Abstract
The diversity among a set of bacterial strains that have the capacity to degrade total petroleum hydrocarbons (TPH) in soil contaminated with oily sludge (hazardous hydrocarbon waste from oil refineries) was determined. TPH is composed of alkane, aromatics, nitrogen-, sulfur-, and oxygen-containing compound, and asphaltene fractions of crude oil. The 150 bacterial isolates which could degrade TPH were isolated from soil samples obtained from diverse geoclimatic regions of India. All the isolates were biochemically characterized and identified with a Biolog microbial identification system and by 16S rDNA sequencing. Pseudomonas citronellolis predominated among the 150 isolates obtained from six different geographically diverse samplings. Of the isolates, 29 strains of P. citronellolis were selected for evaluating their genetic diversity. This was performed by molecular typing with repetitive sequence (Rep)-based PCR with primer sets ERIC (enterobacterial repetitive intergenic consensus), REP (repetitive extragenic palindromes), and BOXAIR and PCR-based ribotyping. Strain-specific and unique genotypic fingerprints were distinguished by these molecular typing strategies. The 29 strains of P. citronellolis were separated into 12 distinguishable genotypic groups by Rep-PCR and into seven genomic patterns by PCR-based ribotyping. The genetic diversity of the strains was related to the different geoclimatic isolation sites, type of oily sludge, and age of contamination of the sites. These results indicate that a combination of Rep-PCR fingerprinting and PCR-based ribotyping can be used as a high-resolution genomic fingerprinting method for elucidating intraspecies diversity among strains of P. citronellolis.
Oily sludge contains several toxic hydrocarbon constituents, making the sites contaminated by it a major environmental concern because many of the constituents of oily sludge are carcinogenic and potent immunotoxicants (19, 22). It is a complex mixture of total petroleum hydrocarbon (TPH), water, and soil particles. TPH is primarily composed of alkane, aromatics, nitrogen-, sulfur-, and oxygen-containing compounds, and asphaltene fractions. Microbial remediation of a hydrocarbon-contaminated site is accomplished with the help of a diverse group of microorganisms, particularly the indigenous bacteria present in the soil. These microorganisms can degrade a wide range of target constituents present in oily sludge (3, 19). Pseudomonas spp., a group of gram-negative motile rods, have a remarkable ability to degrade a wide range of organic pollutants, including polycyclic aromatic hydrocarbons, halogenated derivatives, and recalcitrant organic residues (12). A large number of Pseudomonas strains capable of degrading polycyclic aromatic hydrocarbons have been isolated from soil and aquifers (12, 13). Pseudomonas citronellolis is one such hydrocarbon-degrading bacterial strain, which metabolizes citronellol (isoprenoid) (4) and can also degrade the toxic hydrocarbon constituents present in oily sludge.
A detailed genetic analysis at the species level gives insight into the variability within a bacterial population and helps to generate evidence of genome plasticity and evolution, which enable bacterial adaptation to various environmental conditions (23). Nucleotide sequences of ribosomal operons have been extensively studied for molecular taxonomy because certain microorganisms tend to exhibit unusual phenotypic characteristics. The nucleotide base sequence of the gene coding for 16S rRNA is considered an important standard for bacterial identification and for deriving phylogenetic relationships among different organisms. The bacterial isolates can be identified down to the genus and species level by amplifying and sequencing the 16S rRNA genes and comparing them to the database of known 16S rRNA sequences. A phylogenetic analysis of the 0.5 kb of 5′-terminal region of 16S ribosomal DNA (rDNA) from cultivated strains has indicated a large bacterial diversity (9).
Genetic diversity among microorganisms has been widely studied by PCR-based techniques, which have opened avenues for the development of new and inventive molecular typing methods (28). Genotypic relationships among microorganisms have been determined by analyzing the genomic DNA with PCR-based methods (21). Repetitive sequence-based PCR (Rep-PCR) has been used successfully to generate DNA fingerprints to distinguish between genetically unrelated isolates and closely related bacterial strains (2). It involves the use of primers based on the short repetitive elements derived from highly conserved palindromic inverted repeat regions dispersed throughout the prokaryotic kingdom (11, 15). Amplification of the regions between adjacent repetitive extragenic elements gives strain-specific DNA fingerprints (2). Primer sets based on the repetitive elements present in the bacterial genome, ERIC (enterobacterial repetitive intergenic consensus), REP (repetitive extragenic palindromes), and BOX, yield genomic fingerprints specific to pathovars and strains of gram-negative bacteria (6, 18, 25). The inter-REP and inter-ERIC profiles are specific for bacterial strains within a species (1, 30).
rRNA genes are ubiquitous in living organism (28) and therefore, the rRNA operon forms an attractive locus for molecular typing by PCR-based ribotyping. rRNA operons are present in multiple copies, and amplimers generated by PCR ribotyping indicate the intraspecies genetic diversity in the number and structure of ribosomal operons (28). The sequences of multicopy rRNA genes are nearly identical and homogeneous (7). They are separated by spacer regions which exhibit a large variation in sequence and length at the genus and species levels (1, 10).
The aim of the present study was to evaluate intraspecies genetic diversity among TPH-degrading bacterial strains isolated from soil contaminated with different types of oily sludge. The sampling sites were located in different geoclimatic regions, and the duration of contamination at the sites also varied.
MATERIALS AND METHODS
Isolation of TPH-degrading bacteria and their functional characterization.
The bacterial strains were isolated by the enrichment culture technique from soil samples contaminated with oily sludge procured from oil refineries and oil exploration sites situated in different geoclimatic locations in India (Table 1). The geoclimatic location and the age of contamination at these sites are listed in Table 1. A 5-g sample of soil was inoculated into 100 ml of minimal salt medium (MSM) (16) containing steam-sterilized oily sludge (1%, wt/vol) as the carbon source and incubated at 37°C on a rotary shaker (200 rpm) for 4 days. After five cycles of such enrichment, 1 ml of the culture was diluted up to 108-fold, and 100 μl of all the dilutions was plated on MSM agar plates with oily sludge (1%, wt/vol) and incubated at 37°C. The bacterial colonies obtained were further purified on MSM agar plates containing oily sludge (1%, wt/vol) as the carbon source. The degradation (qualitative and quantitative) of the various fractions of TPH (aliphatic, aromatic, and asphaltene) was estimated as described elsewhere (17). The strains showing extensive TPH degradation were routinely cultured on MSM containing oily sludge (1%, wt/vol) and stored as frozen stock cultures in 25% glycerol at −70°C.
TABLE 1.
Geographic locations of oily sludge-contaminated sampling sites
| Sampling site | Geographic location (latitude and longitude) | Duration of contamination (yr) | Total no. of isolates | No. of isolates identified as P. citronellolis |
|---|---|---|---|---|
| Mathura refinery | Northern India (27° 26′ N, 77° 43′ E) | 30 | 27 | 5 |
| Barauni refinery | Eastern India (25° 28′ N, 85° 59′ E) | 27 | 30 | 3 |
| Haldia refinery | Eastern India (22° 00′ N, 88° 05′ E) | 32 | 21 | 4 |
| Baroda refinery | Western India (22° 16′ N, 73° 14′ E) | 25 | 25 | 7 |
| Jorhat | Northeastern India (26° 40′ N, 95° 35′ E) | 15 | 32 | 5 |
| Digboi refinery | Northeastern India (27° 15′ N, 95° 15′ E) | 100 | 15 | 5 |
Characterization of bacterial strains.
All selected bacterial strains were Gram stained. Microscopic examination (for the shape and size of the bacterial cell) and oxidase, and catalase tests were performed. The isolated strains were characterized by determining their substrate utilization profiles with Biolog GN plates (Biolog Inc., Hayward, Calif.). For this determination, the isolates were grown on Luria-Bertani broth (LB), harvested by centrifugation at 10,000 × g for 10 min, and resuspended in sterile NaCl solution (0.85%). The Biolog GN microplates were inoculated with 150 μl of the cell suspensions that had been adjusted to a density of ≈3 × 10 8 cells per ml by comparison with the turbidity standards supplied by the manufacturer. These plates were then incubated at 30°C for 24 h, omitting the 4-h measurement recommended by Homes et al. (8). The color development in the microplate wells was interpreted visually as positive, negative, or borderline when it was impossible to differentiate positive from negative. The readings were also entered in the Biolog microlog GN release 1 4.01B database to provide identification, which was acknowledged when the similarity index used by Biolog was 5.0 or more (8).
Isolation of genomic DNA.
A loopful of bacterial cells grown on LB was suspended in 245 μl of 0.1 M TE (Tris-EDTA). The cell suspension was incubated with 5 μl (50 mg/ml) of lysozyme solution at 56°C for 45 min. The following reagents were then added: 196.2 μl of 0.1 M TE, 5 μl of dithiothreitol (1 M), 20 μl of EDTA (0.25 M), 25 μl of sodium dodecyl sulfate (10%), and 3.8 μl of proteinase K (20 mg/ml). The reaction mixture was incubated at 37°C for 1 h. Next 500 μl of Prepman solution (PE Applied Biosystems. Foster City, Calif.) was added and incubated at 56°C for 30 min. The reaction mixture was finally heated at 100°C for 8 min and centrifuged at 8,500 × g for 2 min. The supernatant obtained was diluted (1:10) with deionized water and used as the template DNA.
16S rDNA sequencing.
Partial and full gene sequencing of 16S rRNA was performed. The 500-bp 16S rDNA sequences were amplified with Microseq 500 16S rDNA PCR module (PE Applied Biosystems). The reaction mixture (50 μl) contained 25 μl of diluted genomic DNA and 25 μl of the ready reaction mixture. Cycling conditions for the amplification reaction were initial denaturation at 95°C for 10 min, 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s (rapid thermal ramp of 1°C/s between steps), final extension at 72°C for 10 min, and lastly a 4°C soak. The PCR products were purified with a Microcon PCR centrifugal filter device (Millipore Corp. Bedford, Mass.) according to the manufacturer's protocol. The purified DNA was recovered in 25 μl of deionized water. The amplified 16S rDNA was subjected to cycle sequencing with the Microseq 500 16S rDNA sequencing module.
The reaction mixture (20 μl) contained 3 μl of purified PCR product, 4 μl of deionized water, and 13 μl of sequencing reaction mixture (forward and reverse sequencing mixture in separate reactions). The cycling conditions were 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min (rapid thermal ramp of 1°C/s between steps), followed by a 4°C soak. Similarly the 16S rRNA full gene sequencing was also performed with the Microseq 16S rRNA full gene kit (PE Applied Biosystems). The amplification and sequencing reaction was performed according to the manufacturer's protocol. The cycle-sequenced DNA (partial and full gene) was precipitated with ethanol (95%) and 3 M sodium acetate (pH 4.6) and finally analyzed with an ABI Prism 310 genetic analyzer (PE Applied Biosystems).
To identify unknown bacterial isolates, the 16S rDNA sequences obtained were subjected to Blast search with Microseq identification and Microseq analysis software version 1.40, Microseq 16S rDNA sequence databases version 1.01 (PE Applied Biosystems), and Blast search from the NCBI database.
Rep-PCR-based DNA fingerprinting.
Template DNA was prepared according to the protocol given above. A quantity of 2 μl of supernatant was used as the template DNA for the amplification reactions. The primer sets and the amplification cycling conditions for the Rep-PCRs are listed in Table 2. The reaction mixture (15 μl) consisted of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.01% (wt/vol) gelatin, 0.2 mM each dATP, dCTP, dGTP, and dTTP, 1 μM each primer, and 0.45 U of Taq polymerase.
TABLE 2.
Primer sequences and amplification cycling conditions for the different PCR-based genomic DNA fingerprints
| Primer set | Primer | Nucleotide sequence | References | Amplification cycling conditions |
|---|---|---|---|---|
| ERIC | ERIC-1R ERIC-2 | 5′-ATG TAA GCT CCT GGG GAT TCA C-3′ 5′-AAG TAA GTG ACT GGG GTG AGC G-3′ | 1, 19, 30 | Initial denaturation 95°C for 2 min, 92°C for 30 s; 35 cycles of 92°C for 30 s, 50°C for 80 s, and 68°C for 200 s, final extension at 68°C for 8 min, final 4°C soak |
| REP | REP 1R DT REP 2-DT | 5′-III ICG ICG ICA TCI GGC-3′ 5′-ICG ICT TAT CIG GGC TAC-3′ | 1, 30 | Initial denaturation at 95°C for 2 min, 92°C for 30 s, 35 cycles of 92°C for 30 s, 38°C for 80 s, and 68°C for 200 s, final extension at 68°C for 8 min, final 4°C soak |
| BOXAIR | 5′-CTA CGG CAA GGC GAC GCT GAC G-3′ | 2, 24 | Initial denaturation 95°C for 2 min, 94°C for 3 s; 30 cycles of 92°C for 30 s, 50°C for 1 min, and 65°C for 8 min; final extension at 68°C for 8 min, final 4°C soak |
PCR-based ribotyping.
The template DNA and the reaction mixture (15 μl) were prepared according to the protocol given above. The ribotyping primers were GIRRN (5′ GAA GTC GTA ACA AGG 3′) and LIRRN (5′ CAA GGC ATC CAC CGT 3′) (1, 10). The cycling conditions for amplification were initial denaturation at 95°C for 2 min, 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 6 min, final extension at 72°C for 8 min, and finally a 4°C soak.
All amplification reactions were processed in a Geneamp PCR system 2400 (Perkin Elmer). The primers and the Taq polymerase were obtained from Gibco-BRL Life Technologies. The PCR assays were terminated with 1 μl of gel loading solution (15% Ficoll, 0.25% bromophenol blue, and 0.25% xylene cyanol) and resolved on 1.5% agarose gel (with 0.6 μg of ethidium bromide per ml) in 1× TAE (Tris-acetate-EDTA) buffer for 5 to 6 h at 140 V and 25°C.
The gel profiles were visualized (photographed) with UVI gel documentation (UVItec, Cambridge, United Kingdom) and analyzed with UVI photo version 99 and UVI band/map version 99 software (UVItec). Whenever a distinct PCR profile was observed in terms of the number and position of a clearly visible band, the corresponding strains were given a unique number or letter designation. Chemicals used for all the above reactions were of molecular biology grade and obtained from Sigma Chemicals.
Standard bacterial strains.
Four standard bacterial strains, Pseudomonas putida MTCC 978, Pseudomonas citronellolis MTCC 1191, Pseudomonas aeruginosa MTCC 1034, and Pseudomonas aeruginosa MTCC 2642, were obtained from the Microbial Type Culture Collection, situated at the Institute of Microbial Technology, Chandigarh, India. These strains were grown in LB, and the Rep-PCR technique was used to differentiate the Pseudomonas species.
Nucleotide sequence accession numbers.
Following are the accession numbers of the 16S rDNA nucleotide sequences of the strains submitted to GenBank:TERIDB1, AF489934; TERIDB2, AF489935; TERIDB3, AF4889936; TERIDB4, AF489937; TERIDB5, AF489938; TERIDB6, AF489939; TERIDB7, AF489940; TERIDB8, AF489941; TERIDB9, AF489942; TERIDB10, AF489943; TERIDB11, AF489944; TERIDB12, AF489945; AF489946, TERIDB13; AF489947, TERIDB14; TERIDB15, AF489948; TERIDB16, AF489949; TERIDB17, AF489950; TERIDB18, AY0453788; TERIDB19, AY0453792; TERIDB20, AF492391; TERIDB21, AF492392; TERIDB22, AF492393; TERIDB23, AF492394; TERIDB24, AF492395; TERIDB25, AF492396; TERIDB26, AF492397; TERIDB27, AF492398; TERIDB28, AF492399; TERIDB29, AY090561; TERIDB3, AF530069; and TERIDB9, AF530070.
RESULTS
Isolation and identification of TPH-degrading bacterial isolates.
A total of 150 TPH-degrading bacterial isolates were isolated from soil samples contaminated with oily sludge procured from six different geoclimatically diverse sampling sites. The age of contamination at these sites varied from 15 to 100 years (Table 1). Both gram-positive and gram-negative bacterial strains were obtained.
The following strains were identified based on their ability to grow on a variety of substrates in the Biolog GN and GP plates and on partial 16S rDNA sequences. They included Yokenella spp., Alcaligenes spp., Pseudomonas spp., Roseomonas spp., Stenotrophomonas spp., Acinetobacter spp., Flavomonas spp., Cornybacterium spp., Streptococcus spp., Providencia spp., Sphingobacterium spp., Capnocytophaga spp., Moraxella spp., and Bacillus spp. The identification of these strains was randomly confirmed by full-gene 16S rRNA sequencing. Of the total isolates, 29 were Pseudomonas citronellolis. They were obtained from different sampling sites (Table 1). The alignment of the 16S rDNA sequences of the isolates with the Microseq microbial identification and analysis software and nucleotide-nucleotide Blast (BlastN) search of NCBI database recorded up to 97% to 99% similarity.
Functional characters of the strains.
The 29 selected strains of P citronellolis isolated from different oily sludge-contaminated soil samples were functionally characterized. The strains showed TPH degradation in a range from 65% to 96% (Table 3). They degraded the aliphatic fractions of TPH more exclusively than the aromatic and asphaltene fractions (Table 3) because the aliphatic fractions are less toxic and are easily degradable fractions of TPH.
TABLE 3.
Degradation of TPH and its various fractions present in oily sludgea
| Genotypic group | Strain | Degradation (%)
|
|||
|---|---|---|---|---|---|
| TPH | Aliphatic fraction | Aromatic fraction | NSO and asphaltene fraction | ||
| I | TERIDB2 | 85 | 60 | 20 | 5 |
| TERIDB3 | 88 | 69 | 15 | 4 | |
| TERIDB4 | 86 | 61 | 20 | 5 | |
| TERIDB10 | 84 | 59 | 20 | 5 | |
| TERIDB14 | 88 | 68 | 19 | 1 | |
| II | TERIDB8 | 65 | 35 | 25 | 5 |
| III | TERIDB11 | 78 | 44 | 29 | 5 |
| TERIDB12 | 76 | 48 | 25 | 3 | |
| TERIDB13 | 74 | 39 | 28 | 7 | |
| IV | TERIDB5 | 82 | 50 | 29 | 3 |
| TERIDB9 | 84 | 43 | 38 | 3 | |
| V | TERIDB6 | 79 | 40 | 37 | 2 |
| TERIDB7 | 75 | 46 | 26 | 3 | |
| TERIDB22 | 76 | 50 | 24 | 2 | |
| VI | TERIDB25 | 73 | 58 | 12 | 3 |
| VII | TERIDB15 | 67 | 38 | 28 | 1 |
| TERIDB16 | 65 | 40 | 22 | 3 | |
| VIII | TERIDB21 | 68 | 49 | 14 | 5 |
| TERIDB23 | 69 | 51 | 15 | 3 | |
| IX | TERIDB18 | 74 | 45 | 25 | 4 |
| TERIDB20 | 79 | 50 | 25 | 4 | |
| TERIDB26 | 71 | 40 | 28 | 3 | |
| TERIDB27 | 79 | 53 | 20 | 6 | |
| X | TERIDB19 | 63 | 45 | 16 | 2 |
| XI | TERIDB24 | 96 | 68 | 25 | 3 |
| XII | TERIDB27 | 74 | 53 | 16 | 5 |
| TERIDB28 | 75 | 50 | 20 | 5 | |
| TERIDB29 | 79 | 55 | 22 | 2 | |
Values are means of six replicates. NSO, nitrogen-, sulfur-, and oxygen-containing compounds.
Rep-PCR.
Rep-PCR for the 29 strains of P. citronellolis yielded complex genomic fingerprints consisting of 8 to 12 amplified bands of varying intensity. The amplified banding profiles were clearly distinguishable, with sizes ranging from 9,000 bp to 100 bp. Visual inspection of the DNA fingerprints followed by analysis with UVI photo version 99 and UVI band/map version 99 software enabled the identification of novel genotypes. Of the 29 strains of P. citronellolis, ERIC-PCR produced 10 distinct patterns, REP-PCR produced 11 patterns, and BOXAIR-PCR produced five patterns. Among the three primer sets studied, REP-PCR produced the most complex amplified banding patterns, which reflected a high degree of intraspecies diversity among the P citronellolis strains isolated from different oily sludge-contaminated soil samples.
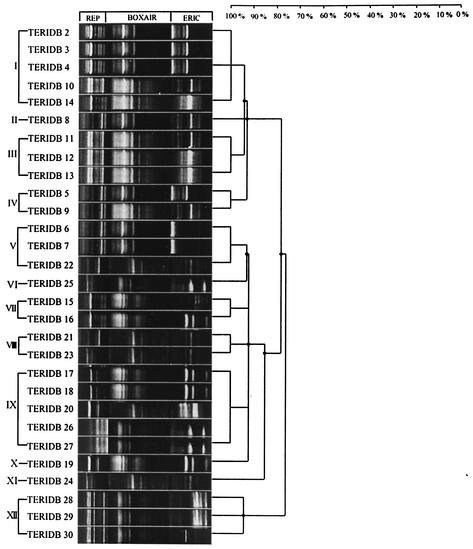
A dendrogram that was calculated with Jaccard's similarity coefficients with unweighted pair group method clustering (UPGMA) for the combination of Rep-PCR results (with the ERIC, REP, and BOXAIR primer sets) segregated the 29 strains into 12 unique genotypic groups (Fig. 1). The isolates from the same sampling sites that displayed identical genomic fingerprints are listed together (Table 4). The DNA fingerprints with the three primer sets were not identical. The isolates TERIDB10, TERIDB11, TERIDB13, and TERIDB14 from Jorhat were distinguished by the REP but not by the ERIC primer set. Similarly, the strains TERIDB15, TERIDB16, TERIDB17, TERIDB18, TERIDB20, and TERIDB21 (isolated from a Baroda refinery) and TERIDB22, TERIDB23, TERIDB24, TERIDB25, and TERIDB26 (isolated from a Digboi refinery) were distinguished by the ERIC and not by the REP primer set (Fig. 1).
FIG. 1.
Cluster analysis of genomic fingerprint patterns of 29 TPH-degrading strains of P. citronellolis. The genomic fingerprints were generated by PCR amplification of the whole-cell suspension with the Rep (ERIC, REP, and BOXAIR) primer sets. The UPGMA algorithm was applied to the similarity matrix with at and above mean Jaccard coefficient (standard deviation) value of 75%. The 29 strains were delineated into 12 genotypic groups.
TABLE 4.
Isolation sites of P. citronellolis strains identified by 16S rDNA sequencing and their genotypic groups
| Strain no. | Isolation site | ERIC type | REP type | BOX type | Ribotype pattern |
|---|---|---|---|---|---|
| TERIDB2 | Barauni refinery | A | RE 1 | 1 | R1 |
| TERIDB3 | Barauni refinery | A | RE 1 | 1 | R1 |
| TERIDB4 | Barauni refinery | B | RE 1 | 1 | R1 |
| TERIDB5 | Mathura refinery | B | RE 2 | 1 | R1 |
| TERIDB6 | Mathura refinery | B | RE 2 | 1 | R1 |
| TERIDB7 | Mathura refinery | B | RE 3 | 1 | R1 |
| TERIDB8 | Mathura refinery | B | RE 3 | 2 | R2 |
| TERIDB9 | Mathura refinery | C | RE 3 | 2 | R2 |
| TERIDB10 | Jorhat | C | RE 3 | 2 | R2 |
| TERIDB11 | Jorhat | C | RE 4 | 2 | R2 |
| TERIDB12 | Jorhat | C | RE 4 | 2 | R2 |
| TERIDB13 | Jorhat | D | RE 4 | 2 | R3 |
| TERIDB14 | Jorhat | D | RE 5 | 3 | R3 |
| TERIDB15 | Baroda refinery | D | RE 6 | 3 | R4 |
| TERIDB16 | Baroda refinery | E | RE 6 | 3 | R4 |
| TERIDB17 | Baroda refinery | E | RE 6 | 3 | R4 |
| TERIDB18 | Baroda refinery | F | RE 6 | 3 | R4 |
| TERIDB19 | Baroda refinery | F | RE 7 | 4 | R5 |
| TERIDB20 | Baroda refinery | G | RE 8 | 4 | R6 |
| TERIDB21 | Baroda refinery | G | RE 8 | 4 | R6 |
| TERIDB22 | Digboi refinery | H | RE 8 | 4 | R6 |
| TERIDB23 | Digboi refinery | H | RE 8 | 4 | R6 |
| TERIDB24 | Digboi refinery | H | RE 9 | 5 | R7 |
| TERIDB25 | Digboi refinery | I | RE 9 | 5 | R7 |
| TERIDB26 | Digboi refinery | I | RE 9 | 5 | R7 |
| TERIDB27 | Haldia refinery | J | RE 10 | 5 | R7 |
| TERIDB28 | Haldia refinery | J | RE 10 | 5 | R7 |
| TERIDB29 | Haldia refinery | J | RE 11 | 5 | R7 |
| TERIDB30 | Haldia refinery | J | RE 11 | 5 | R7 |
Most of the groups obtained by UPGMA cluster analysis were from individual geoclimatic sampling locations except genotypic groups I and IX. Genotypic group I included isolates TERIDB2, TERIDB3, and TERIDB4, isolated from the Barauni refinery, and TERIDB10 and TERIDB14, isolated from the Jorhat oil exploration site (Fig. 1). In group IX, genotypically similar strains isolated from three different sampling locations, Baroda, Digboi, and Haldia, were present (Fig. 1). Multiple genotypic groups were obtained from the Digboi refinery, which included TERIDB22 (genotypic group V), TERIDB23 (genotypic group VIII), TERIDB24 (genotypic group XI), and TERIDB25 (genotypic group VI) (Fig. 1). However, BOXAIR-PCR did not produce many distinguishable fingerprints of these isolates.
The REP-PCR genomic fingerprint of the different species of Pseudomonas produced distinguishable patterns, differentiating these strains from each other (Fig. 2).
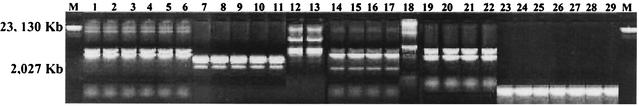
FIG. 2.
REP-PCR genomic fingerprints of the different Pseudomonas species. Lane M is size standards (100-bp ladder). Lane 1, Pseudomonas putida MTCC 978. Lane 2, Pseudomonas citronellolis MTCC 1191. Lane 3, Pseudomonas aeruginosa MTCC 1034. Lane 4, Pseudomonas aeruginosa MTCC 2642.
PCR-based ribotyping.
PCR-based ribotyping characterization of the selected 29 strains of P. citronellolis led to recognition of 7 distinguishable genomic patterns (Table 4). The ribotype patterns of all the strains showed intensely staining multiple amplimers of 9,000 bp to 1,500 bp (Fig. 3). Most of the strains showed more than two amplicons except strains TERIDB24, TERIDB25, and TERIDB26 from Digboi and TERIDB27, TERIDB28, TERIDB29, and TERIDB30 from Haldia, which showed one amplified fragment. This indicated polymorphism in the rRNA operons among the strains of P. citronellolis. The ribotype patterns were not distinct for a particular geographical location except for ribotype patterns R4 of the strains from Baroda.
FIG. 3.
PCR-based ribotype patterns of 29 strains of P. citronellolis. The gel profiles have been arranged so that in general strains of similar ribotype patterns are grouped together. Lane M contained an external size standard, HindIII-digested λ DNA, and lanes 2 to 30 are P. citronellolis strains TERIDB2, TERIDB3, TERIDB4, TERIDB5, TERIDB6, TERIDB7, TERIDB8, TERIDB9, TERIDB10, TERIDB11, TERIDB12, TERIDB13, TERIDB14, TERIDB15, TERIDB16, TERIDB17, TERIDB18, TERIDB19, TERIDB20, TERIDB21, TERIDB22, TERIDB23, TERIDB24, TERIDB25, TERIDB26, TERIDB27, TERIDB28, TERIDB29, and TERIDB30, respectively.
DISCUSSION
The present study was undertaken to develop a molecular framework for evaluation of the intraspecies genetic diversity among TPH-degrading bacterial strains of P. citronellolis. These strains were isolated from oily sludge-contaminated soil samples obtained from six different geoclimatically diverse regions in India. The gene sequences encoding the small ribosomal subunit (16S) rRNA are strictly conserved and are nearly identical for almost all species, and therefore, direct sequencing of the 16S rDNA was performed to differentiate among environmental bacterial strains isolated for the present study (20). Previous reports indicated that identification based on 16S rDNA sequencing has the highest concordance by conventional testing and is superior to identification by analysis of cellular fatty acid profiles and carbon utilization (14, 26). The highly conserved regions of the 16S rDNA sequences have been used to study the relationships among distant taxa (10). DNA sequencing and phylogenetic analysis of rRNA genes obtained by PCR amplification of genomic DNA extracted from environmental samples have also led to detection of diverse unknown microorganisms (31). The variable regions of 16S rDNA have been used mainly for the differentiation of genera and species (10).
In the present study, unique Rep-PCR genotypic fingerprints of different P. citronellolis strains isolated from oily sludge-contaminated soil samples were found that had not been investigated previously. With Rep-PCR, 29 strains of P. citronellolis were separated into 12 genotypic groups distributed among seven distinguishable ribotype patterns. Gardener et al. (5) previously characterized strains of Pseudomonas isolated from eight different soils taken from four different geographic location and elucidated 17 genotypes distributed among three amplified ribosomal DNA restriction analysis groups. In the present study, the strains from the Mathura refinery were separable into genotypic groups II and IV. Similarly, the strains isolated from the Jorhat oil exploration site were distinguishable as genotypic group III, and the strains isolated from the Baroda refinery were distinguishable as genotypic group XI. These genotypic distinctions may reflect the locations of the three sampling sites in different geoclimatic regions of India. The fact that the soil at these three sites was contaminated with different types of oily sludge may also be a contributing factor.
However, the strains obtained from Digboi showed various Rep-PCR genotypes and were clustered in different genotypic groups (genotypic groups V, VI, VIII, and XI). This could be because the soil at the Digboi refinery was contaminated with oily sludge for nearly 100 years, allowing genotypic diversification. In the present work, the REP primer set generated a greater number of distinct genotypic patterns for P. citronellolis. The primer specific for the BOX element was less effective than were the REP and ERIC primer sets in obtaining fingerprints for grouping the strains effectively.
The Rep-PCR genomic fingerprints showed certain intense amplimers, which made the interpretation of the fingerprints relatively simple. These amplimers can serve as distinct molecular markers for P. citronellolis to track them in the environment (24). The banding pattern of the DNA fingerprints of the strains showed many common amplified bands, indicating that the strains were closely related to each other. Visual inspection of the fingerprint pattern indicated that the majority of the amplified bands comigrated. Genotypic analyses are less subject to environmental effects than phenotypic analyses, and the Rep-PCR method was used to elucidate the intraspecies diversity among the different strains of P. citronellolis (2). In addition to genomic fingerprinting, PCR methodologies employing the REP and ERIC sequences as primers binding sites can be used to study the distribution of repetitive sequences in various genomes (27). Advantages of repetitive element-based PCR are its simplicity, accuracy, and speed, which are desirable for high-throughput analysis (2, 29).
PCR-based ribotyping can elucidate the intraspecies polymorphism in the 16S-23S ribosomal spacer region among P. citronellolis strains. The ribotype patterns of the P. citronellolis strains showed multiple amplicons that strongly indicated polymorphism of the rRNA spacer region. Such polymorphism has been reported previously for other bacterial species (1). The ribotype patterns of the P. citronellolis strains also showed differences in the intensity of the amplimers, which suggested the amplification of several rRNA operons (28). The amplification products obtained were of two types, intense, which was reproducible, and weak or variable (10). Jensen et al. (11) suggest that a single species can show a variety of spacer amplification products.
Acknowledgments
We thank R. K. Pachauri, Director General, TERI, and T. P. Singh, Director, Teri SAS, for providing the infrastructure to carry out the present study. We thank Neena Mata for typing the manuscript.
We thank the Department of Biotechnology, Government of India, for funding this research and the Council of Scientific and Industrial Research, New Delhi, India, for providing fellowships to two of us during the work.
REFERENCES
- 1.Appuhamy, S., R. Parton, J. G. Coote, and H. A. Gibbs. 1997. Genomic fingerprinting of Haemophilus somnus by a combination of PCR methods. J. Clin. Microbiol. 35:288-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dombek, P. E., L. A. K. Johnson, S. T. Zimmerley, and M. J. Sadowsky. 2000. Use of repetitive DNA sequences and PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 66:2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson, M., G. Dalhammar, and A.-K. Borg-Karlson. 1999. Aerobic degradation of hydrocarbon mixture in natural uncontaminated potting soil by indigenous microorganisms at 20°C and 6°C. Appl. Microbiol. Biotechnol. 51:532-535. [DOI] [PubMed] [Google Scholar]
- 4.Fall, R. R., J. L. Brown, and T. L. Schaeffer. 1979. Enzyme recruitment allows the biodegradation of recalcitrant-branched hydrocarbons by Pseudomonas citronellolis. Appl. Environ. Microbiol. 38:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardener, B. B. M., K. L. Schroeder, S. E. Kalloger, J. M. RaaijMakers L. S. Thomashow, and D. M. Weller. 2000. Genotypic and phenotypic diversity of phlD-containing Pseudomonas strains isolated from the rhizosphere of wheat. Appl. Environ. Microbiol. 66:1939-1946. [DOI] [PMC free article] [PubMed]
- 6.Giselle, N., and K. Lindstorm. 1994. Use of repetitive sequences and the polymerase chain reaction to fingerprint the genomic DNA of Rhizobium galegae strains and to identify the DNA obtained by sonicating the liquid cultures and the root nodules. Syst. Appl. Microbiol. 17:265-273. [Google Scholar]
- 7.Hillis, D. M., C. Moritz, C. A. Porter, and R. J. Baker. 1991. Evidence of biased gene conversion in concerted evolution of ribosomal DNA. Science 251:308-310. [DOI] [PubMed] [Google Scholar]
- 8.Homes, B., M. Costas, M. Ganner, S. L. W. On, and M. Stevens. 1994. Evaluation of the biolog system for identification of gram-negative bacteria of clinical importance. J. Clin. Microbiol. 32:1970-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter-Cevera, J. C. 1998. The value of microbial diversity. Curr. Opin. Microbiol. 1:278-285. [DOI] [PubMed] [Google Scholar]
- 10.Jensen, M. A., J. A. Webster, and N. Straus. 1993. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphism. Appl. Environ. Microbiol. 59:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jersek, B., P. Gilot, M. Gubina, N. Klun, J. Mehle, E. Tcherneva, N. Rijpens, and L. Herman. 1999. Typing of Listeria monocytogenes strains based on repetitive element sequences-based PCR. J. Clin. Microbiol. 37:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, K., S. Anderson, and C. S. Jacobson. 1996. Phenotypic and genotypic characterization of phenanthrene-degrading fluorescent Pseudomonas biovars. Appl. Environ. Microbiol. 62:3818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyohara, H., N. Takizawa, and K. Nagao. 1992. Natural distribution of bacteria metabolizing many kinds of polyaromatic hydrocarbons. J. Ferment. Bioeng. 74:49-51. [Google Scholar]
- 14.Kolbert, P. C., and D. H. Persing. 1999. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr. Opin. Microbiol. 2:299-305. [DOI] [PubMed] [Google Scholar]
- 15.Laguerre, G., P. Mavingui, M. R. Allard, M. P. Charnay, P. Louvrier, S. I. Mazurier. L. Rigottier-Gois, and N. Amarger. 1996. Typing of rhizobia by PCR and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl. Environ. Microbiol. 62:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal, B., and S. Khanna. 1996. Mineralization of [14C]octacosane by Acinetobacter calcoaceticus S30. Can. J. Microbiol. 42:1225-1231. [Google Scholar]
- 17.Lal, B., and S. Khanna. 1996. Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenes odorans. J. Appl. Bacteriol. 81:335-362. [DOI] [PubMed] [Google Scholar]
- 18.Louws, F. J., D. W. Full bright, C. T. Stephens, and F. J. De Brujin. 1994. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl. Environ. Microbiol. 60:2286-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra, S., J. Jyot, R. C. Kuhad, and B. Lal. 2001. Evaluation of inoculum addition to stimulate in situ bioremediation of oily sludge-contaminated soil. Appl. Environ. Microbiol. 67:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman, P. 1997. A molecular view of microbial diversity and the biosphere. Science 276:734-740. [DOI] [PubMed] [Google Scholar]
- 21.Pooler, M. R., D. F. Ritchie, and J. S. Hartung. 1996. Genetic relationships among strains of Xanthomonas fragariae based on random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR and enterobacterial repetitive intergenic consensus PCR data and generation of multiplexed PCR primers useful for identification of this phytopathogen. Appl. Environ. Microbiol. 62:3121-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Propst, T. L., R. L. Lochmiller, C. W. Qualis, Jr., and K. McBee. 1999. In situ (mesocosm) assessment of immunotoxicity risks to small mammals inhabiting petrochemical waste sites. Chemosphere 38:1049-1067. [DOI] [PubMed] [Google Scholar]
- 23.Renders, N., U. Romling, H. Verbrugh, and A. version Belkum. 1996. Comparative typing of Pseudomonas aeruginosa by random amplification of polymorphic DNA and pulsed field gel electrophoresis of DNA macrorestriction fragments. J. Clin. Microbiol. 34:3190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowsky, M. J., L. L. Kinkel, J. H. Bowers, and J. L. Schottel. 1996. Use of repetitive intergenic DNA sequences to classify pathogenic and disease suppressive Streptomyces strains. Appl. Environ. Microbiol. 62:3489-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, J., J, L. C. Offord, M. Holderness, and G. S. Saddler. 1995. Genetic diversity of Burkholderia solanacearum (synonym Pseudomonas solanacearum) race 3 in Kenya. Appl. Environ. Microbiol. 61:4263-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rossum, D., F. P. Schuurmans, M. Gillis, A. Muyotcha, H. W. van Verseveld, A. H. Stouthamer, and F. C. Boogerd. 1995. Genetic and phenetic analysis of Bradyrhizobium strains nodulating (Arachis hypogaea L.) roots. Appl. Environ. Microbiol. 61:1599-1609. [DOI] [PMC free article] [PubMed]
- 28.Vaughan-Smith, H. C., K. S. Sriprakash, J. D. Mathews, and D. J. Kemp. 1995. Long-range PCR ribotyping of nontypeable Haemophilus influenzae J. Clin. Microbiol. 33:192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versalovic, J., F. J. de Bruijn, and J. R. Lupski. 1998. Repetitive sequences based PCR (Rep-PCR) DNA fingerprinting of bacterial genome, p. 437-454. In F. J. de Bruijn, J. R Lupski, and G. M. Weinstock (ed.), Bacterial genomes: physical structure and analysis. Chapman and Hall, New York, N.Y.
- 30.Versalovic, J., T. Koueth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genome. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, G. C. Y., and Y. Wang. 1997. Frequency of formation of chimeric molecules as consequences of PCR coamplification of 16S rRNA genes from mixed bacterial genomes. Appl. Environ. Microbiol. 63:4645-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]