Abstract
The cyclic nitramine explosive CL-20 (2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane) was examined in soil microcosms to determine whether it is biodegradable. CL-20 was incubated with a variety of soils. The explosive disappeared in all microcosms except the controls in which microbial activity had been inhibited. CL-20 was degraded most rapidly in garden soil. After 2 days of incubation, about 80% of the initial CL-20 had disappeared. A CL-20-degrading bacterial strain, Agrobacterium sp. strain JS71, was isolated from enrichment cultures containing garden soil as an inoculum, succinate as a carbon source, and CL-20 as a nitrogen source. Growth experiments revealed that strain JS71 used 3 mol of nitrogen per mol of CL-20.
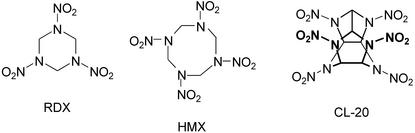
CL-20 (2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane) is a new, highly energetic explosive related to the explosives RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) and HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine) (Fig. 1). Due to its higher energy and its moderate sensitivity, CL-20 is expected to replace earlier explosives (19, 20, 22, 25). The production of CL-20 and its use in munitions and propellants can be expected to lead to environmental contamination. RDX and HMX, common contaminants in soil and groundwater at munition manufacturing sites and firing ranges, are toxic and possibly carcinogenic (7, 8, 11, 12, 16, 23, 27). Because of the structural similarity of CL-20 to RDX and HMX, it is likely that CL-20 has similar effects. So far, there is little information available about the properties of CL-20. In contrast to RDX and HMX, CL-20 is a caged molecule (Fig. 1). The water solubility of CL-20 is 4.8 mg/liter at 25°C (Stevens Institute of Technology [http://www.cee.stevens-tech.edu/ResProj.html]), which is lower than the solubility of the nitramines RDX and HMX (38.4 and 6.6 mg/liter at 20°C, respectively) (27).
FIG. 1.
Molecular structures of the cyclic nitramine explosives RDX, HMX, and CL-20.
The microbial degradation of RDX and HMX under aerobic and anaerobic conditions has been extensively investigated (1, 2, 4-6, 9, 13-15, 17, 21). Under anaerobic conditions, three degradation pathways have been proposed. The first includes the production of nitroso derivatives of RDX and HMX, which are subject to further degradation (15, 21). Hawari et al. suggested a second degradation pathway which includes the direct ring cleavage of RDX and HMX without the initial reduction of the nitro groups (14, 15). The third proposed degradation pathway is based on N denitration prior to ring cleavage (1).
Under aerobic conditions, RDX is degraded by a yet-unknown mechanism. The initial attack seems to be catalyzed by a cytochrome P450 (1a, 6, 24). RDX degradation by Rhodococcus sp. strain DN22 leads to the formation of nitrite, nitrous oxide, ammonia, formaldehyde, and a dead-end product with a molecular weight of 119 which was recently identified as 4-nitro-2,4-diazabutanal (1a, 5, 9). Fournier et al. proposed a degradation pathway which includes an initial denitration of RDX followed by ring cleavage to formaldehyde and the dead-end product (9).
To the best of our knowledge, no biodegradation of CL-20 has been reported to date. It is, therefore, important to determine whether biodegradation might affect the fate and transport of CL-20 in terrestrial and aquatic ecosystems. To that end we evaluated the biodegradation of CL-20 in laboratory microcosms and isolated a bacterial strain that is able to grow on CL-20 as the sole nitrogen source.
CL-20 biodegradation in soil.
Microcosms with dried and sieved garden and agricultural soils were incubated with CL-20 provided by the High Explosives Research and Development Facility at Eglin Air Force Base, Fla.
Microcosm experiments were conducted in 5-ml amber vials containing soil (2 g), sterile water (1.0 to 1.2 ml), and CL-20 (100 nmol). CL-20 was added from a 5 mM stock solution in methanol. The amounts of added water corresponded to the water absorptive capacity of the soils. For inhibiting microbial activity in soils, the added water contained glutaraldehyde (1%) and mercuric chloride (90 mg/liter). The microcosms were vigorously mixed by vortexing them and incubated in the dark at 30°C. For time course studies, one microcosm was sacrificed at each time point. The CL-20 concentrations in the microcosms in acetonitrile extracts were determined by high-pressure liquid chromatography (HPLC) analysis. The mobile phase (65% [vol/vol] acetonitrile, 0.1% [vol/vol] trifluoroacetic acid) was pumped at a flow rate of 1.0 ml/min over a Spherisorb C8 column (250 by 4.6 mm; particle size, 5 μm; Alltech, Deerfield, Ill.) or a Synergi Polar-RP column (150 by 4.6 mm; particle size, 4 μm; Phenomenex, Torrance, Calif.). Absorbance was measured at a wavelength of 230 nm.
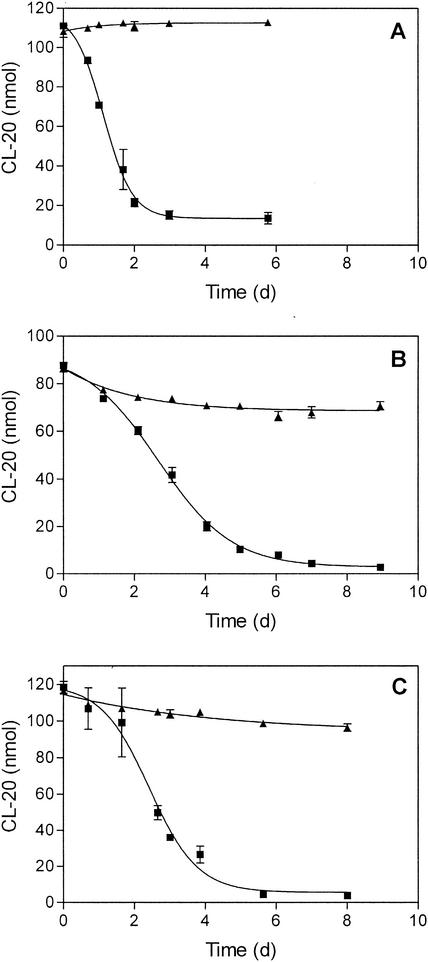
CL-20 was degraded in all three soils (Fig. 2). The persistence of CL-20 in sterile controls indicated that the degradation process in active soil was biological. CL-20 degradation was fastest in the microcosms containing garden soil. CL-20 did not disappear completely in any of the soils. The incomplete degradation of CL-20 might be due to limited bioavailability. Residual amounts of CL-20 may be sorbed to the soil and therefore be unavailable for the microorganisms. In general, the availability of contaminants for microorganisms is dependent on the properties of the soil and the chemical compound, as well as on mass transport (3, 29).
FIG. 2.
CL-20 biodegradation in microcosms. Soils from Florida (A), Iowa (B), and Texas (C) were incubated with CL-20. CL-20 amounts are in nanomoles per microcosm. ▪, active soil; ▴, soil with inhibited microbial activity. Degradation experiments were performed in triplicate. d, days.
During CL-20 degradation in the microcosms with garden soil, traces of a transient metabolite with a molecular weight of 247 could be detected by HPLC-mass spectrometry in the negative electrospray ionization mode. Further investigations are under way to identify the putative CL-20 degradation product.
It is not clear whether CL-20 degradation in the soil microcosms occurred aerobically or anaerobically. No precautions were taken to maintain aerobic conditions. Degradation started relatively quickly, so it can be assumed that CL-20 was degraded under aerobic conditions. On the other hand, it is possible that small anaerobic zones existed in the soil, where microbes could have degraded CL-20 anaerobically. In preliminary experiments CL-20 was also degraded under anaerobic conditions in sewage sludge (data not shown).
Enrichment and isolation of the Agrobacterium strain JS71.
Enrichment cultures were carried out in 10 ml of M medium (pH 7.0) without sodium chloride (5). The medium was supplemented with CL-20 (100 μM) in methanol (5 mM stock solution) as the sole nitrogen source and succinate (20 mM) as the sole carbon source and inoculated with 1 g of Florida garden soil in 50-ml shake flasks. The cultures were incubated on a shaker at 30°C and 250 rpm. Microbial activity in the controls was inhibited by the addition of glutaraldehyde (1%) and mercuric chloride (90 mg/liter). The concentrations of CL-20 in the enrichments were determined by HPLC. Samples were mixed with 1.5 volumes of acetonitrile, and suspended material was removed by centrifugation prior to HPLC analysis.
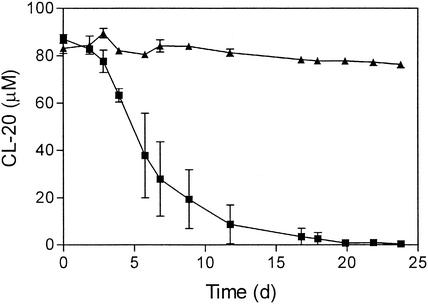
CL-20 degradation in the first enrichment culture started after about 3 days (Fig. 3). The disappearance of CL-20 verified that CL-20 was degraded under aerobic conditions. CL-20 degradation in the enrichments was slower than its degradation in the soil microcosms, probably because of the smaller inoculum and the very low water solubility of CL-20. In contrast to the partial degradation of CL-20 in the soil microcosms, CL-20 was completely degraded in the enrichment cultures. The results support the hypothesis that some of the CL-20 in the microcosms (Fig. 2) was unavailable because of sorption to the soil.
FIG. 3.
CL-20 biodegradation in enrichments with Florida soil. ▪, active soil; ▴, soil with inhibited microbial activity. Minimal medium was supplemented with succinate (20 mM) and CL-20 (100 μM). Enrichments were performed in triplicate. d, days.
After three subcultures of the enrichment, appropriate dilutions were plated on the above-named enrichment medium solidified with 1.5% (wt/vol) agarose (molecular biology grade). Single colonies were tested for the ability to grow on CL-20 as the sole nitrogen source. The selective enrichment yielded a bacterial strain that was able to grow with CL-20 as the sole nitrogen source. The 16S rRNA gene sequence from the isolate was determined and evaluated by Midi Labs (Newark, Del.). The 16S rRNA gene sequence indicated that the strain was most similar to Agrobacterium rubi (0.64% difference) and Agrobacterium tumefaciens (0.84% difference). The isolate was gram negative, motile, and rod shaped. These properties correspond to the characteristics of the genus Agrobacterium (18). The isolate was, therefore, named Agrobacterium sp. strain JS71.
The transformation of explosives by Agrobacterium strains has been described previously. Agrobacterium sp. strain 2PC was able to biotransform 2,4,6-trinitrotoluene to monoaminodinitrotoluenes (10). White et al. isolated an Agrobacterium radiobacter strain that transforms glycerol trinitrate to glycerol dinitrates and finally to glycerol mononitrates. The strain used two nitrogens from glycerol trinitrate as the nitrogen source for growth (28). An NADH-dependent reductase able to remove one nitro group from glycerol trinitrate in the form of nitrite was isolated from the strain (26). The enzyme also denitrated pentaerythrol tetranitrate, isosorbide dinitrate, and ethyleneglycol dinitrate but did not denitrate isopropyl nitrate, 2,4,6-trinitrotoluene, or RDX. Thus, the enzyme was able to reduce nitrate esters but not nitro groups connected to a carbon or nitrogen atom.
Growth studies with Agrobacterium strain JS71.
The Agrobacterium strain JS71 was grown under the same nitrogen-limited conditions as those used for the enrichment cultures but with the addition of 250 μM CL-20. CL-20 was added from a stock solution in methanol (20 mM). The optical densities of the cultures could not be determined because the undissolved CL-20 interfered with the absorption. Therefore, bacterial growth was estimated by determining the protein concentration. Cell suspensions were mixed 1:1 with acetone, and the cells were collected by centrifugation and washed with 100 μl of acetone. The pelleted cells were suspended in 0.1 M NaOH and incubated at 80°C for 5 min to lyse the cells. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.). CL-20 concentrations in the cultures were measured by HPLC after the addition of 4 volumes of acetonitrile to the samples and centrifugation as described above.
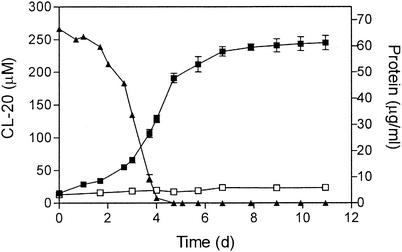
Strain JS71 grew in minimal medium containing succinate and CL-20 at a growth rate of 0.14 per day. The isolate did not grow with CL-20 as the sole nitrogen and carbon source. The slow growth of strain JS71 was probably due to the very low water solubility of CL-20. When the surfactant Tween 80 (0.1% [vol/vol]) was added to the cultures to increase the solubility and therefore the availability of CL-20, the growth rate was 0.59 per day (Fig. 4). Strain JS71 could not grow in media without a nitrogen source (Fig. 4) or in media containing RDX or HMX as the sole nitrogen source (data not shown).
FIG. 4.
Growth of Agrobacterium strain JS71. Aerobic cultures were grown in medium with 20 mM succinate-0.1% (vol/vol) Tween 80 with (▪) and without (□) 250 μM CL-20 as the sole nitrogen source. ▴, CL-20 concentration. The growth studies were performed in triplicate (▪) or duplicate (□). d, days.
The addition of Tween 80 to cultures containing CL-20 facilitated the determination of CL-20 concentrations in addition to increasing the growth rate. The disappearance of CL-20 and the growth of strain JS71 correlated directly, which suggested that initial metabolites of CL-20 were used as the nitrogen source.
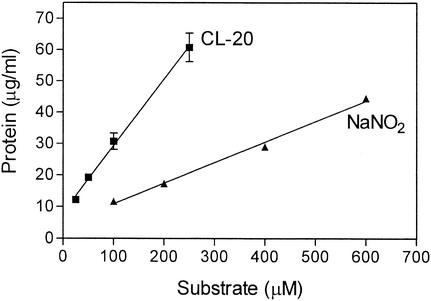
To determine how many moles of nitrogen are assimilated from 1 mol of CL-20 by the Agrobacterium isolate, the strain was grown in various concentrations of CL-20 (25, 50, 100, and 250 μM) or NaNO2 (100, 200, 400, and 600 μM) as the sole nitrogen source. The growth yield of strain JS71 in liquid medium was 213 ± 13 g of protein per mol of CL-20 (Fig. 5). The growth yield in medium with NaNO2 as the sole nitrogen source was 65 ± 3 g of protein per mol of NaNO2 (Fig. 5). Comparison of the growth yields suggests that the Agrobacterium strain JS71 uses 3 of the 12 nitrogen atoms from the CL-20 molecule.
FIG. 5.
Growth yield of Agrobacterium strain JS71 with 20 mM succinate and different concentrations of CL-20 (▪) or NaNO2 (▴). The CL-20 cultures were supplemented with 0.1% (vol/vol) Tween 80. All growth studies were performed in duplicate.
Preliminary HPLC-mass spectrometry negative electrospray ionization mass analysis results indicated the formation of a metabolite with a molecular weight of 88 in cultures degrading CL-20 (data not shown). Further investigations are necessary to identify the structure of the intermediate.
The results indicate clearly that CL-20 is biodegraded readily in soil. Therefore, CL-20 might be less persistent in the environment than RDX and HMX, which accumulate at contaminated sites. The lower water solubility of CL-20 will be a major determinant of its availability for biodegradation. Further investigation is required to elucidate the pathway and final products of CL-20 degradation.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of isolate JS71 was deposited at GenBank under accession number AY174112.
Acknowledgments
This work was funded by the U.S. Air Force Office of Research Science and the U.S. Strategic Environmental Research and Development Program and supported in part by the Oak Ridge Institute for Science and Education (U.S. Department of Energy).
We thank Joe Hughes, Bill Wallace, and Dan Lessner for providing soil samples.
REFERENCES
- 1.Bhushan, B., A. Halasz, J. Spain, and J. Hawari. 2002. Diaphorase catalyzed biotransformation of RDX via N-denitration mechanism. Biochem. Biophys. Res. Commun. 296:779-784. [DOI] [PubMed] [Google Scholar]
- 1a.Bhushan, B., S. Trott, J. C. Spain, A. Halasz, L. Paquet, and J. Hawari. 2003. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a rabbit liver cytochrome P450: insight into the mechanism of RDX biodegradation by Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 69:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binks, P. R., S. Nicklin, and N. C. Bruce. 1995. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl. Environ. Microbiol. 61:1318-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn, J. W., and W. R. Hafker. 1993. The impact of biochemistry, bioavailability and bioactivity on the selection of bioremediation techniques. Trends Biotechnol. 11:328-333. [DOI] [PubMed] [Google Scholar]
- 4.Boopathy, R. 2001. Enhanced biodegradation of cyclotetramethylenetetranitramine (HMX) under mixed electron-acceptor condition. Bioresour. Technol. 76:241-244. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, N. V., D. R. Nelson, and T. Duxbury. 1998. Aerobic biodegradation of the hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol. Biochem. 30:1159-1166. [Google Scholar]
- 6.Coleman, N. V., J. C. Spain, and T. Duxbury. 2002. Evidence that RDX biodegradation by Rhodococcus strain DN22 is plasmid-borne and involves a cytochrome P-450. J. Appl. Microbiol. 93:463-472. [DOI] [PubMed] [Google Scholar]
- 7.Emery, D. D., and P. C. Faessler. 1997. First production-level bioremediation of explosives-contaminated soil in the United States. Ann. N. Y. Acad. Sci. 829:326-340. [DOI] [PubMed] [Google Scholar]
- 8.Etnier, E. L. 1989. Water quality criteria for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Regul. Toxicol. Pharmacol. 9:147-157. [DOI] [PubMed] [Google Scholar]
- 9.Fournier, D., A. Halasz, J. Spain, P. Fiurasek, and J. Hawari. 2002. Determination of key metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine with Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 68:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller, M. E., and J. F. Manning, Jr. 1997. Aerobic gram-positive and gram-negative bacteria exhibit differential sensitivity to and transformation of 2,4,6-trinitrotoluene (TNT). Curr. Microbiol. 35:77-83. [DOI] [PubMed] [Google Scholar]
- 11.Gong, P., J. Hawari, S. Thiboutot, G. Ampleman, and G. I. Sunahara. 2001. Ecotoxicological effects of hexahydro-1,3,5-trinitro-1,3,5-triazine on soil microbial activities. Environ. Toxicol. Chem. 20:947-951. [DOI] [PubMed] [Google Scholar]
- 12.Haas, R., I. Schreiber, E. von Loew, and G. Stork. 1990. Conception for the investigation of contaminated munition plants. Fresenius' J. Anal. Chem. 338:41-45. [Google Scholar]
- 13.Hawari, J. 2000. Biodegradation of RDX and HMX: from basic research to field application. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Boca Raton, Fla.
- 14.Hawari, J., A. Halasz, T. Sheremata, S. Beaudet, C. Groom, L. Paquet, C. Rhofir, G. Ampleman, and S. Thiboutot. 2000. Characterization of metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) with municipal anaerobic sludge. Appl. Environ. Microbiol. 66:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawari, J., A. Halasz, S. Beaudet, L. Paquet, G. Ampleman, and S. Thiboutot. 2001. Biotransformation routes of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine by municipal anaerobic sludge. Environ. Sci. Technol. 35:70-75. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan, A. S., C. F. Berghout, and A. Peczenik. 1965. Human intoxication from RDX. Arch. Environ. Health 10:877-883. [DOI] [PubMed] [Google Scholar]
- 17.Kitts, C. L., D. P. Cunningham, and P. J. Unkefer. 1994. Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Appl. Environ. Microbiol. 60:4608-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieg, N. R., and J. G. Holt (ed.). 1984. Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 19.Larson, S. L., D. R. Felt, L. Escalon, J. D. Davis, and L. D. Hansen. 2001. Analysis of CL-20 in environmental matrices: water and soil. ERDC/EL TR-01-21. U.S. Army Engineer Research and Development Center, Vicksburg, Miss.
- 20.Larson, S. L., D. R. Felt, J. L. Davis, and L. Escalon. 2002. Analysis of CL-20 in environmental matrices: water and soil. J. Chromatogr. Sci. 40:201-206. [DOI] [PubMed] [Google Scholar]
- 21.McCormick, N. G., J. H. Cornell, and A. M. Kaplan. 1981. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Appl. Environ. Microbiol. 42:817-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, A. T., A. P. Chafin, S. L. Christian, D. W. Moore, M. P. Nadler, R. A. Nissan, and D. J. Vanderah. 1998. Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 54:11793-11812. [Google Scholar]
- 23.Robidoux, P. Y., J. Hawari, S. Thiboutot, G. Ampleman, and G. I. Sunahara. 2001. Chronic toxicity of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in soil determined using the earthworm (Eisenia andrei) reproduction test. Environ. Pollut. 111:283-292. [DOI] [PubMed] [Google Scholar]
- 24.Seth-Smith, H. M. B., S. J. Rosser, A. Basran, E. R. Travis, E. R. Dabbs, S. Nicklin, and N. C. Bruce. 2002. Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Appl. Environ. Microbiol. 68:4764-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson, R. L., P. A. Urtiew, D. L. Ornellas, G. L. Moody, K. J. Scribner, and D. M. Hoffman. 1997. CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propel. Explos. Pyrotech. 22:249-255. [Google Scholar]
- 26.Snape, J. R., N. A. Walkley, A. P. Morby, S. Nicklin, and G. F. White. 1997. Purification, properties, and sequence of glycerol trinitrate reductase from Agrobacterium radiobacter. J. Bacteriol. 179:7796-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talmage, S. S., D. M. Opresko, C. J. Maxwell, C. J. E. Welsh, F. M. Cretella, P. H. Reno, and F. B. Daniel. 1999. Nitroaromatic munition compounds: environmental effects and screening values. Rev. Environ. Contam. Toxicol. 161:1-156. [DOI] [PubMed] [Google Scholar]
- 28.White, G. F., J. R. Snape, and S. Nicklin. 1996. Biodegradation of glycerol trinitrate and pentaerythritol tetranitrate by Agrobacterium radiobacter. Appl. Environ. Microbiol. 62:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, J. C., J. W. Kelsey, P. B. Hatzinger, and M. Alexander. 1997. Factors affecting sequestration and bioavailability of phenanthrene in soils. Environ. Toxicol. Chem. 16:2040-2045. [Google Scholar]