Abstract
PCR assays were developed for the direct detection of Paenibacillus larvae in honey samples and compared with isolation and biochemical characterization procedures. Different primer pairs, designed from the 16S rRNA and the metalloproteinase precursor gene regions, and different DNA extraction methods were tested and compared. The sensitivity of the reactions was evaluated by serial dilutions of DNA extracts obtained from P. larvae cultures. The specificity of the primers was assessed by analyzing related Paenibacillus and Bacillus strains isolated from honey. The PCR assays also amplified these related bacteria, but at lower sensitivity. In the next step, the PCR assays were applied to contaminated honey and other bee products originating from 15 countries. Lysozyme treatment followed by proteinase K digestion was determined to be the best DNA extraction method for P. larvae spores. The most sensitive primer pair detected P. larvae in 18 of 23 contaminated honey samples, as well as in pollen, wax, and brood. Honey specimens containing saprophyte bacilli and paenibacilli, but not P. larvae, were PCR negative. Although the isolation and biochemical identification method (BioLog) showed higher sensitivity and specificity, PCR proved to be a valuable technique for large-scale screening of honey samples for American foulbrood, especially considering its rapidity and moderate costs.
American foulbrood is a disease affecting the larval stage of the honeybee (Apis mellifera L.). The causative agent is Paenibacillus larvae subspecies larvae, a gram-positive, spore-forming bacterium which infects only bees. In consequence of its destructive effects, extreme resistance, and worldwide distribution, P. larvae is considered to be one of the most important infectious agents of the honeybee. Thus, American foulbrood is a notifiable disease of high socioeconomic importance and significance in international trade (a list B disease according to the classification of the Office International des Epizooties).
P. larvae (White) subsp. larvae is a slender rod about 2.5 to 5 μm long and about 0.5 μm wide. The bacterium forms large numbers of oval spores in infected larvae within a few days. The spores are highly resistant to heat and chemical agents and can survive in the environment for several years (12). Only the spores are capable of inducing disease.
This bacterium, formerly named Bacillus larvae, was reclassified into the new genus Paenibacillus (5) on the basis of the 16S rRNA sequence diversity. The P. larvae strains are divided into two distinct subspecies, P. larvae subsp. larvae and P. larvae subsp. pulvifaciens (14). Although P. larvae subsp. larvae is considered to be the cause of American foulbrood, P. larvae subsp. pulvifaciens is capable of causing similar but usually milder clinical symptoms.
The spread of the agent between apiaries or countries is often associated with the import of honeybee colonies or the use of contaminated honey (6, 11, 17, 26) for feeding purposes. In most cases, American foulbrood can be diagnosed in the field by visual inspection; however, the diagnosis must be confirmed by laboratory tests. Microscopic identification of stained bacteria can be complemented by the modified hanging-drop technique (19), the Holst milk test (15), or fluorescent-antibody techniques developed for the detection of P. larvae antigens (20, 25, 27). Besides microscopy, the most frequently applied identification method is the isolation of P. larvae using culture media followed by characterization by biochemical tests. The bacterium requires complex media for growth (24). For biochemical diagnosis of suspected P. larvae colonies, the catalase (13) and nitrate reduction (18) tests are widely used.
Detection of P. larvae in honey samples is a valuable method of control of the spread of the disease within and between countries (17, 26). The major problems associated with the diagnosis of P. larvae in honey specimens are the relatively low concentration of P. larvae spores in the honey and the presence of related Paenibacillus or Bacillus species, which often overgrow the P. larvae colonies. Spores can be purified and concentrated by dialysis (22) and/or centrifugation (16) of the diluted honey samples. Heat treatment is used for the inactivation of non-spore-forming bacteria. Several selective media have been developed for the cultivation of P. larvae, such as sheep blood agar containing nalidixic acid (16) or J agar (10) with nalidixic acid and pipermidic acid (1). A virulent mutant bacteriophage from P. larvae has also been used for the identification of P. larvae in honey samples (23). Biochemical identification and characterization systems (e.g., BioLog) are appropriate methods for the recognition of P. larvae as well.
Molecular techniques have also been developed for the identification of P. larvae (2, 3). Govan et al. (8) have described a PCR assay for the detection of P. larvae-specific DNA in bacterial colonies grown on semiselective medium. This technique was suggested for use in the rapid confirmation of the presence of P. larvae strains isolated from honey samples. Dobbelaere et al. (7) also described a PCR assay for the identification of cultivated bacteria and for the detection of P. larvae in DNA extracts of the remains of American foulbrood-diseased larvae. Very recently, Alippi et al. (4) described a method, based on PCR and restriction fragment analysis, which allows the differentiation of P. larvae subsp. larvae strains from all other bacterial species.
The aim of this study was to develop a sensitive and specific PCR assay for the direct detection of P. larvae in contaminated honey samples. Different techniques for the isolation of DNA from bacterial spores were compared, and different sets of oligonucleotide primers were evaluated for their sensitivity and specificity. The PCR assays were compared to the “gold standard” of P. larvae cultivation and biochemical identification methods (BioLog).
MATERIALS AND METHODS
Samples.
Cultures of P. larvae subsp. larvae, P. larvae subsp. pulvifaciens, P. alvei, Bacillus cereus, B. megaterium, and B. subtilis on Columbia blood agar, contaminated honey, pollen, wax samples, and infected brood were investigated. The Paenibacillus strains were isolated from honey samples collected in Australia (1 sample), Austria (10 samples), China (1 sample), France (2 samples), Greece (3 samples), Hungary (1 sample), Iran (1 sample), and Romania (1 sample) within a 6-year period. The honey, pollen, and wax samples were tested for the presence of P. larvae by cultivation. For the origin of the honey samples and other bee products, see Table 4.
TABLE 4.
Comparison of the efficacy of PCR, isolation, and BioLog identification techniques for the detection of P. larvae in bee products
| Sample | Code | Type of sample | Country of origin | PCR results (AF 6 + AF 7) | Isolation (CFU/plate) | BioLog probability (%) |
|---|---|---|---|---|---|---|
| 1 | 2001/36 | Honey | Austria | + | >50 | 100 |
| 2 | 2001/37 | Honey | Austria | − | 3.55 | 98 |
| 3 | 2001/89 | Honey | Austria | + | >50 | 100 |
| 4 | 2001/87 | Honey | Austria | + | 1.25 | 91 |
| 5 | 2001/983 | Honey | Austria | − | 33.8 | 100 |
| 6 | 2000/1019 | Honey | Austria | + | 0.8 | 100 |
| 7 | 2000/1020 | Honey | Austria | + | 1 | 91 |
| 8 | B/768/92 | Honey | Austria | + | 1 | 100 |
| 9 | AZ2000/696 | Honey | Hungary | + | 91 | 100 |
| 10 | AZ2000/695 | Honey | Austria | − | 3.1 | 100 |
| 11 | AZ2000/692 | Honey | United Kingdom | − | 5 | 100 |
| 12 | AZ2000/691 | Honey | France | + | >50 | 100 |
| 13 | AZ2000/497 | Honey | Saudi Arabia | + | 10 | 99 |
| 14 | AZ2000/1112 | Honey | Turkey | + | >50 | 100 |
| 15 | AZ98/76 | Honey | USA | + | 3.5 | 100 |
| 16 | AZ95/5 | Honey | Canada | + | 3.35 | 100 |
| 17 | AZ95/22 | Honey | Romania | + | 39 | 95 |
| 18 | AZ95/4 | Honey | Mexico | − | 17 | 75 |
| 19 | BP10/2001 | Honey | Sardinia/Italy | + | 1.58 | 100 |
| 20 | AZ2001/94 | Honey | Austria | + | 6.2 | 99 |
| 21 | BP28/2001 | Honey | Austria | + | 2 | 96 |
| 22 | BP31/2001 | Honey | Austria | + | >50 | 99 |
| 23 | ÜW3 | Honey | Austria | + | 3.6 | 96 |
| 24 | BP6/2000 | Honey | Austria | − | 0 | 0 |
| 25 | AZ2001/280 | Honey | India | − | 0 | 0 |
| 26 | AZ2001/325 | Honey | India | − | 0 | 0 |
| 27 | ÜW5 | Pollen | Germany | + | 4.5 | 100 |
| 28 | BP79/2000 | Pollen | The Netherlands | + | 0.25 | 100 |
| 29 | W8 | Wax | Austria | + | 0.2 | 91 |
| 30 | Wabe1 | Brood | Austria | + | >50 | 100 |
Sample processing.
Bacterial colonies were collected with a sterile inoculation loop and were dispersed in 500 μl of phosphate-buffered saline. A 100-μl volume of the bacterial suspension was centrifuged at 20,000 × g for 10 min, and the pellet was used for DNA isolation. For honey samples, 10 g of honey was diluted in 10 ml of sterile distilled water and the solutions were incubated at 95°C for 6 min. Thereafter, 10 ml of each solution was centrifuged at 4,000 × g for 30 min and the pellet was used for DNA extraction. The pollen and wax samples were processed similarly, but the initial amount of sample was reduced to 1 g. For testing infected brood, larvae showing clinical signs were homogenized in 500 μl of phosphate-buffered saline; 100 μl of homogenate was centrifuged (14,000 × g for 10 min), and the pellet was used for further analysis.
DNA extractions.
Three different nucleic acid isolation methods were tested comparatively: guanidium thiocyanate treatment (method A), cetyltrimethylammonium bromide treatment (method B), and lysozyme treatment (method C).
(i) DNA extraction method A.
The bacterial pellets were resuspended in denaturing buffer containing guanidinium thiocyanate, N-lauroylsarcosine, sodium citrate, and 2-mercaptoethanol. After mixing, sodium acetate was added, and then nucleic acid was extracted with phenol-chloroform-isoamyl alcohol. The nucleic acids were precipitated with isopropanol, and the precipitates were resolved in elution buffer (Qiagen).
(ii) DNA extraction method B.
The bacterial pellets were resuspended in lysis buffer containing cetyltrimethylammonium bromide, Tris-HCl, NaCl, EDTA, and 2-mercaptoethanol. After vortexing, the mixtures were frozen in liquid nitrogen and thawed by shaking at room temperature; this procedure was repeated twice. Thereafter, nucleic acid was extracted with phenol-chloroform-isoamyl alcohol and precipitated with isopropanol. The precipitates were resolved in elution buffer (Qiagen).
(iii) DNA extraction method C.
The bacterial pellets were resuspended in 200 μl of enzyme solution containing 20 mg of lysozyme per ml, 20 mM Tris-HCl (pH 8.0), 2 mM EDTA, and 1.2% Triton and incubated for 1 h at 37°C. Then 25 μl of proteinase K and 200 μl of buffer AL (Qiagen) were added, and the lysates were incubated at 56°C for 30 min and at 96°C for 5 min. Bacterial DNA was isolated using the QIAamp DNA minikit (Qiagen) as specified by the manufacturer. DNA was eluted with 200 μl of elution buffer and stored at −20°C.
PCR.
Specified DNA fragments of P. larvae and other Paenibacillus and Bacillus species were amplified using PCR.
(i) Primer design.
Oligonucleotide primers were designed on the basis of the published nucleotide sequences of P. larvae. The primers were constructed with the help of the Primer Designer program (Scientific and Educational Software, version 3.0). A previously described P. larvae-specific primer pair (PL 1-PL 2) (8) was also included in our investigations. The primer sequences, orientations, origins, locations, and resulting product sizes are shown in Table 1. Nucleotide positions refer to the P. larvae sequences with accession no. X60619 and AF111421. The primers were synthesized by GibcoBRL Life Technologies, Ltd. (Paisley, Scotland).
TABLE 1.
Oligonucleotide primers selected for P. larvae PCR
| Primera | Sequence (5′ to 3′) | Origin | Nucleotide positions | Product length (bp) |
|---|---|---|---|---|
| AF 1f | GCT CTG TTG CCA AGG AAG AA | 16S rRNA | 436-455 | 451 |
| AF 2r | AGG CGG AAT GCT TAC TGT GT | 868-887 | ||
| AF 3r | TGT CAC CGG CAG TCA TCT TA | 16S rRNA | 1156-1175 | 739b |
| AF 4f | CTG GCG GCG TGC CTA ATA CA | 16S rRNA | 30-49 | 374 |
| AF 5r | GGC GTT GCT CCG TCA GAC TT | 385-404 | ||
| AF 6f | GCA AGT CGA GCG GAC CTT GT | 16S rRNA | 51-70 | 237 |
| AF 7r | GCA TCG TCG CCT TGG TAA GC | 269-289 | ||
| AF 8f | GGA GTA ACT GCC CCT GGA GT | 16S rRNA | 464-483 | 695 |
| AF 9r | CTT AGA GTG CCC ACC TCT GC | 1140-1159 | ||
| AF 10f | TAG GCC ATG AAT TGA CTC AC | Metalloproteinase | 89-108 | 155 |
| AF 11r | TAT TCG GCG TAT ACA CGT CT | 225-244 | ||
| Paeni 30f | CGT ATT CAG AGA CGG TGA TG | Metalloproteinase | 30-49 | 242 |
| Paeni 253r | TCT AAG GAA CGG AGA GCA TC | 253-272 | ||
| PBL 8f | CTT TCT GGA ACG GGC AGC AA | Metalloproteinase | 8-27 | 342 |
| PBL 350r | GTA TGA ACG CCG CCG TTA TC | 331-350 | ||
| PL 1fc | AAG TCG AGC GGA CCT TGT GTT TC | 16S rRNA | 53-75 | 971 |
| PL 2rc | TCT ATC TCA AAA CCG GTC AGA GG | 1002-1024 |
f, forward primer; r, reverse primer. The primer pairs which gave the best results are highlighted in bold.
With the AF 1f primer.
Primers published by Govan et al. (8).
(ii) Amplification.
The 25-μl reaction mixtures contained 2.5 μl of 10× reaction buffer (Perkin-Elmer) which included MgCl2 (final concentration, 1.5 mM), 2 mM deoxynucleoside triphosphate mix, 0.8 μM (each) appropriate forward and reverse primers, 0.625 U of Taq DNA polymerase (Promega), and 5 μl of template DNA. Following an initial denaturation step at 95°C for 5 min, the reaction mixtures were subjected to 40 cycles of heat denaturation at 95°C for 20 s, primer annealing at 50°C for 20 s, and DNA extension at 72°C for 1 min. The reactions were completed by a final extension of 7 min at 72°C. The amplifications were performed in a Perkin-Elmer GeneAmp PCR System 2400 thermocycler. The amplicons were stored at 4°C until electrophoresis was carried out.
Gel electrophoresis.
Samples (5 μl) of the amplicons were electrophoresed in a 1.2% Tris acetate-EDTA-agarose gel at 6 V/cm for 80 min. The gel was stained with ethidium bromide, and the bands were visualized under UV light at 312 nm using a TFX 35M UV transilluminator (Life Technologies) and photographed with a Kodak DS electrophoresis documentation and analysis system using the Kodak Digital Science 1D software program. Product sizes were determined by reference to a 100-bp molecular size ladder (Amersham Pharmacia Biotech).
Nucleotide sequencing and computer analysis.
To confirm the specificity of the PCR assays, PCR amplification products of five selected honey samples positive for P. larvae subsp. larvae and one sample of cultivated P. alvei were sequenced, and the sequences were aligned with the published sequences available in the GenBank database. The method of fluorescence-based sequencing has been described previously by our group (9).
Culture of bacteria.
The bacteria from the aforementioned samples were isolated, identified, and characterized on the basis of their biochemical properties. For the honey samples, 5 g of honey was diluted in 5 ml of sterile distilled water and the solutions were incubated at 90°C for 6 min to inactivate non-spore-forming bacteria. From each sample, 400 μl of diluted honey was inoculated onto each of six Columbia sheep blood agar plates. The plates were incubated at 37°C and were checked for P. larvae growth on days 3 and 6. The bacterial colonies were isolated, and the bacteria were characterized by light microscopy following Gram stain, catalase test (13) (P. larvae is catalase negative), and formation of great whips in the fluid part of Columbia blood slant agar (21).
Metabolic fingerprint technique.
Further identification and characterization of the bacterial isolates were performed using the BioLog GP MicroPlate test panel. The tests were carried out as specified by the manufacturer. The plates were read visually after 4 and 24 h. The results were evaluated using the MicroLog2 system (Biolog, Inc.).
RESULTS
PCR assays applied to cultured bacteria.
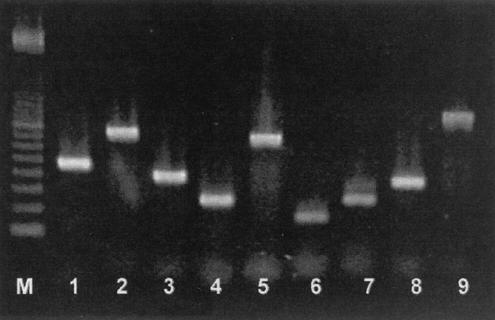
Specific DNA fragments of P. larvae subsp. larvae were amplified by PCR assays with nine different primer pairs (Table 1). The primers were first tested on a P. larvae subsp. larvae strain isolated in Austria (DSMZ 7030). Amplifications with the selected primer pairs resulted in PCR products with the expected molecular weights. The electrophoresis pattern of the amplicons is presented in Fig. 1. Another 19 P. larvae subsp. larvae strains isolated from bee products originating from eight countries were tested by PCR. All strains proved to be clearly positive in the assays (data not shown).
FIG. 1.
Gel electrophoresis of PCR products of P. larvae subsp. larvae amplified with different primer pairs. Lanes: 1, AF 1 plus AF 2; 2, AF 1 plus AF 3; 3, AF 4 plus AF 5; 4, AF 6 plus AF 7; 5, AF 8 plus AF 9; 6, AF 10 plus AF 11; 7, Paeni 30 plus Paeni 253; 8, PBL 8 plus PBL 350; 9, PL 1 plus PL 2; M, molecular size marker (100-bp ladder).
To reveal the specificity of the reactions, P. larvae subsp. pulvifaciens, P. alvei, B. cereus, B. megaterium, and B. subtilis strains were also tested with the selected primer pairs. PCRs applied to undiluted bacterial DNA extracts resulted in amplification products in all involved Paenibacillus and Bacillus species with all primer pairs. The extent of cross-reactivity of the PCR assays with the different Paenibacillus and Bacillus species was determined by titration of the DNA extracts. Tenfold serial dilutions were prepared, and PCRs were performed on these diluted bacterial strains. For this experiment, the primer pairs AF 1 plus AF 2, AF 6 plus AF 7, PBL 8 plus PBL 350, and PL 1 plus PL 2 were selected based on preliminary evaluations (data not shown).
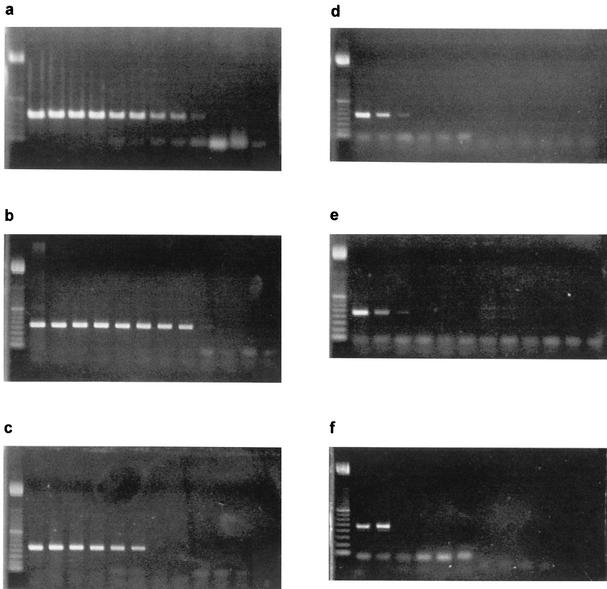
The AF 1 plus AF 2 primers detected P. larvae (subspp. larvae and pulvifaciens) DNA up to a dilution of 10−8, which contained approximately 0.9 to 1.1 CFU of bacteria. The other bacterial species were positive only up to dilutions containing 8 × 106 CFU (B. subtilis), 7 × 105 CFU (B. cereus and B. megaterium), and 9 × 104 CFU (P. alvei), respectively (Fig. 2). The AF 6 plus AF 7 primers detected P. larvae DNA even at a sensitivity level of 0.05 CFU (P. larvae subsp. larvae) and 0.1 CFU (P. larvae subsp. pulvifaciens), respectively; the other tested bacterial species, however, also gave a positive reaction with the AF 6 plus AF 7 primer pair, up to a sensitivity of 2.6 to 7 CFU of bacteria in one case (P. alvei). The other primers detected P. larvae only when large amounts (105 to 107 CFU) were present, and in some cases nonspecific bands of different sizes were produced when other bacterial species were tested (data not shown).
FIG. 2.
Gel electrophoresis of PCR products from serial dilutions of DNA extracts from different bacteria using the AF 1 plus AF 2 primer pair. (a) P. larvae subsp. larvae; (b) P. larvae subsp. pulvifaciens; (c) P. alvei; (d) B. cereus; (e) B. megaterium; (f) B. subtilis. Lanes (from left to right): molecular size marker (100-bp ladder), undiluted DNA, 10-fold dilution steps 10−1 to 10−10, negative control.
PCR assays applied to honey and other bee products.
PCR assays were performed on 26 honey samples originating from 13 different countries (on four continents) to detect P. larvae-specific DNA (see Table 4). Two pollen samples, one wax sample, and one brood sample were also tested.
Because of the resistance of Paenibacillus spores to proteolytic enzymes, three different DNA isolation methods were tested in parallel on seven randomly selected honey samples and on one pollen specimen. The results of the comparison of the DNA isolation techniques are presented in Table 2. All three methods were effective in seven of eight samples; however, following PCR, the sharpness of the bands and the amount of amplified PCR products differed significantly: the largest amount of amplified DNA was obtained when method C was used for DNA extraction. Consequently, extraction method C was applied to all other samples.
TABLE 2.
Comparison of different DNA extraction methods prior to PCR
| Sample | Code | Type of sample | Extraction efficiency of:
|
||
|---|---|---|---|---|---|
| Method A | Method B | Method C | |||
| 1 | 2001/36 | Honey | − | + | + |
| 3 | 2001/89 | Honey | + | − | + |
| 9 | AZ2000/696 | Honey | + | + | + |
| 12 | AZ2000/691 | Honey | + | + | − |
| 14 | AZ2000/1112 | Honey | + | + | + |
| 17 | AZ95/22 | Honey | + | + | + |
| 22 | BP31/2001 | Honey | + | + | + |
| 28 | BP79/2000 | Pollen | + | + | + |
Based on the results of the aforementioned sensitivity tests of the different primers, four primer pairs were compared using DNA extracts of 14 randomly selected honey samples. The results are presented in Table 3. Amplifications with the AF 1 plus AF 2 primers were positive in 8 of 14 samples, while the AF 6 plus AF 7 primers detected P. larvae DNA in 12 of 14 samples. When the PBL 8 plus PBL 350 primers were used, only 1 of the tested 14 samples was positive, and the PL 1 plus PL 2 primers failed to amplify DNA from honey. Based on these results, the AF 6 plus AF 7 primers were used to analyze the other honey samples, as well as selected pollen, wax, and brood samples. Only the honey, pollen, wax, and brood samples from which P. larvae had been isolated were analyzed. The number of bacteria in the samples was classified according to the number of suspected P. larvae colonies on Columbia sheep blood agar plates. The isolates were identified and characterized by BioLog GP MicroPlate test panels. The evaluation of the tests resulted in a probability value for the isolates being P. larvae subsp. larvae. The results of the PCR amplifications, isolations and BioLog tests are shown comparatively in Table 4. Altogether, 23 honey samples containing P. larvae subsp. larvae were tested by PCR, and 18 of them were found to be positive. The PCR-negative samples contained 3.2 to 33.8 CFU of bacteria by isolation, and the identification probability varied between 75 and 100%. The selected pollen, wax, and brood samples were positive by PCR and showed a high BioLog probability value.
TABLE 3.
Comparison of different primer pairs used in the PCR assays on randomly selected honey samples
| Sample | Code | Amplification with primer pair:
|
|||
|---|---|---|---|---|---|
| AF 1 + AF 2 | AF 6 + AF 7 | PBL 8 + PBL 350 | PL 1 + PL 2 | ||
| 2 | 2001/37 | − | + | − | − |
| 4 | 2001/87 | + | + | + | − |
| 5 | 2001/983 | − | + | − | − |
| 6 | 2000/1019 | + | + | − | − |
| 7 | 2000/1020 | + | + | − | − |
| 11 | AZ2000/692 | − | − | − | − |
| 12 | AZ2000/691 | − | + | − | − |
| 13 | AZ2000/497 | + | + | − | − |
| 14 | AZ2000/1112 | + | + | − | − |
| 16 | AZ95/5 | − | + | − | − |
| 17 | AZ95/22 | + | + | − | − |
| 18 | AZ95/4 | − | − | − | − |
| 19 | BP10/2001 | + | + | − | − |
| 21 | BP28/2001 | + | + | − | − |
Another three honey samples (samples 24, 25, and 26), which did not contain P. larvae but from which several other Bacillus species had been isolated, were included in the study. These samples were clearly negative by PCR.
Five PCR products were sequenced, and the sequences were subjected to BLAST search to identify related sequences deposited in the GenBank database. The sequences uniformly exhibited the highest (100%) identity to the P. larvae subsp. larvae 16S rRNA gene partial sequence (accession no. AY030079). The sequence of the cultivated P. alvei nucleic acid showed only 93.13% identity to the P. larvae subsp. larvae sequence, and the highest (99.34%) identity was observed to the P. alvei strain DSM 29T partial 16S rRNA gene sequence (accession no. AJ320491). Because of the high identities of the nucleotide sequences of our strains to already available sequences, the sequences were not submitted to the GenBank database.
DISCUSSION
The detection of contaminated honey plays an important role in the efficient control of American foulbrood. The small numbers of spores and the presence of other Paenibacillus and Bacillus species complicate the identification of P. larvae in honey samples. A large-scale screening of honey samples for P. larvae by classical isolation and identification methods is rather time-consuming, laborious, and expensive.
PCR is a quick and reliable method that is widely used in microbiological diagnostics for the detection of specific nucleic acid sequences in biological materials. In this study, we tested different PCR systems for the detection of P. larvae-specific DNA in honey and compared the sensitivity and specificity of the PCR assays with isolation and biochemical characterization methods.
To date, only two relatively short genomic regions of P. larvae have been sequenced and deposited in the GenBank database. Five primer pairs were selected from the 16S rRNA gene region, and three primer pairs were selected from the metalloproteinase precursor gene region (Table 1). All of them functioned properly on DNA extracts from P. larvae colonies.
The sensitivity of the PCR assays using different primers was determined by serial dilutions of P. larvae DNA isolated from bacterial colonies. The primer pair AF 6 plus AF 7 showed the highest sensitivity, amplifying P. larvae nucleic acid from as little as 0.05 CFU of cultured bacteria; primer pair AF 1 plus AF 2 exhibited a similarly high sensitivity (0.9 CFU). The primer pairs designed in the metalloproteinase precursor gene region showed significantly lower sensitivity (105 to 107 CFU).
To assess the specificity of the primers used, the PCR assays were performed on other Paenibacillus and Bacillus species as well. Although the primers were selected from nucleotide sequences which are relatively specific for P. larvae, they also amplified, to a certain extent, DNA from other Paenibacillus and Bacillus species. The most sensitive primers for the detection of P. larvae (AF 6 plus AF 7; specific amplification as low as 0.05 CFU) also amplified P. alvei and B. subtilis strains at 2.6 and 11 CFU, respectively. The tested primer pair from the metalloproteinase gene region showed a rather low sensitivity for P. larvae (105 CFU) but a relatively high specificity: the other tested bacteria reacted only at 107 CFU.
To investigate the practical value of the assays, honey samples were tested by PCR with the selected primers. Since bacterial spores are more resistant to proteinase K treatment than are vegetative cells, the routine DNA isolation methods have to be complemented by additional treatments to extract nucleic acid from spores. In this study, we compared the DNA isolation efficacy of three extraction methods. Two classical methods, using chemicals (guanidium thiocyanate and cetyltrimethylammonium bromide) for the lysis of the spores followed by phenol-chloroform extraction of the DNA, and one enzymatic (lysozyme) treatment followed by proteinase K digestion were tested. The methods were equally efficient (seven successful isolations from eight P. larvae-containing honey samples); however, with the different methods, different samples were negative. Since the classical methods are rather time-consuming, need special facilities, and result in dangerous waste material, lysozyme treatment followed by proteinase K digestion was chosen for further applications. Also, this DNA extraction method resulted, following PCR, in clear electrophoretic bands with larger amounts of amplified DNA compared to the other methods.
The PCR assays were applied to honey, pollen, wax, and brood samples. Practically only the AF 1 plus AF 2 and AF 6 plus AF 7 primers could detect Paenibacillus-specific nucleotide sequences in these samples (Table 3). The primers AF 6 plus AF 7 showed the highest sensitivity, detecting P. larvae in 18 of 23 contaminated honey samples (78.3%). This primer pair also detected P. larvae from pollen, wax, and brood samples. With honey samples, this PCR technique was specific for P. larvae, because it did not amplify DNA from honey samples which contained saprophytic Bacillus species but no P. larvae (Table 4). The determined nucleotide sequences of selected PCR products also support the specificity of this assay. Although the isolation and biochemical characterization of P. larvae from honey samples were found to be more sensitive and specific than PCR, PCR should still be considered for large-scale screening of American foulbrood in honey samples because of its rapidity and moderate expense.
Govan et al. also described a PCR detection method for rapid identification of P. larvae (8). Their system was developed for the identification of P. larvae cultivated from honey samples in semiselective medium (and not for direct detection in honey). We included their published primers in our studies (PL 1 plus PL 2 primers) for comparison. These primers detected P. larvae nucleic acid at a level of 104 CFU and failed to detect P. larvae DNA in honey samples.
The sensitivity of the PCR assays could be further improved by carrying out a second, nested amplification step. Certain combinations of our primers would allow a second-round PCR. On the other hand, the risk of cross-contamination, a lower specificity, a longer duration, and enhanced costs reduce the usability of nested PCR in large-scale screenings of honey samples.
To summarize, in this study we investigated quite intensively the applicability of PCR for the detection of P. larvae, the causative agent of American foulbrood, and found certain PCR assays to be appropriate for quick screening of honey samples for the presence of P. larvae; the results, however, should be confirmed by isolation and biochemical characterization of the bacteria. The specificity of the PCR assays could be improved by the use of primer sequences from other genomic regions, which are more specific for P. larvae than are the currently available sequences. For this approach, however, large genome segments of P. larvae and related bacteria have to be sequenced beforehand.
Acknowledgments
The project was supported by CEEPUS (Central European Exchange Programme for University Studies) by providing a mobility scholarship to T. Bakonyi.
We cordially thank Jolanta Kolodziejek, Helga Lussy, and Claudia Pallan for their excellent technical assistance and Johanna Kindermann for valuable suggestions.
REFERENCES
- 1.Alippi, A. M. 1995. Detection of Bacillus larvae spores in Argentinean honeys by using a semi-selective medium. Microbiol. Semin. 11:343-350. [PubMed] [Google Scholar]
- 2.Alippi, A. M., and O. M. Aguilar. 1998. Characterization of isolates of Paenibacillus larvae subsp. larvae from diverse geographical origin by the polymerase chain reaction and BOX primers. J. Invertebr. Pathol. 72:21-27. [DOI] [PubMed] [Google Scholar]
- 3.Alippi, A. M., and O. M. Aguilar. 1998. Unique DNA fingerprint patterns of Paenibacillus larvae subsp. larvae strains. J. Apic. Res. 37:273-280. [Google Scholar]
- 4.Alippi, A. M., A. C. López, and O. M. Aguilar. 2002. Differentiation of Paenibacillus larvae subsp. larvae, the cause of American foulbrood of honeybees, by using PCR and restriction fragment analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 68:3655-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ash, C., F. G. Priest, and M. D. Collins. 1993. Molecular identification of rRNA group 3 bacilli (Asc, Farrow, Wallbanks, and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Leeuwenhoek 64:253-260. [DOI] [PubMed] [Google Scholar]
- 6.Aydin, N., H. Bulbul, G. Biyikoglu, C. Yaraly, and M. K. Yavuz. 1998. Isolation of Paenibacillus larvae from honey for human consumption and from samples taken from beehives. Etlik Vet. Mikrobiyol. Dergisi 10:93-100. [Google Scholar]
- 7.Dobbelaere, W., D. C. de Graaf, and J. E. Peeters. 2001. Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 32:363-370. [Google Scholar]
- 8.Govan, V. A., M. H. Allsopp, and S. Davison. 1999. A PCR detection method for rapid identification of Paenibacillus larvae. Appl. Environ. Microbiol. 65:2243-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabensteiner, E., W. Ritter, M. J. Carter, S. Davison, H. Pechhacker, J. Kolodziejek, O. Boecking, I. Derakhshifar, R. Moosbeckhofer, E. Licek, and N. Nowotny. 2001. Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR. Clin. Diagn. Lab. Immunol. 8:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen, H. 1984. Methods for determining the presence of the foulbrood bacterium Bacillus larvae in honey. Dan. J. Plant. Soil Sci. 88:325-328. [Google Scholar]
- 11.Hansen, H., and C. J. Brodsgaard. 1999. American foulbrood: a review of its biology, diagnosis and control. Bee World 80:5-23. [Google Scholar]
- 12.Haseman, L. 1961. How long can spores of American foulbrood live? Am. Bee J. 101:298-299. [Google Scholar]
- 13.Haynes, W. C. 1972. The catalase test. An aid in the identification of Bacillus larvae. Am. Bee J. 112:130-131. [Google Scholar]
- 14.Heyndrickx, M., K. Vandemeulebroecke, B. Hoste, P. Janssen, K. Kersters, P. de Vos, N. A. Logan, N. Ali, and R. C. W. Berkeley. 1996. Reclassification of Paenibacillus (formerly Bacillus) pulvifaciens (Nakamura 1984) Ash et al. 1994, a later subjective synonym of Paenibacillus (formerly Bacillus) larvae (White 1906) Ash et al. 1994, as a subspecies of P. larvae, with emended descriptions of P. larvae as P. larvae subsp. larvae and P. larvae subsp. pulvifaciens. Int. J. Syst. Bacteriol. 46:270-279. [DOI] [PubMed] [Google Scholar]
- 15.Holst, E. C. 1946. A single field test for American foulbrood. Am. Bee J. 86:14-34. [Google Scholar]
- 16.Hornitzky, M. A. Z., and S. Clark. 1991. Culture of Bacillus larvae from bulk honey samples for the detection of American foulbrood. J. Apic. Res. 30:13-16. [Google Scholar]
- 17.Kauko, L., and M. Niskanken. 1995. Occurrence and diagnosis of American foulbrood in SW Finland. Ann. Univ. Marie Curie Sklodowska Sect. DD. 50:249-253. [Google Scholar]
- 18.Lochhead, A. G. 1937. The nitrate reduction test and its significance in the detection of Bacillus larvae. Can. J. Res. C15:79-86. [Google Scholar]
- 19.Michael, A. S. 1957. Droplet method for observation of living unstained bacteria. J. Bacteriol. 74:831-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otte, E. 1973. A contribution of the laboratory diagnosis of American foulbrood of the honey bee with a particular reference to the immunofluorescence method. Apidologie 4:331-339. [Google Scholar]
- 21.Plagemann, O. 1985. Eine einfache Methode zur bakteriologischen Identifizierung von Bacillus larvae mit Columbia-Blut-Schrägagar. Berl. Münch. Tierärztl. Wochenschr. 98:61-62. [PubMed] [Google Scholar]
- 22.Shimanuki, H., and D. A. Knox. 1988. Improved method for the detection of Bacillus larvae spores in honey. Am. Bee J. 128:353-354. [Google Scholar]
- 23.Stahly, D. P., A. M. Alippi, N. Bakhiet, C. F. Campana, C. C. Novak, and R. Cox. 1999. PPL1c, a virulent mutant bacteriophage useful for identification of Paenibacillus larvae subspecies larvae. J. Invertebr. Pathol. 74:295-296. [DOI] [PubMed] [Google Scholar]
- 24.Steinkraus, K. H., and R. A. Morse. 1996. Media for the detection of Bacillus larvae spores in honey. Acta Biotechnol. 16:57-64. [Google Scholar]
- 25.Toshkov, A., T. Valarianov, and A. Tomov. 1970. The immunofluorescence method and quick and specific diagnosis of American foulbrood of beebrood. Bull. Apic. 13:13-18. [Google Scholar]
- 26.von der Ohe, W., and J. H. Dustmann. 1997. Efficient prophylactic measures against American foulbrood by bacteriological analysis of honey for spore contamination. Am. Bee J. 137:603-606. [Google Scholar]
- 27.Zhavnenko, V. M. 1971. Indirect method of immunofluorescence in the diagnosis of foulbrood (American and European). Veterinariia 8:109-111. [PubMed] [Google Scholar]