Abstract
β-Catenin promotes epithelial architecture by forming cell surface complexes with E-cadherin and also interacts with TCF/LEF-1 in the nucleus to control gene expression. By DNA transfection, we overexpressed β-catenin and/or LEF-1 in NIH 3T3 fibroblasts, corneal fibroblasts, corneal epithelia, uveal melanoma cells, and several carcinoma cell lines. In all cases (with or without LEF-1), the abundant exogenous β-catenin localizes to the nucleus and forms distinct nuclear aggregates that are not associated with DNA. Surprisingly, we found that with time (5–8 d after transfection) cells overexpressing β-catenin all undergo apoptosis. LEF-1 does not need to be present. Moreover, LEF-1 overexpression in the absence of exogenous β-catenin does not induce apoptosis, even though some endogenous β-catenin moves with the exogenous LEF-1 into the nucleus. TOPFLASH/FOPFLASH reporter assays showed that full-length β-catenin is able to induce LEF-1–dependent transactivation, whereas Arm β-catenin totally abolishes the transactivating function. However, Arm β-catenin, containing deletions of known LEF-1–transactivating domains, has the same apoptotic effects as full-length β-catenin. Overexpressed β-catenin also induces apoptosis in cells transfected with nuclear localization signal–deleted LEF-1 that localizes only in the cytoplasm. Thus, the apoptotic effects of overexpressed exogenous β-catenin do not rely on its transactivating function with nuclear LEF-1. Overexpressed δ-catenin, containing 10 Arm repeats, induces only minor apoptosis, suggesting that the major apoptotic effect may be due to domains specific to β-catenin as well as to Arm repeats. The absence of p53, Rb, cyclin D1, or E2F1 does not affect the apoptotic effect of overexpressed β-catenin, but Bcl-x(L) reduces it. We hypothesize that in vivo apoptosis of cells overexpressing β-catenin might be a physiological mechanism to eliminate them from the population.
INTRODUCTION
β-Catenin was first identified as a protein binding to E-cadherin in adherent junctions that are required to maintain the architecture of epithelia. β-Catenin can be released from cadherin complexes through several mechanisms, including down-regulation of E-cadherin, and the level of β-catenin in cells is tightly controlled through interactions with other proteins, such as APC, GSK-3β, β-TrCP, and Axin (Aberle et al., 1997; Jiang et al., 1998; Willert et al., 1999). Free cytoplasmic β-catenin is phosphorylated by GSK-3β, then degraded through interaction with β-TrCP (Rubinfeld et al., 1996; Liu et al., 1999). Signaling molecules that affect the stability of cytoplasmic β-catenin include Wnt, Ras, and phosphatidylinositol 3-kinase (Hsu et al., 1998; Espada et al., 1999; Willert et al., 1999). Under some conditions, β-catenin enters the nucleus and affects target gene expression by interacting with TCF/LEF-1 proteins (Behrens et al., 1996; Molenaar et al., 1996). TCF/LEF-1 proteins bind DNA directly, but β-catenin does not. The binding of β-catenin to TCF/LEF-1 is required for the former to transactivate target genes (Hsu et al., 1998; Tetsu and McCormick, 1999).
β-Catenin functions as a signaling molecule vary in different species and in different cell types. In Xenopus, the interaction of β-catenin and TCF/LEF-1 plays a role in dorsal-ventral axis formation, and major downstream targets seem to include a siamois transcription factor and fibronectin (Brannon et al., 1997; Gradl et al., 1999). In mice, E-cadherin and connexin 43 are believed to be controlled by the β-catenin and TCF/LEF-1 pathway (Huber et al., 1996; van der Heyden et al., 1998). Mutations in β-catenin or APC increase free cytoplasmic and nuclear β-catenin in human tumor cells, and the resulting interaction with TCF/LEF-1 may play a role in cell cycle control. With the use of inducible APC cell lines, or cells transfected with β-catenin and/or TCF/LEF-1 DNAs, it was possible to identify c-myc, cyclin D1, PPARδ, and TCF1 as potential targets of β-catenin and TCF/LEF-1 in human tumor cells (He et al., 1998, 1999; Roose et al., 1999; Shtutman et al., 1999; Tetsu and McCormick, 1999).
An additional function recently proposed for the interaction of β-catenin and TCF/LEF-1 involves apoptosis. β-Catenin and TCFs induce apoptosis in Drosophila retinal neurons (Ahmed et al., 1998). Excess β-catenin in NIH 3T3 cells and human H1299 cells mimics the induction by apoptotic stimuli of transcriptionally active p53 (Damalas et al., 1999). Forced expression of N-terminal–deleted β-catenin increases apoptosis and proliferation in mouse intestinal epithelia (Wong et al., 1998). Truncation of the β-catenin–binding domain of VE-cadherin induces endothelial apoptosis in mice, indicating the role of β-catenin signaling in endothelial survival (Carmeliet et al., 1999). There are also reports that neuronal apoptosis is induced by destabilization of β-catenin through missense mutation of presenilin-1, which is the most commonly mutated gene in familial Alzheimer patients (Zhang et al., 1998). An interaction between presenilin-1 and β-catenin may stabilize β-catenin to prevent apoptosis in neuronal cells. However, it is not known whether the effects on apoptosis are direct consequences of β-catenin or secondary compensatory responses to β-catenin–augmented proliferation (Wong et al., 1998). It is also unknown whether or not transactivating domains of β-catenin and functional LEF-1 are required to induce apoptosis in mammalian cells.
Our interest in the effects of β-catenin on apoptosis stems from our efforts to establish stable cell lines expressing exogenous β-catenin, LEF-1, or both. We were able to establish several NIH 3T3 stable cell lines overexpressing LEF-1. As expected, short-term activation of β-catenin and LEF-1 in these cells up-regulates cyclin D1 but not c-myc. Although we failed to establish stable cell lines overexpressing β-catenin, we found that in time overexpressed β-catenin forms distinct nuclear aggregates and induces apoptosis with or without overexpressed LEF-1. Because G1 regulators of the cell cycle, such as Rb, E2F1, p53, and cyclin D1, have been implicated in apoptosis (King and Cidlowski, 1998), we examined the possibility that the apoptotic effects of β-catenin are due to a secondary proliferative response involving these factors. However, we found that fibroblasts deficient in Rb, E2F1, p53, or cyclin D1 show the same apoptotic response to β-catenin as control NIH 3T3 fibroblasts. Thus, the apoptotic effects of β-catenin are not due to effects on proliferation involving these factors. We also found that overexpression of LEF-1 does not induce apoptosis, even when associated with endogenous β-catenin in the nucleus, and that the apoptotic effect of β-catenin is independent of its transactivation function with LEF-1. We suggest that cells overexpressing β-catenin in vivo may be eliminated by this unique apoptotic mechanism under certain circumstances.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
Rb+/+ and Rβ−/− mouse embryo fibroblasts, NIH 3T3 fibroblasts, cyclin D1−/− fibroblasts, p53−/− and E2F1−/− fibroblasts, and stable lines of Rb+/+ 3T3 cells expressing SV40 T antigen and the K1 mutant were kindly provided by Drs. M.E. Ewen and K.Y. Lee (Dana-Farber Cancer Institute, Boston, MA). All mouse embryo fibroblasts and their 3T3 derivatives were maintained in DMEM supplemented with 10% FBS. E8 corneal epithelia and fibroblasts, isolated from chick embryos, were cultured in DMEM/F12 medium supplemented with 5% FBS. M619 human uveal melanomas were derived from surgical tumor specimens of eyes, as described previously (Kim et al., 1998), and cultured in RPMI-1640 supplemented with penicillin, streptomycin, and 10% FBS. SW48, HCT116, SW480, and DLD1 human colon carcinomas were obtained from the American Type Culture Collection (Rockville, MD) and cultured in McCoy's medium. Human HeLa cervical carcinomas and HeLa cells overexpressing Bcl-x(L) were obtained from Dr. Honglin Li (Harvard Medical School, Boston, MA) and cultured in DMEM/F12. Stable NIH 3T3 cell lines overexpressing full-length LEF-1 or nuclear localization signal (NLS)-deleted LEF-1 (pLEF-1ΔNLS) were created by transfection of pcDNA3.1-LEF-1 or pcDNA3.1-LEF-1ΔNLS, respectively, followed by selection in Zeocin (Invitrogen, San Diego, CA).
Plasmid Construction
All β-catenin constructs tagged with blue or green fluorescent protein (BFP or GFP) were produced by PCR amplification with the use of human wild-type β-catenin in a pBAT vector as a template. The resulting PCR products were subcloned into the pEBFP-C1 or pEGFP-C1 vector (Clontech, Palo Alto, CA), respectively. N-terminal–deleted β-catenin (β-cateninΔN) lacks the first 86 amino acids, C-terminal–deleted β-catenin (β-cateninΔC) lacks the last 123 amino acids, and Arm β-catenin lacks both the N-terminal 86 amino acids and the C-terminal 123 amino acids.
A full-length LEF-1 (1–1194 base pairs) construct was kindly provided by Rudolf Grosschedl (University of San Francisco, San Francisco, CA). Full-length LEF-1 tagged with hemagglutinin (HA) was subcloned into pcDNA3.1/Zeo(−) (Invitrogen) with the use of XbaI and Asp-718 restriction enzyme sites to create the selection marker Zeocinr. pLEF-1ΔNLS (1–1065 base pairs) tagged with HA was produced by PCR amplification and subcloning of the resulting PCR products into pcDNA3.1/Zeo(−). After subcloning, the sequences of all β-catenin and LEF-1 constructs were confirmed by DNA sequencing and found to be in frame.
A mouse full-length δ-catenin construct was kindly provided by Qun Lu (East Carolina University, Greenville, NC).
Antibodies
mAbs specific for β-catenin and HA were purchased from Transduction Laboratories (Lexington, KY) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. A polyclonal antibody specific for BFP/GFP was purchased from Clontech. HRP-conjugated secondary antibodies were obtained from Calbiochem (San Diego, CA).
Transfection
Cell were transfected with the use of LipofectAMINE PLUS reagent (Life Technologies, Grand Island, NY). Cells were trypsinized briefly 1 d before transfection and plated on 35-mm-diameter dishes so that they were 50–80% confluent on the day of transfection. One microgram of DNA was diluted in 100 μl of serum-free medium, and 6 μl of LipofectAMINE PLUS regents was added. The DNA-PLUS mixture was incubated at room temperature for 20 min, and 4 μl of LipofectAMINE reagent was added for an additional 20 min of incubation. While complexes were forming, cells were washed with serum-free medium twice and 800 μl of transfection medium without serum and antibiotics. The DNA-PLUS–LipofectAMINE reagent complexes were applied to the cells before incubating at 37°C at 5% CO2 for 3 h. After incubation, recovery medium with 10% FBS was added to bring the final volume to 2 ml. After overnight incubation, the recovery medium was replaced with fresh, complete medium containing serum and antibiotics. DNAs and proteins were extracted at different time intervals for a DNA fragment test and Western blot analysis. Cells were fixed at different times for the TUNEL (TdT-mediated dUTP Nick end-labeling) test, Hoechst staining, and fluorescent observation.
Western Blot Analysis
For Western blot analyses, cells were washed with cold PBS, and the monolayers were extracted in RIPA buffer (1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 150 mM NaCl, 50 mM Tris-Cl, 2 mM EDTA, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM PMSF). Cells were scraped from dishes and incubated in RIPA buffer for 30 min on ice, syringed through 25-gauge needles six times, and centrifuged at 5000 × g for 5 min. Supernatants were stored at −80°C until protein assays were performed.
The titers of the primary antibodies were determined (for β-catenin, 1:1000 dilution; for GFP, 1:100 dilution). For β-catenin and BFP/GFP, 20 μg of protein extract was electrophoresed on 7.5% Tris-glycine gels and blotted onto nitrocellulose. We stained the blot membrane with 0.001% India ink (vol/vol) in PBS to confirm the equal loading of samples after developing blots with the use of ECL detection kits (Amersham, Cleveland, OH).
Quantitation of Apoptotic Cells
For the TUNEL test, we used the in situ cell death detection kit from Boehringer Mannheim (Indianapolis, IN). Briefly, cells were transfected with plasmid containing a specific gene as described above. After culturing cells for different durations (2, 4, and 7 d), they were fixed with 4% paraformaldehyde for 15 min, rinsed with PBS, and incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 min at 4°C. Cells were rinsed with PBS twice, and 50 μl of TUNEL reaction mixture was added to the cells. After incubation for 1 h at 37°C in the dark, cells were rinsed with PBS three times and analyzed under a Zeiss LSM 410 confocal laser scanning microscope (Carl Zeiss, Thornwood, NY).
For Hoechst staining, cells were transfected with β-catenin–GFP. After culturing cells for different durations (2, 4, and 7 days), they were fixed with 4% paraformaldehyde, rinsed with PBS, and incubated with Hoechst 33258 (0.5 μg/ml; Calbiochem) for 10 min at room temperature. Cells were washed with PBS three times and analyzed under a Zeiss LSM 410 confocal laser scanning microscope.
For the DNA fragmentation assay, cells at different times after transfection (2 and 5 d) were harvested and lysed in 500 μl of lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM EDTA, 0.1% SDS, 0.1 mg/ml proteinase K) at 50°C for 16 h followed by an additional incubation with 50 μg/ml RNase A for 1 h. DNA was extracted with phenol/chloroform, precipitated with ethanol, and dissolved in 40 μl of TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA). Four micrograms of extracted DNA was electrophoresed in a 1.8% agarose gel, visualized with ethidium staining, and photographed under a UV transilluminator.
Reverse Transcription PCR
RNAs were extracted from NIH 3T3 fibroblasts and LEF-1–overexpressing stable cell lines with the use of a RNeasy mini kit (Qiagen, Santa Clarita, CA). Reverse transcription (RT)-PCR was performed with the use of Clontech amplimer sets. Sequences of primers specific for lef-1 and c-myc were as follows: for lef-1, 5′CACCTAAGCGACGAGCACT3′ and 5′CGTGTTGAGGCTTCACGTGC3′; for c-myc, 5′CTCTGCCTCTGCCCGCGATCA3′ and 5′CGGTGGAGAA-GTTGCCACC3′. To confirm the even loading, we used β-actin control primer sets (Clontech).
Transient Luciferase Assays
Transient transfections of full-length or mutant β-catenins were performed by the LipofectAMINE PLUS method with the use of NIH 3T3 fibroblasts and A23 cells overexpressing LEF-1, as described above. For TOPFLASH/FOPFLASH reporter assays, A23 cells were transfected with 0.5 μg of pTOPFLASH-Luc or pFOPFLASH-Luc, 0.5 μg of pCMV-βGal, 1 μg of pEGFP-C1 vector containing full-length or mutant β-catenin, or 1 μg of pEGFP-C1 empty vector. For cyclin D1 reporter assays, pCD1-Luc reporter plasmid was used. This pCD1-Luc contained the original fragment of cyclin D1 5′ sequence (−1748CD1) cloned from the PRAD1 breakpoint (Motokura and Arnold, 1993). Cells were transfected as indicated with 1 μg of pCD1-Luc, 1 μg of pCMV-βGal, 1 μg of pEGFP-C1 vector containing full-length or Arm β-catenin, or 1 μg of pEGFP-C1 empty vector. Luciferase and β-galactosidase activities were assayed 1 d (TOPFLASH-Luc/FOPFLASH-Luc) or 2, 6, and 9 d (pCD1-Luc) after transfection.
RESULTS
β-Catenin Overexpression Induces Apoptosis, but LEF-1 Does Not
In the process of establishing β-catenin–overexpressing cell lines, we found that forced expression of β-catenin induces apoptosis in NIH 3T3 fibroblasts. By tagging exogenous β-catenin with BFP (Figure 1A, blue) or GFP (Figure 1B, green), we were able to follow its localization in living cultures with the use of a confocal fluorescence microscope. After transient transfection in NIH 3T3 fibroblasts (Figure 1, A and B), we observed that the full-length wild-type β-catenins coupled to GFP or BFP were mainly localized in the nucleus, where they may have a diffuse and/or reticular staining pattern. Similar data were obtained for primary corneal fibroblasts and corneal epithelia (our unpublished results). Transfection efficiencies of 30 to 40% were observed by counting BFP- or GFP-positive cells within 2 d after transfection. We estimated that cells overexpressing β-catenin within 2 d after transfection have 10- to 12-fold more exogenous β-catenin than endogenous β-catenin based on densitometer analysis and transfection efficiency. However, few if any BFP- or GFP-positive cells could be found 7–10 d after transfection. When proteins were extracted at different times and run on Western blots, we observed the presence of an exogenous β-catenin band (BFP- or GFP-tagged, 119 kDa) in protein extracts 2 d after transfection but not 8 d after transfection (Figure 1C).
Figure 1.
Overexpression of full-length (FL) β-catenin induces apoptosis. (A) NIH 3T3 cells expressing full-length β-catenin–BFP (arrows) are positive for TUNEL staining and show very shrunken apoptotic morphologies. (B) Cells expressing exogenous full-length β-catenin–GFP have bright Hoechst-positive nuclei (arrows), whereas most GFP-negative cells do not (arrowheads). (C) Western blot analysis showed that exogenous full-length β-catenin–GFP/BFP bands were present for protein extracted 2 d after transfection but not at 8 d. (D) DNA fragmentation test. After NIH 3T3 cells were transfected with full-length β-catenin, DNAs extracted at various days were run with DNA ladder (lane 1) and control DNA extracted from mock-transfected NIH 3T3 cells at 5 d after transfection (lane 2). DNA fragments started to appear at 2 d after transfection with β-catenin (lane 3) and intensified at 5 d after transfection (lane 4). Bars, 50 μm.
Because cells overexpressing exogenous β-catenin disappear in time, it seems likely that β-catenin overexpression causes cell death. To determine that cells overexpressing β-catenin undergo apoptosis but not necrosis, we performed TUNEL tests, Hoechst staining, and DNA fragment tests. As shown in Figure 1, A and B, cells overexpressing β-catenin that were GFP or BFP positive were also positive for the TUNEL test and Hoechst staining. DNA ladder, which is the hallmark of apoptosis, was also observed in DNA extracted from cells 5 d after transfection (Figure 1D). The addition of a different exogenous tag (Flag) to full-length β-catenin did not change the apoptotic effects of overexpressed β-catenin (our unpublished results).
As controls, we transfected NIH 3T3 fibroblasts with BFP and GFP empty vectors or LEF-1 DNA under the same cytomegalovirus (CMV) promoter used for β-catenin and did not observe cell death (Figure 2A). We also transfected NIH 3T3 fibroblasts with δ-catenin, a protein related structurally to β-catenin and p120ctn containing 10 Arm repeats and expressed in brain cells (Lu et al., 1999). It induced only minor apoptotic effects (Figure 2A, gray bar). Interestingly, although we were never able to establish stable cell lines overexpressing β-catenin, we were able to establish several stable cell lines overexpressing LEF-1. No apoptosis occurred in these cells. The expression of exogenous LEF-1 was confirmed by RT-PCR, Western blot analysis, and immunostaining (see below).
Figure 2.
Overexpression of full-length β-catenin induces apoptosis. (A) Full-length β-catenin (FL β-catenin, dotted bar) induces significant apoptosis in NIH 3T3 cells, but BFP vector (mock transfection) and LEF-1 do not. δ-Catenin induces only minor apoptosis. Total percentage of BFP-positive cells varies with the transfection efficiency. (B) Overexpression of β-catenin induces apoptosis (hatched bar) in all human colon carcinomas. (C) GFP-positive tumor cells, overexpressing full-length β-catenin, were easily detected 2 d after transfection (arrows). However, cells expressing full-length β-catenin–GFP were noticeably smaller than GFP-negative cells (SW48, HCT116, DLD1, and SW480, human colon carcinomas; HeLa, human cervical carcinomas; and M619, human uveal melanomas). Bar, 50 μm.
Apoptotic Effects of Wild-Type β-Catenin Overexpression Are Also Observed in Several Tumor Cell Lines
Because β-catenin has been suggested to play a positive role in promoting tumorigenesis, we transfected tumor cells with full-length β-catenin–BFP or β-catenin–GFP to determine whether or not the apoptotic effects of overexpressing β-catenin can also be induced in tumor cells. We transfected several human colon carcinomas (SW480, HCT116, DLD1, SW48), an ovarian tumor (HeLa), and a human uveal melanoma (M619). Colon carcinomas contain a mutation in either β-catenin (HCT116-Δ45; SW48-S33Y) or APC (SW480 and DLD1). About two-thirds of the tumor cells expressing full-length β-catenin–BFP were positive for TUNEL on d 2 (Figure 2B, black bar), but by d 4 and 7, the numbers of living cells had decreased greatly (Figure 2B). The transfected tumor cells overexpressing β-catenin undergo the same morphological effects observed in normal cells during apoptosis (NIH 3T3 fibroblasts, primary corneal fibroblasts, and corneal epithelia). GFP-positive tumor cells that overexpress full-length β-catenin (Figure 2C, arrows) are noticeably smaller than GFP-negative tumor cells, indicating that the former are in the process of apoptosis. Hoechst staining of these GFP-positive cells also shows a very condensed or fragmented nuclear staining pattern. As was the case with the NIH 3T3 fibroblasts, we found hardly any GFP-positive β-catenin–transfected tumor cells alive 7–10 d after transfection.
Induction of Apoptosis by β-Catenin Is Independent of Its Transactivating Function and Nuclear Localization of Exogenous LEF-1
As we noted above (see INTRODUCTION), many of the effects of β-catenin involve interactions with nuclear LEF-1. However, the induction by exogenous β-catenin of apoptosis does not appear to involve LEF-1. Endogenous LEF-1 is not detectable in the nuclei of NIH 3T3 cells, corneal epithelia, or Madin-Darby canine kidney cells (K. Kim and E.D. Hay, unpublished data), yet they undergo apoptosis after β-catenin transfection. Moreover, even though they contain endogenous β-catenin, apoptosis does not occur in LEF-1–overexpressing NIH 3T3 cells, corneal epithelia, or Madin-Darby canine kidney epithelia. Indeed, at 3 d after transfection, only a small portion (<5–10%) of these cells positive for exogenous LEF-1 show clear nuclear localization of endogenous β-catenin, which is an essential step for controlling target gene expression. In contrast to transfection with full-length β-catenin (Figure 2A, FL β-catenin), transfection with LEF-1 DNA does not induce apoptosis (Figure 2A, LEF-1), even though the transfected cells express similar levels of exogenous proteins (our unpublished results). The presence of BFP/GFP (mock transfection) and exogenous LEF-1 was confirmed at 2 and 8 d after transfection by Western blot analysis. Transfection with β-catenin of cells already overexpressing LEF-1 does not increase the apoptotic effects of β-catenin (see below).
Because β-catenin transactivates gene expression in a complex with TCF/LEF-1 proteins, we also used several types of β-catenin constructs to determine whether the transactivating function is necessary to induce apoptosis. As shown in Figure 3A, NIH 3T3 cells overexpressing different β-catenin constructs show different staining patterns. Cells with N-terminal–deleted β-catenin (ΔN1–86; no target serine residues for GSK-3β) show clumps of fluorescent staining in the cytoplasm as well as in the nucleus (Figure 3A, lower left). Both C-terminal–deleted β-catenin (ΔC669–781 amino acids) and ARM β-catenin (missing both the N- and C-terminal ΔN1–86 and ΔC669–781) show nuclear punctate fluorescent patterns similar to full-length β-catenin. The nuclear aggregates of exogenous β-catenin are not associated with chromosomal DNA, vary greatly in number and size, and form round to oblong nuclear bodies (Figure 3A).
Figure 3.
The apoptotic effects of β-catenin are independent of its transactivating function with LEF-1. (A) Different β-catenin constructs were expressed in NIH 3T3 fibroblasts. Full-length (FL), C-terminal–deleted (delC), and Arm β-catenin were detected by GFP fluorescence mostly in the nucleus, where they formed distinct aggregates. N-terminal–deleted (delN) β-catenin accumulated mostly in cytoplasm. GFP, control empty GFP transfection. Bar, 50 μm. (B) The presence of exogenous β-catenin was confirmed by Western blot analysis with the use of anti-β-catenin and anti-GFP antibodies. Exogenous full-length and ΔN1–86 β-catenins were detected as bands (119 and 110 kDa, respectively) by these two antibodies. Because anti-β-catenin antibody was raised against the immunogen of C-terminal residues (571–781 amino acids) in β-catenin, ΔC β-catenin (ΔC669–781) and Arm β-catenin (ΔN1–86 and ΔC669–781) showed very faint bands (108 and 99 kDa, respectively) by anti-β-catenin antibody. However, the presence of exogenous ΔC and Arm β-catenin was clearly confirmed with anti-GFP antibody (bottom panel). (C) Full-length and ΔN β-catenin are able to transactivate TOPFLASH-Luc reporter containing TCF/LEF-1–binding motifs, whereas Arm β-catenin totally abolishes the transactivating function. ΔC β-catenin shows only marginal transactivation (1.3-fold). FOPFLASH reporter, containing mutant binding sites, is not activated by any of the β-catenin constructs. (D) Arm β-catenin does not have any transactivating activity with TCF/LEF-1 but still was able to induce apoptosis.
We performed Western blot analyses of these β-catenin–transfected NIH 3T3 fibroblasts with the use of GFP/BFP- or β-catenin–specific antibodies to establish that overexpression of the transfectants was induced (Figure 3B). Exogenous full-length and ΔN1–86 β-catenin were each seen as a band (119 and 110 kDa, respectively) by anti-β-catenin (Figure 3B, top panel) and anti-GFP (Figure 3B, bottom panel) antibodies. ΔC669–781 β-catenin showed a very faint band (108 kDa) by anti-β-catenin antibody but a clear band by anti-GFP antibody, because anti-β-catenin antibody was raised against peptides corresponding to residues 571–781 of β-catenin. For the same reason, Arm β-catenin (missing both the N- and C-terminal ΔN1–86 and ΔC669–781) showed a very faint band (92 kDa) above the endogenous β-catenin (92 kDa) by anti-β-catenin antibody but a clear band by anti-GFP antibody.
To confirm their transactivation activities or inactivities, full-length or mutant β-catenin constructs were introduced into NIH 3T3 cells overexpressing LEF-1 (A23 clones) together with TOPFLASH-Luc, a positive control reporter plasmid containing TCF/LEF-1–binding sites, or with FOPFLASH-Luc, a negative control plasmid having mutant binding sites (Figure 3C). Both full-length and ΔN1–86 β-catenin showed threefold LEF-1–dependent transactivation over mock-transfected cells with empty GFP vector. ΔC669–781 β-catenin showed only 1.3-fold induction, whereas Arm β-catenin (missing both the N and C termini) totally abolished its transactivation activity (Figure 3C, TOPFLASH). As expected, transfection with a negative control plasmid (FOPFLASH) showed no increase in luciferase activity over mock transfection. However, cells overexpressing different β-catenins all undergo apoptosis (Figure 3D), including Arm β-catenin, which has absolutely no LEF-1–dependent transactivating activity (Figure 3C).
We also studied NIH 3T3 cells overexpressing the full-length LEF-1 and NLS-deleted LEF-1 to determine whether the apoptotic effects of β-catenin are affected by the level or localization of the β-catenin DNA-binding partner, LEF-1. As shown in Figure 4A, full-length LEF-1 is localized mainly in the nucleus in a diffuse pattern and NLS-deleted LEF-1 is localized in the cytoplasm. However, after transfection, exogenous full-length β-catenin localized in the nucleus (Figure 4B) regardless of the presence of exogenous LEF-1 or NLS-deleted LEF-1 (Figure 4B). NIH 3T3 stable cell lines overexpressing either full-length LEF-1 or NLS-deleted LEF-1 show the same apoptotic effects as the parental NIH 3T3 fibroblasts after β-catenin transfection (Figure 4C), indicating that neither the level nor the localization of exogenous LEF-1 affects the apoptotic consequence of overexpressed β-catenin. Mutants of β-catenin also induce apoptosis in these A23 cells overexpressing LEF-1 (Figure 4D).
Figure 4.
The apoptotic effects of β-catenin are not affected by the localization of LEF-1. (A) Stable clonal NIH 3T3 cells overexpressing full-length (FL) LEF-1 and NLS-deleted LEF-1 were established. Localization of exogenous LEF-1 was confirmed with the use of anti-HA antibody. Full-length exogenous LEF-1 was localized in the nucleus, but little or no NLS-deleted LEF-1 enters the nucleus. (B) Exogenous full-length β-catenin–GFP in these cells all entered the nucleus, even though some cells overexpressed full-length LEF-1 or NLS-deleted LEF-1. (C) Overexpression of full-length β-catenin induces apoptosis in cells overexpressing either full-length or NLS-deleted LEF-1. (D) Different β-catenins induce apoptosis in A23 cells overexpressing full-length LEF-1. Even Arm β-catenin with no transactivating domains was able to induce apoptosis. Bar, 50 μm.
Short-term activation of the β-catenin/LEF-1 pathway triggers the up-regulation of cyclin D1 but not of c-myc. However, this temporary up-regulation of cyclin D1 is not the cause of apoptotic effects of β-catenin.
The apoptotic effects of β-catenin could be a primary consequence of increased β-catenin pools or a secondary compensatory response to increased proliferation. Because published data suggest that cyclin D1 is a potential target of β-catenin and TCF/LEF-1 (Tetsu and McCormick, 1999), we investigated whether cyclin D1 is up-regulated in NIH 3T3 fibroblasts overexpressing LEF-1 and/or β-catenin in a time-dependent manner.
Reporter assays that used cyclin D1 promoter (pCD1-Luc, containing −1748 cyclin D1 5′ sequence) showed that cyclin D1 is up-regulated by β-catenin and LEF-1 in short-term posttransfection extracts but that this effect disappears with time. Significant increases in cyclin D1 were observed 2 d after transfection by β-catenin (Figure 5A, NIH/FL) and/or LEF-1 (Figure 5A, A23FL and A23Con). Two days after transfection (Figure 5A), cyclin D1 levels increase ∼1.8-fold (NIH/FL) over control (NIHCon), and NIH 3T3 cells overexpressing LEF-1 (A23Con) show a 2.4-fold increase over control (NIHCon). However, cyclin D1 in NIH 3T3 cells overexpressing full-length β-catenin decreases to a normal range 9 d after transfection (Figure 5A) in a time-dependent manner. Cotransfection of β-catenin and LEF-1 (Figure 5A, A23FL) does not induce any synergistic effects on cyclin D1 expression.
Figure 5.
Short-term activation of β-catenin and LEF-1 up-regulates cyclin D1 but not c-myc. However, this up-regulation decreases in a time-dependent manner. (A) Luciferase reporter assays of cyclin D1 promoter. Both β-catenin (NIH/FL) and LEF-1 (A23Con) overexpression increase cyclin D1 reporter activity at 2 d after transfection, as does a combination of exogenous β-catenin and LEF-1 (A23FL). However, the up-regulation of cyclin D1 was maintained at 9 d after transfection only in A23Con (stable clonal NIH 3T3 cells overexpressing full-length LEF-1) or A23FL β-catenin. Arm β-catenin has no transactivating function and is not able to up-regulate cyclin D1 even after short-term activation (d 2, NIHArm and A23Arm). NIHCon, parental NIH 3T3 cells transfected with GFP empty vector. (B) Overexpression of full-length β-catenin induces apoptosis in cyclin D1–deficient fibroblasts. (C) RT-PCR. Three stable clones (A7, A23, and A25) overexpressing full-length LEF-1 were established with the use of NIH 3T3 fibroblasts. Alternatively spliced forms of LEF-1 were detected in NIH 3T3 fibroblasts. However, there was no increase of c-myc mRNA in these clonal cells compared with parental NIH 3T3 fibroblasts. β-Actin primers were used to confirm even loading.
To normalize the transfection efficiency, we cotransfected cells with β-galactosidase in all assays. The background reading of β-galactosidase in full-length β-catenin–transfected cells was considerably lower (0.2-fold) than that of parental NIH 3T3 fibroblasts. In contrast to full-length β-catenin, overexpression of Arm β-catenin, which has no transactivating function with LEF-1, does not up-regulate cyclin D1 even after a short-term transfection in NIH 3T3 cells (Figure 5A, NIH/Arm) or NIH 3T3 cells overexpressing LEF-1 (Figure 5A, A23Arm).
Because cyclin D1 is up-regulated in cells overexpressing β-catenin and/or LEF-1 and also is reported to induce apoptosis as well as proliferation (Janicke et al., 1996; Tetsu and McCormick, 1999), we used cyclin D1–deficient fibroblasts to investigate whether or not the apoptotic effects of β-catenin depend on cyclin D1 levels. Full-length β-catenin overexpression induces apoptosis in cyclin D1–deficient fibroblasts (Figure 5B), ruling out the possibility that the apoptotic effects of β-catenin are a secondary compensatory response to cyclin D1–dependent proliferation.
We performed RT-PCR to determine whether c-myc, another potential target of β-catenin and TCF/LEF-1 and a known inducer of apoptosis (He et al., 1998), is also up-regulated in cell lines overexpressing LEF-1. Even though we analyzed three different stable cell lines overexpressing LEF-1, we did not observe any significant increases in c-myc levels in these cells compared with parental NIH 3T3 fibroblasts (Figure 5C). Therefore, we ruled out the possibility that the apoptotic effects of β-catenin are caused by increases in the levels of c-myc and cyclin D1.
Induction of Apoptosis by β-Catenin Is Independent of p53, Rb, and E2F1 but Is Retarded in Bcl-x(L)–overexpressing Cells
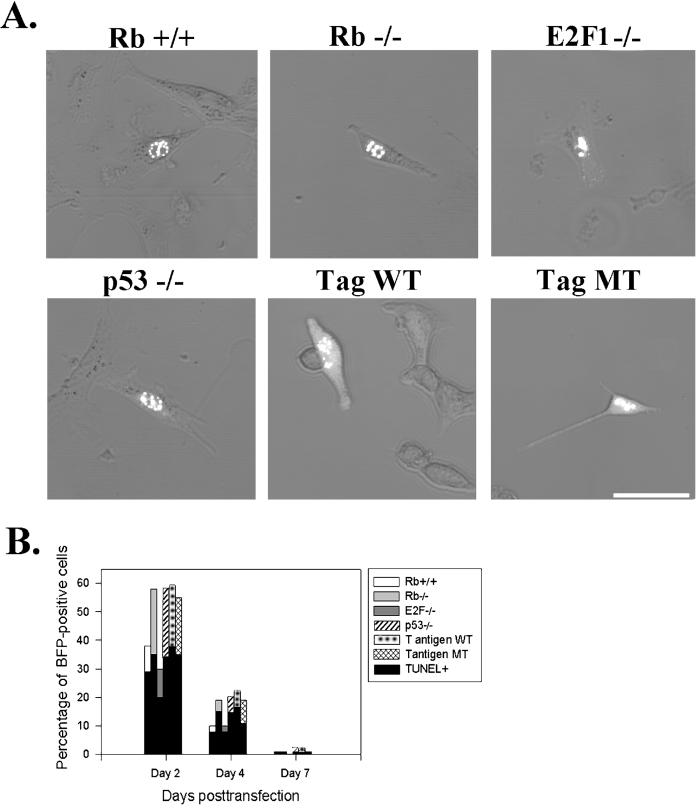
We next investigated whether or not the apoptotic effects of overexpressed β-catenin are mediated by nuclear proteins (p53, Rb, and E2F1) involved in regulating the cell cycle that have also been reported to be key regulators of apoptosis (King and Cidlowski, 1998). Among these, overexpression of p53 and E2F1 promote apoptosis, but overexpression of Rb protects cells from apoptosis. We transfected full-length β-catenin into fibroblasts in which one of the three genes of interest was knocked out (Figure 6). We also examined fibroblasts expressing SV40 large T antigen (Tag), which blocks both p53 and Rb functions (Figure 6A, Tag WT). As a control, we used fibroblasts expressing SV40 mutant Tag (K1 Tag; Zalvide and DeCaprio, 1995), which cannot eliminate p53 functions (Figure 6A, Tag MT). In all of these deficient or SV40 Tag-expressing fibroblasts, full-length β-catenin was transported into the nucleus, where it localized in the distinctive aggregates (Figure 6A) that were illustrated previously (Figure 4B), and induced apoptosis in a temporal pattern (Figure 6B). Thus, p53, Rb, and E2F1 are not required for the apoptotic effects of β-catenin.
Figure 6.
The absence of Rb, E2F1, and p53 does not affect the apoptotic effects of β-catenin. (A) Full-length β-catenin–GFP was transfected into fibroblasts lacking each gene of interest (Rβ−/−, E2F1−/−, or p53−/−). We also used fibroblasts immortalized with SV40 large T antigen wild-type (Tag WT), which blocks both Rb and p53 functions. As controls, we used Rβ-positive fibroblasts (Rb+/+) and fibroblasts expressing SV40 Tag mutant (MT), which abolishes the blocking function on Rb. β-Catenin–GFP localizes in the nucleus in punctate patterns. Bar, 50 μm. (B) Overexpression of β-catenin induces apoptosis in all Rb-, E2F1-, and p53-deficient fibroblasts and in fibroblasts expressing SV40 Tag WT, ruling out the role of these genes in the apoptotic pathways of β-catenin.
Because major apoptotic pathways are blocked or retarded by Bcl overexpression (Adams and Cory, 1998), we compared the effect of full-length and ARM β-catenin overexpression on apoptosis in parental HeLa cells with those in HeLa cells stably overexpressing Bcl-x(L) (Figure 7). HeLa cells overexpressing ARM and full-length β-catenin showed very few GFP-positive cells (expressing exogenous β-catenin) 7 d after transfection (Figure 7, A and B). However, there are more GFP-positive cells at this time (Figure 7B) in transfected HeLa cell lines overexpressing Bcl-x(L). Most GFP-positive cells have either a condensed or fragmented nuclear staining pattern (Figure 7A, arrows), indicating that although Bcl-x(L) retards the apoptotic effects of β-catenin, it is not able to block completely the death effects (Figure 7B). There was no significant difference between full-length and ARM β-catenin in these experiments (Figure 7B).
Figure 7.
The overexpression of Bcl-x(L) partially inhibits the apoptotic effects of β-catenin. (A) Full-length (FL) and Arm β-catenin induce apoptosis in HeLa cells and HeLa cells overexpressing Bcl-x(L). Only a few cells (arrows) contain the exogenous GFP marker after 7 d. Bar, 50 μm. (B) These apoptotic effects are retarded in HeLa cells overexpressing Bcl-x(L). After 7 d of transfection, parental HeLa cells showed very few BFP-positive cells (overexpressing β-catenin), whereas 10–15% of HeLa cells overexpressing Bcl-x(L) (HeLa-Bcl) still showed BFP-positive cells expressing either full-length (FL) or Arm β-catenin. (C) Western blot analyses showed that full-length β-catenin–GFP can still be detected in HeLa cells overexpressing Bcl-x(L) 8 d after transfection, albeit in lesser amounts than on d 2. Because of the lack of a binding epitope of Arm β-catenin and a similar molecular weight with endogenous β-catenin, it was difficult to distinguish Arm β-catenin from endogenous β-catenin by anti-β-catenin antibody (top panel). However, blotting with anti-GFP antibody clearly showed the Arm β-catenin band at d 2 and a decreased band at d 8 in HeLa cells overexpressing Bcl-x(L). Neither band was detectable in HeLa parental cells at d 8. Con, control HeLa cell extracts without transfection; HeLa, HeLa parental cells; Bcl-x(L), HeLa cells overexpressing Bcl-x(L); FL, full-length β-catenin transfection; Ar., Arm β-catenin transfection; Endo., endogenous β -catenin.
The different apoptotic effects of overexpressed β-catenin between HeLa cells and HeLa cells overexpressing Bcl-x(L) were further confirmed by Western blot analysis (Figure 7C). After 2 d of transfection, exogenous full-length β-catenin was clearly detected in both cell lines by anti-β-catenin (Figure 7C, top panel) and anti-GFP (Figure 7C, bottom panel) antibodies, but after 8 d it was detected only in HeLa cells overexpressing Bcl-x(L). Arm β-catenin was difficult to distinguish from endogenous β-catenin (Figure 7C, Endo.) by anti-β-catenin antibody because of the lack of a binding epitope. However, blotting with anti-GFP antibody detected this exogenous Arm β-catenin protein (Figure 7C, bottom panel) in a similar pattern to full-length β-catenin, indicating that both full-length and Arm β-catenin induce apoptosis and that the effects are retarded in HeLa cells overexpressing Bcl-x(L).
DISCUSSION
Previously published data are controversial regarding whether or not β-catenin induces apoptosis and, if so, whether this is a direct or indirect effect (Ahmed et al., 1998; Gat et al., 1998; Wong et al., 1998; Zhang et al., 1998; Orford et al., 1999). Our data make it clear that high levels of β-catenin do cause apoptosis in normal fibroblasts and tumor cells. Moreover, we show that the effects on apoptosis are the primary consequence of the increased β-catenin pools rather than a secondary compensatory response to cyclin D1 activation. The high levels of exogenous β-catenin, rather than its nuclear localization per se after transfection, are critical. Endogenous β-catenin that is nuclear in location does not induce apoptosis. It is possible that there is a certain high β-catenin concentration capable of activating the apoptotic pathway. BFP/GFP mock transfection and LEF-1 transfection do not induce apoptosis, even though they show comparably high levels of exogenous BFP/GFP and LEF-1, respectively. This is so despite the fact that they have the same CMV promoter used for β-catenin. Furthermore, transfection with δ-catenin, which contains 10 Arm repeats (in contrast to 13 Arm repeats of β-catenin and <25% amino acid identity compared with β-catenin) (Lu et al., 1999), induces only minor apoptosis. Thus, the apoptotic effects of overexpressed β-catenin seem to be very specific as well as concentration-dependent.
The possibility that the effects of β-catenin on apoptosis are related to an effect on proliferation is raised by the following considerations. Cyclin D1 and c-myc are reported to be downstream targets of β-catenin and TCF/LEF-1 (He et al., 1998; Tetsu and McCormick, 1999), although there are conflicting data for c-myc (Kolligs et al., 1999). Our data show that the β-catenin and LEF-1 pathways could affect proliferation by up-regulating cyclin D1, but they do not affect c-myc. We observed no significant increase of c-myc in cell lines overexpressing LEF-1 or β-catenin. However, we cannot rule out the possibility that c-myc can be a direct downstream target of β-catenin and other TCF family proteins or that it may require additional factors other than β-catenin and LEF-1. Interestingly, c-myc and cyclin D1 have been shown to induce apoptosis as well as proliferation (Janicke et al., 1996; Kangas et al., 1998). However, our data show that β-catenin overexpression induces apoptosis even in cyclin D1–deficient fibroblasts, making it unlikely that up-regulated cyclin D1 is the cause of the apoptotic events reported here.
As we suggested above, it is reasonable to conclude that a high level of β-catenin expression is critical for triggering apoptosis in both normal cells and tumor cells. N-terminal–truncated β-catenin increases apoptosis in normal colon epithelia and also affects proliferation (Wong et al., 1998), but this report did not distinguish between these effects or prove that increased apoptosis is a direct effect of augmented β-catenin pools. p53, which is often up-regulated during apoptosis, has also been reported to be transcriptionally active after β-catenin overexpression (Damalas et al., 1999). However, p53 expression was not necessary for overexpression of β-catenin to induce apoptosis in our cells. We transfected several tumor cell lines with full-length β-catenin DNA, some of which have been shown to stabilize β-catenin by mutation itself or by APC mutation (Morin et al., 1997). Even though these transfected cells proliferate in response to endogenous β-catenin in the nucleus, only overexpression of exogenous β-catenin induces apoptosis. Thus, the level of β-catenin is critical for inducing apoptosis, rather than just the nuclear localization.
Interestingly, we show here that exogenous overexpressed β-catenin forms distinct nuclear aggregates. Further study reveals that they are not associated with chromosomal DNA and they have a round to oblong shape. These nuclear bodies vary in size and number and can be distinguished from any previously reported nuclear bodies in terms of their sizes and localization patterns. For example, the promyelocytic leukemic protein (PML) body in the nucleus implicated in mediating apoptosis in white blood cells is smaller than exogenous β-catenin aggregates.
To study further the mechanism of induction of apoptosis by overexpressed β-catenin, we tested whether the transactivating function of β-catenin is required. Even though Shtutman et al. (1999) showed that the transactivation function of β-catenin depends on the level of LEF-1, we found that the apoptotic effects of β-catenin are not dependent on nuclear localization of exogenous LEF-1, nor do they differ among parental NIH 3T3 fibroblasts, LEF-1–overexpressing cells, and NLS-deleted LEF-1–overexpressing cells. Furthermore, we showed that Arm β-catenin has no inducing activity in either TOPFLASH or cyclin D1–promoter luciferase assays, supporting reports by others (Prieve and Waterman, 1999) that Arm β-catenin does not transactivate LEF-1–dependent gene expression. However, it still induces apoptosis. Moreover, the ΔC β-catenin (Δ669–781) mutant with very little TOPFLASH activity (1.3-fold) has the same apoptotic effect. Hsu et al. (1998) reported that ΔC β-catenin (Δ696–781) supports LEF-1–dependent transcriptional activation to the same extent as the wild type. This discrepancy may occur because of different deletions and/or assay sensitivities (luciferase assay versus chloram-phenicol acetyl transferase assay).
Ahmed et al. (1998) also showed that the C terminus of d-β-catenin (which is critical for the β-catenin transactivation function) is not required for β-catenin's ability to induce photoreceptor death in the d-APC mutant of Drosophila. However, they also found that reduction of either dTCF or d-β-catenin decreases cell death in d-APC–induced retinal degeneration, leading them to suggest that increased Drosophila β-catenin levels might activate a cell death pathway in the d-APC mutant via the d-β-catenin/dTCF complex. However, reduction of β-catenin in Alzheimer's disease patients with mutated PS-1 is accompanied by neuronal apoptosis (Zhang et al., 1998). These inconsistencies in the effects of β-catenin on apoptosis in different systems and with different primary mutations indicate that complex control mechanisms could be acting. However, our studies make it clear that the apoptotic effects of overexpressed β-catenin in normal mammalian cells do not require its LEF-1 transactivation function.
Because the transactivation function is not required to induce apoptosis, it seemed likely that nuclear proteins that affect apoptosis and cell cycles would not affect the apoptotic effects of β-catenin. As we expected, fibroblasts with p53, Rb, E2F1, or cyclin D1 knockouts are not rescued from the apoptotic effects of β-catenin overexpression. We hypothesize that a protein–protein interaction is responsible for induction of apoptosis by overexpressed β-catenin rather than a transactivating function. Arm repeats are considered to play a role in protein–protein interaction. In Drosophila, deleted Arm mutants (deletion of Arm repeats 5 and 8 or portions of 10 and 11) do disrupt β-catenin's ability to induce cell death in the d-APC mutant (Ahmed et al., 1998), suggesting that the presence of multiple Arm repeats is essential for induction of apoptosis by overexpressed β-catenin. APC, a β-catenin–binding protein with Arm repeat domains, also induces apoptosis when overexpressed in mammalian cells (Morin et al., 1996), whereas our data showed that δ-catenin, which contains 10 Arm repeats (but whose protein sequence is <25% identical to that of β-catenin), induced only minor apoptosis. This suggests that the apoptotic effects of β-catenin and APC depend on their specific conformation and size of Arm repeats, which may confer the tight binding to other proapoptotic proteins. It will be interesting to determine if plakoglobin induces similar apoptotic effects when abnormally overexpressed.
Because some tumor cells contain overexpressed and/or mutated β-catenin (Morin et al., 1997), questions arise regarding the mechanism by which tumor cells that contain overexpressed and/or mutated β-catenin prevent themselves from undergoing apoptosis. Interestingly, among all tumor cell lines we tested, only those overexpressing Bcl-x(L) are able to retard β-catenin–induced apoptotic effects, and Bcl-2 and β-catenin are cooverexpressed in other tumors (McEntee et al., 1999). The mechanism is not yet clear, but several investigations suggest a potential role of Bcl-2 in the regulation of β-catenin function. Bcl-2 and Akt kinase are implicated in VE-cadherin/β-catenin–mediated endothelial survival, in which the truncation of the β-catenin–binding domain of VE-cadherin induces endothelial apoptosis (Carmeliet et al., 1999). Moreover, β-catenin–overexpressing normal cell lines exist that do not undergo apoptosis (Kolligs et al., 1999; Orford et al., 1999; Zhu and Watt, 1999), but possibly these tolerant cell lines contain high levels of rescuing molecules such as Bcl-x(L) or lack binding proteins critical to the manifestation of β-catenin apoptotic effects.
In conclusion, this paper provides new information regarding the effects of β-catenin and LEF-1 on apoptosis and the apoptotic pathway of β-catenin. When LEF-1 is overexpressed, it may trigger cell proliferation by up-regulating cyclin D1, but it does not induce apoptosis of the avian and mammalian cells we studied, even in the presence of endogenous β-catenin. When full-length β-catenin is overexpressed, it up-regulates cyclin D1 but does not require cyclin D1 to induce apoptosis. The absence of functional p53, E2F1, and Rb, whose genetic alterations are reported in tumors, does not affect the apoptotic effects of overexpressed β-catenin. Moreover, the apoptotic effects of overexpressed β-catenin do not require its LEF-1–transactivating function in the cells we studied. We hypothesize that overexpressed β-catenin binds other protein(s) through potential death domain(s) in Arm repeats. Our work also calls attention to a physiological function for the induction of apoptosis by up-regulated β-catenin. In vivo, apoptosis of cells overexpressing β-catenin may serve to remove them from the body. Certainly, these destructive effects of overexpressed β-catenin pose a problem that should be taken into account in future in vitro studies.
ACKNOWLEDGMENTS
We are greatly indebted to Drs. M.E. Ewen and K.Y. Lee for critical comments on the manuscript and gifts of cells (Rb+/+, Rβ−/−, E2F1−/−, cyclin D1−/−, and fibroblasts expressing SV40 wild-type or mutant Tag). We also thank Dr. W. Birchmeier for β-catenin cDNA; Dr. R. Grosschedl for LEF-1-HA cDNA; Dr. Qun Lu for δ-catenin cDNA; Dr. H. Clevers for TOPFLASH and FOPFLASH reporter plasmids; Dr. K. Daniels for providing uveal melanoma cells; and Drs. H. Li and J. Yuan for providing HeLa cells and HeLa cells overexpressing Bcl-x(L). This work was supported by a National Institutes of Health RO1 grant to E.D.H. (EY 09721)
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171–1182. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J, Geiger B, Oren M. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18:3054–3063. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada J, Moreno MP, Braga VM, Rodriguez-Viciana P, Cano A. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of beta-catenin in epidermal keratinocytes. J Cell Biol. 1999;146:967–980. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dyn. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Lin XY, Lee FH, Porter AG. Cyclin D3 sensitizes tumor cells to tumor necrosis factor-induced, c-Myc-dependent apoptosis. Mol Cell Biol. 1996;16:5245–5253. doi: 10.1128/mcb.16.10.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signaling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kangas A, Nicholson DW, Hottla E. Involvement of CPP32/Caspase-3 in c-Myc-induced apoptosis. Oncogene. 1998;16:387–398. doi: 10.1038/sj.onc.1201779. [DOI] [PubMed] [Google Scholar]
- Kim K, Daniels KJ, Hay ED. Tissue-specific expression of beta-catenin in normal mesenchyme and uveal melanomas and its effect on invasiveness. Exp Cell Res. 1998;245:79–90. doi: 10.1006/excr.1998.4238. [DOI] [PubMed] [Google Scholar]
- King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS. δ-Catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol. 1999;144:519–532. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee MF, Chiu CH, Whelan J. Relationship of beta-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–640. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- Molenaar M, et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T, Arnold A. PRAD1/cyclin D1 proto-oncogene: genomic organization, 5′ DNA sequence, and sequence of a tumor-specific rearrangement breakpoint. Genes Chromosomes Cancer. 1993;7:89–95. doi: 10.1002/gcc.2870070205. [DOI] [PubMed] [Google Scholar]
- Orford K, Orford CC, Byers SW. Exogenous expression of beta-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855–867. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin-lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol Cell Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signaling. J Cell Sci. 1998;111:1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998;141:765–777. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalvide J, DeCaprio JA. Role of pRβ-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol. 1995;15:5800–5810. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. beta-Catenin signaling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]