Abstract
Sexual transmission of HIV most closely reflects the concentration of HIV in the genital tract; HIV in the genital tract of subjects with acute HIV and some “classical” STDS is 8–10 times greater than in control subjects. It seems likely that these latter subjects lead to spread of HIV. Accordingly, the state of North Carolina committed to HIV testing that detects subjects with acute, recent, and established infection. We tested 109,500 samples over 9 months. We found 563 people with undiagnosed HIV infection. The majority of subjects were in STD clinics. This included 23 subjects with (pre-seroconversion) acute HIV infection (HIV RNA positive, antibody negative). The median blood HIV was 209,000 copies/ml, more than 10 times higher than in subjects with established HIV infection. Recognizing the increased number of subjects with unrecognized acute HIV infection in STD clinics, we conducted similar studies in STD Clinics in Malawi and South Africa. Between 1 and 2% of subjects had undetected acute HIV infection. The median viral burden in blood of subjects in Malawi was greater than 1,000,000 copies/ml. STDS and HIV are often co-transmitted, and STDS set the stage for subsequent HIV transmission. Prevention of sexual transmission of HIV likely requires maximal suppression of genital tract HIV viral burden, either through treatment of STDS or use of antiretroviral agents.
Introduction
More than 60,000,000 people have been infected with HIV-1 (1). The routes of HIV transmission are extremely well known (2) (Table 1), and most HIV transmission can be ascribed to sexual intercourse. However, the precise biological requirements for transmission are not completely understood. Furthermore, earlier epidemiology studies reported coital transmission efficiency so low as to create confusion (3,4). In the past 5 years increased focus on subjects with acute and early HIV infection and/or sexually transmitted diseases have allowed new insights into HIV transmission, which have changed our understanding of the spread of HIV, and provided critical opportunities for prevention and treatment.
TABLE 1.
Routes of Exposure and HIV Infection Risk
| Infection Route | Risk of Infection |
|---|---|
| Sexual Transmission | |
| a. Female-to-male transmission | 1 in 700 to 1 in 3,000 |
| b. Male-to-female transmission | 1 in 200 to 1 in 2,000 |
| c. Male-to-male transmission | 1 in 10 to 1 in 1,600 |
| d. Fellatio?? | 0–6% |
| Parenteral transmission | |
| a. Transfusion of infected blood | 95 in 100 |
| b. Needle sharing | 1 in 150 |
| c. Needle stick | 1 in 200 |
| d. Needle stick/AZT PEP | 1 in 10,000 |
| Transmission from mother to infant | |
| a. Without AZT treatment | 1 in 4 |
| b. With AZT treatment | Less than 1 in 10 |
The Biology of HIV Transmission
Transmission of HIV requires a sufficiently infectious HIV carrier and a susceptible host (2). While this seems like a simple idea, the details have turned out to be very complex. The concentration of virus (discussed in detail below) and viral genotypic features are critical determinants of transmission. Understanding the virologic requirements for HIV transmission has proven difficult because a very small number of subjects with acute infection [pre-seroconversion (5)] or transmission pairs [a subject with acute infection and the appropriate sexual partner (6)] have been studied. Current ideas about HIV transmission include: i) the virus with the R5 envelope (macrophage trophic) phenotype is preferentially transmitted or expanded after transmission (7); ii) more viral diversity is observed after women or gay men acquire HIV (7,8); iii) viral subtypes (clades) may be transmitted with differential efficiency and clades A and C may enjoy transmission advantages (2,3); iv) HIV variants with short viral envelope sequence and increased susceptibility to neutralizing antibodies may be preferentially transmitted (6).
Not all people are equally susceptible to HIV and differences in hereditary, innate and acquired immunity have been observed (2). CCR5 receptor deletions drastically reduce HIV susceptibility (9), although such deletions are not distributed homogeneously across the human species (10). Furthermore, yet to be discovered genetic forms of resistance are very likely important.
Several forms of innate resistance to HIV have also been described. The female hormonal status and/or use of contraceptives may influence HIV acquisition in women (11). Vaginal flora rich in lactobacilli (the antithesis of the flora of bacterial vaginosis) also favor resistance to HIV (12). Circumcised men appear to resist HIV (13). And a sexual partner with HLA type different than the infected subject may be less likely to acquire HIV (14).
Acquired (immune) resistance has been reported in sex workers in Kenya (15). Resistance has been correlated with the cytotoxic lymphocyte response in peripheral blood cells, and these results are consistent with studies of acquired immunity in macaques (16).
The Viral Concentration
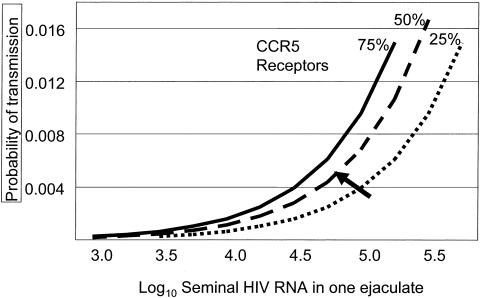
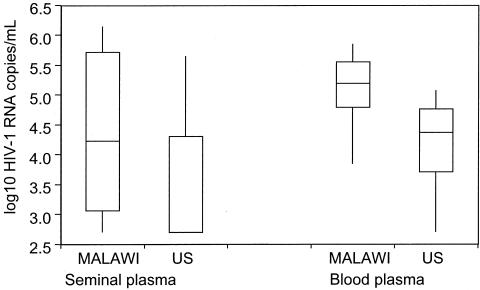
Careful measurement of the HIV concentration in blood and genital secretions has greatly advanced our understanding of HIV transmission (17,18). In their landmark study, investigators working in the Rakai District of Uganda demonstrated that HIV transmission was very unlikely to occur when the concentration in blood fell below 3000 copies/ml blood (19). Modeling results using semen HIV concentration demonstrate an even more precise relationship between HIV concentration and transmission probability (20) (Figure 1). HIV clade (21) and phase of disease affect the HIV concentration as well (22,23). Among subjects studied in the US and Malawi with similar CD4 count we observed much higher concentration of HIV blood and semen in the Malawi group with clade C infection (Figure 2) (21), compared to US subjects with clade B infection.
Fig. 1.
The probability of HIV transmission from a man to a female sexual partner through heterosexual intercourse based on the concentration of HIV in semen (adapted from reference 20). HIV transmission probability is also affected by the density of endocervical CCR5 receptors, represented as percentages in the population.
Fig. 2.
Comparison of the concentration of HIV in blood (n = 22) and semen (n = 33) of men in Malawi and the US at a similar stage of HIV disease and CD4 count. A horizontal bar represents the median concentration and vertical lines the standard error of the mean (adapted from reference 21). The differences between American and Malawian men are significant (p < 0.05).
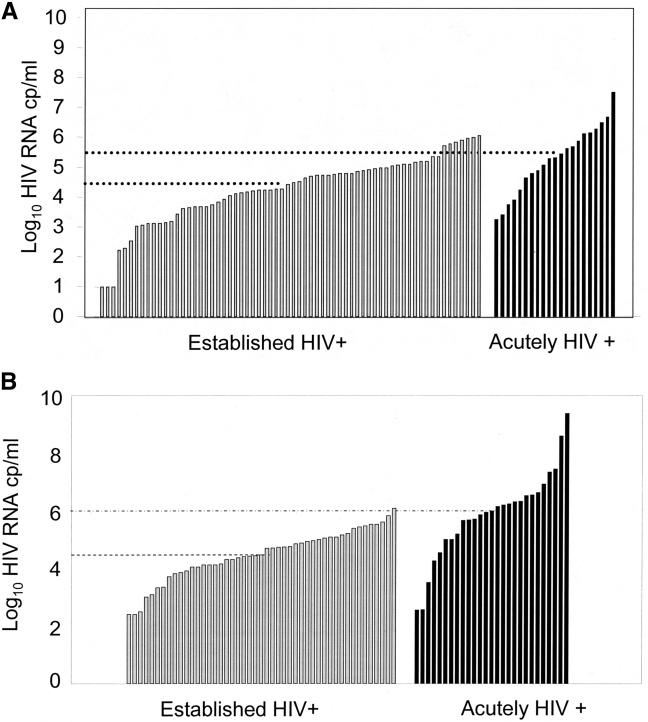
HIV concentration in blood and semen vary greatly with different stages of HIV disease; peak HIV concentrations are observed during acute (pre-seroconversion) HIV infection, and in subjects with AIDS (22). Figures 3A and 3B compare HIV concentration in blood in subjects with acute and established HIV infection in the US and Africa respectively, demonstrating 10 fold differences in HIV concentration (21). These results translate into greatly increased probability of HIV transmission (Figure 1) (22).
Fig. 3.
Comparison of HIV concentration in blood of subjects with established and acute HIV infection in the US (3A) and Malawi (3B) adapted from references 34 and 33, respectively.
The idea that acute HIV infection might be important in the spread of HIV has long been popular (24), supported by early modeling papers (25,26). This hypothesis has received powerful support from recent further interpretation of the Rakai study (27). In retrospective analysis of discordant couples, Wawer et al. found that 43.2% of all transmission events could be related to index (infected) subjects with acute and early infection (23,27).
Finding Patients with Acute HIV Infection
Finding patients with Acute HIV infection (AHI) has become an important goal for HIV prevention and treatment programs. Heretofore, only two strategies have been used to find subjects with AHI. First, investigators have tried to find at-risk patients with a “mono-like illness”. About half of subjects with early HIV developed such symptoms including fever, fatigue and adenopathy around the time of HIV seroconversion (28). Second, very high risk subjects can be followed in a cohort over time, literally waiting for some participants to develop symptoms or seroconvert (29). Neither of these approaches has proven efficient, and they lead to detection of subjects only weeks after infection, too late for optimal prevention or therapy, and certainly too late for accurate biological studies of HIV transmission.
Cross-Sectional Detection of Acute HIV Infection
Bollinger et al. identified subjects with acute HIV infection in STD clinics in India through detection of HIV p24 antigen in blood before antibody seroconversion (30). Recognizing the importance of this strategy, Pilcher et al. (22,31–34) conducted a series of studies designed to detect HIV RNA in HIV antibody-negative blood samples. Given the cost of an individual RNA HIV test this would be prohibitively expensive without a strategy to try to detect HIV in multiple pooled samples (32). The high viral burden associated with acute HIV infection and the increased and exquisite sensitivity of the newer versions of HIV testing make this strategy possible.
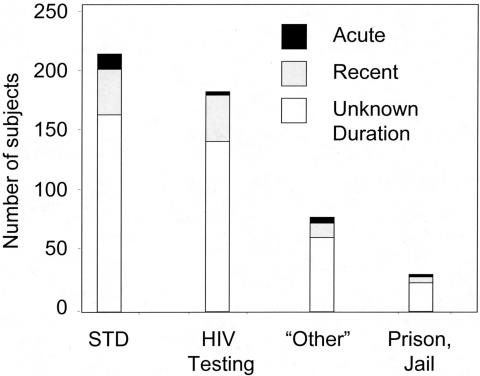
Working with the State of North Carolina, Pilcher et al. screened all agreeable subjects at public testing sites including STD clinics (33). The results are shown in Figure 4. More than 100,000 subjects were studied over 9 months. Four hundred seventy seven subjects had HIV antibodies consistent with established HIV infection. Using an assay that can report “early” infection based on reduced antibody concentration or avidity (35), 106 patients with recent HIV infection were also discovered. Most important, 23 subjects with acute HIV infection were detected in subjects with no HIV antibodies, although all of these subjects ultimately seroconverted. As shown in Figure 3A, subjects with AHI had the expected high concentration of HIV RNA in their blood. Intriguingly, the largest number of subjects were detected in STD Clinics, even though less than half the clients seeking STD screening or care agreed to testing. Since these initial reports investigators in California have applied these methods with similar results (36).
Fig. 4.
The location of subjects with newly detected HIV infections through screening in public testing sites in North Carolina over 9 months (adapted from reference 34). A total of 109,500 people were screened: 45,656 in STD Clinics, 11,658 at local free testing sites, 3,053 in jails, and 7,575 at “other” venues reporting to the State. The majority of subjects with acute HIV infection (16/23) were found in STD Clinics.
To further examine the prevalence of AHI subjects in STD clinics we undertook a series of studies in Southern Africa. Our first study involved retrospective analysis of samples from the STD Clinic in Lilongwe, Malawi (33). Among 1361 men who agreed to join the study, HIV prevalence was 47%; however, 2.1% of HIV antibody negative subjects had AHI. We noted that AHI could be predicted among patients with genital ulcers, adenopathy, and/or exposure to sex workers.
We have recently completed a prospective study that included 1441 men and women (37). Five hundred eighty three subjects (40.2%) with established HIV infection were compared to 20 subjects with AHI. In multivariate analysis we demonstrated extreme risk for AHI in HIV antibody negative subjects with genital ulcers (OR 6.69).
To demonstrate that these findings were not unique to Lilongwe we collaborated with investigators at University of Wittswatersrand (38). Among 1906 subjects screened, established, HIV infection was detected in 672 (35%) subjects with suspected incident infection. Among HIV antibody negative subjects, 12 with AHI were detected, the majority from an STD clinic setting.
Further Consideration of Sexually Transmitted Diseases
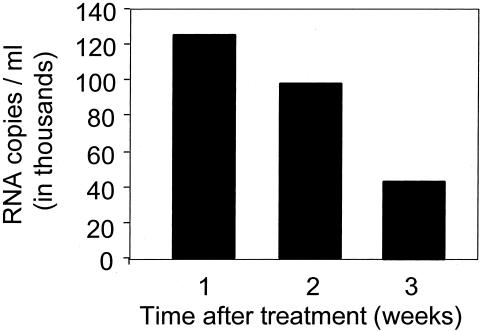
Finding AHI in STD clinics should not be surprising. The results support the well-established synergy between STDS and HIV (3,39). STDs greatly increase the concentration of HIV in genital secretions (40) as well as the diversity of the viral swarm (41). STDs cause mucosal disruption and inflammation that would be expected to facilitate HIV acquisition (3). Recent findings emphasize the importance of genital ulcers and suggest the possibility of co-transmission of HIV and other STD pathogens (3). Genital ulcers caused by HSV appear to be a particularly powerful risk factor for HIV acquisition (42). Treatment of STDS reduces the genital tract excretion of HIV (Figure 5) (40). However, clinical trials designed to use treatment of STDs to reduce incidence of HIV in a general population have had mixed results, almost certainly because of limitations in study design and approach (43).
Fig. 5.
Effects of treatment of urethritis on excretion of HIV in semen in 87 subjects in Malawi. Bars represent the median values (adapted from reference 40).
Implications for Prevention of HIV
The strategies for HIV prevention must take into consideration the method by which the epidemic is sustained. May and Anderson (44) provided a critical frame work with the formula:
Where b is the efficiency of HIV transmission, a product of both infectiousness and susceptibility;
c is the number of partners or partner change rate;
d is the duration of infectiousness
When Ro becomes less than 1 HIV spread should be reduced. Strategies for HIV prevention are shown in Table 2. The combination of condoms, partner number reduction, and aggressive attention to STDs appears to have had great success in Thailand and Uganda (45).
TABLE 2.
Prevention of HIV
| 1. STD control, behavior change, condoms |
| 2. Vaccines (Trials ongoing) |
| 3. Treatment of Bacterial Vaginosis (??) |
| 4. Topical microbicides (Trials ongoing) |
| 5. The diaphragm (Trials Ongoing) |
| 6. Male circumcision (Trials ongoing) |
| 7. Antiviral therapy (Trials Ongoing) |
| 8. Societal (Structural) Change: Incentives for safer sex? |
We have been particularly interested in the use of antiretroviral therapy (ART) for HIV prevention (46,47). Such therapy could be used as pre- or post-exposure prophylaxis, or to reduce the viral burden in the infected person. Animal experiments have been used to support prophylaxis (reviewed in 47). Antiviral therapy provided to HIV infected subjects substantially reduces HIV excretion in both male (48) and female (49) genital secretions, and some drugs actually concentrate in semen (50). Limited evidence suggests that ART can reduce transmission of HIV to a sexual partner (51), and broader use of ART might reduce HIV in a population.
Conclusions
We have an ever better understanding of the requirements for HIV transmission. It seems clear that considerable HIV transmission occurs at the extremes of the infection (during acute infection and as the disease progresses and viral burden increases), but HIV prevention strategies to date have all but ignored such people. In addition, STDs help to drive HIV transmission, and STD clinics are sites where unrecognized infections can be readily detected. Success in HIV prevention demands that we focus on those who are most susceptible and those who are most contagious, so as to maximize effective utilization of resources.
DISCUSSION
Luke, Cincinnati: Why are the levels so much higher in Africa?
Cohen, Chapel Hill: First, African subjects have type (clade) C HIV, whereas U.S. subjects have clade B HIV. Differences in viral genotype and phenotype likely have an effect on viral replication. Second, many African subjects have malaria, and/or tuberculosis and/or worms. Such co-infections drive up the blood viral level. Third, Africans and Americans and Europeans have different genetic backgrounds, and genetic differences in host defenses can affect control of the virus.
Goodenberger, St. Louis: In his book, The Tipping Point, Gladwell talks about an epidemic of syphilis in Baltimore, and asserts that the epidemic can be traced to a remarkably small number of individuals. Is there such a phenomenon that occurs in HIV? That is, are there super transmitters, either by virtue of their immunologic status or their behaviors?
Cohen: HIV “Super shedders” demonstrate increased excretion of the virus in the genital tract, and increased shedding is especially common in people with acute HIV infection or an STD co-infection. There was a man living in up state New York who infected a very large number of female high school students in a very short period of time; it seems likely that he was a “super shedder.”
Branch, Atlanta: Following up on this from the public health point of view, are we back into a model where we identify a case and then surround it with case-detection and treatment?
Cohen: Another great question. So we abandoned case findings in AIDS because of what is called “AIDS exceptionalism.” When HIV first started, we decided it was too traumatic for the person to not have any benefit, personal benefit, and, instead, only suffer the consequences of the stigma. So we never found all the partners. Now in 2005, one of the highest priorities of the CDC, based on this and other work, is, in fact, to find the sexual partners, and we find snowballs. So from the study in North Carolina, we found 23 patients in North Carolina from that study. We then realized that of those 23, about 9 were African American college students. We then, with the help of the CDC, found that there’s an epidemic of HIV being transmitted among African American college students up and down the east coast. So every time we find an acutely-infected patient, we find a mini-epidemic. Demography, place and partners are really important. By the way, I didn’t have a chance to talk about the biology of all this. But, of course, understanding transmission is how you would make a vaccine as well. So this kind of work is critical to vaccine development because it’s what you acquire that tells you how to make a vaccine.
Alexander, Atlanta: I was curious about the high infectivity during the acute episode and was wondering if there was more to it than just copy number. Is there something different about the virus that has recently been transmitted?
Cohen: That was a question for a man named Eric Hunter. For everyone of these studies where we collect semen and do epidemiology, we are also doing biology. And there is some evidence that the virus that’s transmitted to men and women is different, and that the virus that is transmitted is short, and has a lot of glycosolation and is differently responsive to neutralizing antibodies. That’s a very hot topic. However, the investigators at this point in time only have a total of 12 transmission pairs. So one of our big goals is to find more of these. But there’s every reason to believe the virus is different, just as you suggested.
REFERENCES
- 1. UNAIDS. 2004 Report on the Global AIDS Epidemic ( http://www.unaids.org/bangkok2004/report.html)
- 2.Royce AR, Seña A, Cates W, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 3.Galvin SR, Cohen MS. Sexual transmission of HIV. Nature Reviews Microbiology. 2004:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 4.Gisselquist D, Rothenber R, Potterat JJ, Drucker E. HIV infections in sub-Saharan Africa not explained by sexual or vertical transmission. Int J STD AIDS. 2002;13:657–666. doi: 10.1258/095646202760326390. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV Revisited: New Opportunities for Treatment and Prevention. J Clin Invest. 2004;13:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo K, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 7.Moore JP, Kitchens S, Pugach P, Zack J. THE CCR5 and CXCR4 Receptors-Central to the pathogenesis and transmission of HIV-1. AIDS and Human Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 8.Ritola K, Pilcher CD, Fiscus SA, Hoffman NG, Nelson JAE, Kitrinos KM, Hicks CB, Eron JJ, Jr, Swanstrom R. Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J Virol. 2004;78:11208–11218. doi: 10.1128/JVI.78.20.11208-11218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNicholl JM, Smith DK, Qari SH, Hodge T. Host genes and HIV: The role of the chemokine receptor gene CCR5 and its allele (delta32 CCR5) Emerg Infect Dis. 1997;3:261–270. doi: 10.3201/eid0303.970302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng T, Ni A, Yang G, Galvin SR, Hoffman I, Cohen MS. Distribution of the CCR5 gene 32-base pair deletion and CCR5 expression in Chinese minorities. J Acquir Immune Defic Syndr. 2002 Feb 1;32:121–4. doi: 10.1097/00126334-200302010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Baeten JM, Lavreys L, Sagar M, Kreiss JK, Richardson BA, Chohan B, Panteleeff D, Mandaliya K, Ndinya-Achola JO, Overbaugh J, Farley T, Mwachari C, Cohen C, Chipato T, Jaisamrarn U, Kiriwat O, Duerr A. Effect of contraceptive methods on natural history of HIV: studies from the Mombasa cohort. J Acquir Immune Defic Syndr. 2005;38(Suppl 1):S18–21. doi: 10.1097/01.qai.0000167030.18278.0e. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang L, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12(13):1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Siegfried N, Muller M, Volmink J, Deeks J, Egger M, Low N, Weiss H, Walker S, Williamson P. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2003;(3):CD003362. doi: 10.1002/14651858.CD003362. [DOI] [PubMed] [Google Scholar]
- 14.Al Jabri AA. HLA and in vitro susceptibility to HIV infection. Molecular Immunol. 2002;38:959–967. doi: 10.1016/s0161-5890(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 15.Rowland-Jones SL, Dong T, Fowke KR, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J, MacDonald KS, McMichael AJ, Plummer FA. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infection. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 17.Shepard RN, Schock J, Robertson K, Shugars DC, Dyer J, Vernazza P, Hall C, Cohen MS, Fiscus SA. Quantitation of HIV-1 RNA in different biological compartments. J Clin Micro. 2000;38(4):1414–8. doi: 10.1128/jcm.38.4.1414-1418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17(4):455–80. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 19.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li CJ, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, Patterson BK, Coombs RW, Krieger JN, Cohen MS. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15:621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 21.Dyer JR, Vernazza PL, Gilliam B, Zimba D, Hoffman IF, Royce RR, Fiscus SA, Kazembe P, Cohen MS, Eron JJ. High levels of human immunodeficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J Infect Dis. 1998;177:1742–1746. doi: 10.1086/517436. [DOI] [PubMed] [Google Scholar]
- 22.Pilcher CD, Tien HC, Eron JJ, Vernazza PL, Leu S, Stewart PM, Goh L, Cohen MS. Brief but Efficient: Acute HIV Infection and the Sexual Transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 23.Cohen MS, Pilcher C. Amplified HIV transmission and new approaches to HIV prevention. J Infectious Diseases. 2005;191:1391–1393. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 24.Cates W, Chesney M, Cohen MS. Primary HIV disease: A public health emergency. Am J Public Health. 1997;87:1928–1930. doi: 10.2105/ajph.87.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopman Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, Barth-Jones D, Adams AL, Lange K. The role of early HIV infection in the spread of HIV through populations. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Jacquez Jacquez J, Koopman J, Simon C, Longini I. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 27.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 28.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 29.Lavreys L, Thompson ML, Martin HL, Mandaliya K, Jr, Ndinya-Achola JO, Bwayo JJ, Kreiss J. Primary human immunodeficiency virus type 1 infection: clinical manifestations among women in Mombasa, Kenya. Clinical Infectious Diseases. 2000;30:486–90. doi: 10.1086/313718. [DOI] [PubMed] [Google Scholar]
- 30.Bollinger RC, Brookmeyer RS, Mehendale SM, Paranjape RS, Shepherd ME, Gadkari DA, Quinn TC. Risk factors and clinical presentation of acute primary HIV infection in India. JAMA. 1997;278(23):2085–9. [PubMed] [Google Scholar]
- 31.Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, Dean B, Robertson K, Hart CE, Lennox JL, Eron JJ, Hicks CB. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 32.Pilcher CD, McPherson T, Leone PA, Smurzynski M, Owen-O’Dowd J, Peace-Brewer AL, Harris J, Hicks JB, Eron JJ, Jr, Fiscus Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288:216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 33.Pilcher CD, Price MA, Hoffman IF, Galvin SR, Martinson FEA, Kazembe PN, Eron JJ, Miller WC, Fiscus SA, Cohen MS. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:1–8. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 34.Pilcher CD, Fiscus SA, Nguyen NQ, Foust E, Wolf E, Williams D, Ashby R, Owen O, Dowd J, McPherson JT, Stalzer B, Hightow L, Miller WC, Eron JJ, Cohen MS, Leone P. Detection of acute HIV infections in the general HIV testing population in North Carolina. N Engl J Med. 2005;352:1873–83. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 35.Jansen Janssen RS, Holtgrave DR, Valdisserri RO, Shepherd M, Gayle HD, Cock KM. The serostatus approach to fighting the HIV epidemic: prevention strategies for HIV infected individuals. Am J Public Health. 2001;91:1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez M, Kohn RP, Wong W, Harvey S, Kerndt P, Holmberg SD. Detection of acute HIV patients in California. JAIDS in press. [DOI] [PMC free article] [PubMed]
- 37.Fiscus S, Pilcher C, Miller W, Hoffman I, Price M, Chilongozi D, Mapanje C, Krysiak R, Hosseinipour M, Galvin S, Gama G, Martinson F, Cohen M. Real-time Detection of Patients with Acute HIV Infection in Africa. 2005 Aug; 12th Conference on Clinical Retroviruses and Opportunistic Infections. Boston Mass, Abstract 20. [Google Scholar]
- 38.Stevens WS, Akkers E, Myers M, Motlung T, Venter High prevalence of undetected, acute HIV infection in a South African primary care clinic IAS Brazil. 2005 Jul; Abstract no. MoOa0108. [Google Scholar]
- 39.Fleming W, Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Trans Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Zimba D, Vernazza PL, Costello-Daly C, Maida M, Fiscus SA, Eron JJ. Reduction of Concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 41.Ping LH, Cohen MS, Hoffman IF, Vernazza P, Seillier-Moiseiwitsch F, Chakraborty H, Kazembe P, Zimba D, Maida M, Fiscus SA, Eron JJ, Swanstrom R, Nelson JAE. Effects of Genital Tract Inflammation on HIV-1 V3 Populations in Blood and Semen. J Virology. 2000;74:8946–52. doi: 10.1128/jvi.74.19.8946-8952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds SJ, Risbuda, Sheperd ME, Zenilman JM, Brookmeyer RS, Paranjape RS, Divekar AD, Gangakhedkar RR, Ghate MV, Bollinger RC, Mehendale SM. Recent Herpes Simplex Virus Type 2 infection and the risk of HSV-1 in India. J Infect Disease. 2004;187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 43.White RG, Orroth KK, Korenromp EL, Bakker R, Wambura M, Sewankambo NK, Gray RH, Kamali A, Whitworth JA, Grosskurth H, Habbema JD, Hayes RJ. Can Population Differences Explain the Contrasting Results of the Mwanza, Rakai, and Masaka HIV/Sexually Transmitted Disease Intervention Trials?: A Modeling Study. JAIDS. 2004;37:1500–1513. doi: 10.1097/01.qai.0000127062.94627.31. [DOI] [PubMed] [Google Scholar]
- 44.May RM, Anderson RM. Transmission dynamics of HIV infection. Nature. 1987;326:137–142. doi: 10.1038/326137a0. [DOI] [PubMed] [Google Scholar]
- 45.Low-Beer S, Stoneburner RL. Behaviour and communication change in reducing HIV: Is Uganda unique? African J Aids Research. 2003;2:9–21. doi: 10.2989/16085906.2003.9626555. [DOI] [PubMed] [Google Scholar]
- 46.Hosseinipour M, Cohen MS, Vernazza PL, Kashuba ADM. Can antiviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:1391–1395. doi: 10.1086/340403. [DOI] [PubMed] [Google Scholar]
- 47.Cohen MS, Hossenipour M, Butera S, Kashuba AA. In: Antiretroviral therapy to prevent sexual transmission of HIV In Current Clinical Topics in Infectious Disease. Remington J, Swarz M, editors. 2002. [PubMed] [Google Scholar]
- 48.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, Boggian K, Cohen MS, Fiscus SA, Eron JJ, the Swiss HIV Cohort Study Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. AIDS. 2000;14(2):117–21. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 49.Cu-Uvin S, Caliendo AM, Reinert S, Chang A, Juliano-Remollino C, Flanigan TP, Mayer KH, Carpenter CC. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–21. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 50.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. Antiretroviral Drug Concentration in Semen: Implications for Sexual Transmission of HIV-1. Antimicrobial Agents and Chemotherapy. 1999;43(8):1817–1826. doi: 10.1128/aac.43.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musicco M, Lazzarin A, Nicolosi A, Gasparini M, Costigliola P, Arici C, Saracco A. Antiretroviral treatment of men infected with human immunodeficiency virus type 1 reduces the incidence of heterosexual transmission. Arch Intern Med. 1994;154:1971–1976. [PubMed] [Google Scholar]