Abstract
Certain human papillomaviruses (such as HPV-16 and HPV-18) are associated with specific anogenital cancers, most notably cervical cancer. These viruses encode two oncoproteins, E6 and E7, which are expressed in the HPV positive cancers. E7 functions in cellular transformation, at least in part, through inactivation of pRB, and the other pRB related “pocket proteins” p107 and p130. The major target of the E6 oncoprotein encoded by the genital tract, cancer-associated human papillomaviruses is the p53 tumor suppressor protein. E6 binding to p53 is mediated by a cellular protein, the E6-associated protein (E6AP). In the presence of E6, E6AP catalyses the ubiquitylation and proteolysis of p53. E6AP is an E3 ubiquitin protein ligase and is not normally involved in the regulation of p53 stability in the absence of E6. E6AP is the prototype for the HECT domain family of E3 ubiquitin protein ligases.
Introduction
The papillomaviruses are a group of small DNA viruses, which induce squamous epithelial tumors (warts and papillomas). The first papillomavirus described was the cottontail rabbit papillomavirus (1). Subsequently, papillomaviruses have been isolated and characterized from other vertebrate species, including man. Standard virologic approaches to the study of these viruses have been limited, however, due to the lack of a tissue culture system for their in vitro propagation. This failure may, in part, be due to the fact that the productive functions of the papillomaviruses are expressed only in the more fully differentiated squamous epithelial cells. To date, tissue culture systems for keratinocytes have not permitted the full expression of the papillomavirus life cycle. Thus, much of what we know about the papillomaviruses has been learned by reverse genetics.
The productive functions of the papillomaviruses, including vegetative viral DNA synthesis and the expression of late viral genes, occur only in the fully differentiated squamous epithelial cells of the wart (2). Vegetative viral DNA synthesis has been detected by in situ hybridization techniques only in the squamous epithelial cells of the stratum spinosum and of the granular layer of the epidermis, but not in the basal layer nor in the underlying dermal fibroblasts. Viral capsid protein production and virus assembly occur only in the super stratum spinosum and in the granular layer where the epithelial cells are terminally differentiated. Investigators generally believe that the viral genome is present in the epithelial cells of the basal layer, and it is generally thought that the expression of specific viral genes in the basal layer and in the lower layers of the epidermis is responsible for proliferation of the epithelial cells characteristic of a wart or a papilloma. As the cells of the epidermis migrate upward through the stratum spinosum into the granular layer, they undergo a program of differentiation. The control of papillomavirus late gene expression, therefore, appears tightly linked to the state of differentiation of the squamous epithelial cells. The molecular basis for this control is not yet known.
Human Papillomaviruses
There are no serologic reagents yet available to distinguish the various HPVs. Different HPV types are, therefore, distinguished on the basis of their DNA. To date, over 140 different HPVs have been described, and each is associated with specific clinical lesions. Of these viruses approximately 25–30 are associated with genital track lesions and a subset of these viruses (referred to as the “high risk” HPVs) are associated with a risk for malignant progression (3).
Many of the HPV genomes have been completely or partially sequenced. The genomes are double-stranded closed circular DNAs of approximately 8000 base pairs. All of the open reading frames (ORFs) greater than approximately 400 bases in size are located on one strand and are indicated as arcs outside of the circular genome. These ORFs could encode the viral proteins. All of the detectable viral mRNAs are transcribed from one strand (4). The genomic organization of all the papillomaviruses is quite similar. The known HPV gene functions are listed in Table 1.
TABLE 1.
Papillomavirus Genes and Gene Functions
| Genes | Functions |
|---|---|
| E1 | DNA Replication, Helicase activity, ATP binding, ATPase. |
| E2 | Transcription regulation and auxiliary role in DNA replication. |
| Required for genome maintenance in persistent infections. | |
| E3 | None yet assigned. |
| E4 | Abundant cytoplasmic protein in warts. |
| Disrupts keratin filaments. | |
| E5 | Transformation. Prevents the down-regulation of activated receptors. |
| E6* | Transformation. Binds E6AP and promotes ubiquitylation and degradation of p53. Also binds other cellular proteins including paxillin and IRF3. Activates transcription of cellular telomerase. |
| E7* | Transformation. Binds and inactivates pRB. Interferes with centrosome duplication leading to aneuploidy. |
| E8 | None yet assigned. |
| L1 | Major capsid protein. |
| L2 | Minor capsid protein. |
E6 and E7 are the viral genes that are expressed in HPV positive cancers.
Papillomaviruses and Cancer
The Shope cottontail rabbit papillomavirus (CRPV) was the first papillomavirus to be identified (1). It induces benign cutaneous papillomas in rabbits that can progress to invasive squamous cell carcinoma (5,6). Such malignant progression is more frequent and more rapid in lesions painted with a co-carcinogen such as coal-tar or methylcholanthrene (7). The role of cofactors in malignant progression of papillomavirus-induced lesions is a general one and may be important in those cancers associated with papillomaviruses in humans. Despite important early studies with CRPV establishing the carcinogenic potential of the papillomaviruses, an awareness of the potential role of the papillomaviruses in human cancers did not emerge until the 1970s.
The first evidence linking a HPV with human cancer came from studies in the 1970s of skin cancers in patients with epidermodysplasia verruciformis, a rare X-linked genetic disorder in which patients are covered with flat warts or pityriasis-like lesions caused by unusual HPV types (such as 5, 8, or 17) (8). In about 20% of patients, these lesions progress to skin cancers, usually in sun-exposed areas. These same viruses have also been found associated with skin cancers arising in immunosuppressed patients, such as renal transplant patients.
A broader interest in the role of HPVs and human cancer came in the mid-1980s with the discovery of HPV16 and HPV18 in cervical cancers. These same high risk HPVs were also found associated with the preneoplastic dysplasias recognized as precursor lesions to cervical cancer. A subset of the human papillomaviruses has now been associated with anogenital lesions (9). In general, HPV-6 and HPV-11 have been associated with venereal warts, benign lesions that are less likely to progress to carcinoma. HPV types 16, 18, 31, 33, 35, and 39 are found in the invasive carcinomas, as well as dysplasias and in situ carcinomas, which may progress to malignancy. Approximately 95% of human cervical cancers harbor a high risk HPV, usually integrated into the host chromosome (3).
HPV Expression in Cervical Cancer
In general, HPV DNAs are found as extrachromosomal plasmids in benign precursor dysplastic lesions. In contrast, the DNA is often integrated in malignant lesions. Integration generally occurs in a manner that disrupts the integrity of the E2 gene resulting in the loss of E2 expression (2). Since E2 represses E6 and E7 expression, loss of E2 results in the dysregulated expression of E6 and E7, which have transforming activities. It has been postulated that the deregulated expression of E6 and E7 in cervical carcinomas may play an early role in carcinogenic progression. RNA studies have shown that E6 and E7 are invariably expressed in human cervical cancers (3).
Those HPVs that have been associated with lesions with a high risk for malignant progression (i.e., HPV-16 and HPV-18) are capable of transforming a variety of cells in tissue culture, including primary human keratinocytes. Genetic studies have shown that the E7 gene is sufficient for transformation of established rodent cell lines such as NIH 3T3 cells, and that E7 is sufficient for cooperation with an activated ras gene in transforming primary baby rat kidney cells.
E7 is, however, not sufficient for the immortalization of primary human keratinocytes. Both E6 and E7 are necessary (10,11). Interestingly, cells transformed by E6 and E7 are immortalized but are not tumorigenic in nude mice and, therefore, are not considered fully transformed. The dysregulated expression of the HPV viral oncoproteins (E6 and E7) is not sufficient for the full malignant phenotype. Other genetic events are necessary for the progression of an HPV lesion to a cancer. Expression of E6 and E7 likely contributes to genomic instability providing the opportunity for additional mutations to accumulate within the cell. This would explain why it is estimated that it takes approximately 20 years for cervical cancer to develop following the initial infection.
The HPV E7 Oncoprotein
The E7 protein encoded by the “high risk” HPVs is a small nuclear protein of about 100 amino acids, has been shown to bind zinc, and is phosphorylated by casein kinase II (CK II). Insight into its mechanism of action came initially from the recognition that E7 has functional similarities with the adenovirus (Ad) 12S E1A product (12). Like Ad E1A, E7 can transactivate the Ad E2 promoter, induce DNA synthesis in quiescent cells, and cooperate with an activated ras oncogene to transform primary rodent cells.
In addition to these functional similarities, the HPV-16 E7 shares amino acid sequence similarity with portions of the AdE1A proteins and the SV40 large tumor antigen (TAg). The conserved regions in all of these oncoproteins bind cellular proteins, one of which is the product of the retinoblastoma tumor suppressor gene pRB (13,14).
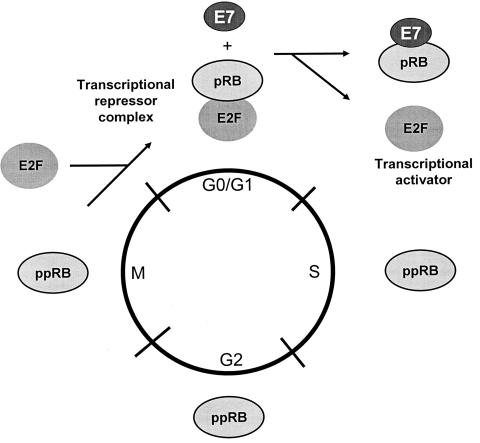
The retinoblastoma protein is a member of a family of cellular proteins that also include p107 and p130 which are homologous in their binding “pockets” for E7, AdE1A, and SV40 TAg. The retinoblastoma protein is the most extensively studied member of this family of proteins. Its phosphorylation state is regulated through the cell cycle, being hypophosphorylated in G0 and G1 and phosphorylated during S, G2, and M. pRB becomes phosphorylated at multiple serine residues by one or more cyclin-dependent kinases (cdk) at the G1/S boundary and remains phosphorylated until late M when it becomes hypophosphorylated again through the action of a specific phosphatase. Since pRB acts as a negative regulator of cell growth at the G1/S border, it follows that the hypophosphorylated form represents the active form with respect to its ability to inhibit cell cycle progression. HPV-16 E7, like SV40 TAg, binds preferentially to the hypophosphorylated form of pRB, consistent with the model that this interaction results in the functional inactivation of pRB and permits progression of the cell into S phase of the cell cycle (15). This property of the viral oncoproteins to complex pRB would appear to account, at least in part, for their ability to induce DNA synthesis. The engagement of pRB by E7 of the cancer associated HPVs results in its degradation (16). Consequently the levels of pRB are reduced in cells expressing E7. Figure 1 depicts a schematic of the cell cycle with pRB being targeted by E7.
Fig. 1.
E7 abrogates the cell cycle regulation mediated by pRB (as well as the related proteins p107 and p130) by complex formation. During the cell cycle, pRB is differentially phosphorylated (indicated as pRB for the under-phosphorylated form and as ppRB for the phosphorylated form). The under-phosphorylated form is detected in G0/G1. This under-phosphorylated form is the active form of pRB, acting as a negative regulator of the cell cycle. During the transition to the S-phase, pRB is phosphorylated by cyclin-dependent kinases (cdk), resulting in the inactivation of its cell cycle regulatory functions. Members of the E2F family of cellular transcription factors are preferentially bound to the under-phosphorylated form of pRB, which functions to repress their function as transcription activators. Phosphorylation of pRB results in the release of the E2F factors allowing them to function as transcriptional activators of cellular genes involved in cellular DNA synthesis and progression into the S phase of the cell cycle. E7 binding to pRB results in its ubiquitylation and degradation, resulting in the consequent release of the E2F family of transcription factors to activate expression of genes necessary to turn on the cellular DNA replication machinery (15).
Genetic studies of HPV-16 E7 have revealed that an intact and high-affinity pRB binding site is necessary for transformation of rodent cells. The amino terminal sequences of E7, which are similar to CR1 and Ad E1A may also effect cellular transformation independent of the pRB binding. Mutational studies have demonstrated the importance of the amino terminus of E7 in its transformation functions. Recent studies have implicated a large cellular protein, p600, as a potential target of E7 through this amino terminal domain (17). In addition to stimulating cell cycle progression through inactivating pRB, E7 has also been shown to cause over-replication of centrosomes and aneuploidy (18).
The HPV E6 Oncoprotein
Although HPV-16 E7 is sufficient for the transformation of established rodent cells such as NIH3T3 cells and for cooperation with ras in the transformation of baby rat kidney cells, E6 together with E7 is required for the efficient immortalization of primary human fibroblasts or keratinocytes. E6 together with E7 can extend the life span of human keratinocytes and lead to the emergence of clones with an immortalized phenotype and resistant to challenges to terminal differentiation. This property is dependent upon the full length E6 gene. How does E6 achieve this function?
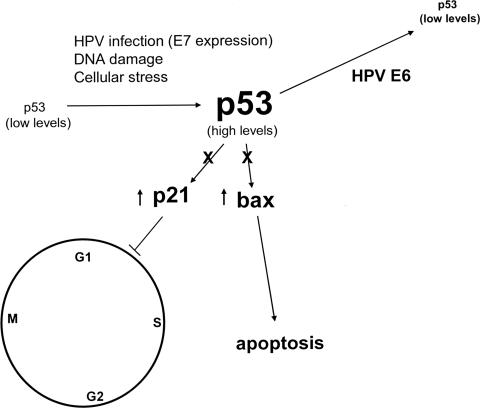
Each of the small DNA tumor viruses encodes mechanisms to complex and functionally inactivate the tumor suppressor gene product p53. Like the SV40 TAg and the Ad5 E1B 55K protein, the E6 proteins encoded by the high risk HPVs can complex with p53 (19). This interaction is specific for the high-risk HPV E6 proteins and not the low risk HPV E6 proteins. p53 is a sequence specific transcriptional transactivator that functions in regulating cell growth and tumor growth suppression. Downstream transcriptional targets of p53 include the cyclin dependent kinase inhibitor p21, as well as several pro-apoptotic genes. By targeting p53, the viral oncoproteins, SV40 TAg, AdE1B 55K and E6 efficiently counter the activation of these cell cycle inhibitor and pro-apoptotic pathways. Shown in Figure 2 is the p53 pathway regulated by the high risk HPV E6 and E7 proteins.
Fig. 2.
The level of p53 in primary cells is generally low. DNA damaging agents, viral infection, and expression of E7 increase the level of p53 through a combination of mechanisms including increased protein stability and translation. Elevated levels of p53 can result in either apoptosis or a cell cycle checkpoint arrest in G1 through the transcriptional activation of pro-apoptotic genes and the cyclin dependent kinase inhibitor, p21. Viral oncoproteins may interfere with this negative growth regulatory function of p53, either by sequestering p53 into a stable, but non-functional complex (such as with SV40 TAg or the Ad5 55 kDa E1B protein) or by ubiquitylation and enhanced proteolysis as observed with the high risk HPV E6 proteins.
Although SV40 TAg, the Ad5 E1B 55-kDa protein, and the high-risk HPV E6 proteins all can complex p53, the consequence of these interactions is different with respect to the stability of the p53 protein. In SV40- and adenovirus-transformed cells, levels of p53 are usually quite high and the half-life of p53 is increased. In contrast, the levels of p53 in HPV-infected cells are low compared to uninfected primary host cells. Unlike TAg and the E1B 55-kDa protein, which may inactivate p53 at least in part by sequestering it into stable complexes, the E6 proteins of the high risk HPVs inactivate p53 by inducing its degradation. This was first demonstrated by in vitro studies that showed that the high-risk HPV E6 proteins could facilitate the rapid degradation of p53 through the ubiquitin-dependent proteolytic system (20). The low-risk HPV E6 proteins, which do not bind detectable amounts of p53, have no effect on p53 stability in vitro.
In addition to the in vitro evidence that the high-risk HPV E6 proteins accelerate the degradation of p53, E6 also appears to affect the stability of intracellular p53 in vivo. Levels of p53 in E6-immortalized cells or in HPV-positive cervical carcinoma cells are, on average, two- to three-fold decreased compared to primary cells. The half-life of p53 is reduced markedly in human keratinocytes expressing E6 (21). In uninfected cells, intracellular p53 levels increase significantly in response to DNA damage induced by gamma irradiation or other agents. The higher levels of p53 are thought to result in growth arrest or apoptosis of the treated cells, which may be a cell defense mechanism that would allow for the DNA damage to be repaired prior to the initiation of a new round of DNA replication. E6-expressing cells, however, do not manifest a p53-mediated cellular response to DNA damage, indicating the ability of E6 to promote the degradation of p53 and prevent the steady level of p53 to rise above a certain threshold level. Under DNA-damaging conditions, the E6-stimulated degradation of p53, therefore, abrogates the negative growth-regulatory effect of p53 and contributes to genomic instability.
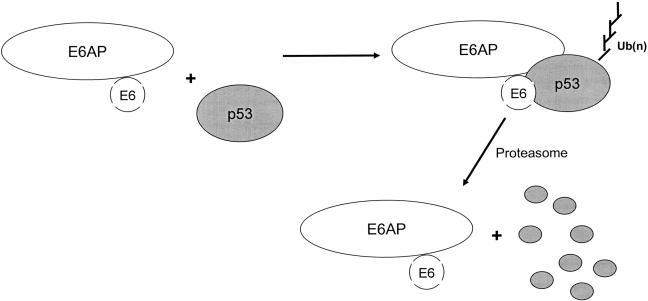
E6 does not bind p53 directly. The binding is mediated by the E6 Associated Protein (E6AP), identified as the cellular protein that allowed the oncogenic E6 protein to bind p53 (Figure 3) (22). Subsequent studies revealed that E6AP is an E3 ubiquitin protein ligase, a class of proteins responsible for substrate recognition by the cellular ubiquitylation machinery (23). E6AP is the founding member of this class of enzymes which are referred to as HECT E3 ligases (24). These are recognized through a conserved C-terminal 350 amino acid domain. Indeed HECT stands for Homologous to the E6 carboxyl terminus. E6AP was the first mammalian E3 enzyme to be identified and characterized. HPV E6 functions by binding to E6AP and directing its ubiquitin protein ligase activity to p53. The biochemical pathway for the E6 and E6AP ubiquitylation of p53 has been determined (Figure 4). In the absence of E6, E6AP does not bind to p53 and is not directly involved in the regulation of p53 protein stability (25). The E6AP gene has also been linked to Angelman syndrome, a human imprinted genetic disorder, characterized by mental retardation, seizures and a number of other neurological characteristics (26). It is postulated that the loss of the E6AP ubiquitin protein ligase activity in specific neurons resulting in a failure of the normal E6AP substrates to be ubiquitylated and degraded may underlie the pathogenesis of this disorder. Several E6-dependent and E6-independent substrates for E6AP have now been identified. In addition the hHR23 and hPLIC families of ubiquitin like proteins have been identified through studies with E6AP as potential links between the ubiquitylation machinery and the proteasome (27).
Fig. 3.
The papillomavirus E6 protein binds the cellular E6AP protein and forms a ternary complex with the p53 tumor suppressor protein. E6AP is an E3 ubiquitin protein ligase that is then redirected to promote the ubiquitylation of p53. Polyubiquitylated p53 is recognized by the proteasome for degradation.
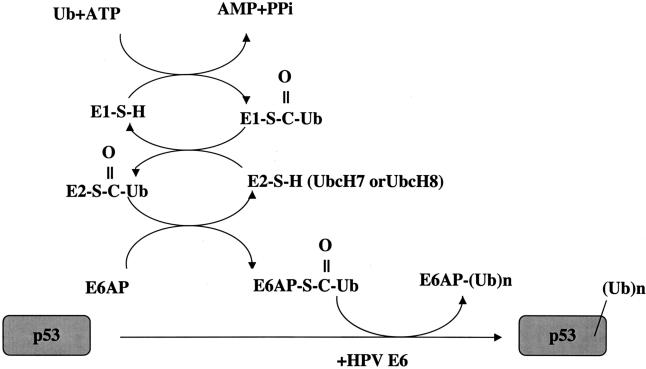
Fig. 4.
HPV E6-mediated ubiquitylation of p53. E6 binds the cellular protein E6AP to functions as an E3 (ubiquitin protein ligase in the ubiquitylation of p53 (23). The ubiquitylation of a protein involves three cellular activities: E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin protein ligase). Ubiquitin is activated in an ATP dependent manner and bound to E1 through a high energy thiolester bond. It is then transferred to the E2 ubiquitin conjugating enzyme, again through a thiolester linkage. Ubiquitin can then be transferred to a cysteine within the HECT domain of E6AP, again as a thiolester linkage (46) through the direct binding of E6AP with UbcH7 or UbcH8 (47). In conjunction with HPV-16 E6, E6AP then recognizes p53 and catalyses the formation of an isopeptide bond between the carboxy-terminal glycine of ubiquitin and a lysine side chain of p53. Noteworthy is that the turnover of E6AP itself is increased in the presence of E6, presumably a result of its increased enzymatic activity mediated by E6.
Although E6 is a small protein, it is pleiotropic, and not all of its transforming activities can be explained through p53. A number of additional cellular targets have now been identified for the high-risk E6 proteins. As an example, the bovine papillomavirus E6 protein has oncogenic properties but does not engage p53. Instead, paxillin, appears to be a relevant target for the BPV-1 E6 transformation function (28,29). Interestingly the high-risk E6 oncoproteins contain a X-(S/T)-X-(V/I/L)-COOH motif at the extreme C-terminus that can mediate the binding of cellular PDZ domain containing proteins. This motif is unique to high-risk HPV E6 proteins and is not present in the E6 proteins of the low risk HPV types. The high risk HPV E6 proteins have been shown to bind a number of cellular PDZ domain containing proteins including hDlg (the human homologue of the Drosophila melanogaster tumor suppressor discs large protein), MUPP1 (the multi-PDZ domain protein) and hScrib (the human homologue of Drosophila scribble) (30–33). PDZ domains are domains that have been recognized in a number of proteins and that are involved in mediating binding to binding to specific peptide regions in proteins. E6 binding to these PDZ-containing proteins results in their E6AP mediated ubiquitylation and proteolysis (33,34). These PDZ containing proteins have been shown to be involved in negatively-regulating cellular proliferation. At least some of the p53 independent transforming activities of the high risk E6 oncoproteins may be linked to their ability to bind and degrade some of these PDZ motif-containing proteins. E6 has also been reported to bind the transcriptional co-activator p300/CBP, a target also of Ad E1A and SV40 large T-antigen (35,36). The physiologic relevance to the transformation functions of the other E6 cellular targets has not yet been elucidated. It is possible that the binding of E6 to some of these targets could contribute to the viral pathogenic functions unrelated to cellular transformation.
E6 has a number of other activities and binding partners. It activates transcription of hTERT, the catalytic subunit of the cellular telomerase, thereby contributing to the immortalization phenotype of a transformed cell (37). A number of models have been proposed involving E6AP, binding of the Myc oncoprotein, and binding of a repressor transcription factor NFX1 (37–42). A mechanistic understanding of how E6 activates TERT, however, is not yet clear. E6 can also bind IRF3, and as such may help the cells evade innate immune responses (43,44).
Discussion and Concluding Comments
Approximately 20% of human cancers are associated with a viral etiology. In addition to HPV, Epstein Barr Virus (EBV) is associated with African Burkitt’s lymphoma, nasopharyngeal carcinoma, some forms of Hodgkins disease, and potentially a number of other cancers. Hepatocellular cancer in man is associated with hepatitis B virus and hepatitis C virus. The only retrovirus to be linked to cancer in humans is HTLV in adult T-cell leukemia. In addition herpesvirus 8 (also known as KSHV) is associated with Kaposi’s sarcoma. For each of these viruses, the best understood from a mechanistic standpoint is HPV. The identification of p53 and pRB as the cellular targets of E6 and E7, helped to establish the etiologic role that HPV plays in human cervical cancer. Vaccination trials using Virus Like Particles (VLPs) are now underway by at least 2 pharmaceutical companies and show extraordinary preliminary results (45). If the vaccine can be taken into under-developed countries, it has the potential to significantly impact the major morbidity and mortality associated with human cervical cancer, the second most common cause of cancer deaths among women worldwide (approximately 250,000 deaths per year).
DISCUSSION
Lemon: Galveston: Peter, a wonderful talk. Can you tell us something about the regulation of E6AP.
Howley: Boston: E6AP is a conserved cellular protein that’s expressed in every cell type. Like many E3 ubiquitin protein ligases, E6AP is more rapidly turned over and degraded when activated. So for instance, in the presence of HPV E6, the half-life is shorter and levels of E6AP are lower due to the fact that E6 stimulates its activity.
Lemon: Is it regulated through the ubiquitin-proteasome pathway also?
Howley: It’s upstream of the proteasome pathway, although E6AP might itself be closely linked to the proteasome through interactions with ubiquitin-like proteins, such as hPLIC. The PLIC proteins are ubiquitin-like proteins that may link the ubiquitylation pathway with the proteasome pathway. So we think that the ubiquitylation pathway and the proteasome may be more hard-wired than was previously thought.
Lemon: But in terms of E6AP abundance and E6AP regulation, is it degraded?
Howley: Yes it is degraded in a ubiquitin- and proteasome-dependent manner.
Lemon: Do we know the E3 ubiquitin ligase for that?
Howley: The presumption is that through its activation, E6AP is auto-ubiquitylated and, therefore, serves as its own E3 ligase.
Hait: New Brunswick: If there’re these limitations on the HPV vaccine, have you thought about, based on these pathways and mechanisms, other approaches to limiting the cervical infection by papillomavirus?
Howley: An aspect of our laboratory work is actually screening for small molecule inhibitors of the E6 degradation of p53 with the rationale that stabilization of p53 in the presence of the proliferation signal provided by E7 would provide a pro-apoptotic signal in the infected cells. Our hope is to identify molecules that could potentially be developed into cancer or antiviral therapeutics.
Tweardy: Houston: You may not know the answer to this, but, in the course of p53 binding to E6AP, does it get sequestered or localized within the cell in a different way than in the absence of that binding?
Howley: That’s a very good question. There are experiments that have been done by Arnold Levine’s laboratory that have looked at the degradation of p53 and have shown that there needs to be shuttling between the nucleus and the cytoplasm for MDM2 degradation of p53, the normal pathway by which p53 is degraded. In those experiments, his laboratory was able to show, using leptomycin B, that shuttling of p53 was also required in the present of E6 and presumably also E6AP for degradation. So, it’s not clear where the degradation of p53 actually occurs, whether it’s cytoplasmic or nuclear, but it is clear that that shuttling between the nucleus and the cytoplasm is required.
REFERENCES
- 1.Shope RE, Hurst EW. Infectious papillomatosis of rabbits; with a note on the histopathology. J Exp Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy DR, Howley PM. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 2231–2264. [Google Scholar]
- 3.zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Phys; 1999. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Howley PM, Lowy DR. Papillomaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 2197–2230. [Google Scholar]
- 5.Rous P, Beard JW. Carcinomatous changes in virus-induced papillomas of rabbits. Proc. Soc. Exp. Bio. Med.; 1935. pp. 578–580. [Google Scholar]
- 6.Rous P, Beard JW. The progression to carcinoma of virus-induced rabbit papillomas (Shope) J. Exp. Med. 1935;62:523–548. doi: 10.1084/jem.62.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rous P, Kidd JG. The carcinogenic effect of a virus upon tarred skin. Science. 1936;83:468–469. doi: 10.1126/science.83.2159.468. [DOI] [PubMed] [Google Scholar]
- 8.Orth G, Favre M, Breitburd F, Croissant O, Jablonska S, Obalek S, Jarzabek CM, Rzesa G. Epidermodysplasia verruciformis: A model for the role of papillomaviruses in human cancer. Cold Spring Harbor Conf Cell Prolif. 1980;7:259–282. [Google Scholar]
- 9.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 10.Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelps WC, Yee CL, Münger K, Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53:539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- 13.Dyson N, Howley PM, Munger K, Harlow E. The human papillomavirus-16 E7 oncoprotein is able to bind the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 14.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Münger K, Basile JR, Duensing S, Eichte A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogne. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 16.Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 17.Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102:11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 20.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 21.Sedman SA, Barbosa MS, Vass WC, Hubbert N, Hass JA, Lowy DR, Schiller JT. The full-length E6 protein of human papillomavirus type 16 has transforming and trans-activating activities and cooperates with E7 to immortalize keratinocytes in culture. J Virol. 1991;65:4860–4866. doi: 10.1128/jvi.65.9.4860-4866.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP: A protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 24.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci U S A. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talis AL, Huibregtse JM, Howley PM. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV) positive and HPV negative cells. J Biol Chem. 1998;273:6439–6445. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL. De novo truncating mutations in E6AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nature Genetics. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 27.Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, Gill G, Howley PM. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Molecular Cell. 2000;6:409–419. doi: 10.1016/s1097-2765(00)00040-x. [DOI] [PubMed] [Google Scholar]
- 28.Tong X, Howley PM. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Nat Acad Sci U S A. 1997;94:4412–4417. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vande Pol SB, Brown MC, Turner CE. Association of bovine papillomavirus type 1 E6 oncoprotein with the focal adhesion protein paxillin through a conserved protein interaction motif. Oncogene. 1998;16:43–52. doi: 10.1038/sj.onc.1201504. [DOI] [PubMed] [Google Scholar]
- 30.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Nat Acad Sci U S A. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74:9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Nat. Acad. Sci. (USA) 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa S, Huibregtse JM. Human scribble (vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18:5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- 35.Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann H, Degenkolbe R, Bernard HU, O’Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–81. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 38.Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci U S A. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75:5559–5566. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronco LV, Karpova AY, Vidal M, Howley PM. The human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirshner JR, Karpova AY, Kops M, Howley PM. Identification of TRAIL as an interferon regulatory factor 3 transcriptional target. J Virol. 2005;79:9320–9324. doi: 10.1128/JVI.79.14.9320-9324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 46.Scheffner M, Nuber U, Huibregtse J. Protein ubiquitination involving an E1–E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S, Kao WH, Howley PM. Physical interaction between specific E2 and HECT E3 enzymes determines functional cooperativity. J Biol Chem. 1997;272:13548–13554. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]