Abstract
Objective: This study was to assess the influence of interaction of combination of immobilized nitrogen cycling bacteria (INCB) with aquatic macrophytes on nitrogen removal from the eutrophic waterbody, and to get insight into different mechanisms involved in nitrogen removal. Methods: The aquatic macrophytes used include Eichhornia crassipes (summer-autumn floating macrophyte), Elodea nuttallii (winter-growing submerged macrophyte), and nitrogen cycling bacteria including ammonifying, nitrosating, nitrifying and denitrifying bacteria isolated from Taihu Lake. The immobilization carriers materials were made from hydrophilic monomers 2-hydroxyethyl acrylate (HEA) and hydrophobic 2-hydroxyethyl methylacrylate (HEMA). Two experiments were conducted to evaluate the roles of macrophytes combined with INCB on nitrogen removal from eutrophic water during different seasons. Results: Eichhornia crassipes and Elodea nuttallii had different potentials in purification of eutrophic water. Floating macrophyte+bacteria (INCB) performed best in improving water quality (during the first experiment) and decreased total nitrogen (TN) by 70.2%, nitrite and ammonium by 92.2% and 50.9%, respectively, during the experimental period, when water transparency increased from 0.5 m to 1.8 m. When INCB was inoculated into the floating macrophyte system, the populations of nitrosating, nitrifying, and denitrifying bacteria increased by 1 to 2 orders of magnitude compared to the un-inoculated treatments, but ammonifying bacteria showed no obvious difference between different treatments. Lower values of chlorophyll a, CODMn, and pH were found in the microbial-plant integrated system, as compared to the control. Highest reduction in N was noted during the treatment with submerged macrophyte+INCB, being 26.1% for TN, 85.2% for nitrite, and 85.2% for ammonium at the end of 2nd experiment. And in the treatment, the populations of ammonifying, nitrosating, nitrifying, and denitrifying bacteria increased by 1 to 3 orders of magnitude, as compared to the un-inoculated treatments. Similar to the first experiment, higher water transparency and lower values of chlorophyll a, CODMn and pH were observed in the plant+INCB integrated system, as compared to other treatments. These results indicated that plant-microbe interaction showed beneficial effects on N removal from the eutrophic waterbody.
Keywords: Nitrogen, Immobilized nitrogen-cycling bacteria (INCB), Eutrophication, Eichhornia crassipes, Elodea nuttallii
INTRODUCTION
Water eutrophication in the surface water system resulted in negative environmental effects in recent years, due to rapid industrialization, urbanization, and population growth. One of the major stresses comes from the excessive input of macronutrients (especially nitrogen and phosphorus) causing a change in the trophic status of a given waterbody and leading to eutrophication. Eutrophic conditions are typically found in relatively shallow (<10 m depth) freshwater ponds, lakes and reservoirs where anthropogenic influence is high. One of major problems was caused by nitrogen compounds.
Aquatic ecosystems play a major role in returning N2 gas back to the atmosphere by bacteria-mediated processes of nitrification and denitrification (Matulewich and Finstein, 1978; Northup et al., 1995). Most aquatic ecosystems, including natural wetlands, have relatively poor inefficiency rates of producing N2 gas, because of the low growth rates of nitrifying and denitrifying bacteria. Such low growth may be improved by inoculation of aquatic ecosystems with these bacteria immobilized on a carrier.
Phytoremediation is one of most promising biotechnologies to reduce trophic water remediation costs. Effective removal of nutrients by floating aquatic macrophyte (Eichhornia crassipes) has been reported for treatment of dairy manure wastewater (Sooknah and Wilkie, 2004), domestic wastewater (Kim et al., 2006), industrial wastewater (Ntengwe, 2005). Eichhornia crassipes is the fast-growing floating macrophyte with outstanding ability to purify polluted water, but can grow only from spring to autumn (May to November in northern latitudes). When this floating macrophyte is harvested, different aquatic plant species (e.g., submerged macrophytes that can grow in winter) are required to maintain the water quality. Submerged macrophytes are very important to many aquatic ecosystems such as estuaries (Neundorfer and Kemp, 1993; Herbert, 1999) and lakes (Kufel and Ozimek, 1994; van Donk and van de Bund, 2002). Widespread reduction of submerged macrophytes has been reported over the past several decades (Livingston et al., 1998; Seddon et al., 2000). This reduction can be related to decline of water transparency by suspended particles and phytoplankton, and excessive nutrient loading (Jeppesen et al., 1991; Lauridsen et al., 1994). Elodea nuttallii is a submerged aquatic plant species inhabiting freshwaters. As an ever-green submerged macrophyte, it can grow in the winter season. This species had been used previously in water purifications studies (Wu, 2003; Yang et al., 2003; Li, 1997; Eighmy and Bishop, 1989). However, little information is available on the relationship between aquatic plants and nitrogen-cycling bacteria in in-situ N removal from eutrophic water.
In the present study, we used immobilized nitrogen-cycling bacteria combined with floating and submerged macrophytes to determine the roles of these bacteria with or without macrophytes in nitrogen removal, and to measure the effectiveness of various microbe-plant systems in removing N from eutrophic water.
MATERIALS AND METHODS
Experimental sites
The experiment was set up in Hua-Jia-Chi pool (120°11′ E, 30°16′ N) situated in Hangzhou City, Zhejiang Province, China. This is a rectangular reservoir with total area of 53360 m2 and average depth of approximately 1.2 m.
Materials
1. Floating macrophyte
Eichhornia crassipes (Mart.) Solms-Laub was collected close to the experimental site and introduced into the treatment enclosures (1000 kg to each plot).
2. Submerged macrophyte
Elodea nuttallii (10 kg to each plot) was collected from Taihu lake, China (119°54′~120°36′ N, 30°56′~31°33′ E) and introduced into treatment enclosures.
3. Nitrogen cycling bacteria
Ammonifying, nitrosating, nitrifying and denitrifying bacteria were prepared according to the methods of Matulewich and Finstein (1978) and Li et al.(1996). We have previously immobilized nitrogen-cycling bacteria using the carrier in which is included four bacterial communities of ammonifying, nitrosating, nitrifying and denitrifying bacteria to treat eutrophic water (Li and Pu, 2001a). The four bacteria communities were separated from Taihu wastewater and inoculated into relevant media with adjusted pH described by Li and Pu (2000), and incubated about 30 d for nitrosating and nitrifying bacteria and 15 d for denitrifying and ammonifying bacteria at 28 °C. Cell suspension at density of OD 600=0.5 was centrifugated at 4000 r/min for 20 min at 4 °C and then kept at 4 °C for use. The four communities mainly include: Bacillus subtilis, Pseudomons fluorescens, Nitrosococcus, Nitrosomonos, Nitrobacter, Nitrococcus, Pseudomonas denitificans, Chromo bacterium denitrificans.
Methods
1. Cell immobilization
Carrier made by hydrophilic monomers 2-hydroxyethyl acrylate (HEA) and hydrophobic 2-hydroxyethyl methylacrylate (HEMA), fully mixed at certain ratio was added into the distilled water, by charging nitrogen into the four mixtures at −78 °C, using 60Co-γ (1×104 Gy) radiated to form the polymer, then cutted into 0.5 cm×0.5 cm immobilized carrier, dipped in distilled water for one week, and sterilized for 30 min at 1.03×105 Pa, so that four carriers could be made (Li and Pu, 2001b) using artificial wastewater to marinate the four carriers, and allowed to quiver 24 h at 28 °C, then kept silent for 24 h, and cycled three times before the collected bacteria was added into the carrier. The aerobic and anaerobic conditions made these bacteria absorbed into the polymer.
2. The first experiment design
The first experiment in the enclosed experimental plot with area of 1000 m2 and depth of 1.8 m was conducted from Aug. 11, 2003 to Sept. 30, 2003. In view of the large area of the experimental plots (1000 m2/plot), there were three treatments: floating macrophyte (Eichhornia crassipes), floating macrophyte (Eichhornia crassipes)+bacteria (INCB) (ammonifying, nitrosating, nitrifying and denitrifying bacteria) and control (no macrophyte and no INCB). Twenty days before the first sampling on Aug. 11, 2003, pre-determined weight (1000 kg fresh weight per plot) of Eichhornia crassipes was placed into specified treatment enclosures, with 1/10 of the water surface covered. Immobilized nitrogen-cycling bacteria carrier was placed into the 0.2 mm aperture nylon bag, then fixed into the plastic basket (0.4 m×0.3 m×0.2 m). Three baskets were placed into each plot, each basket was fixed to two nylon bags loaded with 300 g carrier, and positioned about 0.3 m below water surface 10 d before the first sampling. Fresh supply of bacteria immobilized on the carrier was provided every 7 d during the experiment. For treatment, the initial mean values of TN, nitrite and ammonium in the eutrophic water were 2.17, 0.042, 0.13 mg/L. The pH and transparency were 7.85 and 0.50 m respectively, and the CODMn, chlorophyll a were 11.17 mg/L and 30.85 μg/L, the four communities’ magnitude (ammonifying, nitrosating, nitrifying and denitrifying bacteria) were 5.53, 2.86, 2.65, 3.12 logMPN/ml, respectively.
3. The second experiment design
The experiment was conducted in the pilot enclosed experimental plot with area 25 m2 and depth of 1.8 m from Feb. 2, 2004 to May 13, 2004. The experiment included four treatments: bacteria (INCB), submerged macrophyte (Elodea nuttallii), submerged macrophyte (Elodea nuttallii)+bacteria (INCB) and control (no macrophyte and no bacteria). Each treatment had three repeats. In the experiment, the pre-determined weight (10 kg per plot) of Elodea nuttallii was placed into specified treatment enclosures about 25 d before the first sampling. Elodea nuttallii was planted into the sediment. Similar to the first experiment, immobilized nitrogen-cycling bacteria were placed into a nylon bag and fixed into a plastic basket (same as the first experiment). Each plot had one nylon bag in one basket loaded with 200 g carrier and positioned about 0.3 m below the water surface 10 d before the first sampling. Fresh supply of bacteria immobilized on the carrier was provided every 7 d during the experiment. The initial mean values of TN, nitrite and ammonium in the eutrophic water were 1.21, 0.028, 0.15 mg/L, respectively. The pH and transparency were 9.10 and 0.90 m, respectively, and the CODMn, chlorophyll a were 10.85 mg/L and 58.43 μg/L, the four communities magnitude (ammonifying, nitrosating, nitrifying and denitrifying bacteria) were 4.59, 1.45, 1.62, 1.76 logMPN/ml, respectively.
Sampling and analyses
Water samples were taken at depth of 0.5 m below the water surface, stored at 4 °C and analyzed for TN, NH4-N and NO2-N within a day. Samples were collected every 10 d in the first experiment and every 20 d in the second experiment, respectively. Water samples analysis followed the procedures described by Wei et al.(2002). All analyses were conducted in triplicates. Samples were oxidized with potassium peroxodisulphate followed by ultra-colorimetric determination for TN. NH4-N was analyzed by indophenol blue colorimetry, and NO2-N by N-ethylenediamine colorimetry. The concentration of chemical oxygen demand (CODMn) was analyzed according to GB11892-89 in China. Chlorophyll a was determined colorimetrically. The numbers of four types of nitrogen-cycling bacterial communities were determined using the most probable number method (MPN) (Smith et al., 1968).
Statistical analysis
The data were subjected to one-way analysis of variance (ANOVA). Comparisons of means of parameters were performed with least significant difference (LSD) test.
RESULTS
Effects on the concentration of different nitrogen forms in eutrophic water
The results showed that Eichhornia crassipes, Elodea nuttallii and INCB bacteria significantly affected the concentration of different nitrogen forms in eutrophic water (Fig.1). Content of TN in the treatments of floating macrophyte+bacteria and floating macrophyte decreased by 66.60% and 70.21%, respectively, which reached significant difference (P<0.05) as compared to that in the control at the last sampling date (Fig.1a), The maximum reduction in TN (77.74%) occurred during the floating macrophyte+bacteria treatment on day 30 in the first experiment. Similarly, in winter season, TN was decreased most by submerged macrophyte+bacteria treatment, followed by macrophyte and bacteria treatment, least in the control. The corresponding values were 26.10%, 24.60%, 10.34% and 7.07%, respectively, by the end of the experiment (Fig.1b). These results suggested that macrophytes combined with bacteria the most effective way for removing TN from eutrophic water.
Fig. 1.
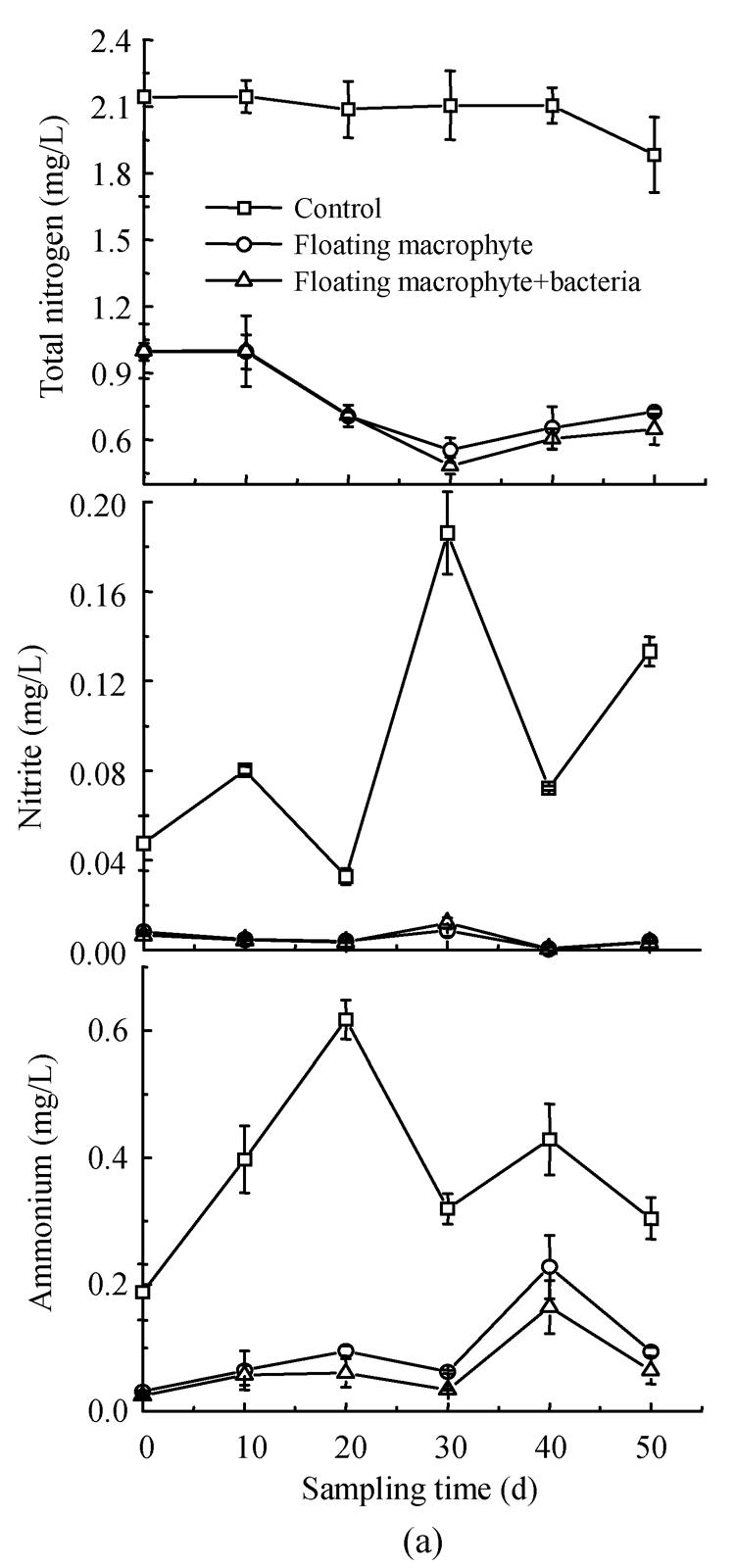
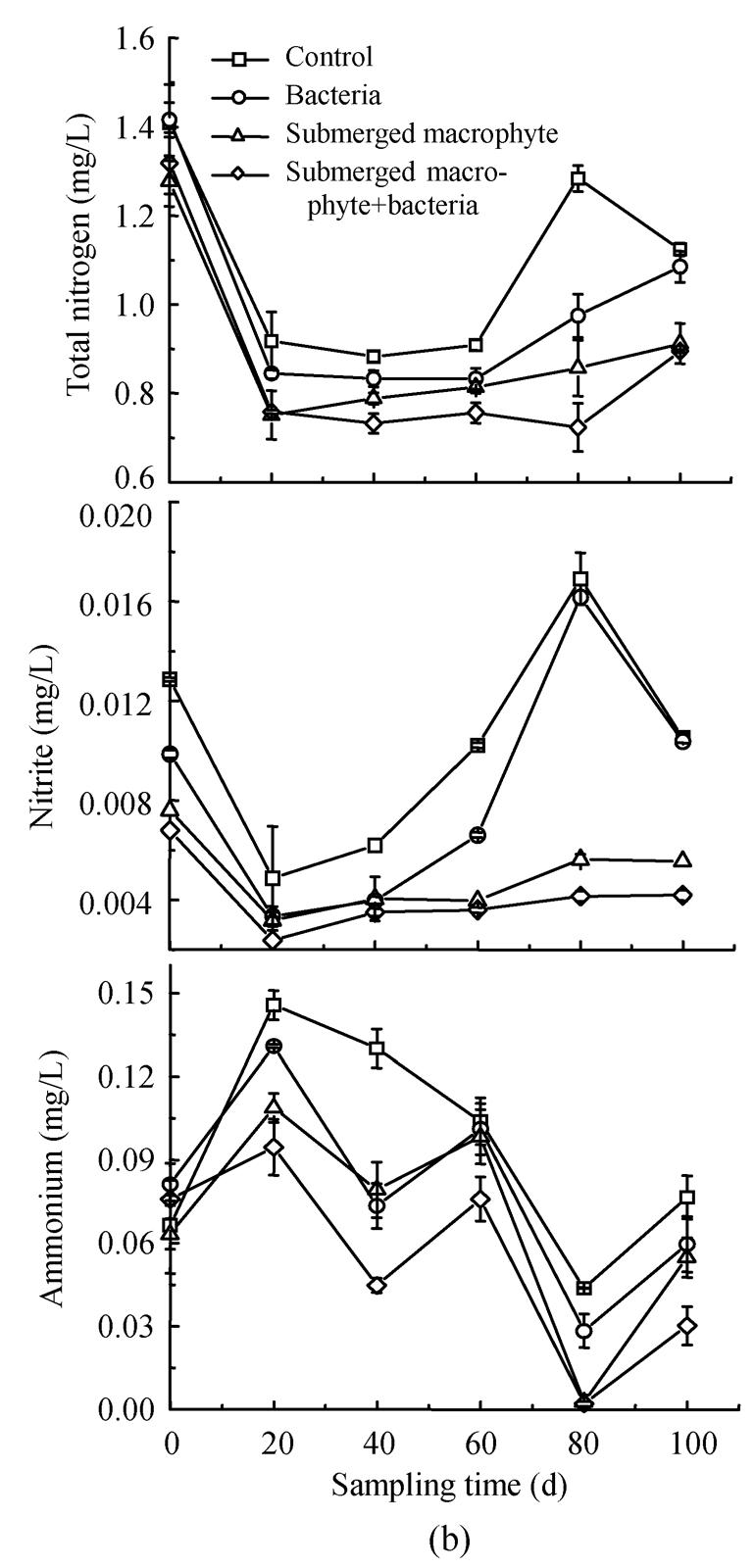
Changes in total nitrogen, nitrite and ammonium concentrations in the eutrophic water with treatment time as affected by (a) floating plant-microbe and (b) submerged plant-microbe interaction system
The treatments with both floating macrophyte+bacteria and submerged macrophyte+bacteria resulted in lowest concentration of nitrite and ammonium in the eutrophic water (Fig.1). The concentration of nitrite with Eichhornia crassipes was significantly lower than that of the control during the experiment, but significant difference (P<0.05) in nitrite reduction was not observed between the treatments of Eichhornia crassipes (91.47%) and Eichhornia crassipes+bacteria (92.21%) (Fig.1a). The concentration of nitrite changed in the order: control>bacteria>submerged macrophyte>submerged macrophyte+bacteria (Fig.1b), and the nitrite reduction changed by 62.79%, 63.36%, 80.42%, 85.23%, respectively. Significant difference was observed between the control and other treatments, except for between the control and bacteria treatment at day 80 and 100. The lower nitrite in bacteria than the control may contribute to the inoculation INCB, which promoted oxidation of nitrite to nitrate and through denitrification to produce N2 gas.
Macrophytes, bacteria and their combination decreased the content of ammonium in eutrophic water (Fig.1). The content of ammonium in the treatments with floating macrophyte and floating macrophyte+bacteria was significantly lower than the control in the first experiment, with the reduction in ammonia being 28.5% and 50.9%, respectively, on final day. However, ammonium concentration in the control increased for 1.4~4.6 folds during 100-day experiment period as compared to the initial value (0.15 mg/L) (Fig.1a). Treatment with submerged macrophyte+bacteria significantly decreased ammonium by 80.01% at the final sampling date. Significant difference (P<0.05) in reducing ammonium was observed between submerged macrophyte+bacteria and other treatments. Similarly, the concentration of ammonium in the second experiment changed in the order: control>bacterial>submerged macrophyte>submerged macrophyte+bacteria. These results showed that macrophyte and inoculated INCB could accelerate ammonium removal from eutrophic water.
Effects on the population size of nitrogen-cycling bacteria in eutrophic water
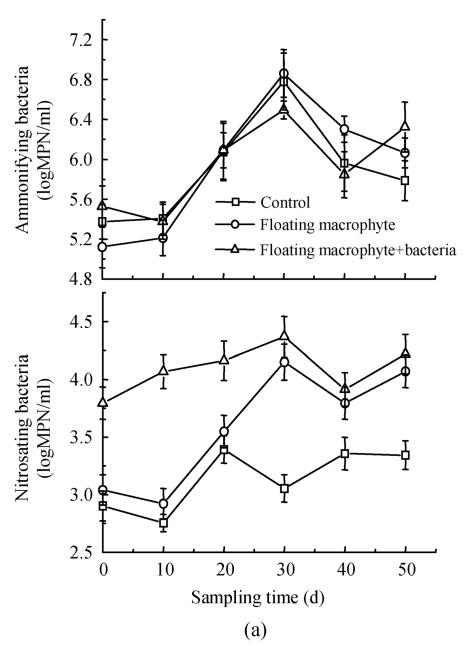
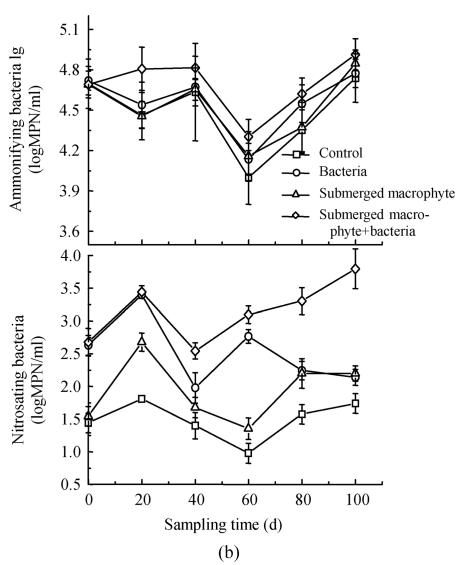
The number of ammonifying bacteria changed from 105 to 107 per milliliter in first experiment (Fig.2a), and fluctuated between 103.5 and 105 per milliliter (Fig.2b) in the second experiment. Populations of ammonifying bacteria increased by about twice from day 10 to 30, and then decreased slightly in the first experiment (Fig.2a). The ammonifying bacteria population, minimized at day 60 in all treatments, and then increased in the second experiment (Fig.2b). Since the populations of ammonifying bacteria kept higher than other populations in the waterbody, and a large number of these heterotrophic bacteria easily dominated the population in the environment. No obvious effect was observed with inoculation of INCB bacteria during the period of experiment (Figs.2a and 2b), and different treatments did not reveal significant difference (P<0.05).
Fig. 2.
Changes in the numbers of ammonifying and nitrosating bacteria in the eutrophic water with treatment time as affected by (a) floating plant-microbe and (b) submerged plant-microbe interaction system
The number of nitrosating bacteria was obviously greater (by about one order of magnitude) in Eichhornia crassipes+bacteria treatment than in other treatments (Fig.2a). Nitrosating bacteria increased steadily from the beginning of the experiment and maximized at day 30 in treatment with Eichhornia crassipes. The treatment with submerged macrophyte+bacteria significantly increased nitrosating by two orders of magnitude compared with the control. The nitrosating bacteria decreased in the order: submerged macrophyte+bacteria>bacteria>submerged macrophyte>control (Fig.2b). Nitrosating bacteria in three treatments revealed significant (P<0.05) variation compared to the control in the second experiment.
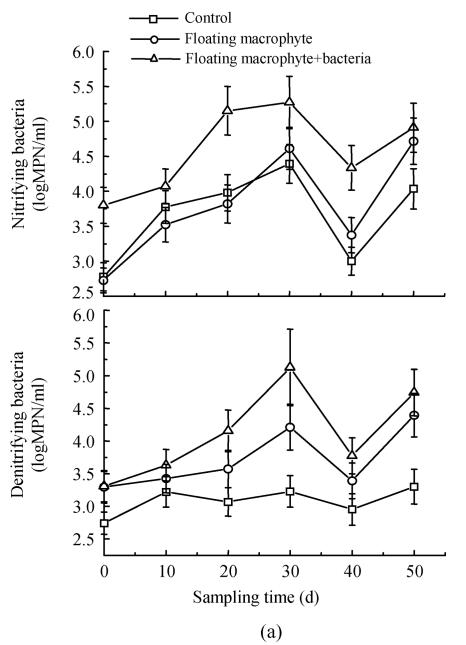
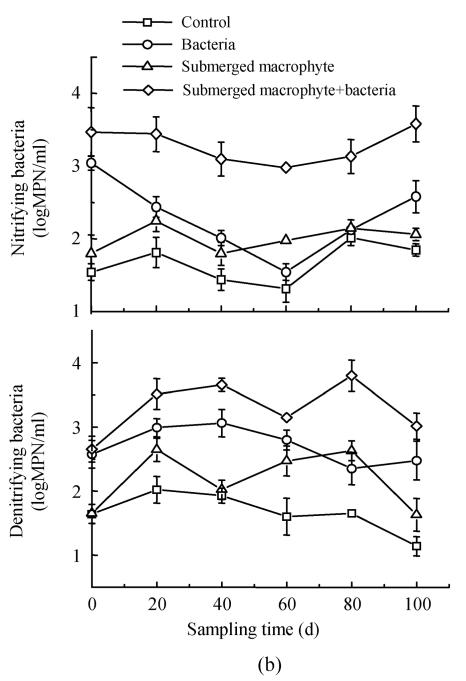
Nitrifying bacteria increased with time, and maximized at day 30, then declined at day 40, and increased again at day 50 in the first experiment (Fig.3a). The number of nitrifying bacteria in the Eichhornia crassipes+bacterial treatment was about one and half orders of magnitude higher than that in the control during the experiment. Analysis of variance revealed significant (P<0.05) difference between submerged macrophyte+bacteria treatment and other treatments for the nitrifying bacteria, and there was about three-fold greater number of nitrifying bacteria in macrophyte+bacteria system compared to control. Bacteria treatment always had more nitrifying bacteria than the treatment only with submerged macrophyte on day 60 (Fig.3b). The numbers of nitrifying bacteria followed the order: submerged macrophyte+bacteria>bacteria>submerged macrophyte>control, and macrophyte+bacteria in the second experiment.
Fig. 3.
Changes in the numbers of nitrifying and denitrifying bacteria in the eutrophic water with treatment time as affected by (a) floating plant-microbe and (b) submerged plant-microbe interaction system
The number of denitrifying bacteria decreased in the order: Eichhornia crassipes+bacterial>Eichhornia crassipes>control, with about one order of magnitude difference between the plant-microbe system and the control (Fig.3a). On day 80, the submerged macrophyte treatment had more denitrifying bacteria than inoculated bacteria treatment. At other sampling times, denitrifying bacteria decreased in the order: submerged macrophyte+bacteria>bacteria>submerged macrophyte>control in the second experiment (Fig.3b). The number of denitrifying bacteria fluctuated from 102.5 to around 105 per milliliter during the first experiment and from 101 to 103.5 per millilitre during the second experiment.
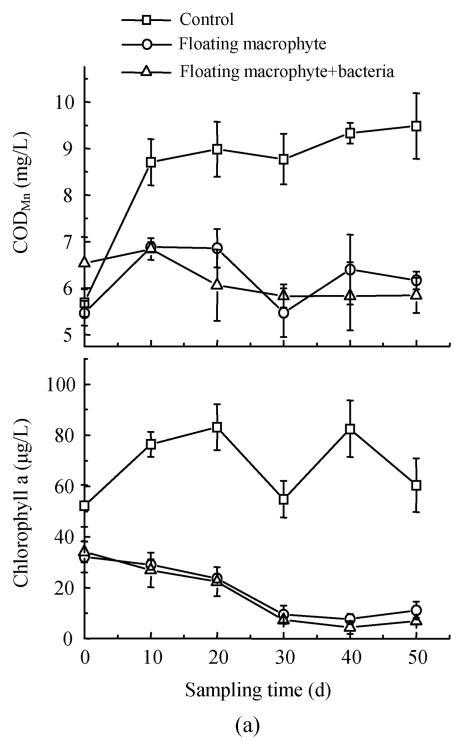
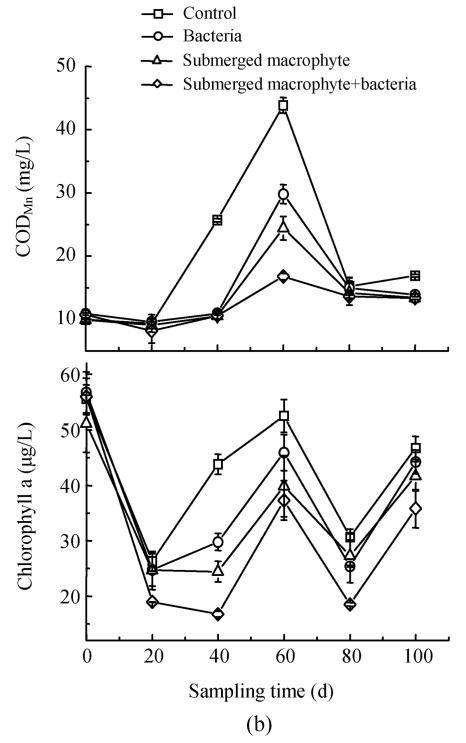
Effects on chlorophyll a, CODMn in eutrophic water
In the first experiment, there was significant (P<0.05) variation between different treatments Eichhornia crassipes treatments with or without bacterial inoculation and control in chlorophyll a, and CODMn. Concentration of chlorophyll a in the control was 2 to 10 times that of other two treatments (Fig.4a). Higher CODMn was found for the control also in comparison with the other two treatment at all sampling times except for the first sampling time (Fig.4a). In the second experiment, we found CODMn in the control was 2~3 folds that in other treatments, especially Elodea nuttallii+bacterial (Fig.4b). As the submerged macrophyte, Elodea nuttallii and algae had different niches, the macrophyte could not dramatically decrease the chlorophyll a concentration. But CODMn could promote microorganism growth as carbon source. In addition, microbe could change CODMn into H2O and CO2 through metabolization.
Fig. 4.
Changes in CODMn and chlorophyll a levels in the eutrophic water with treatment time as affected by (a) floating plant-microbe and (b) submerged plant-microbe interaction system
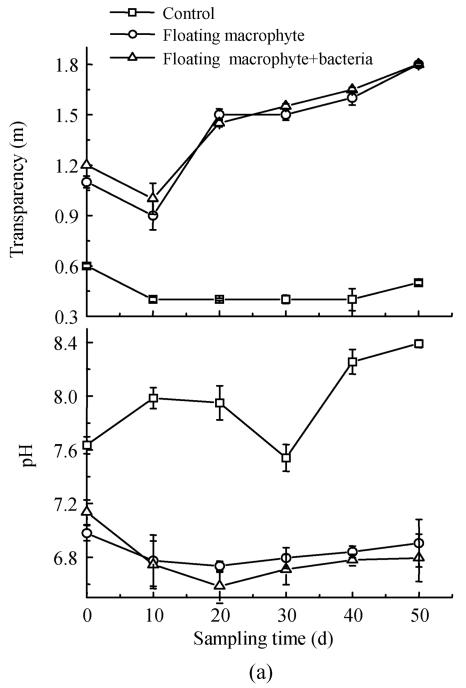
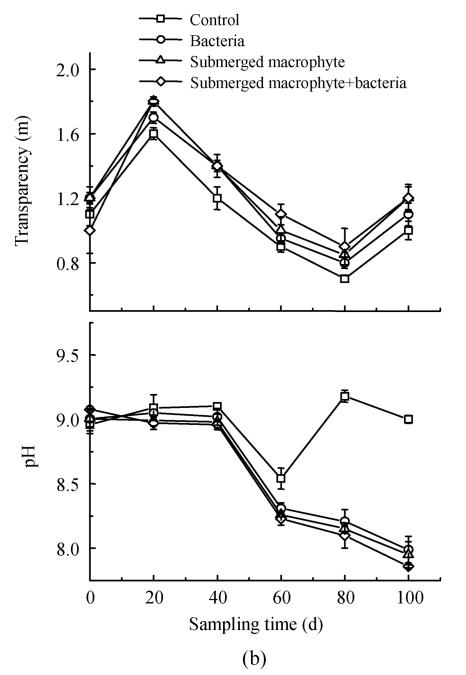
Effects on water transparency and pH in eutrophic water
Alkalinity reduction and transparency increase were observed in macrophytes systems. Transparency in the treatment with macrophyte was about 5 times that in the control at the end of the experiment (Fig.5a). The transparency level in the control was only 0.4~0.6 m during experiment, but that with floating macrophyte+bacteria increased up to 1.8 m. With the floating macrophyte (Eichhornia crassipes), pH kept below 7.2 and became lowest on day 20 (Fig.5a), whereas pH values were 6.73 and 6.58 in the treatments with Eichhornia crassipes and Eichhornia crassipes+bacteria respectively. Treatment with submerged macrophyte+bacteria produced higher transparency and lower pH than in other three treatments at all sampling times (Fig.5b).
Fig. 5.
Changes in transparency and pH in the eutrophic water with treatment time as affected by (a) floating plant-microbe and (b) submerged plant-microbe interaction system
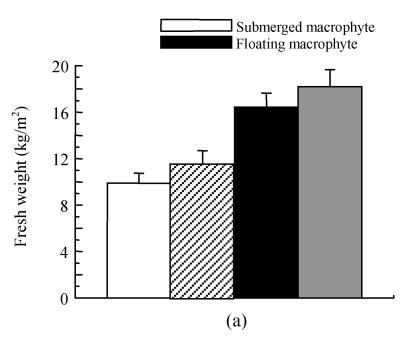
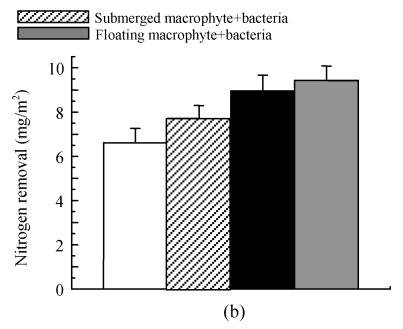
Effects on fresh biomass production and nitrogen removal at the end of experiment
The fresh biomass production and nitrogen removal by macrophytes are shown in Fig.6. Higher fresh matter production (18.21 kg/m2) and nitrogen removal (9.43 mg/m2) were found in the treatment with floating macrophyte+INCB in the first experiment submerged macrophyte with INCB achieved higher fresh matter production (11.52 kg/m2) and greater nitrogen removal (7.71 mg/m2) compared to the treatment with submerged macrophyte alone.
Fig. 6.
Effects of plant-microbe interaction on (a) plant fresh production and (b) nitrogen removal at the final day (after 70 d in the experiment) (Exp. 1) and on May 12, 2004 (after 126 d in the experiment) (Exp. 2), respectively
DISCUSSION
Immobilization of support gel is one way to maintain high cell density and prevent washout, at the same time aerobic surface and anaerobic subsurface layers of the gel provide niches suitable for both aerobic nitrifiers and anaerobic denitrifiers (Cao et al., 2002; dos Santos et al., 1996), and this method was used to remove nitrogen from eutrophic water (Chang et al., 2005), seawater (Sakairi et al., 1996) and domestic wastewater (Takizawa et al., 1996) and proved to be a feasible way for removing nitrogen from wastewater. In addition, aquatic plants can also provide nutrient resources and attachment sites for these bacteria in microbial-plant integrated system.
In our microbial-plant integrated systems, inoculation of INCB into the eutrophic water column could increase by 1 to 2 orders of magnitude compared to non-inoculated control, and the combination with macrophyte resulted in greater increase in these bacteria. These bacteria can distribute in different habitats: sediments, macrophyte and waterbody (Körner, 1999; Matulewich and Finstein, 1978). So the abundance of INCB in different habitats need further study.
Nitrogen removal through plant-micro integrated system has been reported by some scholars (Körner and Vermaat, 1998; Brix, 1994). Plant uptake is often regarded as a major nitrogen removal mechanism. Aquatic macrophytes can acquire nitrogen from both the sediment and the water column (Rattray et al., 1991). They provide attachment surfaces for bacteria colonization, the nitrifiers can utilize photosynthetically derived O2 to oxidize ammonium to nitrate. Dark plant respiration can then reduce dissolved oxygen (DO) levels in the reactors to promote denitrifier activity which can convert nitrate to nitric oxide, nitrous oxide or molecular nitrogen and cause these gases to efflux from the system (Eighmy and Bishop, 1989). But in site plant-micro integrated system, different macrophytes may have different effects on the distribution of the nitrifiers and denitrifier.
In our results two macrophytes significantly decreased nitrogen concentration, more TN could be removed by combining macrophytes with INCB. The main reason was Eichhornia crassipes and Elodea nuttallii could directly uptake ammonium as a nitrogen source for protein synthesis and subsequent production of biomass. Uptake was the most important factor affecting the total nitrogen decrease. Inoculating INCB into the water system also promoted the cycling of nitrogen, the interaction between bacteria and macrophyte benefited nutrient absorption from water systems, so when combined with INCB there was more production than that only by macrophyte treatment. In our two plant-microbial systems, volatilization of ammonia was thought to be a minor mechanism because the pH levels in the water column were almost lower than the pKa of ammonium (9.3). We can draw the conclusion from the results of two experiments that harvesting these macrophytes from water system in time was needed because too much macrophyte grown in water system will not benefit the nitrogen removal.
When Eichhornia crassipes combined with nitrogen cycling bacteria inoculation provided additional benefit in terms of capacity to remove nitrogen and decrease concentrations of ammonium and nitrite. However, the importance of inoculation with nitrogen-cycling bacteria is somewhat greater in combination with Elodea nuttallii. The reason for such a difference may be due to the cold period in which Elodea nuttallii was grown, which might have decreased its growth and metabolic capacity, hence allowing the contribution to purifying eutrophic water by nitrogen cycling bacteria to become more pronounced. Even though inoculation of nitrogen-cycling bacteria in the present study was carried out continuously to improve their competitive ability, with respect to the well-adapted indigenous bacterial populations, the effect of INCB inoculation was relatively small.
The macrophytes in aquatic system suppress algae growth through their competition for nutrient or secreted some substances inhibiting algae growth, INCB combined with Eichhornia crassipes and Elodea nuttallii reduced nutrient more than the control, so there was lower chlorophyll a concentration. The degradation of pollutants (CODMn) is mainly attributed to microorganisms which may establish a symbiotic relationship with the plants and filtration through macrophytes in the aquatic cultures also can reduce CODMn. In our microbial-plant integrated system, the roots of Eichhornia crassipes and Elodea nuttallii may provide more favorable conditions for bacteria to establish themselves in the plant cultures compared to the control. The higher pH level in the control most probably resulted from the consumption of carbonate during algal photosynthesis. Eichhornia crassipes and Elodea nuttallii exudating organic acid also lead to lower pH.
The first experiment (conducted with Eichhornia crassipes during the warm season of the year) was more effective in improving the quality of eutrophic water compared to the second experiment (using Elodea nuttallii grown during winter-spring period). However, such results may not mean that there was an inherent difference in the capacity to remove nitrogen from eutrophic water between the two macrophytes species, because the difference in the ambient water temperature could have been the main factor. However, testing these two plant species under identical temperature conditions in the controlled environment is not recommended, due to their differential temperature requirement. Despite observed differences between the plant species, we concluded that both macrophytes tested are effective in purifying eutrophic water. Further field testing with joint inoculation of the two species and monitoring over several years is needed before conclusions can be reached on the effectiveness of this plant-based year-round water purification system.
Compared to nitrogen removal, there was no significant difference (P<0.05) between macrophyte and macrophyte+bacteria treatments from the two experiments results, though inoculated nitrogen cycling bacteria increased more nitrogen removal. So in the in-situ nitrogen removal process, macrophytes play a more important role than nitrogen cycling bacteria. We need to combine them to achieve better effectiveness. Though inoculated frequently, the number of four communities did not increase persistently, and was of a relatively low magnitude. So further work is needed to clarify the relationship between nitrogen-cycling bacteria and aquatic macrophytes, and take into account the natural abundance of nitrogen cycling bacteria and their dynamics in eutrophic water during the year.
Footnotes
Project supported by the Ministry of Science and Technology of China, the Education Ministry of China (No. 20305), Australia Government’s Innovation Statement Backing Australia’s Ability (No. [2002]68), and the Science and Technology Bureau of Zhejiang Province (No. 2005C22020), China
References
- 1.Brix H. Functions of macrophytes in constructed wetlands. Wat Sci Tech. 1994;29(4):71–78. [Google Scholar]
- 2.Cao GM, Zhao QX, Sun XB, Zhang T. Characterization of nitrifying and denitrifying bacteria coimmobilized in PVA and kinetics model of biological nitrogen removal by coimmobilized cells. Enzyme Microb Technol. 2002;30(1):49–55. doi: 10.1016/S0141-0229(01)00458-6. [DOI] [Google Scholar]
- 3.Chang HQ, Yang XE, Fang YY. Effects on nutrient of eutrophicated water by Elodea nuttallii and immobilized bacteria. Journal of Soil and Water Conservation. 2005;19(3):114–117. [Google Scholar]
- 4.dos Santos VMPM, Bruijnse M, Tramper J, Wijffels RH. The magic-beads concept: an integrated approach to nitrogen removal with co-immobilized micro-organisms. Appl Microbiol Biotechnol. 1996;45(4):447–453. doi: 10.1007/BF00578454. [DOI] [Google Scholar]
- 5.Eighmy TT, Bishop PL. Distribution and role of bacterial nitrifying populations in nitrogen removal in aquatic treatment systems. Wat Res. 1989;23(8):947–955. doi: 10.1016/0043-1354(89)90167-X. [DOI] [Google Scholar]
- 6.Herbert RA. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev. 1999;23(5):563–590. doi: 10.1016/S0168-6445(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen E, Kristensen P, Jensen JP, Søndergaard M, Mortensen E, Lauridsen T. Recovery resilience following a reduction in external phosphorus loading of shallow, eutrophic Danish lakes: duration, regulating factors and methods for overcoming resilience. Mem Ist Ital Idrobiol. 1991;48(1):127–148. [Google Scholar]
- 8.Kim Y, Giokas DL, Lee JW, Paraskevas PA. Potential of natural treatment systems for the reclamation of domestic sewage in irrigated agriculture. Desalination. 2006;189(1-3):229–242. doi: 10.1016/j.desal.2005.07.007. [DOI] [Google Scholar]
- 9.Körner S. Nitrifying and denitrifying bacteria in epiphytic communities of submerged macrophytes in a treated sewage channel. Acta Hydrochim Hydrobiol. 1999;27(1):27–31. doi: 10.1002/(SICI)1521-401X(199901)27:1<27::AID-AHEH27>3.0.CO;2-1. [DOI] [Google Scholar]
- 10.Körner S, Vermaat JE. The relative importance of Lemna gibba L., bacteria and algae for the nitrogen and phosphorus removal in duckweed-covered domestic wastewater. Water Research. 1998;32(12):3651–3661. doi: 10.1016/S0043-1354(98)00166-3. [DOI] [Google Scholar]
- 11.Kufel L, Ozimek T. Can Chara control phosphorus cycling in Lake Luknajno (Poland) Hydrobiologia. 1994;275/276(1):277–283. [Google Scholar]
- 12.Lauridsen TL, Jeppesen E, Søndergaard M. Colonization and succession of submerged macrophytes in shallow Lake Vaeng during the first 5 years following fish manipulation. Hydrobiologia. 1994;275/276(1):233–242. [Google Scholar]
- 13.Li WC. Construction and purification efficiency test of an ever-green aquatic vegetation in an eutrophic lake. China Enviromental Science. 1997;17(1):53–57. (in Chinese) [Google Scholar]
- 14.Li ZK, Pu PM. The dynamic mode of INCB purifying the polluted lake by nitrogen compound in the autumn-winter seasons. Lake Science. 2000;1-2(4):321–326. (in Chinese) [Google Scholar]
- 15.Li ZK, Pu PM. The dynamic mode of INCB purifying the polluted lake by nitrogen compound in the autumn-winter seasons. Lake Science. 2001;12(4):321–326. (in Chinese) [Google Scholar]
- 16.Li ZK, Pu PM. The technics immobilized nitration bacteria removal ammonium in low temperature radiation. Jiangsu Agriculture. 2001;16(2):115–117. (in Chinese) [Google Scholar]
- 17.Li FD, Yu ZN, He SJ. Experimental Techniques of Agricultural Microorganism. Beijing, China: Chinese Agriculture Press; 1996. pp. 44–50. (in Chinese) [Google Scholar]
- 18.Livingston RJ, McGlynn SE, Niu X. Factors controlling seagrass growth in a gulf coastal system: water quality and light. Aquat Bot. 1998;60(2):135–159. doi: 10.1016/S0304-3770(97)00079-X. [DOI] [Google Scholar]
- 19.Matulewich VA, Finstein MS. Distribution of autotrophic nitrifying bacteria in a polluted river (the Passaic) Applied and Environmental Microbiology. 1978;35(1):67–71. doi: 10.1128/aem.35.1.67-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neundorfer JV, Kemp WM. Nitrogen versus phosphorus enrichment of brackish waters: responses of the submersed plant Potamogeton perfoliatus and its associated algal community. Mar Ecol Prog Ser. 1993;94:71–82. [Google Scholar]
- 21.Northup RR, Zengshou YU, Randy A. Polyphenol control of nitrogen release from pine litter. Nature. 1995;377(21):227–229. doi: 10.1038/377227a0. [DOI] [Google Scholar]
- 22.Ntengwe FW. An overview of industrial wastewater treatment and analysis as means of preventing pollution of surface and underground water bodies―the case of Nkana Mine in Zambia. Physics and Chemistry of the Earth. 2005;30(11-16):726–734. [Google Scholar]
- 23.Rattray MR, Howard-Williams C, Brown JMA. Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquat Bot. 1991;40(3):225–237. doi: 10.1016/0304-3770(91)90060-I. [DOI] [Google Scholar]
- 24.Sakairi MAC, Kimiaki Yasuda K, Matsumura M. Nitrogen removal in seawater using nitrifying and denitrifying bacteria immobilized in porous cellulose carrier. Water Science and Technology. 1996;34(7-8):267–274. doi: 10.1016/S0273-1223(96)00754-8. [DOI] [Google Scholar]
- 25.Seddon S, Connolly RS, Edyvane KS. Large-scale seagrass die back in northern Spencer Gulf, South Australia. Aquat Bot. 2000;66(4):297–310. doi: 10.1016/S0304-3770(99)00080-7. [DOI] [Google Scholar]
- 26.Smith WH, Bormann FH, Likens GE. Response of chemoautotrophic nitrifiers to forest cutting. Soil Sci. 1968;106(6):471–473. [Google Scholar]
- 27.Sooknah RD, Wilkie AC. Nutrient removal by floating aquatic macrophytes cultured in anaerobically digested flushed dairy manure wastewater. Ecological Engineering. 2004;22(1):27–42. doi: 10.1016/j.ecoleng.2004.01.004. [DOI] [Google Scholar]
- 28.Takizawa S, Aravinthan V, Fujita K. Nitrogen removal from domestic wastewater using immobilized bacteria. Water Science and Technology. 1996;34(1-2):431–440. doi: 10.1016/0273-1223(96)00532-X. [DOI] [Google Scholar]
- 29.van Donk E, van de Bund WJ. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot. 2002;72(3-4):261–274. doi: 10.1016/S0304-3770(01)00205-4. [DOI] [Google Scholar]
- 30.Wei FS, Qi WQ, Bi T. Inspectorial and Analytical Methods of Water and Wastewater Transaction. Beijing: Chinese Environmental Sciences Press; 2002. pp. 1254–1284. (in Chinese) [Google Scholar]
- 31.Wu ZB. The effect of submerge plant recovery on the nitrogen and phosphorus of the eutrophic water. Chinese Journal of Applied Ecology. 2003;14(8):1351–1353. (in Chinese) [PubMed] [Google Scholar]
- 32.Yang XE, Tong CH, Pu PM. The effects and mechanism of aquatic plant control the nutrient release from the lake sediment. Agriculture Environment Science Journal. 2003;22(6):673–676. (in Chinese) [Google Scholar]