Abstract
Previous studies have suggested that members of the Geobacteraceae can use electrodes as electron acceptors for anaerobic respiration. In order to better understand this electron transfer process for energy production, Geobacter sulfurreducens was inoculated into chambers in which a graphite electrode served as the sole electron acceptor and acetate or hydrogen was the electron donor. The electron-accepting electrodes were maintained at oxidizing potentials by connecting them to similar electrodes in oxygenated medium (fuel cells) or to potentiostats that poised electrodes at +0.2 V versus an Ag/AgCl reference electrode (poised potential). When a small inoculum of G. sulfurreducens was introduced into electrode-containing chambers, electrical current production was dependent upon oxidation of acetate to carbon dioxide and increased exponentially, indicating for the first time that electrode reduction supported the growth of this organism. When the medium was replaced with an anaerobic buffer lacking nutrients required for growth, acetate-dependent electrical current production was unaffected and cells attached to these electrodes continued to generate electrical current for weeks. This represents the first report of microbial electricity production solely by cells attached to an electrode. Electrode-attached cells completely oxidized acetate to levels below detection (<10 μM), and hydrogen was metabolized to a threshold of 3 Pa. The rates of electron transfer to electrodes (0.21 to 1.2 μmol of electrons/mg of protein/min) were similar to those observed for respiration with Fe(III) citrate as the electron acceptor (Eo′ =+0.37 V). The production of current in microbial fuel cell (65 mA/m2 of electrode surface) or poised-potential (163 to 1,143 mA/m2) mode was greater than what has been reported for other microbial systems, even those that employed higher cell densities and electron-shuttling compounds. Since acetate was completely oxidized, the efficiency of conversion of organic electron donor to electricity was significantly higher than in previously described microbial fuel cells. These results suggest that the effectiveness of microbial fuel cells can be increased with organisms such as G. sulfurreducens that can attach to electrodes and remain viable for long periods of time while completely oxidizing organic substrates with quantitative transfer of electrons to an electrode.
Fuel cells theoretically bypass the inefficiencies of internal combustion engines by directly oxidizing and reducing compounds at electrode surfaces, the most common example being the hydrogen fuel cell, which oxidizes hydrogen at the anode surface and passes electrons to a cathode, where they are used to reduce molecular oxygen (10). So-called microbial fuel cells seek to add the diversity of microbial catalytic abilities to this high-efficiency design, allowing organic compounds, from simple carbohydrates to waste organic matter, to be converted into electricity (28).
Previous studies on the microbial conversion of organic matter to electricity have noted several problems. A major shortcoming is that most microorganisms studied to date only partially oxidize their organic substrates and transfer a portion of these electrons to electrodes (8, 9, 11, 12, 24, 25). Furthermore, electrical current was generated only when soluble mediator compounds were added to these microbial cultures to facilitate electron transfer from the bacteria to the electrodes. Examples of mediators include potassium ferricyanide (9), anthraquinone 2,6-disulfonic acid, cobalt sepulchrate (7), thionine (13), neutral red (24), and azure A (3). The requirement for mediators is problematic, because many of these mediators are toxic and cannot be used when electrical energy is harvested from organic matter in an open environment.
A new concept in the construction of microbial fuel cells resulted from the observation (26) that if graphite or platinum electrodes were placed into anoxic marine sediments and connected to similar electrodes in the overlying oxic water, sustained electrical power could be harvested (on the order of 0.01 W/m2 of electrode). Analysis of the microbial community firmly attached to anodes harvesting electricity from a variety of sediments demonstrated that microorganisms in the family Geobacteraceae were highly enriched on these anodes (1, 27; D. Holmes, D. Bond, L. M. Tender, and D. R. Lovley, submitted for publication). Given the ability of Geobacteraceae to transfer electrons to other insoluble electron acceptors, such as Fe(III) oxides (14), these results suggested that the electrode surfaces were serving as terminal electron acceptors for Geobacteraceae.
Studies with Desulfuromonas acetoxidans, a marine representative of the Geobacteraceae, demonstrated that suspensions of this organism could oxidize acetate in a two-electrode fuel cell that simulated the marine sediment fuel cells, with no added mediator compounds (1). Furthermore, a freshwater representative of the Geobacteraceae, Geobacter metallireducens, oxidized aromatic compounds, such as benzoate and toluene, to carbon dioxide in a three-electrode poised-potential system, where an electrode maintained at +0.2 V (versus an Ag/AgCl reference electrode) served as the sole electron acceptor (1). Since Geobacteraceae are not known to produce any soluble electron shuttles (20), it was hypothesized that these Geobacteraceae were directly transferring electrons to the electrode surface. However, the mechanisms for this electron transfer, the possibility that this form of electron transport could support cell growth, and the role of attached versus unattached cells have not been previously investigated.
The study of Geobacteraceae has been facilitated by the availability of the genome sequence of Geobacter sulfurreducens and the development of a genetic system for this organism (4). In order to take advantage of these tools in elucidating cell-electrode interactions in the Geobacteraceae, studies were initiated with G. sulfurreducens. Here we report that (i) G. sulfurreducens can completely oxidize electron donors by using only an electrode as the electron acceptor, (ii) it can quantitatively transfer electrons to electrodes in the absence of electron mediators, and (iii) this electron transfer is due to a population of cells that attach to the electrode and are capable of rates of electron transport to electrodes that are similar to those observed for electron transport to Fe(III) citrate.
MATERIALS AND METHODS
Media and growth conditions.
G. sulfurreducens strain PCA (ATCC 51573) was obtained from our laboratory culture collection. All incubations were done at 30°C. Growth medium contained the following (per liter): 0.1 g of KCl, 0.2 g of NH4Cl, 0.6 g of NaH2PO4, 10 ml of vitamin mix (16), and 10 ml of trace mineral mix (16). The medium was adjusted to pH 6.8, 2 g of NaHCO3 was added, and the medium was flushed with N2-CO2 (80:20) to remove oxygen before autoclaving in sealed bottles. Acetate served as the electron donor unless otherwise indicated. To obtain cells well adapted to utilizing insoluble electron acceptors, cells were maintained on this medium amended with 100 to 120 mM poorly crystalline Fe(III) oxide (17) as the electron acceptor. The cells were then were transferred (10% inoculum) three times in medium containing 40 mM fumarate as the electron acceptor prior to inoculation into electrode-containing chambers. The growth medium in electrode-containing chambers was amended with 2.9 g of NaCl to minimize differences in osmolarity between the fumarate medium and the electrode growth medium, which lacked fumarate. When growth medium was replaced with an anaerobic salts buffer in electrode experiments, the buffer contained the following (per liter): 0.1 g of KCl, 0.6 g of NaH2PO4, 2.9 g of NaCl, and 2 g of NaHCO3.
Electrodes and electrode chambers.
A dual-chambered fuel cell was constructed with 54-mm-outside-diameter glass tubing and a 22-mm-outside-diameter pinch clamp assembly. The top of each chamber was sealed with a glass dome attached to a ground glass fitting, and the junction was sealed with silicone grease and thick glove box tape. Sampling ports sealed with butyl stoppers, and aluminum crimps were added to the sides and top of each chamber, while electrodes were introduced from the top by feeding a wire through a butyl stopper in the sampling port. The volume of each chamber, with the electrode, was approximately 225 ml of medium with a 150-ml headspace. The chambers were separated with a cation-selective membrane (Nafion 117; Electrosynthesis, Lancaster, N.Y.). The electrodes for fuel cells were 1- by 3- by 0.5-in. sticks of unpolished graphite (grade G10; Graphite Engineering and Sales, Greenville, Mich.). New electrodes were soaked in 1 N HCl that was changed daily until extractable Fe(II) was below detection. After each use, the electrodes were washed in 1 N HCl and 1 N NaOH to remove possible metal and biomass contamination. Connections were made with threaded watertight connectors using no. 20 AWG marine-grade wire (Impulse, San Diego, Calif.) screwed into holes drilled directly in the graphite electrodes. Holes were filled with silver epoxy (Epoxy Technology, Billerica, Mass.) and sealed with epoxy (type 730; Epoxy Technology). A reference electrode (BAS, West Lafayette, Ind.) was introduced into the anode-working electrode chamber by embedding it in a butyl rubber stopper and was sterilized by immersing the electrode and stopper in 5 N HCl for 5 min, rinsing in ethanol, and allowing the electrode to fully dry before placing it in a sampling port.
When electrodes were operated as fuel cells, the anode chamber (where cells were to be grown and used to donate electrons to the anode) was sterilized, flushed with anaerobic grade gas, and filled with anaerobic growth medium. The cathode chamber (aerobic chamber where oxygen was used as the electron acceptor for the electrode) was filled with a similar medium in which NaHCO3 was replaced with 30 mM Tris-HCl as a buffering agent. The cathode chamber was provided with air that was passed through a 0.45-μm-pore-size filter, and the anode chamber was mixed slowly (200 rpm) with a magnetic stir bar. Bubbling and mixing in each chamber was kept to a minimum to prevent excessive exchange of oxygen across the semipermeable membrane separating the two chambers. When the electrodes were poised with a potentiostat, both chambers were filled with identical growth media and the counter electrode chamber was flushed with a slow stream of N2-CO2 (80:20). As the counter electrode chamber was anaerobic, small amounts of hydrogen were produced at the counter electrode as the potentiostat disposed of electrons donated to the working electrode by microorganisms. Slow flushing of this chamber prevented hydrogen from diffusing into the working electrode chamber and serving as an electron donor for bacteria.
Current and voltage measurements for long-term studies were collected directly from potentiostat outputs every 10 s with a Power Lab 4SP unit connected to a Power Macintosh computer, and data was logged with Chart 4.0 software (ADInstruments, Mountain View, Calif.). Fuel cell power output was monitored by measuring the voltage across a known resistance (500 ohms) in the fuel cell. For current-voltage analysis, fuel cells were allowed to equilibrate at open circuit for ∼2 to 3 h, until the open circuit potential stabilized. The resistance between electrodes was lowered stepwise, pausing at each resistance setting for 5 min. Current (in milliamperes) was integrated over time and converted to electrons recovered by using the following conversions: 1 C = 1 A × 1 s, 1 C = 6.24 × 1018 electrons, and 1 mol = 6.02 × 1023 electrons (96,500 C/mol). Background current (current at the working electrode in the absence of cells, typically 0.03 to 0.04 mA) was determined for each experiment and subtracted from all values before calculating the total electron recovery.
SEM.
Electrodes were removed from electrode chambers, rinsed with sterile medium, and immersed in sterile growth medium (buffer) plus 1% glutaraldehyde and 1% formaldehyde. A saw was used to cut 20 to 40 mm beneath the surface of the electrode, allowing the surface to be removed as a thin plate without disturbing attached organisms or exposing them to graphite powder from the saw. Subsamples were postfixed overnight in 1% osmium tetroxide in buffer on ice, rinsed three times in buffer and then once in distilled water, and dehydrated by a graded ethanol series (30, 50, 70, 80, 95, 100, 100, and 100%; 30 min each stage with very gentle periodic agitation). The electrode was CO2-critical point dried from ethanol transitional solvent with a 3-h slow, continuous exchange. Electrode pieces were mounted on aluminum specimen mounts with contact adhesive, and conducting bridges were applied with isopropanol-based colloidal graphite paint. The samples were sputter coated in a Polaron E-5100 Sputter Coater (2 min at 2.2 kV) with argon at 13 Pa by using a gold-palladium target and observed in a JEOL JSM-5400 scanning electron microscope (SEM). The SEM was operated at 15 kV, and images were captured digitally.
Other analyses.
To extract protein from electrodes, the electrodes were shaken to remove free medium and then placed in a petri dish with 3 ml of 0.2 N NaOH. The NaOH solution was withdrawn into a 3-ml syringe and flushed over the surface of the electrode six to eight times over a 1-h extraction period. This liquid was removed and weighed, and the electrode was further rinsed with an equivalent amount of deionized water. The liquids were pooled, yielding a sample in 0.1 N NaOH, which was frozen (−20°C). Planktonic biomass was measured by collecting 10 ml of medium from culture chambers, centrifuging the sample at 10,000 × g for 5 min, removing the supernatant, and adding 1 ml of 0.1 NaOH to the centrifuge tube for resuspension of cells and freezing (−20°C). Thawed samples were heated at 100°C for 10 min, and protein was measured by the bicinchoninic acid method against a bovine serum albumin standard in 0.1 N NaOH with reagents obtained from Sigma Chemical Company (St. Louis, Mo.).
Acetate and organic acids were determined via high-pressure liquid chromatography with a fast acid analysis column (Bio-Rad, Hercules, Calif.) operated with 0.002 N H2SO4 as the eluant and UV detection. For hydrogen analysis, gas samples were injected into a 0.5-ml sampling loop, separated on a Supelco 100/120 Carbosieve S-II column at room temperature with N2 gas as the carrier, and detected with a reduction gas analyzer (RGD2; Trace Analytical, Melno Park, Calif.) (15).
RESULTS
Fuel cells.
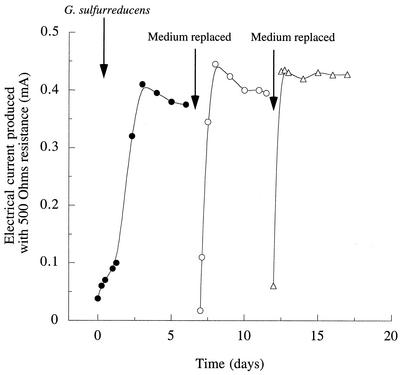
To initiate growth on graphite electrodes, sterile anaerobic chambers (225 ml) containing a graphite electrode (61.2-cm2 surface area) were inoculated with stationary-phase cultures of G. sulfurreducens that had been grown with fumarate as the electron acceptor (10% inoculum). The electrodes were connected via a 500-ohm fixed resistor to a similar electrode in a second sterile chamber which was bubbled continuously with air, and the chambers were separated by a cation-selective membrane. This fuel cell apparatus was designed to be similar to the conditions used to harvest electricity from sediments. Acetate (5 mM) was provided as the electron donor, and no electron acceptors other than the electrode were present. Electron flow began to increase soon after inoculation (Fig. 1). When anaerobically grown cultures of Escherichia coli were placed in similar chambers with glucose as the electron donor, even at levels equivalent to 100% inoculum no current flow was observed.
FIG. 1.
Current production by G. sulfurreducens in a microbial fuel cell. Cells were inoculated into an anaerobic chamber containing growth medium (5 mM acetate) and a graphite electrode connected to another electrode in an aerobic chamber. At the indicated times, medium was removed and replaced with sterile, anaerobic salts buffer plus 5 mM acetate.
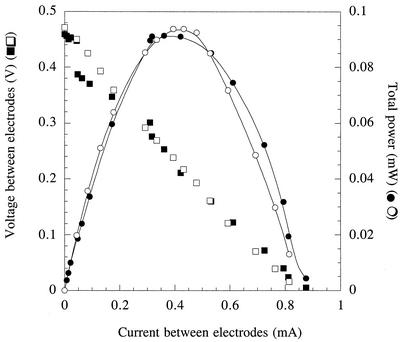
When electrical current production became stable (0.4 mA, or 65 mA/m2 of electrode surface) (Fig. 1), data were collected to determine the voltage and power production sustained across a range of current densities obtained by varying the resistance between the electrodes (Fig. 2). To determine if this power production was affected by free cells, or by soluble medium components, growth medium in the anaerobic chambers was removed under sterile, anaerobic conditions. Chambers were refilled with a sterile, anaerobic buffer that did not contain any vitamins, minerals, or N or S sources in order to remove any soluble compounds and limit further growth of cells. When acetate was again added as the electron donor, electrical current production rapidly rose to a maximum and stabilized at levels similar to those observed prior to medium replacement (Fig. 1). After 5 days of operation, the medium was again removed and replaced, and upon addition of 5 mM acetate, the current returned to similar levels.
FIG. 2.
Current-voltage and current-power (watts = amperes × volts) relationships for fuel cells containing G. sulfurreducens shown in Fig. 1. Open symbols represent the current produced from the oxidation of acetate in a fuel cell after the initial growth of cells in the electrode chamber. Closed symbols represent current produced from the oxidation of acetate in the fuel cell after the medium was replaced the second time.
While the power produced by a fuel cell at high rates of current flow (e.g., >0.2 mA) is largely limited by ohmic (transport of ionic species through the medium) and mass transfer (transport of donor or acceptor to the electrode surface) factors, power production at low rates of current flow is largely influenced by the rate of charge transfer at the electrode surface. If soluble mediators or cells were facilitating electron transfer, significant differences in the current-power relationship at low current densities would be expected following medium replacement. Analysis of the voltage and power production sustained over a range of current densities after the second medium change resulted in a current-power profile that was almost identical to that initially observed (Fig. 2), even though this represented the second time that the medium had been replaced in the electrode chamber, planktonic biomass was below detection limits, and electrode-attached cells had been deprived of growth nutrients for over 7 days.
Poised-potential growth.
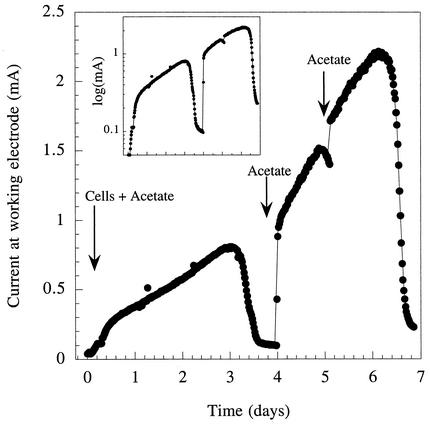
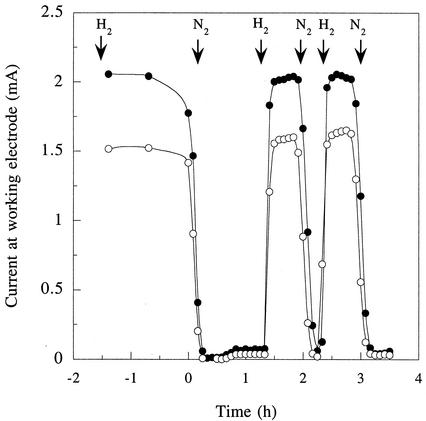
To study growth under more-defined conditions and remove the effects of electron transfer reactions at the cathode, similar chambers and graphite electrodes were used in conjunction with a potentiostat to poise the working electrode (anode) at a constant potential (+200 mV versus an Ag/AgCl reference electrode). As in fuel cell experiments, chambers were inoculated with stationary-phase cultures (5%) of G. sulfurreducens grown with fumarate as the electron acceptor. There was an acetate-dependent exponential increase in current production following inoculation (Fig. 3). Rates of current increase averaged 0.04 h−1. When the acetate was exhausted, current production fell to a basal rate. Analysis of organic acids in the electrode chamber indicated that slow oxidation of compounds present in the inoculum, such as succinate (primarily to fumarate), was responsible for this current production in acetate-depleted cultures.
FIG. 3.
Growth and current production by G. sulfurreducens inoculated into a chamber containing a graphite electrode poised at +200 mV versus an Ag/AgCl reference. Acetate (1 mM) was provided with the initial inoculum, and pulses of 1 mM acetate were given at the times indicated to demonstrate acetate-dependent growth. Inset gives data for current on a semilogarithmic scale to show exponential growth.
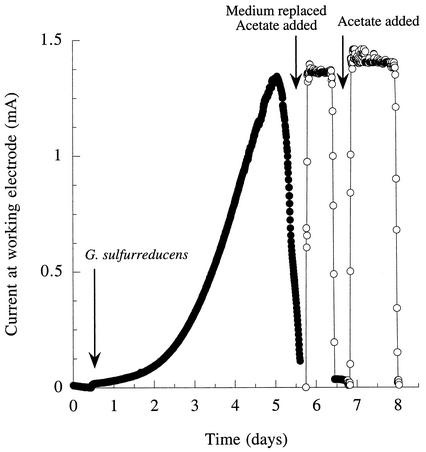
When 2 mM acetate was used to establish electrode-attached cultures, an average of 3.2 mg (n = 2) of cell protein (0.052 mg/cm2 of electrode surface area) could be extracted from the electrodes with NaOH. To determine if current production could be attributed solely to the biomass attached to the electrode, new cultures were established with electrodes and the growth medium was replaced with buffer, as described in the experiments with fuel cells. When the growth medium was replaced with the anaerobic salts buffer, the production level of electrical current was similar to previous levels as soon as acetate was added as an electron donor (Fig. 4). In addition, current levels did not increase, which is consistent with the nongrowth nature of the medium. When the acetate was depleted, current production fell to background levels but was restored with the addition of more acetate. These electrodes, with their attached populations, were maintained in this nongrowth state for as long as 4 weeks with little deterioration in performance.
FIG. 4.
Growth and current production by G. sulfurreducens in a chamber containing a graphite electrode poised at +200 mV versus an Ag/AgCl reference and effect of removing the growth medium and replacing it with anaerobic buffer plus acetate as the electron donor. Acetate (0.5 mM) was provided after replacing the medium, followed by a second pulse of 1 mM.
At the end of these incubations, electrodes were removed and the biomass was extracted. The attached biomass averaged 2.9 mg (standard deviation = 1.7; n = 3) or 0.047 mg/cm2 of electrode. SEM of electrode surfaces recovered at this stage revealed nearly full coverage of the electrode surface by a layer of cells, which was rarely more than a few cells thick. A sample SEM field is shown in Fig. 5.
FIG. 5.
SEM image of an electrode surface following growth of G. sulfurreducens with acetate as an electron donor (2 mM) under poised-potential conditions. Over 75% of viewed fields at this magnification had no exposed electrode; however, this image was chosen to provide an example of electrode surface characteristics and show individual bacterial attachment.
Rates of current production from cultures of G. sulfurreducens attached to electrodes prepared in this manner and exposed to acetate concentrations at or below 2 mM were typically 1 to 3 mA, but rates of 5 to 7 mA have been obtained at higher (10 mM) acetate concentrations. This represents a range of 163 to 1,143 mA/m2 of electrode surface area. When rates of current production (by attached populations) were corrected for biomass extracted from these same electrodes, rates of electron transport to electrodes ranged from 0.21 to 1.2 μmol of electrons/mg of protein/min. In comparison, the rates of electron transport to soluble Fe(III) citrate by G. sulfurreducens have been estimated to be 1 to 3 μmol of electrons/mg of protein/min (A. Esteve-Nunez, M. M. Rothermich, M. L. Sharma, and D. R. Lovley, submitted for publication). As rates of growth by G. sulfurreducens are significantly slower when insoluble Fe(III) substrates are used [6- to 8-h doubling times when using Fe(III) citrate versus 12- to 24-h doubling times when using Fe(III) oxides], rates of electron transport to solid electrodes that are slower than those with Fe(III) citrate are expected.
Stoichiometry and energetics.
Comparisons of acetate disappearance with electron recovery both in fuel cell experiments and with poised electrodes were consistent with eight electrons harvested per mole of acetate oxidized (average, 95% recovery), indicating that acetate was completely oxidized to CO2. For example, in the acetate pulse shown in Fig. 4, 85.8 C of charge (0.889 mmol) was recovered at the working electrode (when corrected for background current). During this period, 0.51 mM acetate was oxidized, and the working volume of the chamber was 225 ml; thus, 0.114 mmol of acetate was oxidized (yielding 0.918 mmol of electrons). These values resulted in the following electron recovery: 0.889/0.918 = 96.8%. No other organic acids were produced in the electrode chambers when acetate was consumed as the electron donor, and acetate was consumed until it reached levels below detection (<10 μM). Hydrogen levels in the headspace of acetate-consuming cultures ranged from 18 to 50 Pa, consistent with the low levels of H2 production by acetate-oxidizing G. sulfurreducens that had been observed previously (5). Temporarily flushing the headspace with N2-CO2 to reduce H2 concentrations below 1 Pa did not significantly affect the rate of current production, but constant flushing of the headspace to remove evolved hydrogen diminished total electron recovery.
Hydrogen alone as an electron donor (as H2-CO2 [80:20]) supported rates of electrical current production by electrode-attached cells that were similar to those obtained with acetate as the electron donor (Fig. 6). Hydrogen did not react with sterile graphite electrodes or produce current when cells were not present. When hydrogen was the sole electron donor available for electricity production, cells metabolized hydrogen to an apparent threshold of 3 Pa after a 7-day equilibration, which is equivalent to a ΔE′ for the 2H+-H2 redox couple of −0.27 V (versus a SHE).
FIG. 6.
Hydrogen-dependent current production by attached populations of G. sulfurreducens in two different chambers (open and closed symbols) containing graphite electrodes poised at +200 mV versus an Ag/AgCl reference electrode. Hydrogen-carbon dioxide (80:20) and nitrogen-carbon dioxide (80:20) gas mixtures were bubbled directly into the electrode chambers where indicated.
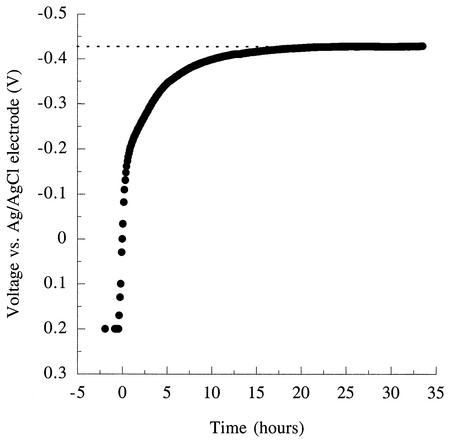
When the potentiostat was turned off in the presence of excess electron donor (acetate) and allowed to come to equilibrium, the potential of the electrode rapidly decreased (Fig. 7). Equilibrium potentials reached at these electrodes averaged −0.42 V versus the Ag/AgCl reference electrodes. As reference electrodes were found to produce potentials of +0.25 V when tested in the medium used for these studies, these equilibrium values were equivalent to a potential of −0.17 V (versus SHE) [for comparison, the potential of Fe(III) citrate, a common electron acceptor for G. sulfurreducens, is Eo′=+0.37 V].
FIG. 7.
Response of the graphite electrode potential to an attached population of G. sulfurreducens metabolizing acetate as the electron donor, after electrode control (poising by potentiostat) was switched off.
DISCUSSION
These studies demonstrate that G. sulfurreducens grows on the surface of energy-harvesting anodes in mediator-free microbial fuel cells, forming a stable, attached population that can continually produce electrical current via the oxidation of organic matter or hydrogen coupled to electron transfer to the electrode. Recovery of acetate as electrical current is quantitative, and both acetate and hydrogen can be metabolized to low concentrations. As discussed below, these findings greatly expand the potential for using microorganisms to convert organic matter to electricity because previously described microbial fuel cells did not effectively oxidize the organic fuel and most required the addition of electron transfer-mediating compounds and/or large amounts of biomass.
Electron transport to electrodes.
The results show that G. sulfurreducens can effectively catalyze the transfer of electrons from the oxidation of acetate or hydrogen to graphite electrodes. Rates of electron transfer to the electrode were comparable to those achieved by G. sulfurreducens with Fe(III)-citrate as an electron acceptor (Esteve-Nunez et al., submitted). This ability is consistent with the previous finding that two other Geobacteraceae, D. acetoxidans and G. metallireducens, were able to use electrodes as electron acceptors for organic matter oxidation (1). However, in those previous studies it was not clear whether the electron transfer to the electrodes resulted from planktonic cells that intermittently interacted with the electrode or whether the cells became firmly attached to the electrode surface. For example, recent studies have suggested that although G. metallireducens directly attaches to Fe(III) oxides in order to reduce them, it is motile during Fe(III) oxide reduction, suggesting that attachment to Fe(III) oxides is temporary (2).
The finding that the medium surrounding the electrodes could be completely replaced without significantly altering the capacity for power production demonstrated that G. sulfurreducens cells attached to the electrode surface were responsible for power production. The attached populations were stable and, even when maintained in medium which lacked nutrients essential to support growth, consistently continued to produce power for weeks. Attached cells were capable of transferring electrons under both fuel cell and poised-potential conditions.
The direct attachment of the cells to the electrode is consistent with the known physiology of Geobacteraceae. Unlike other Fe(III)-reducing bacteria such as Shewanella (22, 23) and Geothrix (21), Geobacteraceae do not appear to produce soluble electron-shuttling compounds to assist electron transfer to metals (20). This suggests that G. sulfurreducens would need to directly contact the electrode surface in order to transfer electrons to the electrode. SEM images of the electrode surface after growth under poised-potential conditions also revealed a community densely coating the electrode surface.
The reduction of Fe(III) oxides by G. sulfurreducens is thought to involve numerous electron transport proteins, including an 89-kDa c-type cytochrome that was a component of a membrane-bound Fe(III) reductase complex purified from G. sulfurreducens (18, 19) and is required for effective Fe(III) reduction in vivo (13a). Purified preparations of this cytochrome have a midpoint potential of −0.19 V (19). This is similar to the estimated equilibrium potential of G. sulfurreducens cultures attached to graphite electrodes (−0.17 V) and suggests that the terminal reductase for the electrode at the outer membrane surface may lie near this potential.
In a fuel cell with oxygen as the acceptor at the cathode, oxidation of a donor with a potential of −0.17 V would predict an open circuit potential of +0.99 V if the acceptor (cathode) reaction was O2/H2O (ΔEo′ = +0.82 V). If the cathode reaction was O2/H2O2 (ΔEo′ = +0.3 V), the open circuit potential would be expected to be only +0.47 V. Based on the open circuit potentials obtained in the fuel cells (shown in Fig. 2), these calculations suggest that the cathode reaction in graphite-electrode Geobacter fuel cells is more likely to involve hydrogen peroxide formation.
The ability of G. sulfurreducens to colonize the electrode surface and conserve energy to support growth from electron transport to the electrode provides a possible explanation for the predominance of Geobacteraceae on electrodes harvesting electricity from a variety of aquatic sediments (1, 27; Holmes et al., submitted). Geobacteraceae are able to oxidize acetate, the primary organic intermediate in the degradation of organic matter in anoxic aquatic sediments, with an electrode as the sole electron acceptor. This may provide them with a competitive advantage over other microorganisms that cannot use acetate as an electron donor in anaerobic respiration and/or cannot directly transfer electrons to electrodes in a manner that generates energy.
Comparison with previously described microbial fuel cells.
The electron transfer from G. sulfurreducens to electrodes is different from previously described microbial fuel cells. For instance, in microbial fuel cells where the metabolism of sugars or organic acids was shifted to end products more oxidized than the products formed during fermentation in the absence of an electrode, a significant fraction of the electrons available in the initial electron donor remained in the end products (8, 9, 12, 24). Of the electrons released from this partial metabolism, typically only 30 to 60% were recovered by electrodes (6, 12, 13). In all of these cases, electron transfer mediator compounds, such as thionine, potassium ferricyanide, or neutral red, had to be added to obtain any current production (9, 13, 24) and there was no evidence that electron transfer to the mediator compound provided energy to support growth of the microorganisms. Most previously described microbial fuel cells were operated for a single pulse of substrate addition, and performance significantly declined over time (6, 12). In contrast, G. sulfurreducens was able to completely oxidize its organic electron donor with nearly quantitative transfer of electrons to the electrode, without the need for an electron transfer mediator, and the G. sulfurreducens systems remained stable for weeks.
Exceptions to experiments using electron mediators are recent reports that Shewanella putrefaciens and a Clostridium isolate can transfer electrons to electrodes without the addition of exogenous electron-shuttling compounds (11, 12, 25). In fuel cells containing dense washed-cell suspensions (200 mg [dry weight]/liter) of S. putrefaciens (12) and graphite felt electrodes of sizes similar to those used with G. sulfurreducens (50 cm2), a current density of 8 mA/m2 of electrode surface area, which decayed to 4 mA/m2 after 7 days, was observed (compared to the 65 mA/m2 obtained in the G. sulfurreducens fuel cell). Recovery of the 4 electrons generated per lactate oxidized to acetate ranged from 3.5 to 9%, (or, as complete oxidation of lactate could yield 12 electrons, 1.2 to 3% of electrons were recovered as electricity). Lower recovery in a similar mediatorless system was reported for a Clostridium strain isolated from an electricity-harvesting electrode; approximately 0.04% of electrons available from glucose oxidation were transferred to an electrode during a fuel cell incubation (25).
Differences between G. sulfurreducens and organisms believed to be active in the absence of added electron mediators are also apparent from reports of poised-potential systems. With a 1,400-cm2 electrode poised at +1.0 V (versus an Ag/AgCl reference electrode) (11), current production by S. putrefaciens reached a peak value of 0.003 mA (0.02 mA/m2), and electron recovery from lactate oxidation was <0.03%. This contrasts with >3,000-fold-higher electron recovery (95% versus 0.03%) and more than 10,000-fold-higher rates of current flow (326 to 1,143 mA/m2 versus 0.003 mA/m2) in the poised-potential systems with G. sulfurreducens described here. As it is known that Shewanella species can release electron-shuttling compounds into the medium (22, 23), it is not clear how S. putrefaciens was transferring electrons to the electrodes in these experiments, but it is clear that significant differences between these organisms exist.
In summary, G. sulfurreducens shows significant potential for the harvesting of energy from organic compounds in the form of electricity, especially in comparison to organisms previously studied for this purpose. However, optimization of this process will require a better understanding of the interactions of G. sulfurreducens with the electrode surface. Such studies should be facilitated by the availability of its complete genome and new tools allowing the study of the G. sulfurreducens proteome and transcriptome, as well as a genetic system (4) that will permit more-detailed functional genomic studies with this organism.
Acknowledgments
This research was supported by the Office of Naval Research (ONR; grant N00014-00-0776), the Defense Advanced Research Projects Agency (DARPA) Defense Sciences Office (DSO) (grant N66001-02-C-8044), and the Office of Science (BER), U.S. Department of Energy (cooperative agreement DE-FC02-02ER63446).
REFERENCES
- 1.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms harvesting energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 2.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens access Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 3.Choi, Y., J. Song, S. Jung, and S. Kim. 2001. Optimization of the performance of microbial fuel cells containing alkalophilic Bacillus sp. J. Microbiol. Biotechnol. 11:863-869. [Google Scholar]
- 4.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cord-Ruwisch, R., D. R. Lovley, and B. Schink. 1998. Growth of Geobacter sulfurreducens with acetate in syntrophic cooperation with hydrogen-oxidizing anaerobic partners. Appl. Environ. Microbiol. 64:2232-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaney, G. M., H. P. Bennetto, J. R. Mason, S. D. Roller, J. L. Stirling, and C. F. Thurston. 1984. Electron-transfer coupling in microbial fuel cells. II. Performance of fuel cells containing selected microorganism-mediator combinations. J. Chem. Technol. Biotechnol. B Biotechnol. 34:13-27. [Google Scholar]
- 7.Emde, R., and B. Schink. 1990. Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Appl. Environ. Microbiol. 56:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emde, R., and B. Schink. 1990. Oxidation of glycerol, lactate, and propionate by Propionibacterium freudenreichii in a poised-potential amperometric culture system. Arch. Microbiol. 153:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emde, R., A. Swain, and B. Schink. 1989. Anaerobic oxidation of glycerol by Escherichia coli in an amperometric poised-potential culture system. Appl. Microbiol. Biotechnol. 32:170-175. [Google Scholar]
- 10.Haynes, C. 2001. Clarifying reversible efficiency misconceptions of high temperature fuel cells in relation to heat engines. J. Power Sources 92:199-203. [Google Scholar]
- 11.Kim, B. H., H. J. Kim, M. S. Hyun, and D. H. Park. 1999. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 12.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 13.Kim, N., Y. Choi, S. Jung, and S. Kim. 2000. Effect of initial carbon sources on the performance of microbial fuel cells containing Proteus vulgaris. Biotechnol. Bioeng. 70:109-114. [DOI] [PubMed] [Google Scholar]
- 13a.Leang, D., M. V. Coppi, and D. R. Lovley. OmcB, a c-type polyheme cytochrome involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 14.Lonergan, D. J., H. Jenter, J. D. Coates, E. J. P. Phillips, T. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovley, D. R., and S. Goodwin. 1988. Hydrogen concentrations as an indicator of the predominant terminal electron accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52:2993-3003. [Google Scholar]
- 16.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnuson, T. S., A. L. Hodges-Myerson, and D. R. Lovley. 2000. Characterization of a membrane bound NADH-dependent Fe3+ reductase from the dissimilatory Fe3+ reducing bacterium Geobacter sulfurreducens. FEMS Microbiol. Lett. 185:205-211. [DOI] [PubMed] [Google Scholar]
- 19.Magnuson, T. S., N. Isoyama, A. L. Hodges-Myerson, G. Davidson, M. J. Maroney, G. G. Geesey, and D. R. Lovley. 2001. Isolation, characterization, and gene sequence analysis of a membrane associated 89 kDa Fe(III) reducing cytochrome from Geobacter sulfurreducens. Biochem. J. 359:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevin, K. P., and D. R. Lovley. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66:2248-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl. Environ. Microbiol. 68:2294-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19:141-159. [Google Scholar]
- 23.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 24.Park, D. H., and J. G. Zeikus. 2000. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 66:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. H. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297-306. [Google Scholar]
- 26.Reimers, C. E., L. M. Tender, S. Fertig, and W. Wang. 2001. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 35:192-195. [DOI] [PubMed] [Google Scholar]
- 27.Tender, L. M., C. E. Reimers, H. A. Stecher, D. E. Holmes, D. R. Bond, D. L. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Buried treasure; harnessing microbial power generation on the seafloor. Nat. Biotechnol. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 28.Wingard, L. B., Jr., C. H. Shaw, and J. F. Castner. 1982. Bioelectrochemical fuel cells. Enzyme Microb. Technol. 4:137-142. [Google Scholar]