Abstract
The P-glycoprotein homolog of the human malaria parasite Plasmodium falciparum (Pgh-1) has been implicated in decreased susceptibility to several antimalarial drugs, including quinine, mefloquine and artemisinin. Pgh-1 mainly resides within the parasite's food vacuolar membrane. Here, we describe a surrogate assay for Pgh-1 function based on the subcellular distribution of Fluo-4 acetoxymethylester and its free fluorochrome. We identified two distinct Fluo-4 staining phenotypes: preferential staining of the food vacuole versus a more diffuse staining of the entire parasite. Genetic, positional cloning and pharmacological data causatively link the food vacuolar Fluo-4 phenotype to those Pgh-1 variants that are associated with altered drug responses. On the basis of our data, we propose that Pgh-1 imports solutes, including certain antimalarial drugs, into the parasite's food vacuole. The implications of our findings for drug resistance mechanisms and testing are discussed.
Keywords: drug transport, Fluo-4, malaria, P-glycoprotein
Introduction
Many developing countries are engaged in a battle against malaria, an infectious disease that is endemic throughout most of Africa, Southeast Asia and Latin America, where it causes an estimated 515 million clinical cases annually (Snow et al, 2005). Options to control the spread of malaria are increasingly limited as widely used antimalarials are losing their efficacy, including the 4-aminoquinoline drug chloroquine and the folate antagonists pyrimethamine and sulfadoxine (Baird, 2005). Moreover, reduced susceptibility has emerged to other antimalarials, including quinine, mefloquine, and possibly artemether (Baird, 2005; Jambou et al, 2005).
In both microorganisms and tumors, drug resistance can arise from the presence of P-glycoproteins (P-gp) that are capable of extruding a broad range of structurally and functionally unrelated cytotoxic agents (Borges-Walmsley et al, 2003). P-gp belong to the ABC (ATP-binding cassette) transporter superfamily and are encoded by mdr genes. The human malaria parasite Plasmodium falciparum possesses an mdr homologue (pfmdr1) whose gene product, Pgh-1, is expressed during intraerythrocytic development of the parasite (Foote et al, 1989; Wilson et al, 1989). Pgh-1 has a domain structure typical of P-gp, with two homologous domains, each comprising a hydrophobic membrane-associated segment with six transmembrane domains followed by a hydrophilic nucleotide binding fold. Pgh-1 is mainly localized to the membrane of the parasite's acidic food vacuole (Cowman et al, 1991; Cremer et al, 1995), with its ATP binding domain facing the cytoplasm (Cowman et al, 1991; Karcz et al, 1993).
pfmdr1 has been implicated in several drug resistance phenotypes. For example, a comprehensive clinical study has shown a strong association between pfmdr1 amplification and mefloquine treatment failure and in vitro resistance (Price et al, 2004). pfmdr1 amplification is further associated with in vitro resistance to halofantrine and quinine (Wilson et al, 1993; Cowman et al, 1994). Furthermore, polymorphisms at amino-acid residues 86, 184, 1034, 1042 and 1246 have been associated with altered in vitro susceptibility to chloroquine, quinine, mefloquine and artemisinin (Duraisingh et al, 2000; Reed et al, 2000; Ngo et al, 2003; Pickard et al, 2003; Sidhu et al, 2005). In particular, the N1042D substitution seems to play a prominent role in low-level quinine resistance (Sidhu et al, 2005), while the N86Y substitution has been implicated in contributing to lumefantrine and high level chloroquine resistance (Duraisingh and Cowman, 2005; Sisowath et al, 2005). How Pgh-1 affects drug phenotypes is largely unclear. Pgh-1 may directly transport drugs, either out of or into the food vacuole, or alternatively, Pgh-1 may indirectly influence drug partitioning as a result of altered transport of other substrates (reviewed in Duraisingh and Refour, 2005).
Fluorochromes such as fluorescein acetoxymethylester (AM) derivates have been widely used in imaging and in surrogate assays of P-gp function in normal and malignant cells (Szakacs et al, 1998). Fluorescein AM derivates are hydrophobic, cell-permeant substances that only fluoresce after AM hydrolysis by intracellular esterases (Szakacs et al, 1998). Cells expressing P-gp expel the nonfluorescent probe, resulting in decreased accumulation of the fluorescent dye in the cytoplasmic compartment (Szakacs et al, 1998). As the free fluorochrome is a poor substrate, P-gp transport activity can be quantitatively assessed by measuring the net accumulation of intracellular fluorescence, thereby providing information regarding P-gp protein levels and the directionality of transport (Szakacs et al, 1998). Here, we present genetic, positional cloning and pharmacological evidence suggesting that Pgh-1 mediates solute import into, as opposed to solute efflux from, the food vacuole. Moreover, our data suggest that the Fluo-4 staining pattern provides a live cell surrogate assay for Pgh-1 variants associated with altered drug responses.
Results
Clonal variation in Fluo-4 live cell imaging
Figure 1A depicts fluorescent images of different P. falciparum parasites loaded with Fluo-4 AM under standardized conditions. While the Dd2, K1 and FCB parasites showed a bright Fluo-4 fluorescence in the food vacuole and a weak fluorescence in the cytoplasm, the HB3, NF54 and 7G8 parasites revealed a distinctly more diffuse staining pattern of the entire parasite. The acidic food vacuole of the parasite was localized using the acidotropic dye LysoSensor Blue DND-192 (LS Blue) (Wissing et al, 2002; Rohrbach et al, 2005). The clonal variations in Fluo-4 staining were confirmed by quantifying the fluorescence intensities in the food vacuole and cytoplasm (Figure 1B). Both values differed significantly between the two sets of parasites, with Dd2, K1 and FCB having higher food vacuolar and lower cytoplasmic Fluo-4 fluorescence as compared to HB3, NF54 and 7G8. Accordingly, the ratio of the food vacuolar over cytoplasmic fluorescence (Rvac/cyt) differed two- to three-fold between these two sets of parasites (Figure 1C).
Figure 1.
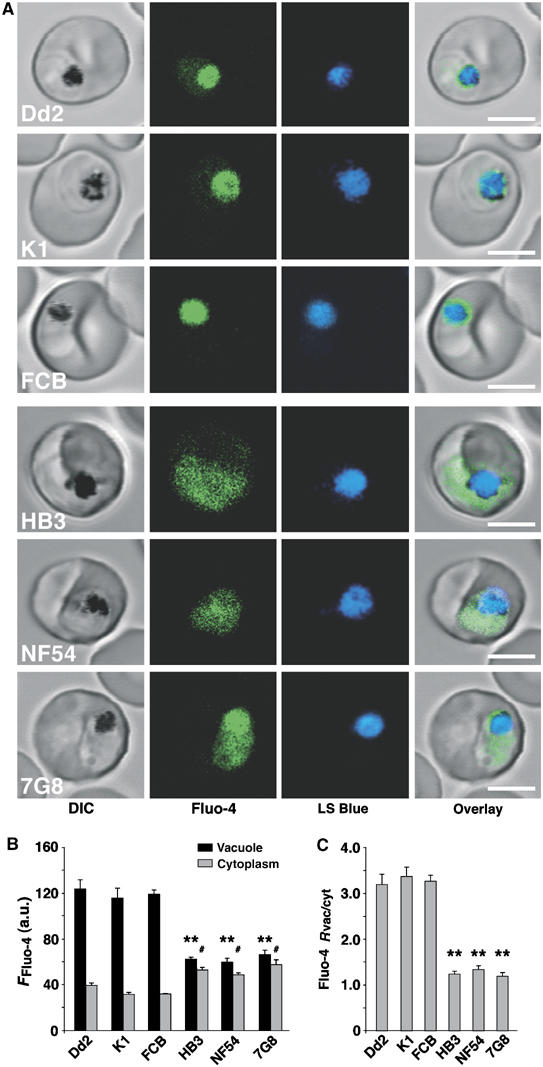
Fluo-4 fluorescence in different P. falciparum parasites. (A) Single images of P. falciparum-infected erythrocytes stained with Fluo-4 AM (green) and the acidotropic dye LS Blue (blue). Bar, 5 μm. (B) Mean Fluo-4 fluorescence quantified from the food vacuolar and cytoplasmic regions. (C) Ratios of the mean Fluo-4 fluorescence signals in the vacuolar and cytoplasmic regions (Rvac/cyt). The means±s.e. of over 15 independent determinations collected over several days are shown in (B) and (C). **P<0.001 and #P<0.05, comparing the vacuole and cytoplasm of the two groups of parasites, respectively.
As Fluo-4 fluorescence is calcium-dependent, with the fluorescence increasing with rising free calcium concentrations ([Ca2+]i), we wondered whether the differences observed in Fluo-4 live cell imaging were due to variations in intracellular Ca2+ homeostasis. Since Fluo-4 is a nonratiometric Ca2+ indicator, calibrating the fluorescence signals is difficult. In a previous study, we used the ratiometric fluorophore Fura-Red AM to quantify free [Ca2+]i in different subcellular compartments of the parasite and observed that Fura-Red, in a confocal setting, provided reliable and robust recordings of both steady-state and dynamic free [Ca2+]i (Rohrbach et al, 2005). We applied this method to a representative number of parasites. No significant differences in cytoplasmic or food vacuolar free [Ca2+]i were found between the examined parasites Dd2, K1, HB3 and NF54 (P>0.05) (Table I). The free [Ca2+]i values measured were consistent with previous determinations (Biagini et al, 2003, 2005; Rohrbach et al, 2005).
Table 1.
Steady-state free food vacuolar and cytoplasmic [Ca2+]i in different P. falciparum parasites
| Parasite | Vacuolar [Ca2+]i (nM) | Cytoplasmic [Ca2+]i (nM) |
|---|---|---|
| Dd2 | 454±60 (n=54) | 352±42 (n=73) |
| K1 | 375±57 (n=46) | 289±39 (n=45) |
| HB3 | 450±52 (n=64) | 397±34 (n=81) |
| NF54 | 441±91 (n=23) | 302±29 (n=40) |
Genetic linkage of the Fluo-4 phenotype to pfmdr1
We next investigated the Fluo-4 fluorescence pattern in 16 genetically distinct progeny from a cross between Dd2 and HB3 (Wellems et al, 1990; Su et al, 1999) (Figure 2A). Quantitative trait locus (QTL) analysis revealed a locus on chromosome 5, with the highest LOD score of >6 associated with the pfmdr1 marker (Figure 2B). In fact, all progeny that displayed an intense food vacuolar Fluo-4 staining phenotype contained the Dd2 pfmdr1 allele (Y86, Y184, S1034, N1042 and D1246), whereas progeny with a diffuse Fluo-4 staining inherited the HB3 pfmdr1 allele (N86, F184, S1034, D1042 and D1246) (Figure 2A). To assess the possible linkage with other loci, a secondary scan was performed with the effect of pfmdr1 removed. No other significant QTL was found (Figure 2C).
Figure 2.
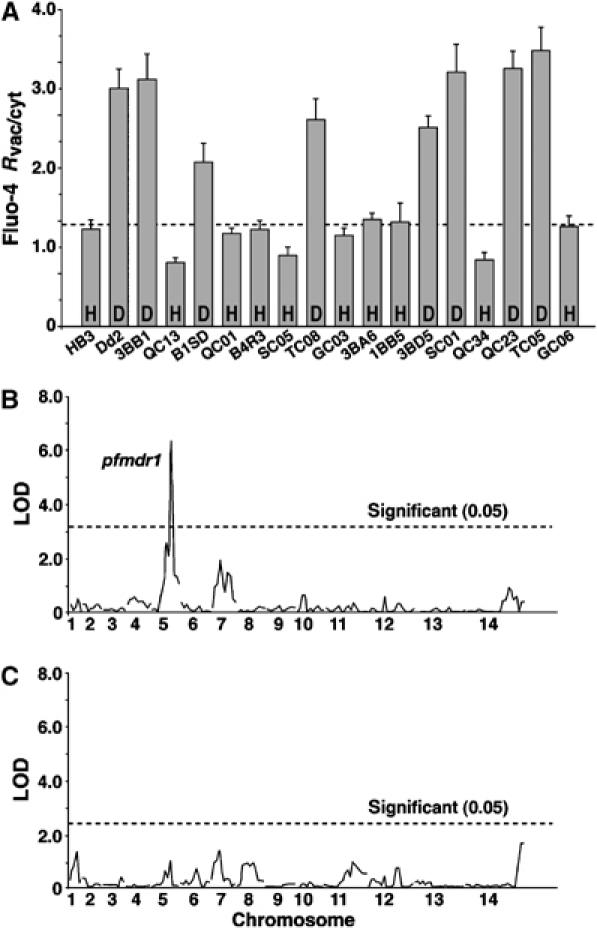
Linkage of the Fluo-4 phenotype to pfmdr1. (A) Fluo-4 Rvac/cyt in the progeny from the HB3 × Dd2 cross. The dotted line indicates the cutoff level of fluorescence ratio, as defined by HB3. H and D indicate the pfmdr1 haplotypes of HB3 and Dd2, respectively. The mean±s.e. of over 60 independent determinations are shown. (B) QTL analysis of Fluo-4 Rvac/cyt using Pseudomaker. (C) Secondary scan using Pseudomaker to search for minor QTL. Dashed lines represent the threshold values calculated from 1000 permutations (Churchill and Doerge, 1994). The P. falciparum chromosomes are indicated.
To investigate whether pfmdr1 itself plays a role in the Fluo-4 phenotype, we examined the effect of established P-gp inhibitors on the subcellular Fluo-4 fluorescence pattern. For Dd2, addition of P-gp inhibitors before and during loading with Fluo-4 AM significantly altered the Fluo-4 fluorescence pattern. The preferential staining of the food vacuole over the cytoplasm was substantially reduced in the presence of cyclosporine A (CSA, 10 μM), a first generation P-gp inhibitor, and was completely ablated in the presence of the third generation P-gp inhibitors ONT-093 (ONT, 1 and 10 μM) and XR-9576 (XR, 3 nM and 3 μM) (Figure 3A and B). In the case of XR-9576, a concentration of as low as 3 nM sufficed to render the Fluo-4 fluorescence image similar to that of HB3. Verapamil (VP, 30 μM) did not significantly affect the Fluo-4 staining pattern (Figure 3A and B). For HB3, P-gp inhibitors had no significant effect on the Fluo-4 staining pattern (Figure 3B) and a diffuse staining of the entire parasite remained.
Figure 3.
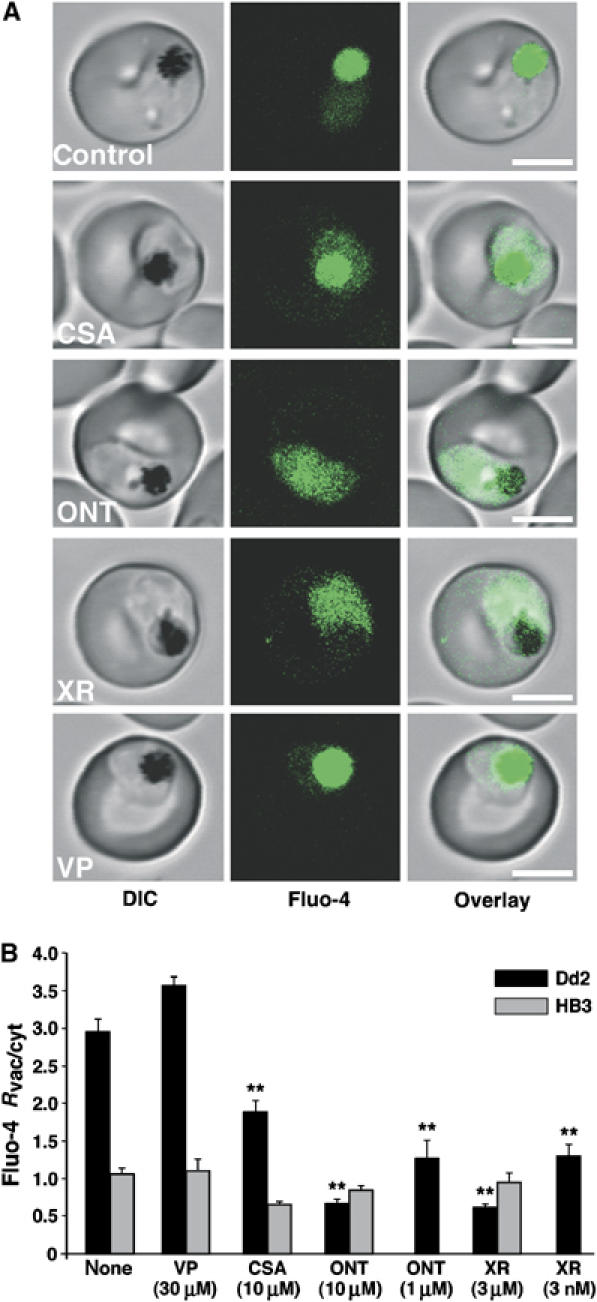
Confocal Fluo-4 AM imaging of vacuolar and cytosolic fluorescence in P. falciparum in the presence of various inhibitors. (A) Images of Dd2 parasites were obtained after loading with Fluo-4 AM in the presence of the P-gp inhibitors CSA, ONT-093 (ONT), XR-9576 (XR) or verapamil (VP). Bar, 5 μm. (B) Ratio of the vacuolar and cytosolic fluorescence (Fluo-4 Rvac/cyt) measured in Dd2 and HB3 in the presence of various P-gp inhibitors. The concentrations used are indicated. The mean±s.e. of over 10 independent determinations are shown. **P<0.001.
Pgh-1 overexpression enhances food vacuolar Fluo-4 staining
The pfmdr1 loci of HB3 and Dd2 differ not only regarding polymorphisms (see above) but also regarding copy number (1 versus 3–4, respectively). Taking this into account, we found that the food vacuolar phenotype, in addition to being linked with the Dd2 pfmdr1 allele, was further associated with an increased pfmdr1 copy number in the progeny of the HB3 × Dd2 cross (Figure 4A), with the exception of TC08, which contains only one pfmdr1 gene. Using quantitative real-time PCR, we generally confirmed the published pfmdr1 copy numbers (Wellems et al, 1990), although quantitative real-time PCR indicated lower values for B1SD, 3BB1 and Dd2 (Figure 4A, Supplementary Table I).
Figure 4.
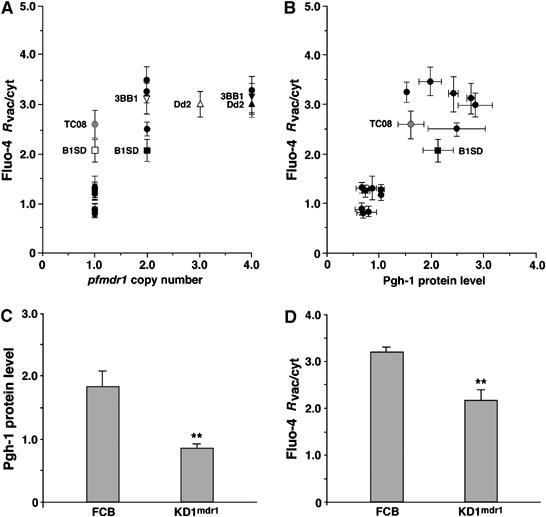
pfmdr1 overexpression contributes to the food vacuolar Fluo-4 phenotype. (A) The pfmdr1 copy number of the progeny, analyzed as a function of Fluo-4 Rvac/cyt. The solid circles represent copy numbers that are in agreement using both real-time PCR and Southern analysis. For Dd2, 3BB1 and B1SD, diverging results were obtained (solid triangles and solid squares, Southern analysis; corresponding open symbols, real-time PCR). TC08 (grey circle) represents a clone that harbors one pfmdr1 gene copy number measured in both methods, yet shows a high Fluo-4 Rvac/cyt ratio. (B) Pgh-1 protein levels analyzed as a function of Fluo-4 Rvac/cyt values. Symbols are as in (A). (C) Pgh-1 protein levels evaluated in FCB (harboring two pfmdr1 gene copies) and its isogenic clone KD1mdr1 (harboring one pfmdr1 copy). (D) Fluo-4 Rvac/cyt ratios quantified in FCB and KD1mdr1. Means±s.e. of over 40 independent determinations collected over several days are shown. **P<0.001.
The correlation between pfmdr1 copy number and the Fluo-4 phenotype improved when directly assessing the Pgh-1 protein level. Pgh-1 amounts were quantified by Western analysis, normalized against α-tubulin and expressed in relation to the Pgh-1 level of HB3. All progeny with a diffuse Fluo-4 staining pattern contained the HB3 pfmdr1 allele and had Pgh-1 protein levels comparable to that of HB3, whereas all progeny with a bright food vacuolar staining contained the Dd2 pfmdr1 allele and had 1.5- to 3.0-fold higher Pgh-1 protein levels, including TC08 (Figure 4B, Supplementary Table I).
To better assess the contribution of Pgh-1 protein levels to the Fluo-4 phenotype, we examined the parasite FCB and its isogenic clone KD1mdr1, in which one of the two pfmdr1 gene copies present were destroyed by insertional knockout mutagenesis (Sidhu et al, in press). Both FCB and KD1mdr1 contain the Y86, Y184, S1034, N1042 and D1246 allelic form of pfmdr1, also present in Dd2. FCB revealed a Pgh-1 protein level of 1.8±0.3 (in reference to that of HB3) and a food vacuolar staining phenotype with a high Fluo-4 Rvac/cyt value of 3.2±0.1 (Figure 4C and D). In KD1mdr1, the Pgh-1 protein level was reduced to 0.9±0.1 and the Fluo-4 Rvac/cyt value to 2.2±0.1, yet the food vacuolar staining phenotype remained (Figure 4C and D). This finding suggests that Pgh-1 levels contribute to the extent of the food vacuolar Fluo-4 staining by increasing the Fluo-4 Rvac/cyt values. However, Pgh-1 levels do not determine the Fluo-4 staining phenotype, as parasites with comparable low levels of Pgh-1 can have different Fluo-4 staining patterns (compare KD1mdr1 and K1 with HB3 in Supplementary Table I).
The Fluo-4 phenotype is linked with pfmdr1 polymorphisms
We next examined genetically engineered mutants of pfmdr1 (Reed et al, 2000; Sidhu et al, 2005), which encode amino-acid substitutions in positions 86, 184, 1034, 1042 and 1246 that have been associated with various drug responses. These include pfmdr1 mutants generated from GC03 and 3BA6, which are both progeny of the HB3 × Dd2 cross and which share the same pfmdr1 allele as HB3 (N86, F184, S1034, D1042 and D1246). GC03, 3BA6 and their corresponding recombinant controls SDDGC03 and SDD3BA6 that retain the original HB3 pfmdr1 allele revealed the diffuse Fluo-4 staining phenotype of HB3 and a similarly low Rvac/cyt value (Figure 5A). The clones SNDGC03 and SND3BA6, which no longer harbor the N1042D polymorphism in Pgh-1, showed the food vacuolar Fluo-4 phenotype and a significantly higher Rvac/cyt value (Figure 5A). The clones CDYGC03 and CDY3BA6, which encode the three C-terminal polymorphisms S1034C, N1042D and D1246Y, revealed the diffuse Fluo-4 staining pattern of HB3, albeit their Fluo-4 Rvac/cyt values were significantly lower than those of the parental clones GC03 and 3BA6 (Figure 5A).
Figure 5.
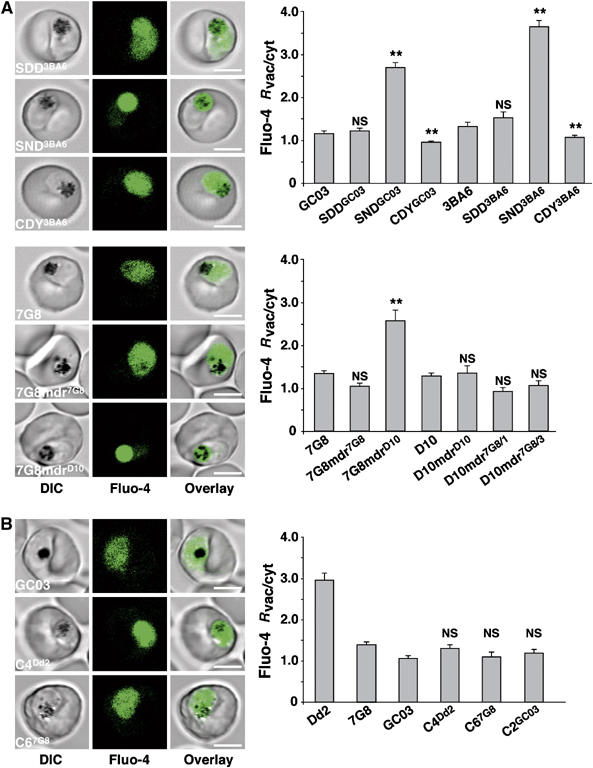
Association of the Fluo-4 phenotype with pfmdr1 polymorphisms. (A) Fluo-4 staining images and ratios of the mean fluorescence signals in the vacuolar and cytoplasmic regions (Fluo-4 Rvac/cyt), observed in different pfmdr1 allelic exchange mutants generated on either a GC03, 3BA6, 7G8 or a D10 genetic background (Reed et al, 2000; Sidhu et al, 2005; Supplementary Table II). NS, not significant as compared to GC03, 3BA6, 7G8 or D10. Means±s.e. of over 12 independent determinations collected over several days are shown. (B) Fluo-4 staining images and Fluo-4 Rvac/cyt ratios observed in different pfcrt allelic exchange mutants (Sidhu et al, 2002). C4Dd2 and C67G8 contain the Southeast Asian/African and Latin American pfcrt alleles from Dd2 and 7G8, respectively (Sidhu et al, 2002). GC03 is the parental clone and C2GC03 is a control retaining the pfcrt allele of GC03. NS, not significant (compared to GC03). The means±s.e. of over 14 independent determinations collected over several days are shown. **P<0.001; NS, not significant. Bar, 5 μm.
We also examined pfmdr1 mutants engineered in D10 and 7G8 parasites. The pfmdr1 allele of 7G8 encodes four amino-acid substitutions with respect to that of D10: Y184F, S1034C, N1042D, and D1246Y (Supplementary Table II). The allelic exchange mutants investigated were D10-mdrD10, which retained the wild-type D10 pfmdr1 sequence; D10-mdr7G8/3 encoding the S1034C, N1042D, and D1246Y substitutions; D10-mdr7G8/1 encoding only the D1246Y substitution; 7G8-mdr7G8, which retained the pfmdr1 allele of 7G8; and 7G8-mdrD10 encoding F184, S1034, N1042 and D1246 residues. Only 7G8-mdrD10 exhibited the food vacuolar Fluo-4 staining phenotype with a high Fluo-4 Rvac/cyt value of 2.6±0.2 (Figure 5A), whereas all other mutants revealed the diffuse Fluo-4 staining pattern with low Fluo-4 Rvac/cyt values of approximately 1.0 (Figure 5A), comparable to that of the parental clones D10 and 7G8. Interestingly, 7G8-mdrD10, SNDGC03 and SND3BA6, which all displayed elevated Fluo-4 Rvac/cyt values, encode the same polymorphisms in pfmdr1, namely N86, F184, S1034, N1042, D1246.
As controls we investigated previously described pfcrt allelic exchange mutants that have a GC03 background and that encode different pfcrt alleles found in chloroquine resistant parasites (Sidhu et al, 2002). No significant changes in the Fluo-4 staining phenotype were observed as compared to the parental clone GC03 (Figure 5B). This finding was in agreement with the QTL analysis that did not reveal significant linkage of the Fluo-4 phenotype with polymorphisms within pfcrt (present on chromosome 7; Figure 2C).
Substrate specificity of Pgh-1 and competition of Fluo-4 accumulation by antimalarial drugs
When erythrocytes infected with Dd2 were incubated with Fluo-4 salt instead of Fluo-4 AM, no fluorescence was observed in the parasite's cytoplasm or food vacuole (Figure 6A). This finding is consistent with the membrane-impermeable nature of the dye (Gee et al, 2000). However, in saponin and digitonin permeabilized Dd2 infected erythrocytes (Saliba et al, 2003), a strong fluorescence was seen in the food vacuole using both Fluo-4 salt and Fluo-4 AM (Figure 6B). The food vacuolar fluorescence depended on the addition of 2 mM ATP (in the case of the membrane permeable Fluo-4 AM fluorescence increased seven-fold), and could be inhibited by XR-9576 (3 μM) as shown for Fluo-4 salt (Figure 6B). Cell permeability was verified using trypan blue, which stained the cytosol of permeabilized cells but was excluded from the food vacuole under the experimental conditions used (data not shown).
Figure 6.
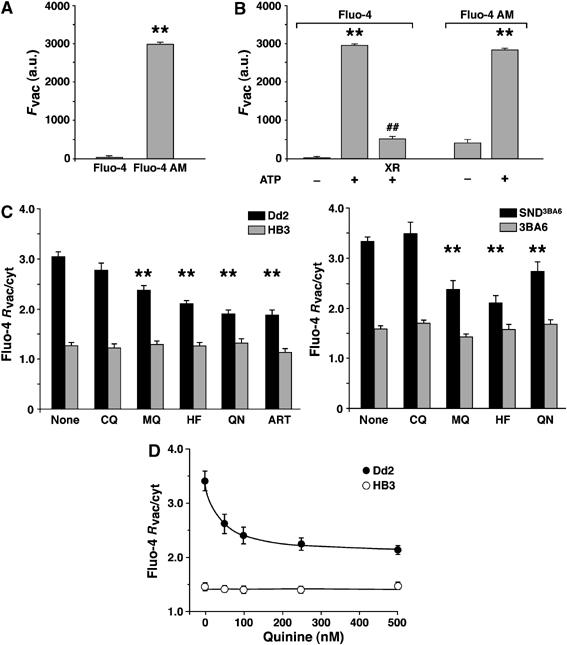
Substrate specificity of Pgh-1. (A) Food vacuolar fluorescence of intact erythrocytes infected with Dd2 after incubation with the membrane impermeable fluorochrome Fluo-4 salt and the membrane permeable variant Fluo-4 AM. Means±s.e. of over 15 independent determinations are shown. a.u., arbitrary units. **P<0.001. (B) Food vacuolar fluorescence of permeabilized erythrocytes infected with Dd2 using Fluo-4 salt and Fluo-4 AM in the presence and absence of 2 mM ATP. Where indicated XR-9576 (3 μM) was added. Means±s.e. of over 20 independent determinations are shown. **P<0.001 without and with ATP; ##P<0.001 without and with XR-9576. (C) Competition of food vacuolar Fluo-4 fluorescence with different antimalarial drugs. Erythrocytes infected with either Dd2 or HB3 or the pfmdr1 allelic exchange mutant SND3BA6 and its parental clone 3BA6 were incubated with Fluo-4 AM (5 μM) in the presence of 100 nM of chloroquine (CQ), mefloquine (MQ), halofantrine (HF), quinine (QN) or artemisinin (ART) and Fluo-4 Rvac/cyt ratios were determined. Means±s.e. of over 20 independent determinations are shown. **P<0.001. (D) Erythrocytes infected with Dd2 or HB3 were incubated with Fluo-4 AM (5 μM) and increasing concentrations of quinine and the Fluo-4 Rvac/cyt ratios were determined. Means±s.e. of over 20 independent determinations are shown.
We tested whether Fluo-4 fluorescence was affected by the presence of antimalarial drugs implicated as potential Pgh-1 substrates. For this, we incubated infected erythrocytes with 5 μM Fluo-4 AM and 100 nM of chloroquine, mefloquine, halofantrine, quinine or artemisinin, corresponding to a 50-fold excess of Fluo-4 AM. The Fluo-4 AM concentration was kept at 5 μM to maintain experimental consistency throughout the study. In HB3 and 3BA6, the Fluo-4 fluorescence was unaffected by the addition of these antimalarial drugs (Figure 6C). In comparison, significant decreases in Fluo-4 Rvac/cyt values were observed for Dd2 and SND3BA6 parasites in the presence of mefloquine, halofantrine, quinine or artemisinin (the latter was only tested for Dd2 and HB3), whereas no significant change occurred in the presence of chloroquine (Figure 6C). To validate this finding, we selected quinine and tested different concentrations ranging from 0 to 500 nM. The Fluo-4 Rvac/cyt value responded to increasing concentrations of quinine in Dd2, but not in HB3 (Figure 6D).
Discussion
We have identified and characterized two distinct Fluo-4 staining phenotypes in P. falciparum-infected erythrocytes. Some parasites, including HB3, showed a diffuse fluorescence of the entire parasite, whereas other parasites, such as Dd2, revealed an intense Fluo-4 staining of the food vacuole and only a weak cytoplasmic fluorescence (Figure 1).
As Fluo-4 is a non-ratiometric Ca2+ indicator, we considered the possibility that the differences in Fluo-4 staining patterns reflect strain variations in Ca2+ homeostasis. However, no significant differences in food vacuolar or cytoplasmic free Ca2+ concentrations were observed in the four representative parasites investigated. Our quantitative Ca2+ determinations relied on Fura-Red AM, a ratiometric Ca2+ indicator that provides reliable recordings of steady-state free [Ca2+]i in different subcellular compartments of P. falciparum-infected erythrocytes (Rohrbach et al, 2005). The steady-state food vacuolar and cytoplasmic free [Ca2+]i values reported herein are consistent with previous determinations (Biagini et al, 2003, 2005; Rohrbach et al, 2005).
The fluorescence intensity of Fluo-4, unlike that of Fura-Red, is pH sensitive (Rohrbach et al, 2005). There are reports suggesting differences in the standing food vacuolar pH between HB3 and Dd2 of 5.7 and 5.2, respectively (Dzekunov et al, 2000; Bennett et al, 2004), although these studies have been controversial (Bray et al, 2002; Wissing et al, 2002; Hayward et al, 2006). Even if these food vacuolar pH values are considered, this cannot explain the two-fold higher food vacuolar Fluo-4 fluorescence of Dd2. In the acidic range up to pH 6.0, the Fluo-4 fluorescence intensity increases with pH (Rohrbach et al, 2005), thus predicting a lower food vacuolar fluorescence for Dd2, which is contrary to our observation.
An alternative model to explain the Fluo-4 phenotypes involves altered fluorochrome handling. Fluo-4 belongs to a group of structurally related fluorescein derivatives that, when present as AM, are known substrates of multidrug efflux systems (Homolya et al, 1993; Brezden et al, 1994; Nelson et al, 1998; Szakacs et al, 1998) (Figure 7A). Our finding that, in Dd2, the Fluo-4 staining pattern responded to several established P-gp inhibitors, yielding a staining pattern similar to that of HB3, provided evidence for mechanistic similarities to dye transport in tumor cells. The very characteristic pharmacological profile—partially sensitive to CSA and highly sensitive to the third generation P-gp inhibitors ONT-093 and XR-9576 at concentrations as low as 3 nM (for XR-9576)—suggests that the proposed carrier belongs to the family of multidrug resistance transporters. The observed segregation of the Fluo-4 staining phenotype with pfmdr1 in the genetic cross between HB3 and Dd2 supports this model. The LOD score of >6 suggests strong linkage with pfmdr1. Indeed, all progeny displaying a food vacuolar staining phenotype have the Dd2 pfmdr1 allele whereas all progeny with a diffuse staining pattern inherited the HB3 pfmdr1 allele. Although verapamil, at a concentration of 30 μM and within a 40 min incubation time, did not affect the Fluo-4 phenotype, proliferation assays have found that polymorphisms within Pgh-1 can influence the parasite's susceptibility to verapamil (Hayward et al, 2005), suggesting a possible interaction between verapamil and Pgh-1.
Figure 7.
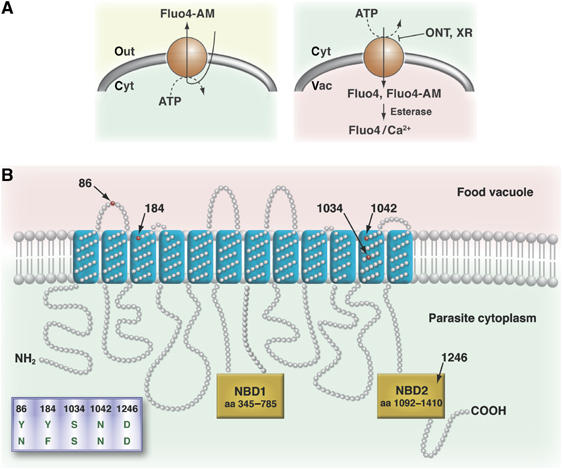
Models of Fluo-4 AM transport in tumor cells and P. falciparum. (A) Fluo-4 AM passively diffuses through membranes and can be actively extruded by P-gp in tumor cells (left). In parasites, Fluo-4 AM enters by passive diffusion and is converted to the free fluorochrome Fluo-4. Some Pgh-1 variants are capable of pumping both Fluo-4 AM and the membrane impermeable Fluo-4 into the food vacuole. Fluo-4 AM entering the food vacuole is de-esterified by esterases present in this organelle (right) (Krugliak et al, 2003). (B) Predicted topology of Pgh-1. Polymorphisms associated with both increased Fluo-4 AM/Fluo-4 import into the food vacuole and altered drug responses are indicated. NBD, nucleotide-binding domain.
Investigating several genetically engineered pfmdr1 mutants confirmed the causative linkage of the food vacuolar Fluo-4 staining phenotype with certain pfmdr1 polymorphisms. The single amino-acid replacement D1042N within the HB3 pfmdr1 allele altered the Fluo-4 phenotype of a diffuse to a food vacuolar staining pattern. Similarly, replacing the amino acids C1034S, D1042N, Y1246D within the pfmdr1 of 7G8 resulted in a food vacuolar Fluo-4 phenotype from the original diffuse Fluo-4 staining pattern displayed by 7G8. Interestingly, all mutants that have a food vacuolar staining pattern maintain the N86, F184, S1034, N1042, D1246 allelic form of pfmdr1 (Figure 5A). Thus, two allelic forms of pfmdr1 are associated with a food vacuolar Fluo-4 phenotype, namely Y86, Y184, S1034, N1042, D1246 (Dd2, K1 and FCB) and N86, F184, S1034, N1042, D1246 (allelic exchange mutants) (Figure 5A). The related allelic form N86, F184, S1034, D1042, D1246, which differs by a single amino-acid substitution at position 1042, replacing the negatively charged aspartic acid by the amide asparagine, showed a diffuse staining pattern. Similarly, a diffuse staining pattern was observed for N86, Y184, S1034, N1042, D1246, which differs by single amino-acid substitutions at positions 86 or 184. This finding suggests a specific interaction with Fluo-4 (Figure 7B). The clones CDYGC03 and CDY3BA6, encoding the N86, F184, C1034, D1042, Y1246 allelic form of pfmdr1, showed an interesting phenotype in that their Fluo-4 Rvac/cyt values were significantly lower than those of the parental clones GC03 and 3BA6 or that of HB3 (Figure 5A).
We further noted that the Pgh-1 protein level contributes to the food vacuolar Fluo-4 phenotype. There is a trend towards this effect in the progeny from the genetic cross, which was genetically validated by investigating FCB and its isogenic clone KD1mdr1. Disruption of one of the two pfmdr1 copies decreased Pgh-1 protein level in KD1mdr1 and to the same extent reduced the Fluo-4 Rvac/cyt value, albeit the overall staining pattern remained that of a food vacuolar phenotype (Figure 4C and D).
While our data causatively link the Fluo-4 phenotype to pfmdr1, there are clear differences to mdr-mediated fluorochrome efflux in cancer cells. In tumor cells, P-gp expel the nonfluorescent esterified probe, resulting in reduced intracellular fluorescence (Homolya et al, 1993) (Figure 7A, left scheme), while in some P. falciparum parasites the fluorochrome accumulates in an intracellular compartment. These apparent differences can be reconciled when the different subcellular localizations of P-gp and Pgh-1 are considered. In tumor cells, P-gp are localized to the plasma membrane, whereas Pgh-1 mainly resides within the parasite's food vacuolar membrane (Cowman et al, 1991; Cremer et al, 1995). On the basis of these considerations, we propose that Pgh-1, in a variant-dependent manner, pumps solutes into the food vacuole (Figure 7A, right scheme). Inwardly directed transport is fully consistent with the predicted topology of Pgh-1, with its ATP-binding domain facing the cytoplasm (Cowman et al, 1991; Karcz et al, 1993). Our model is supported by several independent lines of evidence: (i) the food vacuolar phenotype intensified with increasing pfmdr1 copy number (Figure 4C and D); (ii) Fluo-4 AM, but more importantly, the membrane-impermeable form, Fluo-4 salt, accumulated in the food vacuoles of permeabilized erythrocytes infected with Dd2 in an ATP-dependent manner (Figure 6B); and (iii) accumulation of Fluo-4 salt (permeabilized cells, Figure 6B) and Fluo-4 AM (live cells, Figure 3) could be inhibited by the established P-gp inhibitor XR-9576. While P-gp transports Fluo-4 AM and only poorly the de-esterified form (Szakacs et al, 1998), Pgh-1 seems to use both Fluo-4 salt and Fluo-4 AM as substrates.
The conclusion that Pgh-1 can transport solutes into the food vacuole has major implications for the interpretation of antimalarial drug responses. The Pgh-1 variants that most effectively concentrate Fluo-4 salt/Fluo-4 AM in the food vacuole are those that have been linked to reduced susceptibility to artemisinin, halofantrine and mefloquine and increased susceptibility to quinine (Reed et al, 2000; Sidhu et al, 2005). Conversely, parasites expressing the N86, F184, C1034, D1042, Y1246 allelic form of pfmdr1 concentrate the least Fluo-4 salt/Fluo-4 AM in their food vacuoles and concomitantly have an increased susceptibility to mefloquine, halofantrine and possibly artemisinin (Reed et al, 2000; Sidhu et al, 2005). Thus, the Fluo-4 staining pattern directly correlates with certain drug responses, providing a rapid cell-based diagnostic assay for pfmdr1 polymorphisms associated with various drugs. Indeed, accumulation of Fluo-4 into the food vacuole could be competed by mefloquine, halofantrine, quinine and artemisinin, but not chloroquine (Figure 6C). For quinine, a concentration-dependent response was established (Figure 6D), albeit Fluo-4 Rvac/cyt values could not be reduced to the level observed in parasites with a diffuse fluorescence staining pattern, possibly because Pgh-1, like human P-gp (Shapiro et al, 1999; Shapiro and Ling, 1997), may possess multiple transport-active and substrate-specific binding sites (in this case one for Fluo-4 salt/Fluo-4 AM and one for quinine) that may interact in a cooperative fashion. Chloroquine, a highly acidotropic compound that accumulates in the parasite's food vacuole (Yayon et al, 1984), and possibly alkalinizing this compartment (Yayon et al, 1985), had no effect on the food vacuolar Fluo-4 fluorescence, providing further evidence for our model that the Fluo-4 staining pattern is unrelated to pH. Our finding that chloroquine does not compete with Fluo-4 in HB3 and Dd2 is consistent with genetic and positional cloning experiments that dissociated chloroquine resistance from the Pgh-1 polymorphisms displayed by HB3 and Dd2 (Wellems et al, 1990; Sidhu et al, 2005), although other Pgh-1 variant may directly or indirectly interact with chloroquine (Reed et al, 2000).
On the basis of these findings, it is tempting to speculate that various forms of Pgh-1 transport several antimalarial drugs into the food vacuole. For mefloquine, halofantrine and artemisinin, it may be advantageous to the parasite to sequester these drugs in a compartment where they are less harmful. Recent evidence of possible cytoplasmic targets for artemisinin is fully consistent with this concept (Eckstein-Ludwig et al, 2003). Alternatively, Pgh-1 itself may be the target of antimalarial drugs, as suggested for mefloquine (Rubio and Cowman, 1996). In the case of quinine, its site of action is believed to be the food vacuole (Hawley et al, 1998; Mungthin et al, 1998) and concentrating the drug in this organelle may be disadvantageous; consequently, pfmdr1 polymorphisms are selected that mediate reduced drug import. Importantly, the N1042D substitution that has been associated with low-level quinine resistance (Sidhu et al, 2005) abrogates accumulation of Fluo-4 in the food vacuole (Figure 5A). In summary, our study provides evidence for Pgh-1 mediated solute import into the parasite's food vacuole in a manner that may involve interactions at several, if not all, polymorphic residues.
Materials and methods
Chemicals and antibodies
Fluo-4 AM, Fluo-4 pentapotassium salt, Fura-Red AM, LysoSensor Blue DND-192, Pluronic F-127, Alexa Fluor 680 goat anti-rabbit IgG and Alexa Fluor goat anti-mouse IgG were purchased from Molecular Probes (Netherlands). The Ca2+-ionophore nigericin was purchased from Calbiochem (Germany). The P-gp inhibitor Cyclosporin A (CSA) was obtained from Sigma (Germany), ONT-093 was kindly supplied by Ontogen and XR-9576 by Xenova. Chloroquine, artemisinin and quinine were purchased from Sigma (Germany), mefloquine from Roche (Germany), and halofantrine from GalaxoSmithKline (UK). α-Tubulin clone B-5-1-2 was purchased from Sigma (Germany) and α-Pgh-1 was a kind gift from A Cowman.
P. falciparum culture
P. falciparum parasites were maintained in continuous in vitro cultures (adapted from Trager and Jensen, 1976). Live cell experiments were carried out using synchronized P. falciparum trophozoites harvested 28–34 h post invasion.
Dye loading of P. falciparum parasites
Cells were washed twice with Ringer's solution (122.5 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 11 mM D-glucose, 10 mM HEPES, 1 mM NaH2PO4, pH 7.4) and loaded with 5 μM Fluo-4 AM in Ringer's solution with Pluronic F-127 (0.1% v/v) or Fluo-4 for 40 min at 37°C. Dye loaded parasites were settled onto poly-L-lysine coated coverslips in a microperfusion chamber as previously described (Wunsch et al, 1998). Unbound parasites and remaining dye were washed away by perfusion with Ringer's solution. For the double labeling experiments, Ringer's solution was supplemented with 1 μM of the fluorescent dye LysoSensor Blue DND-192. The P-gp inhibitors verapamil (30 μM), CSA (10 μM), ONT-093 (10 and 1 μM) and XR-9576 (3 μM and 3 nM) were added to P. falciparum-infected erythrocytes 10 min prior to loading with Fluo-4 AM. For the competition assays, 100 nM of the antimalarial drugs chloroquine, mefloquine, halofantrine, quinine or artemisinin were incubated with 5 μM Fluo-4 AM for 40 min at 37°C.
Selective permeabilization of the erythrocyte and parasite plasma membranes
Infected erythrocytes were permeabilized by brief exposure to saponin (0.01% w/v), permeabilizing both the host cell membrane and the parasitophorous vacuole membrane (Ansorge et al, 1997; Saliba et al, 2003) and giving extracellular solutes access to the parasite plasma membrane. The plasma membrane of the parasite was permeabilized using digitonin (Krogstad et al, 1985), permitting solutes added to the extracellular medium access to the food vacuolar membrane (Saliba et al, 2003). Isolated parasites were suspended in 2 ml of ice-cold buffer (110 mM KCl, 30 mM NaCl, 2 mM MgCl2, 5 mM HEPES, pH 7.3). The cell suspension was kept on ice for 5–10 min before adding digitonin (0.02% w/v). The cells were mixed gently and returned to ice for additional 4 min, after which time 1 ml of ice-cold buffer containing 1 mg/ml bovine serum albumin (BSA) was added. The permeabilized parasites were immediately centrifuged (15 800 g) for 1 min, washed twice (1 min at 15 800 g) with 1 ml of the same BSA-containing solution, and then once in buffer without BSA. Digitonin-permeabilized parasites were suspended in buffer and placed at 37°C until use (within 30 min to 1 h). Permeabilized parasites were loaded with 5 μM of either Fluo-4 AM or Fluo-4, and ATP was supplemented at 2 mM where mentioned. XR-9576 (3 μM) was added to permeabilized erythrocytes, maintained at 37°C, 10 min prior to addition of the fluorochrome.
Live cell imaging
Confocal scanning fluorescence microscopy was performed using a Zeiss LSM510 (Carl Zeiss, Germany) equipped with UV and visible laser lines. Multi-Track mode was used for LysoSensor Blue DND-192 (excited at 364 nm with emission detected using the BP 385–470 nm filter, blue channel) and Fluo-4 AM or Fluo-4 (excited at 488 nm with emission using LP 505 nm filter, green channel). Optimized laser settings were excitation 488 nm with 1% transmission, and 364 nm with 2% transmission. Single images were obtained using a × 63 lens (C-APO, N.A.=1.2) with an eight-fold software zoom at 512 × 512 pixel. For Fluo-4 AM (or Fluo-4) labeling alone, Single-Track mode was applied using the above-mentioned laser and filter. Regions of interest restricted to either the vacuole or the cytosol of the parasite were defined and fluorescence intensity quantified, using the LSM510 v3.2 software.
In situ Ca2+-calibration of Fura-Red
The fluorescent calcium indicator Fura-Red AM was used to measure the intracellular free calcium concentration, as described (Rohrbach et al, 2005). Mean resting free [Ca2+]i were compared between the vacuole and the cytoplasmic area using a Student's t-test at the P=0.05 level.
Genome-wide scans
The Dd2 × HB3 genetic cross and genetic linkage map have been previously described (Wellems et al, 1990; Su et al, 1999). QTL linked to Fluo-4 fluorescence were analyzed using Pseudomaker (Sen and Churchill, 2001). Significance thresholds were determined by permutation analyses (Churchill and Doerge, 1994; Lander and Kruglyak, 1995). A secondary scan was used to search for minor QTL (Doerge and Churchill, 1996).
Real-time PCR
pfmdr1 copy number was determined by TaqMan real-time PCR using an ABI 7700. The pfmdr1 probe was FAM™ (6-carboxyfluorescein) labeled at the 5′-end, and the α-tubulin probe was VIC™ labeled. Both probes had a TAMRA™ label at the 3′-end. Amplification reactions were carried out in MicroAmp 96-well plates in 25 μl volumes, containing TaqMan buffer, passive reference dye ROX (5-carboxy-X-rhodamine), 300 nM forward and reverse primer, 100 nM of each probe, and 100 ng purified parasite genomic DNA. Forty cycles were performed (95°C for 15 s and at 58°C for 1 min). Fluorescence data were expressed as normalized reporter signals, calculated by dividing the amount of reporter signal by the passive reference signal. The optimal detection threshold was determined for the assay conditions and used for every run. Results were analyzed by a delta–delta Ct method. The assay was replicated four independent times and normalized to HB3, which was included in every run. Primers and probes used were:

Pgh-1 expression levels
Magnet-purified trophozoite-infected erythrocytes were isolated as described (Sanchez et al, 2003). After purification, erythrocytes were lysed by hypotonic shock at 4°C (Herwaldt et al, 1990). Protein amounts were determined using Bradford assays (BioRad, Germany). Samples were run on NuPAGE Novex Tris-Acetate gels (Invitrogen, Germany) and transferred onto a 0.2 μm PDVF membrane (BioRad, Germany). Membranes were blocked overnight at 4°C using 5% milk in PBS. Primary antibodies (α-Pgh-1 and α-tubulin, both diluted 1:1000) were incubated for 1 h at RT in 1% BSA/PBS. Membranes were washed three times using PBS/0.1% Tween for 10 min at RT and then blocked again in 5% milk in PBS for 1 h. Secondary antibodies (Alexa Fluor 680 goat anti-rabbit IgG, or Alexa Fluor goat anti-mouse IgG, both diluted 1:10 000) were added to 1% BSA/PBS for 30 min at RT. After washing four times in PBS/0.1% Tween for 5 min at RT, signals were read using an Odyssey-Li-cor infrared imaging system (Li-cor Biosciences). Fluorescence intensities for Pgh-1 were normalized using fluorescence intensities measured for α-tubulin. The resulting values were then expressed in relation to HB3.
Statistical analysis
Statistical significance (P-value) was tested using the Student's t-test (Sigma Plot, SPSS software).
Online supplemental material
Supplementary Table I shows pfmdr1 inheritance and copy number in various parasites. Supplementary Table II shows relevant point mutations in Pgh-1.
Supplementary Material
Supplementary Tables
Acknowledgments
This work was supported by the SFB 544 ‘Control of Tropical Infectious Diseases' (ML), the European Network of Excellence BioMalPar (ML) and a Blood Disease Research Grant from the New York Community Trust (DF). We thank Anne-Catrin Uhlemann, Sanjeev Krishna and Stephanie Gaw-Volderramos for their contribution to generating and characterizing the pfmdr1 knockdown lines, as well as Elisabeth Wilken, Marina Müller and Christine Reinhardt for technical help. We are grateful to Tom Wellems for the progeny of the HB3 × Dd2 cross and Alan Cowman for pfmdr1 allelic exchange mutants and Pgh-1 antiserum.
References
- Ansorge I, Paprotka K, Bhakdi S, Lingelbach K (1997) Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol Biochem Parasitol 84: 259–261 [DOI] [PubMed] [Google Scholar]
- Baird JK (2005) Effectiveness of antimalarial drugs. N Engl J Med 352: 1565–1577 [DOI] [PubMed] [Google Scholar]
- Bennett TN, Kosar AD, Ursos LM, Dzekunov S, Singh Sidhu AB, Fidock DA, Roepe PD (2004) Drug resistance-associated pfcrt mutations confer decreased Plasmodium falciparum digestive vacuolar pH. Mol Biochem Parasitol 133: 99–114 [DOI] [PubMed] [Google Scholar]
- Biagini GA, Bray PG, Spiller DG, White MR, Ward SA (2003) The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J Biol Chem 278: 27910–27915 [DOI] [PubMed] [Google Scholar]
- Biagini GA, Fidock DA, Bray PG, Ward SA (2005) Mutations conferring drug resistance in malaria parasite drug transporters Pgh1 and Pfcrt do not affect steady-state vacuolar Ca2+. Antimicrob Agents Chemother 49: 4807–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges-Walmsley MI, McKeegan KS, Walmsley AR (2003) Structure and function of efflux pumps that confer resistance to drugs. Biochem J 376: 313–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray PG, Saliba KJ, Davies JD, Spiller DG, White MR, Kirk K, Ward SA (2002) Distribution of acridine orange fluorescence in Plasmodium falciparum-infected erythrocytes and its implications for the evaluation of digestive vacuole pH. Mol Biochem Parasitol 119: 301–304; discussion 307–309, 311–313 [DOI] [PubMed] [Google Scholar]
- Brezden CB, Hedley DW, Rauth AM (1994) Constitutive expression of P-glycoprotein as a determinant of loading with fluorescent calcium probes. Cytometry 17: 343–348 [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Galatis D, Thompson JK (1994) Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc Natl Acad Sci USA 91: 1143–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Karcz S, Galatis D, Culvenor JG (1991) A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J Cell Biol 113: 1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer G, Basco LK, Le Bras J, Camus D, Slomianny C (1995) Plasmodium falciparum: detection of P-glycoprotein in chloroquine-susceptible and chloroquine-resistant clones and isolates. Exp Parasitol 81: 1–8 [DOI] [PubMed] [Google Scholar]
- Doerge RW, Churchill GA (1996) Permutation tests for multiple loci affecting a quantitative character. Genetics 142: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Cowman AF (2005) Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop 94: 181–190 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Refour P (2005) Multiple drug resistance genes in malaria—from epistasis to epidemiology. Mol Microbiol 57: 874–877 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Roper C, Walliker D, Warhurst DC (2000) Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol 36: 955–961 [DOI] [PubMed] [Google Scholar]
- Dzekunov SM, Ursos LM, Roepe PD (2000) Digestive vacuolar pH of intact intraerythrocytic P. falciparum either sensitive or resistant to chloroquine. Mol Biochem Parasitol 110: 107–124 [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424: 957–961 [DOI] [PubMed] [Google Scholar]
- Foote SJ, Thompson JK, Cowman AF, Kemp DJ (1989) Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell 57: 921–930 [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I (2000) Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium 27: 97–106 [DOI] [PubMed] [Google Scholar]
- Hawley SR, Bray PG, Mungthin M, Atkinson JD, O'Neill PM, Ward SA (1998) Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob Agents Chemother 42: 682–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R, Saliba KJ, Kirk K (2005) Mutations in pfmdr1 modulate the sensitivity of Plasmodium falciparum to the intrinsic antiplasmodial activity of verapamil. Antimicrob Agents Chemother 49: 840–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R, Saliba KJ, Kirk K (2006) The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J Cell Sci 119: 1016–1025 [DOI] [PubMed] [Google Scholar]
- Herwaldt BL, Schlesinger PH, Krogstad DJ (1990) Accumulation of chloroquine by membrane preparations from Plasmodium falciparum. Mol Biochem Parasitol 42: 257–267 [DOI] [PubMed] [Google Scholar]
- Homolya L, Hollo Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B (1993) Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem 268: 21493–21496 [PubMed] [Google Scholar]
- Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O (2005) Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366: 1960–1963 [DOI] [PubMed] [Google Scholar]
- Karcz SR, Galatis D, Cowman AF (1993) Nucleotide binding properties of a P-glycoprotein homologue from Plasmodium falciparum. Mol Biochem Parasitol 58: 269–276 [DOI] [PubMed] [Google Scholar]
- Krogstad DJ, Schlesinger PH, Gluzman IY (1985) Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol 101: 2302–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugliak M, Zhang J, Nissani E, Steiner-Mordoch S, Ginsburg H (2003) Killing of intraerythrocytic Plasmodium falciparum by lysosomotropic amino acid esters. Parasitol Res 89: 451–458 [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11: 241–247 [DOI] [PubMed] [Google Scholar]
- Mungthin M, Bray PG, Ridley RG, Ward SA (1998) Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob Agents Chemother 42: 2973–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EJ, Zinkin NT, Hinkle PM (1998) Fluorescence methods to assess multidrug resistance in individual cells. Cancer Chemother Pharmacol 42: 292–299 [DOI] [PubMed] [Google Scholar]
- Ngo T, Duraisingh M, Reed M, Hipgrave D, Biggs B, Cowman AF (2003) Analysis of pfcrt, pfmdr1, dhfr, and dhps mutations and drug sensitivities in Plasmodium falciparum isolates from patients in Vietnam before and after treatment with artemisinin. Am J Trop Med Hyg 68: 350–356 [PubMed] [Google Scholar]
- Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR (2003) Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother 47: 2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S (2004) Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364: 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF (2000) Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403: 906–909 [DOI] [PubMed] [Google Scholar]
- Rohrbach P, Friedrich O, Hentschel J, Plattner H, Fink RH, Lanzer M (2005) Quantitative calcium measurements in subcellular compartments of P. falciparum-infected erythrocytes. J Biol Chem 280: 27960–27969 [DOI] [PubMed] [Google Scholar]
- Rubio JP, Cowman AF (1996) The ATP-binding cassette (ABC) gene family of Plasmodium falciparum. Parasitol Today 12: 135–140 [DOI] [PubMed] [Google Scholar]
- Saliba KJ, Allen RJ, Zissis S, Bray PG, Ward SA, Kirk K (2003) Acidification of the malaria parasite's digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J Biol Chem 278: 5605–5612 [DOI] [PubMed] [Google Scholar]
- Sanchez CP, Stein W, Lanzer M (2003) Trans stimulation provides evidence for a drug efflux carrier as the mechanism of chloroquine resistance in Plasmodium falciparum. Biochemistry 42: 9383–9394 [DOI] [PubMed] [Google Scholar]
- Sen S, Churchill GA (2001) A statistical framework for quantitative trait mapping. Genetics 159: 371–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AB, Fox K, Lam P, Ling V (1999) Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur J Biochem 259: 841–850 [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Ling V (1997) Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur J Biochem 250: 130–137 [DOI] [PubMed] [Google Scholar]
- Sidhu ABS, Uhlemann A-C, Valderramos SG, Valderramos J-C, Krishna S, Fidock DA (2006) Decreasing pfmdr1 copy number in Plasmodium falciparum heightens sensitivity to mefloquine, lumefantrine, halofantrine, quinine and artemisinin. Journal Infect Dis (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu AB, Valderramos SG, Fidock DA (2005) pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol Microbiol 57: 913–926 [DOI] [PubMed] [Google Scholar]
- Sidhu AB, Verdier-Pinard D, Fidock DA (2002) Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298: 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisowath C, Stromberg J, Martensson A, Msellem M, Obondo C, Bjorkman A, Gil JP (2005) In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J Infect Dis 191: 1014–1017 [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434: 214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ferdig MT, Huang Y, Huynh CQ, Liu A, You J, Wootton JC, Wellems TE (1999) A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science 286: 1351–1353 [DOI] [PubMed] [Google Scholar]
- Szakacs G, Jakab K, Antal F, Sarkadi B (1998) Diagnostics of multidrug resistance in cancer. Pathol Oncol Res 4: 251–257 [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193: 673–675 [DOI] [PubMed] [Google Scholar]
- Wellems TE, Panton LJ, Gluzman IY, do Rosario VE, Gwadz RW, Walker-Jonah A, Krogstad DJ (1990) Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature 345: 253–255 [DOI] [PubMed] [Google Scholar]
- Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, Wirth DF (1989) Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science 244: 1184–1186 [DOI] [PubMed] [Google Scholar]
- Wilson CM, Volkman SK, Thaithong S, Martin RK, Kyle DE, Milhous WK, Wirth DF (1993) Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol Biochem Parasitol 57: 151–160 [DOI] [PubMed] [Google Scholar]
- Wissing F, Sanchez CP, Rohrbach P, Ricken S, Lanzer M (2002) Illumination of the malaria parasite Plasmodium falciparum alters intracellular pH. Implications for live cell imaging. J Biol Chem 277: 37747–37755 [DOI] [PubMed] [Google Scholar]
- Wunsch S, Sanchez CP, Gekle M, Grosse-Wortmann L, Wiesner J, Lanzer M (1998) Differential stimulation of the Na+/H+ exchanger determines chloroquine uptake in Plasmodium falciparum. J Cell Biol 140: 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Cabantchik ZI, Ginsburg H (1984) Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J 3: 2695–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Cabantchik ZI, Ginsburg H (1985) Susceptibility of human malaria parasites to chloroquine is pH dependent. Proc Natl Acad Sci USA 82: 2784–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables


