslie, and M. E. Parsons. 1978. Characterization and development of cimetidine as a histamine H2-receptor antagonist. Gastroenterology 74:339-347. [PubMed] [Google Scholar]
- 6.Brooks, D. P., T. A. Fredrickson, J. Weinstock, R. R., Jr. Ruffolo, R. M. Edwards, and M. Gellai. 1992. Antihypertensive activity of the nonpeptide angiotensin II receptor antagonist, SK&F 108566, in rats and dogs. Naunyn-Schmiedeberg's Arch. Pharmacol. 345:673-678. [DOI] [PubMed] [Google Scholar]
- 7.Cardini, G., and P. Jurtshuk. 1968. Cytochrome P-450 involvement in the oxidation of n-octane by cell-free extracts of Corynebacterium sp. strain 7E1C. J. Biol. Chem. 243:6070-6072. [PubMed] [Google Scholar]
- 8.Carini, D. J., J. V. Duncia, A. L. Johnson, A. T. Chiu, W. A. Price, P. C. Wong, and P. B. M. W. Timmermans. 1990. Part VI. Nonpeptide angiotensin II receptor antagonists: N-[(benzyloxy)benzyl]imidazoles and related compounds as potent antihypertensives. J. Med. Chem. 33:1330-1336. [DOI] [PubMed] [Google Scholar]
- 9.Catto, A., A. Rossi, A. Leonardi, R. Testa, and D. Nardi. 1989. Synthesis and anticonvulsant evaluation of 1,2-diphenylethane derivatives, potential metabolites of denzimol. Farmaco (Lausanne) 44:595-607. [PubMed]
- 10.Dale, H. H., and P. P. Laidlaw. 1910. The physiological action of β-iminazolylethylamine. J. Physiol. Lond. 41:318-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale, H. H., and P. P. Laidlaw. 1911. Further observations on the action of β-iminazolylethylamine. J. Physiol. Lond. 43:182-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, J. B., and R. L. Raymond. 1961. Oxidation of alcylsubstituted cyclic hydrocarbons by a Nocardia during growth on n-alkanes. Appl. Microbiol. 9:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean-Raymond, D., and M. Alexander. 1977. Bacterial metabolism of quaternary ammonium compounds. Appl. Environ. Microbiol. 33:1037-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.do Socorro de Souza Furtado, M., and P. S. Minami. 1997. In vitro susceptibility tests of dermatophytes to griseofulvin and imidazole derivatives. Rev. Microbiol. 28:110-115. [Google Scholar]
- 15.Dubini, F., L. Riviera, C. Cocuzza, and M. G. Bellotti. 1992. Antibacterial, antimycotic and trichomonicidal activity of a new nitroimidazole (EU 11100). J. Chemother. 6:342-346. [DOI] [PubMed] [Google Scholar]
- 16.Dutta, T. K., and S. Harayama. 2001. Biodegradation of n-alkylcycloalkanes and n-alkylbenzenes via new pathways in Alcanivorax sp. strain MBIC 4326. Appl. Environ. Microbiol. 67:1970-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedorak, P. M., D. L. Coy, and T. M. Peakman. 1996. Microbial metabolism of some 2,5-substituted thiophenes. Biodegradation 7:313-327. [Google Scholar]
- 18.Fritsche, W. 1968. Der Einfluβ der Kohlenstoffquelle auf das Wachstum, den Proteingehalt und das Enzymmuster von Candida guilliermondii. Z. Allg. Mikrobiol. 8:91-99. [DOI] [PubMed] [Google Scholar]
- 19.Ganellin, C. R., A. Fkyerat, B. Bang-Andersen, S. Athmani, W. Tertiuk, M. Garbarg, X. Ligneau, and J. C. Schwartz. 1996. A novel series of (phenoxyalkyl)imidazoles as potent H3-receptor histamine antagonists. J. Med. Chem. 39:3806-3813. [DOI] [PubMed] [Google Scholar]
- 20.Goodfellow, M. 1971. Numerical taxonomy of some Nocardioform bacteria. J. Gen. Microbiol. 69:33-80. [DOI] [PubMed] [Google Scholar]
- 21.Graziani, G., P. Cazzulani, C. Luca, G. Nava, and R. Testa. 1983. Denzimol, a new anticonvulsant drug. II. General pharmacological activities. Arzneim.-Forsch. 33:1161-1168. [PubMed] [Google Scholar]
- 22.Graziani, G., F. Tirone, E. Barbadoro, and R. Testa. 1983. Denzimol, a new anticonvulsant drug. I. General anticonvulsant profile. Arzneim.-Forsch. 33:1155-1160. [PubMed] [Google Scholar]
- 23.Groth, I., P. Schumann, N. Weiss, K. Martin, and F. A. Rainey. 1996. Agrococcus jenensis gen. nov., sp. nov., a new genus of actinomycetes with diaminobutyric acid in the cell wall. Int. J. Syst. Bacteriol. 46:234-239. [DOI] [PubMed] [Google Scholar]
- 24.Hetzheim, A., C. Leschke, C. Kerstan, B. Weiher, E. Morgenstern, and E. Göres. August1989. Verfahren zur Synthese neuer 1-substituierter 2-Morpholino-4-aryl-imidazole mit anticonvulsiver Wirkung. German patent 290,194.
- 25.Hetzheim, A., A. Irmscher, and B. Weiher. August1990. Neue anticonvulsiv wirksame 1-substituierte 2-Arylamino-4-aryl-imidazole und ihr Syntheseverfahren. German patent 296,918.
- 26.Hetzheim, A., C. Kerstan, B. Weiher, and E. Morgenstern. June1990. Verfahren zur Synthese neuer 1-substituierter 2-Piperidino-4-aryl-imidazole mit anticonvulsiver Wirkung. German patent 294,940.
- 27.Hetzheim, A., O. Peters, and H. Beyer. 1967. Über die Ringumwandlung von 2-Amino-3-phenacyl-1.3. 4-oxadiazoliumhalogeniden mit Aminen zu 1.2.-Diamino-imidazol-Derivaten. Chem. Ber. 100:3418-3426. [Google Scholar]
- 28.Hill, D. T., G. R. Girard, J. Weinstock, R. M. Edwards, E. F. Weidley, E. Ohlstein, C. E. Peishoff, E. Baker, and N. Aiyar. 1995. D- and L-N-[(1-benzyl-1H-imidazol-5-yl)-alkyl]-amino acids as angiotensin II AT-1 antagonists. Bioorg. Med. Chem. Lett. 5:19-24. [Google Scholar]
- 29.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1994. Bergey's manual of determinative bacteriology. The Williams & Wilkins Co., Baltimore, Md.
- 30.Hommel, R., and C. Ratledge. 1992. Biosynthetic mechanisms to low molecular weight surfactants and their precursor molecules, p. 3-63. In N. Kosaric (ed.), Biosurfactants: production—properties—application. Marcel Dekker, New York, N.Y.
- 31.Hundt, K., M. Wagner, D. Becher, E. Hammer, and F. Schauer. 1998. Effect of selected environmental factors on degradation and mineralization of biaryl compounds by the bacterium Ralstonia pickettii in soil and compost. Chemosphere 36:2321-2335. [DOI] [PubMed] [Google Scholar]
- 32.Keenan, R. M., J. Weinstock, J. A. Finkelstein, R. G. Franz, D. E. Gaitanopoulos, D. R. Girard, D. T. Hill, T. M. Morgan, J. M. Samanen, et al. 1993. Potent nonpeptide angiotensin II receptor antagonists. 2. 1-(Carboxybenzyl)imidazole-5-acrylic acids. J. Med. Chem. 36:1880-1892. [DOI] [PubMed] [Google Scholar]
- 33.Kreisel, H., and F. Schauer. 1987. Methoden des mykologischen Laboratoriums, p. 103-104. Gustav Fischer Verlag, Stuttgart, Germany.
- 34.Lechevalier, M. P., and H. A. Lechevalier. 1985. Biology of actinomycetes not belonging to the genus Streptomyces, p. 315-358. In A. L. Demain and N. A. Solomon (ed.), Biology of industrial microorganisms. Benjamin-Cummings, San Francisco, Calif.
- 35.Malachowsky, K. J., T. J. Phelps, A. B. Teboli, D. E. Minnikin, and D. C. White. 1994. Aerobic mineralization of trichloroethylene, vinyl chloride, and aromatic compounds by Rhodococcus species. Appl. Environ. Microbiol. 60:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merlos, M., A. Casas, A. Grauβ, and J. Castaner. 1997. Eprosartan. Antihypertensive angiotensin II antagonist. Drugs Future 22:1079-1085. [Google Scholar]
- 37.Morgan, P., and R. J. Watkinson. 1994. Biodegradation of components of petroleum, p. 1-31. In C. Ratledge (ed.), Biochemistry of microbial degradation. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 38.Nardi, D., A. Tajana, A. Leonardi, R. Pennini, F. Portioli, M. J. Magistretti, and A. Subissi. 1981. Synthesis and anticonvulsant activity of N-(benzoylalkyl)imidazoles and N-(ω-phenyl-ω-hydroxyalkyl)imidazoles. J. Med. Chem. 24:727-731. [DOI] [PubMed] [Google Scholar]
- 39.Petersen, M., M. Edelsten, V. F. Nielsen, A. Scarpellini, S. Skytte, and C. Slot. 1993. Formation and antimycotic effect of cyclodextrin inclusion complexes of econazole and miconazole. Int. J. Pharm. (Amsterdam) 90:247-254. [Google Scholar]
- 40.Poole, P. R., and G. Whitaker. 1997. Biotransformation of 6-pentyl-2-pyrone by Botrytis cinerea in liquid cultures. J. Agric. Food Chem. 45:249-252. [Google Scholar]
- 41.Ratledge, C. 1978. Degradation of aliphatic hydrocarbons, p. 1-46. In R. J. Watkinson (ed.), Developments in biodegradation of hydrocarbons—1. Applied Science, London, United Kingdom.
- 42.Raymond, R. L., V. W. Jamison, and J. O. Hudson. 1967. Microbial hydrocarbon co-oxidation I. Oxidation of mono- and dicyclic hydrocarbons by soil isolates of the genus Nocardia. Appl. Microbiol. 15:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson, D. W., J. H. Krushinski, E. E. Beedle, J. D. Leander, D. T. Wong, and R. C. Rathbun. 1986. Structure-activity relationships of (arylalkyl)imidazole anticonvulsants: comparison of the (fluorenylalkyl)imidazoles with nafimidone and denzimol. J. Med. Chem. 29:1577-1586. [DOI] [PubMed] [Google Scholar]
- 44.Roth, H. J., and H. Fenner. 1994. Pharmazeutische Chemie III Arzneistoffe. Georg Thieme Verlag, Stuttgart, Germany.
- 45.Schauer, F. 1981. Untersuchungen zum Abbau von n-Dodecan durch Pseudomonas aeruginosa 7./4II. Ph.D. thesis. University of Greifswald, Greifswald, Germany.
- 46.Schwartz, J. C., J. M. Arrang, M. Garbarg, H. Pollard, and M. Ruat. 1991. Histaminergic transmission in the mammalian brain. Physiol. Rev. 71:1-51. [DOI] [PubMed] [Google Scholar]
- 47.Smits, T. H. M., M. Röthlisberger, B. Witholt, and J. B. van Beilen. 1999. Molecular screening for alkane hydroxylase genes in Gram-negative and Gram-positive strains. Environ. Microbiol. 1:307-317. [DOI] [PubMed] [Google Scholar]
- 48.Tsukamura, M. 1967. Identification of Mycobacteria. Tubercle (London) 48:311-338. [DOI] [PubMed] [Google Scholar]
- 49.Urbanik, E., J. Zabielska-Matejuk, A. Skrzypczak, and J. Pernak. 1998. Antifungal properties of new imidazolium chlories against Coniophora puteana (Schum.: Fr.) Karst, Trametes versicolor (L.: Fr.) Pilat and Chaetomium globosum (Kunze: Fr.). Mater. Org. (Berlin) 31:247-263.
- 50.Warhurst, A. M., and C. A. Fewson. 1994. Biotransformation catalyzed by the genus Rhodococcus. Crit. Rev. Biotechnol. 14:29-73. [DOI] [PubMed] [Google Scholar]
- 51.Webley, D. M., R. B. Duff, and V. C. Farmer. 1956. Evidence for β-oxidation in the metabolism of saturated aliphatic hydrocarbons by soil species of Nocardia. Nature 178:1467-1468.13387737 [Google Scholar]
- 52.Weide, H., and F. Schauer. 1981. Induction of enzymes for n-alkane degradation in two strains of Pseudomonas aeruginosa, p. 167-170. In M. Moo-Young, C. W. Robinson, and C. Vezina (ed.), Advances in biotechnology, vol. 1. Pergamon Press, Toronto, Canada.
- 53.Werbrouck, S. P. O., and P. C. Debergh. 1996. Imidazole fungicides and paclobutrazol enhance cytokinin-induced adventitious shoot proliferation in araceae. J. Plant Growth Regul. 15:81-85. [Google Scholar]
- 54.Werbrouck, S. P. O., P. Redig, H. A. Van Onckelen, and P. C. Debergh. 1996. Gibberellins play a role in the interaction between imidazole fungicides and cytokinins in araceae. J. Plant Growth Regul. 15:87-93. [Google Scholar]
- 55.White, G. F., and N. J. Russell. 1994. Biodegradation of anionic surfactants and related molecules, p. 143-177. In C. Ratledge (ed.), Biochemistry of microbial degradation. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 56.Whyte, L. G., J. Hawari, E. Zhou, L. Bourbonniere, W. E. Inniss, and C. W. Greer. 1998. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 64:2578-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]