Case Presentation
In February, 2005, I was asked by the Department of Anesthesia at the Brigham and Women’s Hospital in Boston to attend their Morbidity and Mortality Weekly Report rounds to discuss a fall-asleep motor vehicle crash that had occurred on February 20, 2005 in one of their trainees. The trainee was a 39-year-old white male who was in his fifth postgraduate year of training as an anesthesia fellow (Case A.F.). At about 3 pm on February 20, 2005, A.F. fell asleep at the wheel and collided with a stopped vehicle while he was en route home from a 7-hour work shift at the Brigham and Women’s Hospital. There was no injury to the fellow, to the other driver or to her 5-year-old child—and hence there is no litigation involved—which is why I am able to present Case A.F. to you today. A.F. reported a similar incident four years earlier in which he had fallen asleep at the wheel while driving his car at high speed on an expressway during a commute home from a >30-hour extended duration work shift in his first postgraduate year of training. In that incident, A.F. was awoken by a rumble strip on the expressway and thereby avoided a potentially catastrophic motor vehicle crash. He said that he understood that the >30-hour shift had made him vulnerable to the fall-asleep incident while driving on the highway during his PGY1 year. However, he wondered why he experienced a fall-asleep crash during his PGY5 year, after working a much shorter, seven-hour shift. To address this question, I initiated a series of steps comparable to those which might be undertaken by investigators (such as local or state police departments or the National Transportation Safety Board) evaluating if sleepiness or sleep deprivation were a probable contributing factor to a transportation accident.
Work-hour and Sleep-wake History
As a PGY-5 anesthesia fellow, A.F.’s work schedule required that he be “on call” from home for the intensive care unit for two out of every three weeks. He reported that his sleep habits were as follows: (1) during non-call/non-work nights (which occurred 6 times per month) his nightly sleep episode was from 10 pm to 6:30 am; (2) during non-call/work nights (which occurred 7–14 times per month) his nightly sleep episode was from 10 pm to 4:30 am; (3) during on-call/work nights (which occurred 7–21 times per month) his nightly sleep episode was from 10 pm to 4:30 am and he was awoken by 3–4 pages from the intensive care unit per night. Finally, A.F. noted that he and his wife were typically awoken once per night by one of their three young children for about 20 minutes per night.
Pharmacologic History
A.F. denied alcohol or hypnotic drug use prior to the crash. He admitted using caffeine, which he reportedly administered in the form of “three shots of espresso” each morning and which he said he “titrated to effects” during the remainder of the day (typically two more small cups of coffee). He reported that he avoided caffeine most afternoons, including the day of the crash, in order to avoid disturbing his subsequent night of sleep.
Hospital Paging Records
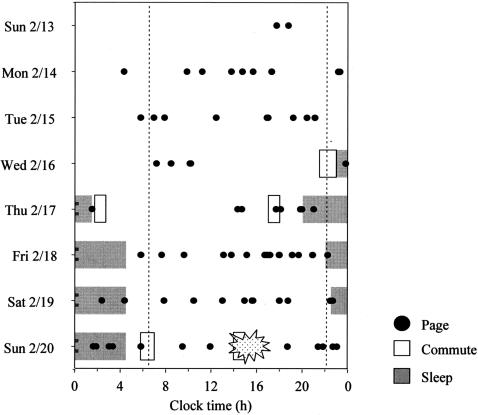
The times at which A.F. was paged were downloaded from the Brigham and Women’s Hospital paging system records and are illustrated together with his reported sleep-wake times, as recollected by history, during the prior four nights, in Figure 1. On the night before the crash, A.F. was paged twice at the beginning of his sleep episode, then awoken by a page from the ICU again at about 1:30 am, then awoken by a page just before 2 am and then awoken by a page again at approximately 3 am before his final page during that sleep episode, which was at about 3:30 am. He arose at 4:30 am, showered, dressed and ate breakfast (including three shots of espresso) prior to commencing a 50-mile commute into the hospital at 5:45 am.
Fig. 1.
The sleep-wake times, commute times and pages (from the Brigham and Women’s Hospital paging records) of a PGY-5 Anesthesia Fellow leading up to motor vehicle crash.
Sleep Disorders Evaluation
A.F. was referred to Dr. David White, who then directed the Brigham and Women’s Hospital Sleep Disorders Service, for an evaluation. He reported a history of loud snoring, particularly when sleep deprived and when sleeping on his back. He denied weight change, restless legs, history of sleep attacks or limb weakness with extreme emotion (cataplexy) or a history of difficulty falling asleep or staying asleep. His physical examination was unremarkable (Height: 6′3″; Weight: 180 lbs; BMI: 22.5). Dr. White ordered a polysomnographic recording, which revealed an hourly apnea/hypopnea index (AHI) of 20.5, with an AHI of 47 per hour when supine and 2 per hour when sleeping on his side. Oxygen desaturations to 86 percent were recorded during the apneic events. Dr. White diagnosed A.F. with positional sleep-related breathing disorder and behaviorally induced insufficient sleep syndrome.
In order to put the results of this evaluation into context, it is important to understand the physiology of sleep and wakefulness.
Background on Determinants of Alertness and Performance
Multiple factors influence the ability to sustain effective waking neurocognitive performance in young healthy individuals not taking medications. These include consecutive waking hours; nightly sleep duration; biological time of day (i.e., circadian phase); and the recency of the last sleep episode (i.e., sleep inertia). While the effects of these circadian and homeostatic sleep regulatory processes can be modified by environmental conditions, physical activity and pharmacological agents (i.e., stimulant and hypnotic agents), there is no countermeasure known that can consistently overcome the impact of adverse circadian phase and/or sleep deprivation on performance. The interaction of these regulatory processes can create an imposing biological force which can overpower an individual’s ability to sustain alert wakefulness and remain attentive. This leads to impaired neurocognitive performance, including reduced memory consolidation, and deterioration of waking performance marked by increased rates of attentional failures (1). These consequences of misalignment of circadian phase and the wake-sleep schedule, cumulative sleep deprivation and lengthy prior wakefulness are particularly evident while attempting to sustain attention for a continuous duration of time (e.g., for 10–20 minutes or more) while performing a routine, highly over-learned task such as driving a motor vehicle.
The Sleep Homeostat
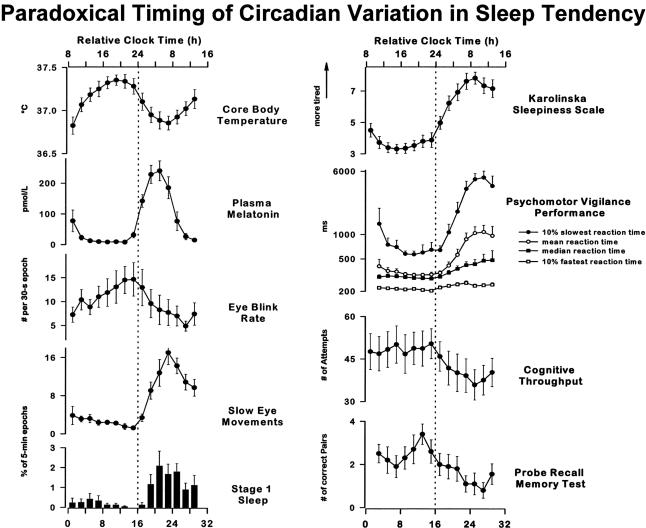
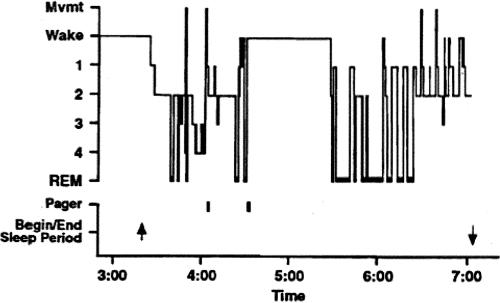
Without sleep, alertness and neurocognitive performance exhibit a steady deterioration attributable to sleep loss, onto which a rhythmic circadian variation is superimposed (2–18). Among individuals who win the struggle to remain awake, 24 hours of sleep deprivation has been shown to impair neurobehavioral performance to an extent that is comparable to a level of 0.10 percent blood alcohol content (19). In fact, the duration of time it takes to react to a visual stimulus (simple reaction time) averages three times longer after 24 hours of wakefulness than before an individual has stayed up all night (20) (Figure 2). Moreover, this is when the risk of attentional failures—in which the eyes begin rolling around in their sockets at the transition from wakefulness to sleep—is greatest. Within several days, chronic sleep restriction has been demonstrated to yield impairment in neurobehavioral performance and a risk of attentional failures that increased to a level comparable to that seen with acute total sleep deprivation (21,22). In fact, six hours of time in bed per night for a week or two brings the average young adult to the same level of impairment as 24 hours of wakefulness, whereas 4 hours of time in bed per night gets there in four to six days and induces a level of impairment comparable with 48 hours of wakefulness (i.e., two consecutive days and nights without sleep) after 10 days of restriction. As with alcohol intoxication, chronically sleep-deprived individuals tend to underestimate the extent to which their performance is impaired, despite increasing impairment evident in objective recordings of the rate of lapses of attention (23). Objective measures of performance, including reaction time and memory, worsen. The effects of recurrent nights of sleep restriction are not overcome with a single night of sleep. Increasing sleep deprivation leads to an increased probability of experiencing lapses of attention, episodes of automatic behavior and/or falling asleep while attempting to remain awake. In a condition of chronic sleep deprivation, even when wakefulness is scheduled during an appropriate circadian phase, the probability of a sleep-related attentional failure or neurocognitive performance failure while waking is markedly increased (23,24). Moreover, repeated interruptions of sleep, such as is experienced by physicians when they are on call, degrade the restorative quality of sleep compared to an equal amount of consolidated sleep. This is thought to be a primary basis for the excessive daytime sleepiness associated with sleep disorder breathing, which induces many brief arousals during the night. Interestingly, just being on call disturbs sleep, even when the individual is not called (25,26). Dr. Marshall Wolf and I learned this firsthand when recording the sleep of interns in a study that we did with Dr. Gary Richardson and that Dr. Wolf presented to this group fifteen years ago (27). As shown in Figure 3, the sleep of this young intern was acutely disturbed while he was on call, with little deep slow wave sleep and many awakenings that were not accounted for by the two pages that were received. This intern remained awake for almost an hour after being awakened by a page the second time. The impact on the brain of resulting insufficient sleep is only beginning to be appreciated. Positron Emission Tomography (PET) imaging has revealed that sleep deprivation is associated with decreased metabolism in the thalamus, the pre-frontal cortex and the parietal cortex (28). Metabolic studies have demonstrated that such sleep curtailment also has adverse effects on the metabolic and immune systems.
Fig. 2.
Laft-hand column: Time courses of core body temperature, endogenous plasma melatonin, mean eye blink rate per 30-s epoch during Karolinska drowsiness test (KDT), incidence of slow eye movements (SEMs, percentage of 30-s epochs containing at least 1 SEM/5-min interval), and incidence of stage 1 sleep (% of 30-s epochs containing at least 15 s of stage 1 sleep per 5-min interval) are shown, averaged across 10 subjects+ SE. Right-hand column: Time course of subjective sleepiness as assessed on Karolinska sleepiness scale (KSS; highest possible score = 9, lowest possible score = 1), psychomotor vigilance performance [mean, median, 10% slowest and fastest reaction times in ms (logarithmic scale)], cognitive performance (numbers of attempt in 4-min 2-digit addition task), and memory performance (number of correct word pairs in probed recall memory task) are shown averaged across 10 subjects+ SE. All data were binned in 2-h intervals and expressed with respect to elapsed time since scheduled waketime. Vertical reference line indicates subject’s habitual bedtime. Reprinted with permission from Cajochen et al (20). Copyright 1999 American Physiological Society.
Fig. 3.
Polysomnographic recording of sleep in an intern on call. The figure depicts the hypnogram and the effects of two pages during the night in an intern from the group not covered by a night float. “Pager” indicates an electronically recorded page was issued to the intern’s beeper at the time indicated on the X-axis. “Begin/End sleep period” indicate the times recorded by the intern for these events in the diary. This record demonstrates the variable impact of pages during sleep. The first page, from one of the nursing stations, occurred at approximately 4.08 am. The page occurred while the intern was in stage 4 and produced only a brief movement without unambiguous awakening. Approximately 28 minutes later, the same nursing station paged again, this time awakening the intern from REM sleep. The intern subsequently remained awake for almost an hour despite no additional pager activity. Reprinted with permission from Richardson et al (26). Copyright 1996 Associated Professional Sleep Societies, LLC.
Sleep Attacks and Sleep Drunkenness
Individuals struggling to stay awake in the face of elevated sleep pressure—whether due to acute total sleep deprivation, chronic sleep restriction or repeated interruption of sleep (due to external interruptions or the presence of a sleep disorder)—are not always able to do so by the sheer force of will. Sleep deprivation greatly increases the risk that an individual will succumb to the increased sleep pressure when their brain initiates an involuntary transition from wakefulness to sleep. This transition is initiated by the ventrolateral preoptic (VLPO) area of the hypothalamus, which Saper et al. have identified as the brain’s “sleep switch” (29). Another region of the hypothalamus, the suprachiasmatic nucleus (SCN), which serves as the central neural pacemaker of the circadian timing system, interacts with the VLPO such that there are two times of day at which such an involuntary transition from wakefulness to sleep is most probable: in the latter half of the night near the habitual wake time and in the mid-afternoon. Of course, once an individual has lost the struggle to stay awake and makes the transition from wakefulness to sleep, driving performance is much worse than that of a drunk driver, as the individual is unresponsive to the environment. Moreover, sometimes drowsy drivers linger in an intermediate state between sleep and wakefulness. The operator of a motor vehicle in this sleep-like condition—which probably represents a transitional state in which part of the brain is asleep while part of the brain remains awake—may maintain full pressure on the accelerator pedal and proceed for a considerable distance, even negotiating gradual turns, but fail to heed stop signals or respond appropriately to traffic conditions in a timely manner. This intermediate state, which has been termed “automatic behavior syndrome” or “sleep drunkenness” is characterized by retention of the ability to turn the steering wheel and to carry out rudimentary tasks and to provide semi-automatic responses to stimuli without appropriate cortical integration, often resulting in a complete loss of situational awareness and judgment (30). Some of you may have experienced this state when you suddenly realize that you have no idea how you went from point A to point B on the expressway—as if there were a missing segment in the video of life. One drowsy driver who steered his car toward an oncoming car and then tracked it as the other driver swerved to avoid him reported “waking up” from this state just in time to observe vividly through his windshield a terrified look on the face of the other driver only a moment before he killed her in a head-on collision (31). A similarly impaired NASA ground controller working in the Mission Control Room of the Johnson Space Center during the middle of the night shift sent a Space Shuttle careening into a spin while crew members were asleep when he repeatedly overrode automatically generated coordinates designed to keep the shuttle on track.
Circadian Rhythmicity
Circadian rhythms, i.e., biological rhythms oscillating with an approximate period of twenty-four hours (from the Latin words: circa—about and dies—a day), are present at all levels of biological complexity from unicellular organisms to humans. Circadian rhythms are endogenous (i.e., internally generated), self-sustaining oscillations; therefore, rhythmicity persists in the absence of periodic external time cues. In humans, many physiological processes, including the body temperature cycle, endocrine patterns, renal and cardiac function, subjective alertness, sleep-wake behavior and performance vary according to the time of day (4,20,32–37). The circadian contribution to variations in alertness and performance is generated by a light-sensitive circadian pacemaker that also drives the circadian rhythms of core body temperature, plasma cortisol and plasma melatonin (5,38–50). The endogenous circadian clock is a major determinant of the timing and internal architecture of sleep in humans (5,6). Spontaneous sleep duration, sleepiness, REM sleep propensity, and both the ability and the tendency to sleep vary markedly with circadian phase or biological time of day and interact with a homeostatic process to regulate sleep propensity and daytime alertness and neurocognitive performance (7–10,51–53). As noted above, deep within the brain, two bilaterally paired clusters of hypothalamic neurons comprising the SCN of the hypothalamus act as the central neural pacemaker for the generation and/or synchronization of circadian rhythms (54–60). This endogenous circadian pacemaker is a major determinant of daily variations in subjective alertness and cognitive performance (4,9,11,12,20,24,38). These and other studies have shown that there is a prominent circadian variation in objective and subjective measures of alertness, performance (psychomotor, vigilance, memory) and attention or ability to concentrate, with a nadir in the latter half of the usual sleep time, just before our usual wake time (Figure 2). Similarly, the peak drive for waking emanating from the hypothalamic circadian pacemaker occurs a couple of hours before our habitual bedtime. This paradoxical relationship between the output of the circadian pacemaker and the timing of the sleep-wake cycle is thought to help consolidate sleep and wakefulness in humans (8). Thus, under ordinary circumstances as the homeostatic drive for sleep increases throughout the 16-hour waking day, the circadian pacemaker sends out a stronger and stronger drive for waking during the latter half of the habitual waking day. Then, near the peak of the circadian drive for waking, about 1–2 hours before the habitual bedtime, the hormone melatonin is released. Activation of melatonin receptors on the surface of human SCN neurons suppresses the firing of those neurons. Since the SCN continues to oscillate with a near-24-hour period in the absence of SCN neuronal firing, this action of melatonin is thought to quiet the wake-promoting signal emanating from the SCN, thereby allowing us to fall asleep at our habitual hour. Similarly, the SCN sends out a strong drive promoting sleep in the latter half of the night, which helps to consolidate sleep once the pressure for deep slow-wave sleep is satiated in the first half of the night. The latter half of the night is richest in REM sleep, which has a very prominent circadian rhythm in its propensity. When one stays awake all night, in the latter half of the night near the habitual wake time, elevated homeostatic drive for sleep interacts with the circadian peak of sleep propensity to create a critical zone of vulnerability.
In the absence of periodic time cues, the period of the human circadian pacemaker is slightly longer than 24 hours (61,62). In order for the biological clock to coordinate its function with the timing of events in the external world that operates on a 24 hour schedule, daily information from the environment must therefore reach the circadian pacemaker. The circadian pacemaker is essentially reset by a small amount each day by this external stimulus in order to maintain synchrony with the 24-hour day (5,40,61).
Light is the principal environmental synchronizer of the mammalian SCN. The SCN is connected to specialized intrinsically photosensitive retinal ganglion cells containing the newly discovered photopigment melanopsin via a monosynaptic pathway called the retinohypothalamic tract (RHT), the presumed conduit by which information from the external light-dark cycle reaches the circadian clock. Properly timed exposure to light and darkness can rapidly shift the phase of the endogenous circadian pacemaker in humans (34,38–50,63,64). Both the magnitude and direction of the phase shifts induced by light are dependent on the timing, duration, intensity and wavelength of the light exposure (39,40,46–49,64–70). On average, light exposure occurring during the first half of the biological night resets the circadian clock to a later hour; light received in the latter half of the biological night resets the circadian clock to an earlier hour. Thus, the circadian pacemaker of an individual living on a conventional schedule of day work and night sleep is synchronized by the naturally occurring light- dark cycle to oscillate at the same period as the Earth’s solar day, i.e., 24 hours.
Sleep Inertia
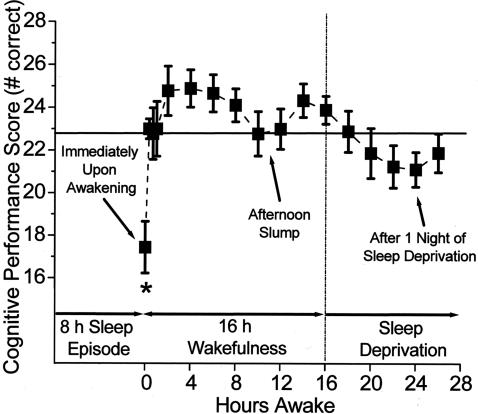
Fifty years ago, following a crash inquiry, scientists in the U. S. Air Force Laboratory discovered that performance is markedly degraded during the transition from wakefulness to sleep (71–83). The extent to which this phenomenon, now called sleep inertia, interferes with neurobehavioral performance is related to the depth of the prior sleep episode (78). Thus, agents that interfere with sleep, such as caffeine, can mute the effect of sleep inertia (84). Remarkably, as we recently reported, the adverse impact of sleep inertia on performance can far exceed the impact of total sleep deprivation (83) (Figure 4). Once residents are able to get to sleep, these house officers—who are often subjected to acute total sleep deprivation after days, weeks or months of chronic sleep restriction—often experience very deep sleep. When this occurs while residents are on call in the hospital, they may be required to make critical care decisions or perform life-saving medical procedures in an impaired state due to profound sleep inertia (15,83). Moreover, at times of night when they have the greatest patient care responsibilities due to the lack of on-site faculty supervision, resident physicians—who sleep on average just 2.6 hours while working 30-hour extended duration “on-call” shifts (85)—are likely to descend rapidly into deep stages 3 and 4 sleep during those two-hour naps, and then attempt to care for patients in an impaired confusional state—characterized by grogginess, disorientation in time or space, slowed mentation and slowed speech—immediately upon awakening.
Fig. 4.
Impact of sleep inertia on cognitive performance upon awakening compared with 24 hours without sleep. Reprinted with permission from Wertz et al. (83). Copyright 2006 American Medical Association.
There are a number of other factors that can have an impact on alertness and performance. These include the length of time on task, the level of environmental stimulation, the level of physical activity, posture, the level of task stimulation/novelty, and the use of pharmacologic agents with stimulant or hypnotic properties. Caffeine is the most widely used drug in the world. In fact, coffee beans are the second most widely traded commodity in the world, second only to oil. Caffeine administration can reduce the adverse impact of misalignment of circadian phase as well as increased homeostatic pressure on neurobehavioral performance (86). However, the dose and timing of administration is not always optimal. Specialty brand coffee, such as that available from retail outlets such as Starbucks and Dunkin’ Donuts, contain 3–4 times as much caffeine per ounce as does home brewed coffee. Super-size (e.g., 24 ounce) servings can thus contain more than a gram of caffeine, as much caffeine as an entire 10-cup pot of home-brewed coffee. When taken in the morning, these large doses of caffeine are being administered when sleep pressure is lowest, with levels declining throughout the day. When taken in the evening, caffeine—which has a 6- to 9-hour half life—may interfere with recovery sleep. When used as a wake-promoting therapeutic, the minimum effective dose of caffeine should be taken at the optimal time to help sustain performance when adequate sleep cannot be obtained. However, neither caffeine nor other wake-promoting therapeutics are a substitute for sleep. In fact, in a study of fatal-to-the-driver truck crashes, the National Transportation Safety Board (NTSB) found that plasma caffeine levels were highest in drivers involved in fatigue-related crashes (87). The NTSB interpreted high plasma caffeine data from those drivers as indicating that the drivers were taking caffeine to try to combat their fatigue. Unfortunately, even high levels of caffeine were insufficient to save those drivers from the effects of fatigue, which the NTSB found to be the leading cause of fatal crashes in those trucker drivers, equal to the fraction of crashes caused by both drugs and alcohol combined (87).
Underlying Medical Condition and Age
A number of medical conditions and/or the medications used to treat those conditions are associated with increased sleep tendency, increased risk of lapses of attention and increased risk of sleep-related accidents (88). These include primary sleep disorders, such as narcolepsy and sleep apnea, as well as sleep disturbance secondary to a medical condition or its treatment. Obstructive sleep apnea patients, for example, with an apnea/hypopnea index greater than 10, have a 6-fold increase in risk of traffic accidents (89). Age decreases the risk of sleep-related lapses of attention at night; in fact, young people are at the greatest risk of the hazards of sleep loss (90). Thus, at first blush, one might hypothesize that senior staff rather than new trainees should be assigned to work marathon overnight shifts. However, before you hastily assign all extended duration night work to your chiefs of service, I would caution you to recognize that as we get older, it becomes more and more difficult to obtain the recovery sleep that is needed following sleep deprivation. In fact, even when sleep deprived, older people have a great deal of difficulty sleeping at an adverse circadian phase (10,16,91).
Graduate Medical Education and Sleep
Extended duration work shifts, like many other features of graduate medical education in the United States, were the product of the postgraduate medical education curriculum developed by William Steward Halsted, M.D. Professor Halsted, who was Surgeon-in-Chief at the Johns Hopkins Hospital, was internationally renowned for his innovations in medical education. He founded the surgical training program at the Johns Hopkins Hospital in the 1890s, which served as a model for postgraduate medical education. He required physicians-in-training to live in the hospital (they were quite literally residents) and discouraged them from marrying so that they could devote themselves to medicine (92). He required residents to be on a “q1” call schedule, i.e. they were on call 362 of 365 days per year. He taught devotion to the profession by example, working heroic hours with his trainees. Only recently was it revealed how he maintained this grueling sleep-deprived schedule. Professor Halsted was in fact addicted to cocaine, an addiction that was an unfortunate by-product of his pioneering work developing cocaine as a surgical anesthetic (93). He spent more than a year in a rehabilitation program at a Rhode Island hospital trying to shed his cocaine addiction prior to his appointment as the first Professor of Surgery at Johns Hopkins Medical School. However he only gained an addiction to morphine there, which had been used to treat his cocaine addiction (93).
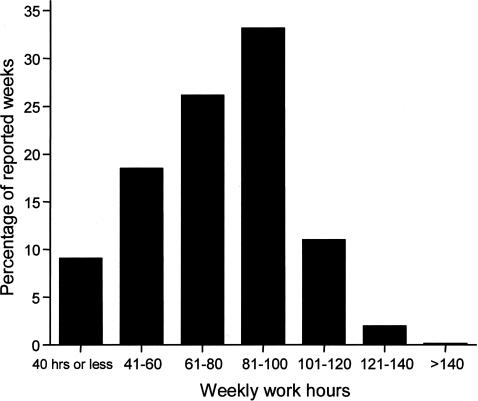
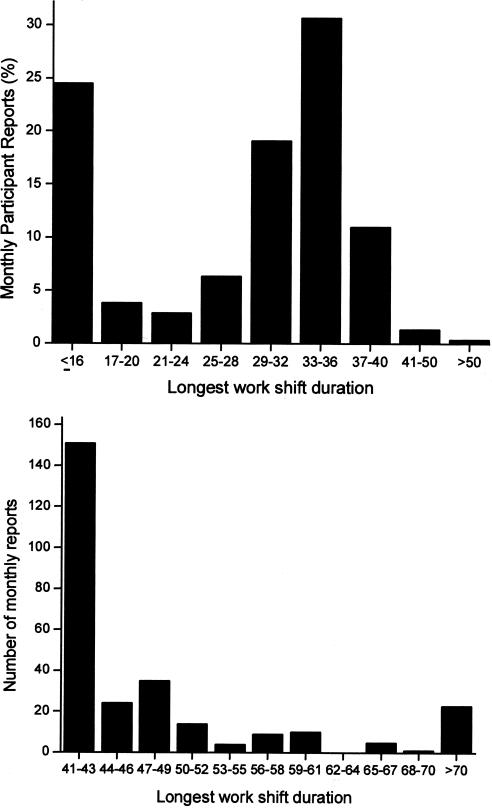
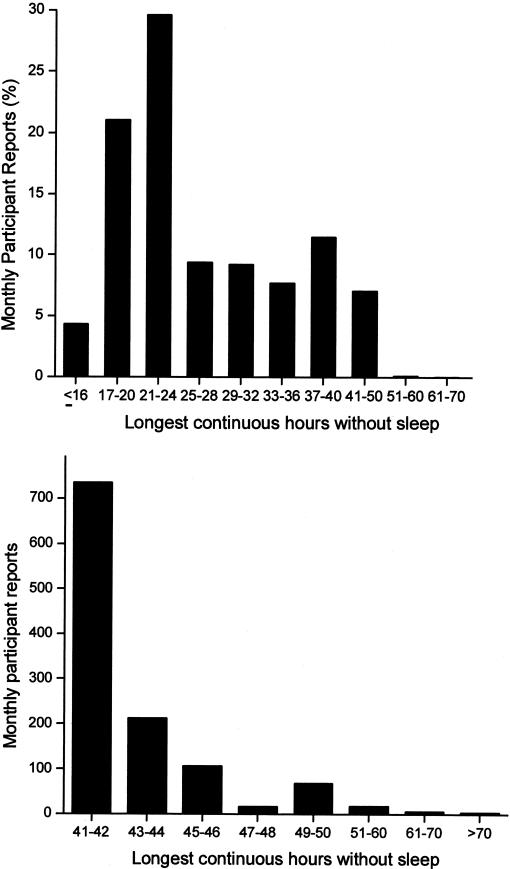
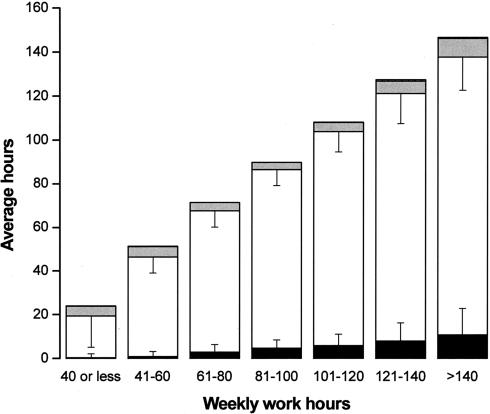
Today, the use of powerful stimulants to allow personnel to remain awake during extended duration work shifts is restricted to certain branches of the military, such as the United States Air Force, which still distributes methamphetamine routinely to pilots required to fly lengthy missions and to other critical military personnel. Of course, neither medical school faculty nor their hospital trainees are allowed to use cocaine or methamphetamine to stay awake for long hours, nor should they be allowed to do so. Yet, based on a 19th century tradition dated to the time of Halsted, academic medical centers continue to single out physicians-in-training as the only group of hospital employees who are required to work for 30 consecutive hours and to work more than double the standard work week. Using a validated survey instrument, we found that in 2002–2003, half of the interns we studied worked more than 80 hours per week, with 11% working more than 100 hours per week (Figure 5). Some were required to work more than 120 hours per week (85), which is remarkable given that there are only 168 hours in a week. During that year, we estimate that physicians-in-training worked 20,000 shifts in the United States that exceeded 40 consecutive hours, with about 2,000 shifts exceeding 64 consecutive hours (85) (Figure 6). Theoretically, the work-hour reforms instituted by the Accreditation Council of Graduate Medical Education (ACGME) in 2003 should have reduced the duration of the longest of these shifts to 30 consecutive hours, although there has been no independent verification that these voluntary guidelines are being consistently enforced. Moreover, the new ACGME guidelines largely sanctioned what was already the status quo in most hospitals, since interns averaged 70.7+ 26.0 work hours per week (85) before the ACGME instituted an 80- to 88-hour limit (averaged over four weeks) and the average duration of the extended duration “on-call” shifts was 32.0+ 3.7 hours before the ACGME instituted their limit of 30 consecutive work hours per shift. On many of these marathon ∼30-hour shifts, we found that trainees obtained little or no sleep (85) (Figure 7). Even among interns who spent the greatest amount of time in the hospital per week, less than 5% of that time was spent sleeping (85) (Figure 8). In fact, we found that interns averaged only 2.6+ 1.7h of sleep on extended duration (>24-hour) work shifts, and that those who were covered by night floats only obtained an additional half hour of sleep (85).
Fig. 5.
A total of 17,003 person-months of data were collected from a nationwide sample during 2002–2003. The distribution of the percentages of reported weeks with a given range of work hours is shown in this chart. Reprinted with permission from Barger et al (85). Copyright 2005 Massachusetts Medical Society.
Fig. 6.
The longest shifts reported by participants are shown in the chart at the top. The chart at the bottom shows the number of surveys in which participants reported shifts that exceeded 40 hours. Reprinted with permission from Barger et al (85). Copyright 2005 Massachusetts Medical Society.
Fig. 7.
The greatest number of hours without sleep as a percentage of monthly reports is shown in the chart at the top. The chart at the bottom shows the number of surveys in which participants reported more than 40 continuous hours without sleep. Reprinted with permission from Barger et al (85). Copyright 2005 Massachusetts Medical Society.
Fig. 8.
This chart shows the average number of hours that interns spent asleep in the hospital, awake in the hospital, studying or working in relation to their program but outside the hospital, and working outside of the program. T bars indicate standard deviations. Interns reported spending an average of 193.4+ 88.8 of their waking hours in the hospital each month participating in direct patient care (e.g., examining patients; writing progress notes; interpreting diagnostic tests, radiographic studies, and pathological specimens; and consulting with other physicians), 43.3+ 47.0 hours in duties not directly related to patient care (e.g., completing other paperwork and scheduling tests), 23.5+ 20.1 hours in structured learning sessions (including classes, laboratories, and grand rounds), and 7.3+ 16.4 hours teaching students or house staff. Reprinted with permission from Barger et al (85). Copyright 2005 Massachusetts Medical Society.
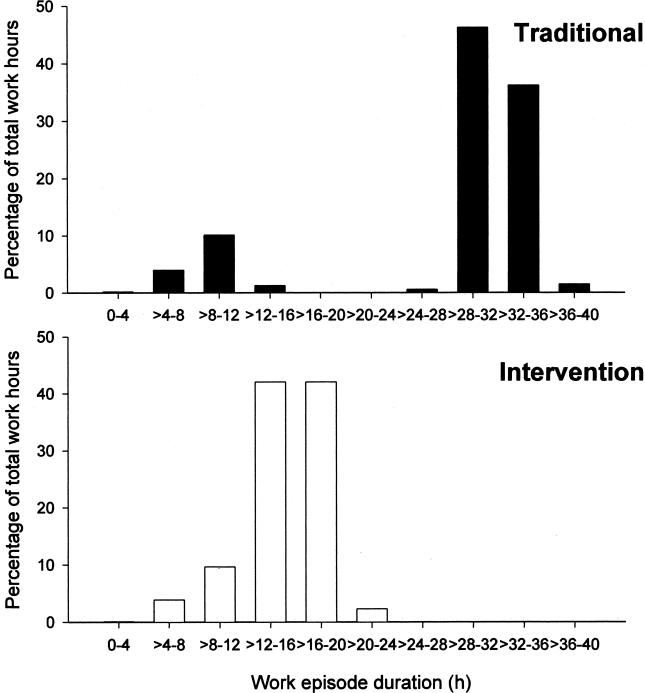
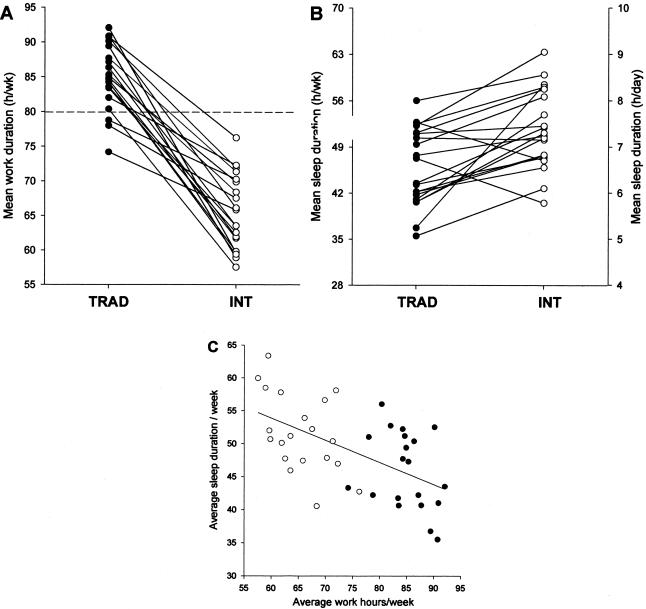
The recent discovery that memory consolidation and learning depends on the sleep obtained after training on a task has called into question the wisdom of keeping residents awake all night as part of their education (94–98). In order to address the impact of such schedules on patient care, the Harvard Work Hours, Health and Safety Group (HWHHSG) conducted a clinical trial for the Medical Intensive Care (MICU) and the Coronary Care (CCU) Units at the Brigham and Women’s Hospital in Boston (1, 99). Interns were randomly assigned to work a three week rotation in the CCU or the MICU either on the traditional “q3” schedule, in which they worked an extended duration (∼30 hour) work shift every other shift, or an intervention schedule in which the 30-hour extended duration work shift was split in half such that no scheduled work shift exceeded 16 consecutive hours. Dr. Steven Lockley, Dr. Daniel Aeschbach and our colleagues in the HWHHSG examined the relationship between work hours, sleep and the ability to sustain attention in these trainees. We found that on the traditional schedule, 85% of all work hours occurred on extended duration (>24h) work shifts, whereas none of their work time was spent on extended duration shifts on the intervention schedule (1) (Figure 9). Moreover, on the traditional schedule, interns were twice as likely to have slept less than 2 hours in the 24 hours prior to each hour worked, whereas on the intervention schedule, they were twice as likely to have obtained at least 8 hours of sleep in the prior 24 hours (1). We found that the interns worked fewer hours and slept more hours per week on the interventional schedule, and that there was a negative correlation between hours worked and hours slept (1) (Figure 10). We found that interns working extended duration shifts were twice as likely to experience attentional failures while working at night than were interns scheduled to work no more than 16 consecutive hours (1) (Figure 11).
Fig. 9.
Proportion of total work hours plotted against the duration of the shift during the Traditional Schedule (top chart) and the Intervention Schedule (bottom chart). Reprinted with permission from Lockley et al (1). Copyright 2004 Massachusetts Medical Society.
Fig. 10.
Subjective mean hours of work per week (top left), duration of sleep (top right), and the relationship between the duration of work and the duration of sleep (bottom chart) for 20 interns during the Traditional schedule and the Intervention schedule (open symbols). Reprinted with permission from Lockley et al (1). Copyright 2004 Massachusetts Medical Society.
Fig. 11.
Mean (+SE) number of attentional failures among the 20 interns as a group and individually while working overnight (11 pm to 7 am) during the Traditional schedule (filled bar) and the Intervention schedule (open bar). Reprinted with permission from Lockley et al (1). Copyright 2004 Massachusetts Medical Society.
But what about patient care? Prior research at the Brigham and Women’s Hospital had found that discontinuity in patient care (patient handoffs) increased the risk of preventable adverse events (100), although this increase could be eliminated by use of a computerized sign out system (101). Reduction in the duration of extended work shifts by half would necessarily double the number of required handoffs of care. Thus, we wondered which was safer for the patient, a tired intern who remained with the patient throughout the night after admission, or a better-rested intern to whom care of the patient was transferred. In order to answer that question, we teamed up with the Patient Safety Center for Excellence led by Dr. David Bates at the Brigham and Women’s Hospital and obtained research support for a study from the Agency for Healthcare Research and Quality and the National Institute for Occupational Safety and Health. Dr. Christopher Landrigan, Dr. Jeffrey Rothschild and our colleagues in the HWHHSG then hired a team of physicians to monitor these interns around the clock while they worked in the MICU and CCU (99). In addition, we hired research nurses to review the medical records of their patients and search for medical errors. Finally, we presented all events noted by these observers to a panel of reviewers who were blind to condition and rated each event. Only serious medical errors were analyzed.
Interns working extended duration “on call” shifts made 35.9% more serious medical errors, including more than 5 times as many serious diagnostic mistakes, as interns scheduled to work no more than 16 consecutive hours (99). We found that interns working extended duration shifts made more serious mistakes that reached the patients than those who worked shorter shifts, notwithstanding the fact that there were twice as many handoffs of patient care with the intervention schedule (99). We found that most of the serious medical errors were due to slips and lapses, i.e., failures to carry out intended plans of action, rather than knowledge-based mistakes (105).
In a companion nationwide survey study supported by the National Institute of Occupational Safety and Health, Dr. Laura Barger, Dr. Najib Ayas and our colleagues in the Harvard Work Hours, Sleep and Safety Group evaluated the impact of extended duration work shifts on the risk of motor vehicle crashes among interns. We gathered 1,417 person-years of monthly survey data from 2,737 interns nationwide in 2002–2003. During that time, interns reported 320 motor vehicle crashes. Eighty-two percent of those crashes were documented by a police report, insurance claim, automobile repair record, medical record, photograph of the damaged vehicle or a written description of the motor vehicle crash. More than forty percent of the motor vehicle crashes were judged to be consequential, i.e., leading to treatment in an emergency department, more than one thousand dollars property damage or filing of a police report. No additional motor vehicle crashes were identified by a search of the Social Security Death Index or through participant’s emergency contacts.
In a prospective analysis of scheduled shifts (across individuals), we found that for each extended duration work shift scheduled per month, interns had an 8.8 percent (CI: 3.2%–14.4%) increased monthly risk of any reported motor vehicle crash, and a 16% (95% CI: 7.6% to 24.4%) increased monthly risk of a reported motor vehicle crash on the commute from work. Trainees who work 10 extended duration shifts per month on a “q3” schedule would thus have a 160% increased monthly risk of a reported motor vehicle crash on the commute from work. In a separate case-controlled analysis of the same data set, we found that the odds ratio for reporting a motor vehicle crash during the commute from the hospital was 2.3 times (95% CI: 1.6 to 3.3) greater after an extended duration (>24h) work shift than after a non-extended work shift (85). The odds ratio for reporting a near miss crash was 5.9 (95% CI: 5.4 to 6.3) during the commute from work after an extended duration work shift as compared to the commute from a non-extended duration work shift (85).
Overall, based on this work, we found that the traditional practice of scheduling interns very long work weeks and extended duration work shifts was hazardous both to the intern themselves as well as to their patients (106). For this reason, the Sleep Research Society (SRS) which represents more than a thousand sleep scientists in the United States, together with the National Sleep Foundation (NSF) in Washington have endorsed legislation in Massachusetts that would limit resident work hours. This legislation is sponsored by Massachusetts State Senator Richard Moore, who requested guidance from the SRS regarding establishment of safer work hours for medical and surgical trainees. Table 1 illustrates the features of the pending legislation. Of note, the SRS and NSF recommend that all physicians be required to notify their patients and receive permission from the patients before caring for them if the physician has slept less than 2 hours in the prior 24 hours. In fact, a nationwide NSF poll found that 86 percent of people would feel anxious about their safety if they learned that their surgeons had been on duty more than 24 hours, and 70% would likely ask for a different doctor (102). Given the findings that I have reported to you today, the SRS and NSF have concluded that a patient has the right to know if his/her physician were sleep deprived, so that the patient can decide whether or not be treated by that physician.
TABLE 1.
Pending Massachusetts Legislation endorsed by Sleep Research Society and National Sleep Foundation
| Schedule Feature | Proposed Directive |
|---|---|
| Weekly Work Hours | Optimal maximum: 60 hours per week |
| Fixed limit: 80 hours in any week | |
| Consecutive Work Hours | Optimal maximum of 10 consecutive hours |
| Fixed maximum of 18 consecutive hours | |
| Maximal frequency of 18-hour night shifts | No more than one 18-hour overnight shift every 3 days |
| Minimum Hours Off per Day | Minimum Hours Off |
| - after 10-hour shift | - Optimum ≥12 consecutive hours off/day; minimum of 10 hours off per day |
| - after ≥18-hour shift | - Minimum of 16 consecutive hours off/day |
| High-intensity settings | Optimal limits required |
| Patient notification | Caring physician awake 22 of prior 24 hours |
| Consecutive Hours Off Duty per Week | Optimum ≥48 consecutive hours off/week |
| Minimum 36 consecutive hours off/week, including two consecutive nights weekly. | |
| Minimum of 60 consecutive hours off duty each month. |
Probable Cause of Sleep-related Crash in Case A.F.
As shown in the initial case I presented today, there are many pathways that can lead physicians to become sleep deprived. The practice of scheduling physicians to be on call for weeks at a time covering busy services from home may seem innocuous compared to the practice of requiring physicians-in-training to work 30 consecutive hours. However, both sleep curtailment and frequent interruptions of sleep, alone or in combination as in this case, can lead to chronic cumulative sleep deprivation that can severely degrade performance and increase the risk of harm or injury. Case A.F. fell asleep at the wheel and crashed into a car with a 5-year old passenger after having been awake for only 10 hours, but he had been averaging only 5.8 hours per night of interrupted on-call sleep, and had worked seven consecutive days without a day off. The crash occurred in the mid-afternoon nap zone, which made him more vulnerable to the impact of sleep deprivation. In addition to behaviorally induced insufficient sleep syndrome, the trainee suffered from positional sleep-related breathing disorder. Taken together, this crash was certainly not an accident. A comprehensive fatigue management program that includes education regarding the principles of sleep and circadian physiology, implementation of safer policies regarding work hours (such as the limits endorsed by the SRS and NSF), and a comprehensive program to screen for sleep disorders would greatly reduce the risk of sleep-related errors and accidents like this one. Like alcohol-related crashes, sleep-related motor vehicle crashes are a preventable cause of injury. The National Highway Transportation Safety Administration estimates that there are more than 250,000 drowsy driver crashes annually; the Institute of Medicine estimates that drowsy driver crashes account for 20 percent of all injuries in motor vehicle crashes (107). With approximately 100,000 residents in training nationwide, steps should be taken to eliminate this preventable cause of injury.
Legal, Moral and Ethical Considerations
Given that drivers in the United States (Colorado, Massachusetts, Michigan and Florida) and Britain have been convicted of driving when impaired by sleepiness, and that the State of New Jersey has recently amended its vehicular homicide statute to add driving after 24 hours without sleep to the definition of “reckless,” the legal and ethical question is raised as to who should be held responsible for the next motor vehicle crash in which a sleep-deprived resident kills or maims another motorist or pedestrian. No one falls asleep at the wheel without having struggled to stay awake beforehand, so the driver certainly bears legal and moral responsibility just as a drunk driver would. However, institutions that have put their trainees in harm’s way by requiring them to work for so many consecutive hours that they are virtually incapacitated by sleep deprivation cannot reasonably walk away from their responsibility to care for those who have been injured by sleep-deprived trainees. Take for example the case of Dr. Sook Im Hong. Dr. Hong was in the second week of her internship at Rush-Presbyterian-St. Luke’s Medical Center in Chicago when she fell asleep at the wheel after a 36-hour hospital shift and rear-ended another car, causing massive brain injuries to Heather Brewster (103). It is shocking to me that Rush Medical Center Attorney George F. Galland, Jr. succeeded in arguing that under Illinois law, the institution that required Dr. Hong to work for 36 consecutive hours does not bear any responsibility for the lifelong care of Ms. Brewster, a former college volleyball star who was a graduate student in physical therapy at the time of the crash. By arguing, on one hand, that the intern alone bears responsibility for sleep-related errors and accidents, and by requiring, on the other hand, that interns work 30-hour shifts as a condition of their employment, interns are being put in a “Catch 22” bind while they are at work. Moreover, given that appeals courts in two states (Oregon and West Virginia) have ruled that an employer’s responsibility for fatigue-related crashes can continue even after they have left work—similar in concept to the dramshop tort liability incurred by establishments serving alcohol to drivers subsequently involved in alcohol-related motor vehicle crashes—it is likely that courts will eventually hold hospitals that require trainees to work extended duration work shifts, notwithstanding evidence of the hazards of this practice, similarly liable. What about the trainees’ physicians? In California, physicians have an obligation to report to the Department of Motor Vehicles patients with conditions characterized by lapses of consciousness. Given that the interns working traditional 30-hour shifts in our study averaged more than five lapses of attention per night (99), do California physicians therefore have an obligation to report all house staff with behaviorally induced insufficient sleep syndrome under their care to the California Department of Motor Vehicles? All these questions will be left for the courts to decide.
But let us move beyond the legal questions. What moral or ethical responsibility should be borne by the program director who required a trainee to work an extended duration shift that resulted in such an accident? What about the department chair or hospital president who requires or allows the program director to do so, knowing that such schedules increase the risk of motor vehicle crashes by more than 160%? Does the ACGME bear responsibility for continuing to sanction extended duration work shifts in the face of evidence that has revealed the hazards of such shifts? In my view, too many trainees on the thresholds of a medical career have already been killed or seriously injured in sleep-related crashes—or have killed or seriously injured others in sleep-related crashes—while attempting to commute home after working extended duration shifts. Even Professor Halsted never envisioned one of his charges getting behind the wheel of a car and attempting to drive at a speed of 60 miles per hour after working for 30 consecutive hours. These marathon work shifts are a vestige of a bygone era in which the pace of the hospital was much slower at night. There were no intensive care units in 19th century hospitals. There were no all night hospital laboratories. Resident physicians were not routinely expected to stay up all night admitting patients who had been kept in a holding area of the Emergency Department until the late evening. Hospitals today are round-the-clock operations focused on reducing patient length of stay by admitting patients only during the most acute phase of their illnesses. The work schedules to which our trainees are scheduled must now be changed to recognize that trainees can no longer sleep in the hospital. Moreover, resident physicians are now allowed to leave the hospital, live in the community and raise families. Our institutional training practices must therefore allow our trainees to take on these additional responsibilities during their training without endangering themselves, their families or others. Given the dictum “Physician, do no harm,” I would urge you as the leaders of American medicine to implement policies that eliminate the practice of scheduling trainees to work extended duration shifts. In the meantime, until this practice has been eliminated, our data indicate that institutions have an obligation—at the very least—to provide round trip transportation to trainees who are required to work extended duration shifts, as it is unsafe to expect or even allow them to drive with inadequate sleep. For this reason, the SRS, NSF and American Academy of Sleep Medicine have recently endorsed model drowsy driver legislation in Massachusetts. Of course, provision of transportation for trainees after extended duration work shifts begs the question as to whether it is appropriate for physicians who are too tired to drive home from work to be caring for patients (104).
Summary
The work schedules of physicians in training require them to work extraordinarily long work shifts and long work weeks. These schedules, which are based on a tradition that dates back to the 19th century, result in acute and chronic sleep deprivation. Sleep deprivation, misalignment of circadian phase and sleep inertia adversely impact cognitive performance and increase the risk of error and accident. Interns working extended duration shifts make significantly more serious medical errors while caring for patients in intensive care units, and make five times as many serious diagnostic mistakes. In addition to the deleterious effects of extended duration work shifts on patient safety, we also found that the risk of motor vehicle crashes is more than doubled driving home from work after such shifts. We conclude that the practice of working extended duration work shifts, which continues to be allowed by new ACGME regulations, are hazardous to both interns and their patients. Academic medical centers are urged to eliminate this now-dangerous tradition.
ACKNOWLEDGMENTS
Supported by grants from the National Institute for Occupational Safety and Health (1 R01 OH07567), which provided a certificate of confidentiality for data protection, and by the Agency for Healthcare Research and Quality (R01 HS12032 and F32 HS14130); Brigham and Women’s Hospital General Clinical Research Center supported by the National Center for Research Resources (M01 RR02635); National Heart, Lung, and Blood Institute fellowships in the Program and Training in Sleep, Circadian, and Respiratory Neurobiology at Brigham and Women’s Hospital (T32 HL-79010); Wellcome Trust, United Kingdom (060018/B/99/Z); Canadian Institutes of Health research/British Columbia Lung Association. Dr. Czeisler is supported in part by the National Space Biomedical Research Institute through the National Aeronautics and Space Administration (NCC9-58).
Special thanks to collaborators Steven W. Lockley, PhD, Christopher P. Landrigan, MD, MPH, Laura K. Barger, PhD, Najib T. Ayas, MD, MPH, Jeffrey M. Rothschild, MD, David W. Bates, MD, Bernard Rosner, PhD, Frank E. Speizer, MD, Joel T. Katz, MD, Craig M. Lilly, MD, Peter H. Stone, MD, Daniel Aeschbach, PhD, John W. Cronin, MD, Erin E. Evans, RPSGT, Brian E. Cade, MS, Clark J. Lee, JD, Elisabeth Burdick, MS and Rainu Kaushal, MD, MPH. I am indebted to the interns who took time from their busy work schedules to participate in this study; the National Resident Matching Program and the Association of American Medical Colleges (especially Jordan J. Cohen, MD, Paul Jolly, PhD) for providing access to the interns; DeWitt C. Baldwin, Jr., MD, Steven R. Daugherty, PhD and Steven K. Howard, MD for their advice in the design of these studies;Victor J. Dzau, MD, Anthony D. Whittemore, MD, Jeffrey Otten, Matthew Van Vranken, Gary L. Gottlieb, MD, MBA, and Joseph B. Martin, MD, PhD, for their support and encouragement of this work.
REFERENCES
- 1.Lockley SW, Cronin JW, Evans EE, et al. fect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 2.Jewett ME. Models of circadian and homeostatic regulation of human performance and alertness. Harvard University. 1997 [Google Scholar]
- 3.Jewett ME, Borbély AA, Czeisler CA. oceedings of the workshop on biomathematical models of circadian rhythmicity, sleep regulations, and neurobehavioral function in humans. J Biol Rhythms. 1999;14:429–630. [PubMed] [Google Scholar]
- 4.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. hort-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 5.Czeisler CA, Khalsa SBS. The human circadian timing system and sleep-wake regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders Company; 2000. pp. 353–374. [Google Scholar]
- 6.Czeisler CA, Dijk DJ. Human circadian physiology and sleep-wake regulation. In: Takahashi JS, Turek FW, Moore RY, editors. Handbook of Behavioral Neurobiology: Circadian Clocks. New York: Plenum Publishing Co; 2001. pp. 531–569. [Google Scholar]
- 7.Dijk DJ, Czeisler CA. ontribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516.2:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Dijk DJ, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh JR, editors. Sleep Onset: Normal and Abnormal Processes. Washington, D.C: American Psychological Association; 1994. pp. 89–110. [Google Scholar]
- 13.Klein T, Martens H, Dijk DJ, Kronauer RE, Seely EW, Czeisler CA. Chronic non-24-hour circadian rhythm sleep disorder in a blind man with a regular 24-hour sleep-wake schedule. Sleep. 1993;16:333–343. doi: 10.1093/sleep/16.4.333. [DOI] [PubMed] [Google Scholar]
- 14.Boivin DB, Czeisler CA, Dijk DJ, et al. omplex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 15.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 16.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 17.Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;289:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 18.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–1060. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 19.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 20.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 21.Van Dongen HP, Dinges D. Sleep debt and cumulative excess wakefulness. Sleep. 2003;26:249–250. [Google Scholar]
- 22.Czeisler CA. Quantifying consequences of chronic sleep restriction. Sleep. 2003;26:247–248. [PubMed] [Google Scholar]
- 23.van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 24.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 25.Torsvall L, Akerstedt T. Disturbed sleep while being on call: an EEG study of ships’ engineers. Sleep. 1988;11:35–38. doi: 10.1093/sleep/11.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Richardson GS, Wyatt JK, Sullivan JP, et al. bjective assessment of sleep and alertness in medical house-staff and the impact of protected time for sleep. Sleep. 1996;19:718–726. [PubMed] [Google Scholar]
- 27.Wolf MA, Richardson GS, Czeisler CA. New York: Waverly Press; 1990. Improved Sleep: a means of reducing the stress of internship. The transactions of the American Clinical and Climatological Association; pp. 255–231. [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas M, Sing H, Belenky G, et al. eural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 29.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Phillips R, Dement WC. A syndrome of hypersomnia with automatic behavior. Electroenceph Clin Neurophysiol. 1975;38:403–413. doi: 10.1016/0013-4694(75)90264-3. [DOI] [PubMed] [Google Scholar]
- 31.State of Florida v. Jorge Rosario. 2004:2D04–4561. District Court of Appeal of Florida, Second District. [Google Scholar]
- 32.Czeisler CA, Winkelman JW, Richardson GS. Harrison’s Principles of Internal Medicine. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Disorders of sleep and circadian rhythms. New York: McGraw-Hill, Inc; 2000. pp. 1–78. [Google Scholar]
- 33.Czeisler CA. Human circadian physiology: internal organization of temperature, sleep-wake, and neuroendocrine rhythms monitored in an environment free of time cues. Stanford University. 1978 [Google Scholar]
- 34.Allan JS, Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab. 1994;79:508–512. doi: 10.1210/jcem.79.2.8045970. [DOI] [PubMed] [Google Scholar]
- 35.El Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous—a general clinical research center study. J Clin Endocrinol Metab. 1997;82:281–286. doi: 10.1210/jcem.82.1.3683. [DOI] [PubMed] [Google Scholar]
- 36.Waldstreicher J, Duffy JF, Brown EN, Rogacz S, Allan JS, Czeisler CA. Gender differences in the temporal organization of prolactin (PRL) secretion: evidence for a sleep-independent circadian rhythm of circulating PRL levels—a clinical research center study. J Clin Endocrinol Metab. 1996;81:1483–1487. doi: 10.1210/jcem.81.4.8636355. [DOI] [PubMed] [Google Scholar]
- 37.Czeisler CA, Jewett ME. Human circadian physiology: Interaction of the behavioral rest—activity cycle with the output of the endogenous circadian pacemaker. In: Thorpy MJ, editor. Handbook of Sleep Disorders. New York: Marcel Dekker, Inc; 1990. pp. 117–137. [Google Scholar]
- 38.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 39.Czeisler CA. The effect of light on the human circadian pacemaker. In: Waterhouse JM, editor. Circadian Clocks and Their Adjustment. Chichester (Ciba Found. Symp. 183): John Wiley and Sons, Inc; 1995. pp. 254–302. [DOI] [PubMed] [Google Scholar]
- 40.Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. New York: Marcel Dekker, Inc; 1999. pp. 149–180. [Google Scholar]
- 41.Czeisler CA, Kronauer RE, Allan JS, et al. right light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 42.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- 43.Czeisler CA, Chiasera AJ, Duffy JF. Research on sleep, circadian rhythms and aging: Applications to manned spaceflight. Exp Gerontol. 1991;26:217–232. doi: 10.1016/0531-5565(91)90014-d. [DOI] [PubMed] [Google Scholar]
- 44.Shanahan TL, Czeisler CA. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab. 1991;73:227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- 45.Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: Influence of sleep timing, social contact and light exposure. J Physiol (Lond) 1996;495:289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: A further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- 47.Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–R1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- 48.Shanahan TL, Zeitzer JM, Czeisler CA. Resetting the melatonin rhythm with light in humans. J Biol Rhythms. 1997;12:556–567. doi: 10.1177/074873049701200610. [DOI] [PubMed] [Google Scholar]
- 49.Boivin DB, Czeisler CA. Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport. 1998;9:779–782. doi: 10.1097/00001756-199803300-00002. [DOI] [PubMed] [Google Scholar]
- 50.Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–253. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- 51.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 52.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2:329–346. [PubMed] [Google Scholar]
- 53.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol (Lond) 1997;505.3:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 55.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein DC, Moore RY, Reppert SM. New York: Oxford University Press; 1991. Suprachiasmatic nucleus: The mind’s clock. [Google Scholar]
- 57.Mumford GK, Benowitz NL, Evans SM, et al. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur J Clin Pharmacol. 1996;51:319–325. doi: 10.1007/s002280050205. [DOI] [PubMed] [Google Scholar]
- 58.Weaver DR. The suprachiasmatic nucleus: A 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 59.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 60.Moore RY. Organization of the mammalian circadian system. In: Waterhouse JM, editor. Circadian clocks and their adjustment. Chichester (Ciba Foundation Symp 183): John Wiley and Sons, Inc; 1994. pp. 88–99. [PubMed] [Google Scholar]
- 61.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 62.Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 64.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Sensitivity of the human circadian pacemaker to moderately bright light. J Biol Rhythms. 1994;9:315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- 66.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 67.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol (Lond) 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanahan TL, Czeisler CA. Physiological effects of light on the human circadian pacemaker. Seminars in Perinatology. 2000;24:299–320. doi: 10.1053/sper.2000.9123. [DOI] [PubMed] [Google Scholar]
- 69.Klerman EB, Shanahan TL, Brotman DJ, et al. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythms. 2002;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 70.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 71.Langdon DE, Hartman B. Performance upon sudden awakening. School of Aerospace Medicine. 1961;62–17:1–8. [PubMed] [Google Scholar]
- 72.Hartman BO, Langdon DE. A second study on performance upon sudden awakening. 1965 TR-65–61, 1–10. Brooks AFB, TX, U.S. Air Force. [PubMed] [Google Scholar]
- 73.Hartman BO, Langdon DE, McKenzie RE. A third study on performance upon sudden awakening. 1965 TR-65–63, 1–4, Brooks, TX, U.S. Air Force. [PubMed] [Google Scholar]
- 74.Koulack D, Schultz KJ. Task performance after awakenings from different stages of sleep. Percept Mot Skills. 1974;39:792–794. doi: 10.2466/pms.1974.39.2.792. [DOI] [PubMed] [Google Scholar]
- 75.Dinges DF, Orne MT, Orne EC. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behavior Research Methods, Instruments Computers. 1985;17:37–45. [Google Scholar]
- 76.Balkin TJ, Badia P. Relationship between sleep inertia and sleepiness: Cumulative effects of four nights of sleep disruption/restriction on performance following abrupt nocturnal awakenings. Biol Psychol. 1988;27:245–258. doi: 10.1016/0301-0511(88)90034-8. [DOI] [PubMed] [Google Scholar]
- 77.Balkin TJ, Braun AR, Wesensten NJ, et al. The process of awakening: A PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 78.Dinges DF. Sleep Inertia. In: Carskadon MA, editor. Encyclopedia of Sleep and Dreaming. New York: Macmillillan Publishing Company; 1993. pp. 553–554. [Google Scholar]
- 79.Ferrara M, De Gennaro L. The sleep inertia phenomenon during the sleep-wake transition: theoretical and operational issues. Aviat Space Environ Med. 2000;71:843–848. [PubMed] [Google Scholar]
- 80.Bruck D, Pisani D. The effects of sleep inertia on decision-making performance. J Sleep Res. 1997;8:95–103. doi: 10.1046/j.1365-2869.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 81.Achermann P,. Werth, E, Dijk DJ, Borbély AA. Time course of sleep inertia after nighttime and daytime sleep episodes. Archives Italiennes de Biologie. 1995;134:109–119. [PubMed] [Google Scholar]
- 82.Naitoh P, Kelly T, Babkoff H. Sleep inertia: Best time not to wake up. Chronobiol Int. 1993;10:109–118. doi: 10.1080/07420529309059699. [DOI] [PubMed] [Google Scholar]
- 83.Wertz AT, Ronda JM, Czeisler CA, Wright KP., Jr Effects of sleep inertia on cognition. JAMA. 2006;295:163–164. doi: 10.1001/jama.295.2.163. [DOI] [PubMed] [Google Scholar]
- 84.van Dongen HPA, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–819. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- 85.Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–134. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 86.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose, repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 87.National Transportation Safety Board. Safety Study: Fatigue, Alcohol, Other Drugs, and Medical Factors in Fatal-to-the-Driver Heavy Truck Crashes (Volume 1) Washington, DC: National Transportation Safety Board; 1990. pp. 1–181. NTSB/SS-90/01. [Google Scholar]
- 88.Carter N, Ulfberg J, Nystrom B, Edling C. Sleep debt, sleepiness and accidents among males in the general population and male professional drivers. Accid Anal Prev. 2003;35:613–617. doi: 10.1016/s0001-4575(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 89.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 90.Akerstedt T, Czeisler CA, Dinges DF, Horne JA. Accidents and Sleepiness: A consensus statement from the International Conference on Work Hours, Sleep and Accidents, Stockholm. J Sleep Res. 1994;3:195. 8–10 September 1994. [Google Scholar]
- 91.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 92.Farley P. Recreating the residency. Yale Medicine. 2004:30–36. [Google Scholar]
- 93.Markel H. The accidental addict. N Engl J Med. 2005;352:966–968. doi: 10.1056/NEJMp048240. [DOI] [PubMed] [Google Scholar]
- 94.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 95.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cognitive Neurosci. 1999;11:182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 96.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual Discrimination task improvement: A multi-step process occurring during sleep. J Cognitive Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 97.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 98.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 99.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in Intensive Care Units. N Engl J Med. 2004;351:1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 100.Petersen LA, Brennan TA, O’Neil AC, Cook EF, Lee TH. Does housestaff discontinuity of care increase the risk for preventable adverse events? [see comments] Ann Intern Med. 1994;121:866–872. doi: 10.7326/0003-4819-121-11-199412010-00008. [DOI] [PubMed] [Google Scholar]
- 101.Petersen LA, Orav EJ, Teich JM, O’Neil AC. Using a computerized sign-out program to improve continuity of inpatient care and prevent adverse events. Joint Commission Journal on Quality Improvement. 1998;24:77–87. doi: 10.1016/s1070-3241(16)30363-7. [DOI] [PubMed] [Google Scholar]
- 102.National Sleep Foundation. Executive summary of the 2002 “Sleep in America” poll. WB&A Market Research, editor. 1–43. 2002. Washington, D.C., National Sleep Foundation. 4-2-2002. [Google Scholar]
- 103.Safety of Medical Residents’ Long Hours Questioned. Washington, D.C: National Public Radio; 2005. Feb 28, (Accessed March 25, 2006 at http://www.npr.org/templates/story/story.php?storyId=4512366) [Google Scholar]
- 104.Hunt KE. Post-Call Accidents. N Engl J Med. 2005;352:1491. [PubMed] [Google Scholar]
- 105.Rothschild JM, Landrigan CP, Cronin JW, et al. The critical care safety study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694–1700. doi: 10.1097/01.ccm.0000171609.91035.bd. [DOI] [PubMed] [Google Scholar]
- 106.Lee CJ. Federal regulation of hospital resident work hours: Enforcement with real teeth. J Health Care Law and Policy. Univ Maryland, in press. [Google Scholar]
- 107.Colten HR, Altevogt BM, editors. Institute of Medicine, Committee on Sleep Medicine and Research. 2006 Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. The National Academies Press, Washington, D.C. [Google Scholar]