Abstract
The endosymbiotic bacteria Spiroplasma spp. are vertically transmitted through female hosts and are known to cause selective death of male offspring in insects. One strain of spiroplasma, NSRO, causes male killing in Drosophila species, and a non-male-killing variant of NSRO, designated NSRO-A, has been isolated. It is not known why NSRO-A does not kill males. In an attempt to understand the mechanism of male killing, we investigated the population dynamics of NSRO and NSRO-A throughout the developmental course of the laboratory host Drosophila melanogaster by using a quantitative PCR technique. In the early development of the host insect, the titers of NSRO were significantly higher than those of NSRO-A at the first- and second-instar stages, whereas at the egg, third-instar, and pupal stages, the titers of the two spiroplasmas were almost the same. Upon adult emergence, the titers of the two spiroplasmas were similar, around 2 × 108 dnaA copy equivalents. However, throughout host aging, the two spiroplasmas showed strikingly different population growth patterns. The titers of NSRO increased exponentially for 3 weeks, attained a peak value of around 4 × 109 dnaA copy equivalents per insect, and then decreased. In contrast, the titers of NSRO-A were almost constant throughout the adult portion of the life cycle. In adult females, consequently, the titer of NSRO was significantly higher than the titer of NSRO-A except for a short period just after emergence. Although infection of adult females with NSRO resulted in almost 100% male killing, production of some male offspring was observed within 4 days after emergence when the titers of NSRO were as low as those of NSRO-A. Based on these results, we proposed a threshold density hypothesis for the expression of male killing caused by the spiroplasma. The extents of the bottleneck in the vertical transmission through host generations were estimated to be 5 × 10−5 for NSRO and 3 × 10−4 for NSRO-A.
Occasionally, female-biased sex ratios have been found in the progeny of wild-caught female insects. This trait has been referred to as the sex ratio (SR) trait. The mated daughters of such females were found to produce daughters and few or no sons. In many cases, the female-biased sex ratios are expressed through selective death of male offspring. Such SR traits have been reported for a wide variety of insect taxa (reviewed in references 22, 26, and 28). In some of these cases, it has been demonstrated that the SR traits are caused by maternally inherited endosymbiotic bacteria. These microorganisms are called male-killing bacteria and have been found in diverse bacterial taxa, such as the genus Spiroplasma of the class Mollicutes (24, 60), the genera Rickettsia and Wolbachia of the α subdivision of the division Proteobacteria (25, 57), the genus Arsenophonus of the γ subdivision of the Proteobacteria (15), an unidentified bacterium belonging to the Flavobacterium-Bacteroides group (23), and others.
Among fruit flies of the genus Drosophila, SR traits have been reported in the following 10 species: D. bifasciata (34), D. prosaltans (7), D. borealis (6), D. willistoni, D. paulistorum (35, 37), D. equinoxialis (36), D. nebulosa (50), D. robusta (60), D. roehrae (56), and D. melanogaster (44). In the D. willistoni group of the genus Drosophila, the causative agents of the SR trait have traditionally been termed sex ratio organisms and have been identified as endosymbiotic bacteria belonging to the genus Spiroplasma (60). Cells of the male-killing spiroplasmas are helical and actively motile, and they are abundant in the hemolymph of adult flies (51, 52, 60). The spiroplasmas are vertically transmitted from mothers to eggs through host generations. Male eggs produced by infected females do not hatch, whereas female eggs hatch and develop normally. The spiroplasmas can be experimentally transferred from infected insects to uninfected insects by injection of hemolymph. Successful transfer results in a stable inherited infection and expression of a female-biased sex ratio in the recipients. Intra- and interspecific transfer experiments have revealed the potential host ranges of the spiroplasmas. Usually, a strain of spiroplasma can establish a stable infection and express male killing in several Drosophila species (38, 39, 48, 49, 50, 51, 53, 54, 59, 60). Several strains of male-killing spiroplasmas have been maintained in stocks of laboratory flies and have been used for studies of male-killing mechanisms; these strains include NSRO from D. nebulosa, WSRO from D. willistoni, ESRO from D. equinoxialis, and PSRO from D. paulistorum. The male-killing spiroplasmas are generally difficult to culture in vitro. Only one case of successful cultivation of WSRO has been reported (17). Based on this isolate, a new species, Spiroplasma poulsonii, was described (61).
Needless to say, male-specific lethality has been the focus of studies on the spiroplasmas. Embryological studies showed that male eggs die at early developmental stages before gastrulation (8). Genetic and mutant analyses demonstrated that insects with the male karyotype are selectively killed, regardless of their sexual phenotype (43, 53). Mosaic contour analysis and in vitro tissue culture suggested that the lethal target tissues might be the primordial mesoderm and nervous system of male embryos (30, 55). In spite of these intensive studies, however, the mechanism of male killing still remains largely unknown.
Some strains of spiroplasmas do not express male killing in the host flies (47). Notably, during maintenance of male-killing strain NSRO, Yamada et al. (62) serendipitously isolated a non-male-killing strain of spiroplasma, NSRO-A. To understand the mechanism of male killing, it will be of great interest to compare various biological aspects of the closely related spiroplasma strains NSRO and NSRO-A. In this study, we investigated the population dynamics of NSRO and NSRO-A throughout the developmental course of the laboratory host, D. melanogaster, using quantitative PCR analysis.
MATERIALS AND METHODS
Materials.
An Oregon-R (OR) strain of D. melanogaster, supplied by T. Murata (Institute of Physical and Chemical Research [RIKEN]), was used as the standard wild-type host strain. T. Koana (Railway Technical Research Institute) provided two fly stocks carrying either NSRO or NSRO-A. By hemolymph injection into the same OR strain, we generated two fly strains, an OR strain carrying male-killing strain NSRO and an OR strain carrying non-male-killing strain NSRO-A. These fly strains were reared in plastic vials with a standard cornmeal medium at 25°C by using a long-day regimen (16 h of light and 8 h of darkness). Since the NSRO-infected OR strain produced an all-female brood, males of an uninfected OR strain were supplied to maintain the fly stock.
Hemolymph injection.
Collection and injection of spiroplasma-laden hemolymph were conducted under a dissecting microscope by using thin glass capillary tubes made with a microelectrode maker (PN-3; Narishige Scientific Instrument) connected to an air pump. On a sheet of Parafilm membrane, a physiological saline solution for Drosophila (0.7% NaCl, 0.02% KCl, 0.002% CaCl2 · H2O, 0.01% MgCl2 · 6H2O, 0.005% NaHCO3, 0.02% NaH2PO4 · 2H2O, 0.08% glucose [pH 6.8]) was injected into the thoraces of adult donor flies. Hemolymph diluted with the saline leaking out of the injury was collected with a micropipette and was sucked into a glass capillary tube. The tip of the capillary was inserted into the thoraces of adult recipient flies that were anesthetized on ice. Approximately 0.2 μl of the solution was injected into each of the recipients. Successful infection of the offspring of the recipients with the spiroplasmas was checked by PCR detection, microscopic observation of hemolymph, and/or expression of the male-killing phenotype in three successive generations.
Collection of samples of different developmental stages of insects.
To collect samples of different developmental stages of insects, 10 female and 5 male adult flies were reared in each plastic vial. Eggs and first-, second-, and third-instar larvae were harvested 12, 36, 84, and 108 h after the start of oviposition, respectively. Early and late pupae were collected 156 and 204 h after the start of oviposition. Adult insects were collected within 12 h after emergence and also 1, 2, 3, 4, 5, and 7 weeks after emergence. Collected samples were immediately preserved in acetone until DNA analysis was performed (10).
DNA extraction.
The samples preserved in acetone were briefly dried in air. Adults and pupae were weighed individually. Total DNA was extracted from the samples with a QIAamp tissue kit (Qiagen). Adults, pupae, and second- and third-instar larvae were extracted individually. Because eggs and first-instar nymphs were too small to be treated individually, 100 individuals were combined and subjected to the DNA extraction procedure. Purified DNA from a sample was eluted with 400 μl of AE buffer supplied in the kit.
PCR, cloning, and sequencing.
A 0.5-kb segment of the dnaA gene of Spiroplasma spp. was amplified by PCR by using primers SRdnaAF1 (5′-GGAGAYTCTGGAYTAGGAAA-3′) and SRdnaAR1 (5′-CCYTCTAWYTTTCTRACATCA-3′) and the following temperature profile: 94°C for 2 min, followed by 30 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Cloning and sequencing of the PCR products were performed as previously described (11).
Diagnostic PCR.
PCR detection of the spiroplasmas was conducted by using the specific reverse primer TKSSsp in combination with the universal forward primer 16SA1 as previously described (12). To differentiate the sexes of host insects at larval and pupal stages, diagnostic PCR targeted to a Y chromosome-specific gene of D. melanogaster, kl5 (14, 16), was conducted. The specific primers kl5-1 (5′-GCTATAAACTTTAACGCAGTC-3′) and kl5-2 (5′-GCAAGCAATATGCTCTC-3′) were provided by G. D. D. Hurst (University College London). PCRs were performed by using a temperature profile consisting of 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min.
Quantitative PCR.
To monitor populations of NSRO and NSRO-A throughout the developmental phases of host insects, real-time fluorescence detection quantitative PCR was performed by using a TaqMan PCR and ABI PRISM 7700 sequence detection system (PE Applied Biosystems) essentially as previously described (33). As an index of the titer of the spiroplasmas, the copy number of the dnaA gene was determined by using the probe and primers DnaA180T (5′-FAM-AGCTTCAAATCCACCAAGATCATCAGGA-TAMRA-3′), DnaA109F (5′-TTAAGAGCAGTTTCAAAATCGGG-3′), and DnaA246R (5′-TGAAAAAAACAAACAAATTGTTATTACTTC-3′). To estimate the density of the spiroplasmas, the copy number of a host gene, the elongation factor 1α (ef1α) gene, was determined for the same samples by using the probe and primers EF157T (5′-FAM-CAAGTCGACGACCACCGGCCAC-TAMRA-3′), EF23F (5′-TTAACATTGTGGTCATTGGCCA-3′), and EF123R (5′-CTTCTCAATCGTACGCTTGTCG-3′).
Monitoring of male-killing expression.
Fifty adult females infected with NSRO were collected within 12 h after emergence. Ten females were placed in five plastic vials, mated with five uninfected males, and allowed to oviposit. During the first 8 days the flies were transferred to new vials every day; after this they were transferred every 2 to 4 days. The vials were kept at 25°C, and the sex ratios of the emerging offspring were recorded.
Statistical analysis.
The Mann-Whitney U test was performed to compare the densities of the spiroplasmas in the NSRO-infected females, the NSRO-A-infected females, and the NSRO-A-infected males by using the software program StatView, version J-4.5 (Abacus Concepts, Berkeley, Calif.).
Nucleotide sequence accession numbers.
The nucleotide sequences of dnaA gene fragments from NSRO and NSRO-A determined in this study have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB089822 and AB089823.
RESULTS
Infection dynamics of male-killing and non-male-killing spiroplasmas through early developmental phases of the host insect.
Populations of NSRO and NSRO-A were monitored during the development of the host insect by using a quantitative PCR technique. Below, we principally describe the results of comparisons between female insects infected with NSRO and female insects infected with NSRO-A; on occasion involvement of male insects infected with NSRO-A is mentioned.
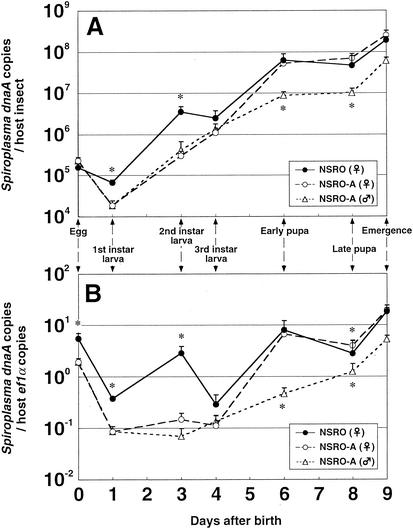
Figure 1A shows the population dynamics of the spiroplasmas expressed in terms of the number of dnaA copies per host insect. In eggs, the titers of NSRO and NSRO-A were almost the same, around 2 × 105 dnaA copy equivalents per insect. The titers of the spiroplasmas increased as host larval development proceeded, although a transient decrease in the titers was observed at the first-instar stage. At the first- and second-instar stages, the titers of NSRO were significantly higher than those of NSRO-A, whereas at the third-instar and pupal stages, the titers of the two strains were similar. In early and late pupae, the titers of the spiroplasmas reached a plateau of around 5 × 107 dnaA copy equivalents per insect. The titers of NSRO-A in male insects were comparable to those in female insects in larvae. However, at the pupal stages the titers of NSRO-A in males were significantly lower than those in females.
FIG. 1.
Infection dynamics of male-killing and non-male-killing spiroplasmas throughout the early developmental stages of D. melanogaster, expressed in terms of the number of dnaA copies per individual insect (A) or per host ef1α gene copy (B). Five replicated measurements for the egg and first-instar stages and 10 replicated measurements for the later stages were obtained. Note that for the tiny eggs and first-instar larvae five samples, each containing 100 individuals, were used for DNA extraction and quantitative PCR analysis. The error bars indicate standard errors. An asterisk indicates that there is a significant difference between NSRO-infected females and NSRO-A-infected females (asterisks above the lines) or between NSRO-A-infected females and males (asterisks below the lines) (P < 0.05, as determined by the Mann-Whitney U test).
Figure 1B shows the density dynamics of the spiroplasmas expressed in terms of the number of dnaA copies per ef1α copy. The densities of the spiroplasmas were generally higher in eggs and pupae than in larvae. However, the density dynamics patterns were different between NSRO and NSRO-A. In larvae the densities of NSRO-A were consistently low, whereas the densities of NSRO exhibited a transient increase at the second-instar stage. At the egg, first-instar, and second-instar stages, the densities of NSRO were significantly higher than those of NSRO-A, whereas at the third-instar and pupal stages, the densities of the two strains were similar. The densities of NSRO-A in male insects were comparable to those in female insects in larvae but were significantly lower in pupae.
Infection dynamics of male-killing and non-male-killing spiroplasmas during aging of adult insects.
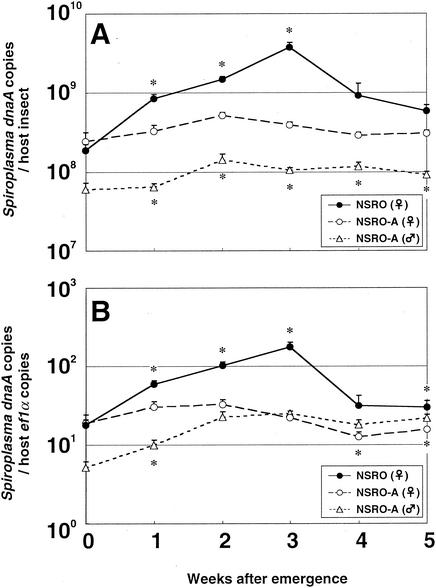
Figure 2A shows the population dynamics of the spiroplasmas expressed in terms of the number of dnaA copies per host insect. Upon adult emergence, the titers of NSRO and NSRO-A were similar, around 2 × 108 dnaA copy equivalents per insect. As the host aged, however, the two spiroplasmas showed strikingly different population growth patterns. The titers of NSRO increased exponentially for 3 weeks, attained a peak value of around 4 × 109 dnaA copy equivalents per insect, and then decreased. In contrast, the titers of NSRO-A were almost constant throughout the adult phase. In adult females, consequently, the titers of NSRO were significantly higher than the titers of NSRO-A except for a short period just after emergence. The titers of NSRO-A in adult males were significantly lower than those in adult females, and they were almost constant throughout the adult phase.
FIG. 2.
Infection dynamics for male-killing and non-male-killing spiroplasmas during aging of D. melanogaster adults, expressed in terms of the number of dnaA copies per insect (A) or per host ef1α gene copy (B). Ten replicated measurements were obtained. The error bars indicate standard errors. An asterisk indicates that there is a significant difference between NSRO-infected females and NSRO-A-infected females (asterisks above the lines) or between NSRO-A-infected females and males (asterisks below the lines) (P < 0.05, as determined by the Mann-Whitney U test).
Figure 2B shows the density dynamics of the spiroplasmas expressed in terms of the number of dnaA copies per ef1α copy. The density dynamics were quite similar to those shown in Fig. 2A. The densities of NSRO increased exponentially for 3 weeks and then decreased, whereas the densities of NSRO-A were almost constant. The densities of NSRO-A in adult males were lower than those in adult females for 2 weeks after emergence. In older insects, however, the densities of NSRO-A were higher in males than in females.
Expression of the male-killing phenotype during aging of adult insects.
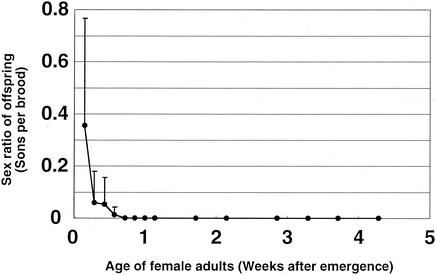
NSRO-infected female adults were examined to determine the sex ratio of their offspring in relation to their age (Fig. 3). Newly emerged infected females produced an average of 36% male offspring on the day of emergence. Starting on the next day, however, extremely female-biased sex ratios were observed. Several days later, no males were produced, and only female offspring were produced throughout the rest of adult phase.
FIG. 3.
Relationship between the age of female adults of D. melanogaster infected with male-killing spiroplasma strain NSRO and the sex ratio of their offspring. Five replicated measurements were obtained. The error bars indicate standard deviations.
DISCUSSION
This study provides the first detailed quantitative analysis of the population dynamics of male-killing and non-male-killing spiroplasmas throughout the developmental phases of a host insect. There were several pioneer studies in which the researchers attempted to estimate the amounts of male-killing spiroplasmas in their hosts. In several Drosophila species, observation by dark-field microscopy showed that male-killing spiroplasmas are particularly abundant in the adult hemolymph (38, 51, 52), although accurate direct counts of small and actively motile spiroplasma cells could not be obtained. Sakaguchi and Poulson (52) developed a bioassay to assess the spiroplasma titer based on the male-killing activity induced by injection of tissue homogenate into uninfected host insects. By using this technique, it was demonstrated that the male-killing activity was very high in adult hemolymph, whereas weak activities were detected in adult fat bodies, thoracic muscles, and ovaries and in larval hemolymph. However, the injection assay is tedious and requires many donor and recipient insects for each assay, and the sensitivity and resolution are low. The quantitative PCR assay developed in this study enabled us to reliably and efficiently quantify the spiroplasmas in individual host insects.
The non-male-killing spiroplasma strain, NSRO-A, was established from the male-killing spiroplasma strain, NSRO, in 1979. Strains NSRO and NSRO-A exhibited the same properties, such as clump-forming spectrum and associating virus (62). Therefore, NSRO and NSRO-A are no doubt genetically very similar. The only difference between them identified previously is the male-killing phenotype. In this study, we demonstrated that NSRO and NSRO-A exhibited differences not only in the male-killing phenotype but also in population dynamics throughout host development (Fig. 1 and 2). These results suggest that the difference in the proliferation profiles of NSRO and NSRO-A might be related to the difference in the male-killing activities of the organisms.
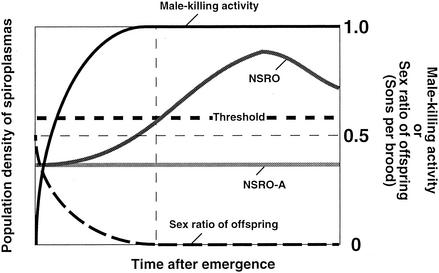
Although it is not understood why NSRO-A does not kill males, one possible explanation is that NSRO-A is a variant of NSRO that is deficient in the male-killing mechanism. Alternatively, even if the male-killing mechanism is intact, NSRO-A may not kill males because it cannot proliferate in the host body. If it is assumed that a threshold density of spiroplasma cells in adult females is required for the expression of male killing, all of the following results obtained in this study are consistently explained: (i) in adult females the density of NSRO was consistently higher than that of NSRO-A; (ii) the density of NSRO was the lowest in newly emerged females and increased exponentially as the insects aged; (iii) the density of NSRO-A was almost constant throughout the adult phase; (iv) the density of NSRO was as low as that of NSRO-A only for a short period just after emergence; and (v) females infected with NSRO failed to express a complete male-killing phenotype only for a short period just after emergence. Figure 4 shows a schematic illustration of the threshold density hypothesis. With this hypothesis, the threshold density of spiroplasmas required for expression of the male-killing phenotype is estimated to be around 6 × 108 dnaA copy equivalents per adult female. However, whether the lower proliferation of NSRO-A in adult females is causally related to the loss of the male-killing phenotype requires further confirmation. Several studies have suggested that in the Wolbachia-insect endosymbiosis, the bacterial density in the host tends to be positively correlated with the intensity of cytoplasmic incompatibility (2, 4, 5, 45) or male-killing (27) phenotypes of the hosts.
FIG. 4.
Threshold density hypothesis for the expression of male killing caused by spiroplasmas.
It is not known why NSRO-A does not proliferate as well as NSRO in the adult host body. NSRO-A may simply have an intrinsic proliferation rate that is lower than that of NSRO. Alternatively, an interaction with the host immune system may be responsible for the difference in proliferation. In the hemolymph of Drosophila and other insects, elaborate innate immune mechanisms, such as a phenol oxidase cascade and various antimicrobial peptides, are active against potentially pathogenic microorganisms (19, 20). The possibility that endosymbiotic microorganisms are also affected by the host immune mechanisms appears to be plausible (3, 18). Considering that the main location of the spiroplasmas is the adult hemolymph, it is conceivable that NSRO-A may be deficient in some mechanism which allows organisms to tolerate the innate immune system of the host insect. If this is so, the density of NSRO-A might be greater in some Drosophila mutants that are deficient in innate immune mechanisms, which could provide a crucial approach for testing the threshold density hypothesis for expression of male killing.
In this study, the results suggested that the density of the spiroplasmas in the maternal body may be related to the expression of male killing. There are two possible ways to explain how a high level of infection in mothers leads to the expression of male killing. One possibility is that higher levels of maternal infection result in higher levels of spiroplasma cells transmitted to the eggs, and the activity of live bacteria at densities above a threshold density in the eggs causes specific death of male embryos. The other possibility is that higher levels of maternal infection result in accumulation of a male-killing factor in the eggs, and activity of the factor at concentrations above a threshold concentration kills male embryos. To gain insight into which of these possibilities is more plausible, the relationships between the level of spiroplasma infection in eggs and the intensity of the male-killing phenotype should be investigated.
In eggs of D. melanogaster, the titers of NSRO and NSRO-A were both around 2 × 105 dnaA copy equivalents per egg (Fig. 1A), suggesting that there is not a significant difference in vertical transmission efficiency between these spiroplasma variants. It has also been suggested that the difference in the male-killing phenotype between the strains is not due to different levels of bacterial infection in eggs.
Vertically transmitted endosymbiotic microorganisms inevitably experience strong bottlenecks between host generations. Theory predicts that the extent of the bottleneck has important consequences for the evolutionary genetics and ecology of the bacterium, such as the amount of genetic drift, the rate of accumulation of deleterious and advantageous mutations, and the genetic structure of the population, and other consequences (42). However, the extent of the bottleneck for endosymbiotic bacteria during vertical transmission has been quantitatively estimated in only a few studies. There have been a number of studies in which the population sizes of endosymbiotic bacteria were assessed by using histological or molecular techniques (1, 2, 4, 5, 21, 29, 33, 41, 42, 46, 58). However, to calculate the severity of a bottleneck, the numbers of endosymbionts in both adults and eggs must be accurately estimated.
In this study, we showed that the titers of NSRO and NSRO-A in eggs were both around 2 × 105 dnaA copy equivalents per egg (Fig. 1A), whereas the maximum titers of NSRO and NSRO-A in adult females were around 4 × 109 and 6 × 108 dnaA copy equivalents per insect, respectively (Fig. 2A). Based on these values, the bottlenecks per generation were roughly calculated to be around 5 × 10−5 for NSRO and 3 × 10−4 for NSRO-A. To our knowledge, this is the first reliable estimate of the bottleneck for an endosymbiotic bacterium through host generations. It should be noted, however, that these values are based on several uncertainties which are inherent in this sort of analysis. First, vertical transmission of the spiroplasma cells occurs during oogenesis. Therefore, the titers obtained from mature eggs should lead to an overestimate of the real values. Second, the spiroplasma cells must turn over in vivo; some cells divide, some die or are already dead, and some are consumed by the host. The rate of turnover might be dependent on the tissues and developmental stages, which would lead to unpredictable biases of the estimated values.
For vertical transmission of NSRO into the host eggs, the results of an ultrastructural study were described previously (45). The spiroplasma cells break through the tunica propria, the noncellular membrane surrounding the egg chamber, and move toward oocytes, passing through the intracellular space of the follicle cell layer at the previtellogenic stages. After reaching the surface of an oocyte, the bacterial cells are incorporated into the ooplasm by endocytosis during vitellogenesis. By the final stage of oogenesis, most of the spiroplasma cells become infolded in intracellular vesicles and yolk granules. In this study, we found that the titers of NSRO and NSRO-A in first-instar larvae were significantly lower than those in eggs (Fig. 1A). In the process of embryonic development, yolk in the egg is consumed to construct the embryo body. The reduction in the spiroplasma titer at the first-instar stage may be explained in this context. If so, the process would further contribute to the extent of the bottleneck.
Infection with spiroplasmas may affect fitness components of the host insects, such as body size, reproduction, and longevity (9, 13, 40). In this study, the titers of ef1α, an index of the cell number in the host insect, were for the most part similar for female insects infected with NSRO and female insects infected with NSRO-A throughout the developmental course (data not shown), suggesting that the fitness effects, if there are any, are not drastically different between NSRO and NSRO-A.
Notably, however, a significant difference in ef1α copy number was detected only at the egg stage; the titer of ef1α in NSRO-infected eggs was around fourfold lower than that in NSRO-A-infected eggs. Consequently, at the egg stage, the density of NSRO was estimated to be significantly higher than the density of NSRO-A (Fig. 1B), despite the fact that there was no significant difference in bacterial titer between NSRO- and NSRO-A-infected eggs (Fig. 1A). The lower titer of ef1α in NSRO-infected eggs probably reflected the developmental arrest in eggs destined to be male. It was demonstrated previously that in eggs produced by spiroplasma-infected females, development of male embryos stops at early stages before gastrulation (8). It should be noted that in this study 100 eggs, one-half of which should have been male, were combined and used for DNA extraction.
Spiroplasmas from Drosophila species are in general difficult to culture, although a successful case was reported by Hackett et al. (17). Direct counting of spiroplasma cells is also difficult because of their small size and active motility. Therefore, quantification of gene copy number provides a practical and reliable approach for estimating the amount of spiroplasmas. It should be noted, however, that gene copy numbers are not always in strict agreement with microbial cell numbers obtained by standard quantification methods, such as CFU and direct cell counting, for a number of reasons (29, 31, 32, 33).
Acknowledgments
We thank A. Sugimura, S. Kumagai, S. Tatsuno, H. Ouchi, and K. Sato for technical and secretarial assistance; T. Koana and T. Murata for providing fly strains; and G. D. D. Hurst for providing kl5 primers and for reading the manuscript.
This research was supported by the Program for Promotion of Basic Research Activities for Innovation Biosciences (ProBRAIN) of the Bio-Oriented Technology Research Advancement Institution.
REFERENCES
- 1.Baumann, L., and P. Baumann. 1994. Growth kinetics of the endosymbiont Buchnera aphidicola in the aphid Schizaphis graminum. Appl. Environ. Microbiol. 60:3440-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourtzis, K., A. Nirgianaki, G. Markakis, and C. Savakis. 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144:1063-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourtzis, K., M. M. Pettigrew, and S. L. O'Neill. 2000. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol. Biol. 9:635-639. [DOI] [PubMed] [Google Scholar]
- 4.Breeuwer, J. A. J., and J. H. Werren. 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bressac, C., and F. Rousset. 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invertebr. Pathol. 61:226-230. [DOI] [PubMed] [Google Scholar]
- 6.Carson, H. L. 1956. A female-producing strain of Drosophila borealis Patterson. Drosoph. Infect. Serv. 30:109-110. [Google Scholar]
- 7.Cavalcanti, A. G. L., D. N. Falcão, and L. E. Castro. 1957. “Sex-ratio” in Drosophila prosaltans—a character due to interaction between nuclear genes and cytoplasmic factors. Am. Nat. 91:321-325. [Google Scholar]
- 8.Counce, S. J., and D. F. Poulson. 1962. Developmental effects of the sex-ratio agent in embryos of Drosophila willistoni. J. Exp. Zool. 151:17-31. [DOI] [PubMed] [Google Scholar]
- 9.Ebbert, M. A. 1991. The interaction phenotype in the Drosophila willistoni-spiroplasma symbiosis. Evolution 45:971-988. [DOI] [PubMed] [Google Scholar]
- 10.Fukatsu, T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935-1945. [DOI] [PubMed] [Google Scholar]
- 11.Fukatsu, T., and N. Nikoh. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 64:3599-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukatsu, T., and N. Nikoh. 2000. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl. Environ. Microbiol. 66:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukatsu, T., T. Tsuchida, N. Nikoh, and R. Koga. 2001. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 67:1284-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gepner, J., and T. S. Hays. 1993. A fertility region on the Y chromosome of Drosophila melanogaster encodes a dynein microtubule motor. Proc. Natl. Acad. Sci. USA 90:11132-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherna, R. L., J. H. Werren, W. Weisburg, R. Cote, C. R. Woese, L. Mandelco, and D. J. Brenner. 1991. Arsenophonus nasoniae gen. nov., sp. nov., the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. Int. J. Syst. Bacteriol. 41:563-565. [Google Scholar]
- 16.Goldstein, L. S. B., R. W. Hardy, and D. L. Lindsley. 1982. Structural genes on the Y chromosome of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 79:7405-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackett, K. J., D. E. Lynn, D. L. Williamson, A. S. Ginsberg, and R. F. Whitcomb. 1986. Cultivation of the Drosophila sex-ratio spiroplasma. Science 232:1253-1255. [DOI] [PubMed] [Google Scholar]
- 18.Hao, Z., I. Kasumba, M. J. Lehane, W. C. Gibson, J. Kwon, and S. Aksoy. 2001. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc. Natl. Acad. Sci. USA 98:12648-12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann, J. A. 1995. Innate immunity of insects. Curr. Opin. Immunol. 7:4-10. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, J. A., J. M. Reichart, and C. Hetru. 1996. Innate immunity in higher insects. Curr. Opin. Immunol. 8:8-13. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys, N. J., and A. E. Douglas. 1997. Partitioning of symbiotic bacteria between generations of an insect: a quantitative study of a Buchnera sp. in the pea aphid (Acyrthosiphon pisum) reared at different temperatures. Appl. Environ. Microbiol. 63:3294-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst, G. D. D., and M. E. N. Majerus. 1993. Why do maternally inherited microorganisms kill males? Heredity 71:81-95. [Google Scholar]
- 23.Hurst, G. D. D., T. C. Hammarton, T. M. O. Majerus, D. Bertrand, C. Bandi, and M. E. N. Majerus. 1997. Close relationship of the inherited parasite of the ladybird, Coleomegilla maculata, to Blattabacterium, the beneficial symbiont of the cockroach. Genet. Res. 70:1-6. [Google Scholar]
- 24.Hurst, G. D. D., J. H. G. von der Schulenburg, T. M. O. Majerus, D. Bertrand, I. A. Zakharov, J. Baungaard, W. Völkl, R. Stouthamer, and M. E. N. Majerus. 1999. Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol. Biol. 8:133-139. [DOI] [PubMed]
- 25.Hurst, G. D. D., F. M. Jiggins, J. H. G. von der Schulenburg, D. Bertrand, S. A. West, I. I. Goriacheva, I. A. Zakharov, J. H. Werren, R. Stouthamer, and M. E. N. Majerus. 1999. Male-killing Wolbachia in two species of insect. Proc. R. Soc. Lond. B 266:735-740.
- 26.Hurst, G. D. D., and F. M. Jiggins. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6:329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurst, G. D. D., A. P. Johnson, J. H. G. von der Schulenburg, and Y. Fuyama. 2000. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst, L. D. 1993. The incidences, mechanisms and evolution of cytoplasmic sex ratio distorters in animals. Biol. Rev. 68:121-193. [Google Scholar]
- 29.Ijichi, N., N. Kondo, R. Matsumoto, M. Shimada, H. Ishikawa, and T. Fukatsu. 2002. Internal spatiotemporal population dynamics of infection with three Wolbachia strains in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Appl. Environ. Microbiol. 68:4074-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koana, T., and T. Miyake. 1983. Effects of the sex ratio organism on in vitro differentiation of Drosophila embryonic cells. Genetics 104:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komaki, K., and H. Ishikawa. 1999. Intracellular bacterial symbionts of aphids possess many genomic copies per bacterium. J. Mol. Evol. 48:717-722. [DOI] [PubMed] [Google Scholar]
- 32.Komaki, K., and H. Ishikawa. 2000. Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem. Mol. Biol. 30:253-258. [DOI] [PubMed] [Google Scholar]
- 33.Kondo, N., N. Ijichi, M. Shimada, and T. Fukatsu. 2002. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 11:167-180. [DOI] [PubMed] [Google Scholar]
- 34.Magni, G. E. 1953. ′Sex-ratio': a non-Mendelian character in Drosophila bifasciata. Nature 4367:81.. [DOI] [PubMed] [Google Scholar]
- 35.Malogolowkin, C. 1958. Maternally inherited “sex ratio” conditions in Drosophila willistoni and Drosophila paulistorum. Genetics 43:274-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malogolowkin, C. 1959. Temperature effects on maternally inherited “sex ratio” conditions in Drosophila willistoni and Drosophila equinoxialis. Am. Nat. 93:365-368. [Google Scholar]
- 37.Malogolowkin, C., and D. F. Poulson. 1957. Infective transfer of maternally inherited abnormal sex-ratio in Drosophila willistoni. Science 126:32.. [DOI] [PubMed] [Google Scholar]
- 38.Malogolowkin, C., and G. G. Carvalho. 1961. Direct and indirect transfer of the “sex-ratio” condition in different species of Drosophila. Genetics 46:1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malogolowkin, C., G. G. Carvalho, and M. C. da Paz. 1960. Interspecific transfer of the “sex-ratio” condition in Drosophila. Genetics 45:1553-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malogolowkin-Cohen, C., and M. A. Q. Rodriguez-Pereira. 1975. Sexual drive of normal and SR flies of Drosophila nebulosa. Evolution 29:579-580. [DOI] [PubMed] [Google Scholar]
- 41.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mira, A., and N. A. Moran. 2002. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44:137-143. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto, C., and K. Oishi. 1975. Effects of SR-spirochete infection on Drosophila melanogaster carrying intersex genes. Genetics 79:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montenegro, H., W. N. Souza, D. D. S. Leite, and L. B. Klaczko. 2000. Male-killing selfish cytoplasmic element causes sex-ratio distortion in Drosophila melanogaster. Heredity 85:465-470. [DOI] [PubMed] [Google Scholar]
- 45.Niki, Y. 1988. Ultrastructural study of the sex ratio organism (SRO) transmission into oocytes during oogenesis in Drosophila melanogaster. Jpn. J. Genet. 63:11-21. [Google Scholar]
- 46.Noda, H., Y. Koizumi, Q. Zhang, and K. Deng. 2001. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 31:727-737. [DOI] [PubMed] [Google Scholar]
- 47.Ota, T., M. Kawabe, K. Oishi, and D. F. Poulson. 1979. Non-male-killing spiroplasmas in Drosophila hydei. J. Hered. 70:211-213. [Google Scholar]
- 48.Poulson, D. F. 1968. Nature, stability, and expression of hereditary SR infections in Drosophila, p. 91-92. In Proceedings of the XII International Congress of Genetics, vol. 2. The Science Council of Japan, Tokyo.
- 49.Poulson, D. F., and B. Sakaguchi. 1960. Evidence concerning the nature of the “sex-ratio” agent in Drosophila. Anat. Rec. 138:376-377. [Google Scholar]
- 50.Poulson, D. F., and B. Sakaguchi. 1961. Hereditary infections in Drosophila. Genetics 46:890-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi, B., and D. F. Poulson. 1960. Transfer of the “sex-ratio” condition from Drosophila willistoni to D. melanogaster. Anat. Rec. 138:381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaguchi, B., and D. F. Poulson. 1961. Distribution of “sex- ratio” agent in tissues of Drosophila willistoni. Genetics 46:1665-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi, B., and D. F. Poulson. 1963. Interspecific transfer of the “sex-ratio” condition from Drosophila willistoni to D. melanogaster. Genetics 48:841-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakaguchi, B., H. Chikushi, D. F. Poulson, and K. Oishi. 1968. Genetic diversities and chemical properties of SR spirochetes in Drosophila, p. 88-89. In Proceedings of the XII International Congress of Genetics, vol. 2. The Science Council of Japan, Tokyo.
- 55.Tsuchiyama, S., B. Sakaguchi, and K. Oishi. 1978. Analysis of gynandromorph survivals in Drosophila melanogaster infected with the male-killing SR organisms. Genetics 89:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaz, S. C., M. D. Vibranovski, and A. B. Carvalho. 1998. Sex-ratio citoplasmático em Drosophila roehrae. Genet. Mol. Biol. 21(Suppl.):260. [Google Scholar]
- 57.Werren, J. H., G. D. D. Hurst, W. Zhang, J. A. J. Breeuwer, R. Stouthamer, and M. E. N. Majerus. 1994. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 176:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson, T. L., and A. E. Douglas. 1998. Host cell allometry and regulation of the symbiosis between pea aphids, Acyrthosiphon pisum, and bacteria, Buchnera. J. Insect Physiol. 44:629-635. [DOI] [PubMed] [Google Scholar]
- 59.Williamson, D. L. 1965. Kinetic studies of “sex ratio” spirochetes in Drosophila melanogaster Meigen females. J. Invertebr. Pathol. 7:493-501. [DOI] [PubMed] [Google Scholar]
- 60.Williamson, D. L., and D. F. Poulson. 1979. Sex ratio organisms (spiroplasmas) of Drosophila, p. 175-208. In R. F. Whitcomb and J. G. Tully (ed.), The mycoplasmas, vol. 3. Academic Press, New York, N.Y.
- 61.Williamson, D. L., B. Sakaguchi, K. J. Hackett, R. F. Whitcomb, J. G. Tully, P. Carle, J. M. Bové, J. R. Adams, M. Konai, and R. B. Henegar. 1999. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int. J. Syst. Bacteriol. 49:611-618. [DOI] [PubMed] [Google Scholar]
- 62.Yamada, M., S. Nawa, and T. K. Watanabe. 1982. A mutant of SR organism (SRO) in Drosophila that does not kill the host males. Jpn. J. Genet. 57:301-305. [Google Scholar]