Abstract
Background: Tissue-tagged magnetic resonance imaging (MRI) with 3-dimensional (3D) myocardial strain analysis allows quantitative assessment of myocardial contractility. We assessed the hypothesis that 3D strain determination at rest and with low-dose dobutamine would discriminate between viable and nonviable myocardium in patients with ischemic cardiomyopathy (ICM).
Methods: MRI with radiofrequency tissue-tagging at rest and with low-dose dobutamine was performed in 16 normal volunteers and 14 patients with ICM. Three-dimensional global and regional circumferential strains (Ecc) were computed for all subjects at rest and with dobutamine. Results were compared to clinically indicated conventional viability studies.
Results: Compared to normal volunteers, global left ventricular Ecc was significantly decreased in patients with ICM at rest (−0.15 ± 0.06 vs. −0.27 ± 0.03, p<0.001) and with dobutamine (−0.17 ± 0.08 vs. −0.37 ± 0.10, p<0.001). Ecc was significantly decreased in nonviable regions compared to viable segments at rest (−0.08 ± 0.06 vs. −0.17 ± 0.10, p<0.001) and with dobutamine (−0.07 ± 0.06 vs. −0.21 ± 0.11, p<0.001). Ecc in viable segments increased significantly in response to dobutamine (p=0.04) whereas Ecc did not change in nonviable segments (p=0.50). Normal controls (96 segments) had increased Ecc at rest (−0.27 ± 0.07) and with dobutamine (−0.37 ± 0.15) compared to both viable and nonviable regions in ICM patients (p<0.001).
Conclusions: Noninvasive dobutamine tissue-tagged MRI with calculation of 3D strain allows the identification, quantification and display of regionally varying ventricular function. The response of systolic strain to low-dose dobutamine has significant promise in discriminating between viable and nonviable myocardium.
Keywords: computers, coronary disease, magnetic resonance imaging, myocardial contraction, revascularization
INTRODUCTION
Patients with severe ischemic cardiomyopathy (ICM) have high rates of adverse events associated with revascularization procedures. Reported perioperative mortality rates from coronary artery bypass grafting (CABG) in this patient subset range from 5% to greater than 30%1. Furthermore, revascularization of nonviable myocardium has not proven to be beneficial in terms of either mortality benefit2 or global left ventricular (LV) functional improvement3. In contrast, revascularization of viable myocardium is believed to be beneficial in multiple regards. Observational data has suggested increased ejection fraction (EF)1,3, decreased congestive heart failure (CHF) symptoms1, and improved survival2 in patients with viability who underwent revascularization over those treated medically. Patients with viability who did not undergo revascularization had annual mortality rates greater than double the rates of patients without viability, regardless of treatment2.
The associated increased risk of revascularization procedures combined with the lack of benefit of revascularizing nonviable segments emphasizes the importance of accurate determination of viability in patients with ICM who are considered for revascularization procedures. Presently used tests for viability are helpful in this assessment. However, many of the testing methods employed today are highly dependent upon the assessment by the observer, while also having a high degree of interobserver variability. Furthermore, most methods provide an assessment that is nonquantitative and lacks accurate regional and transmural characterization of viability. In contrast, three-dimensional LV strain can be semi-automatically and objectively quantified using computer analysis of patient-specific, tissue-tagged MRI-derived displacement data. We hypothesized that this MRI strain analysis at rest and with low-dose dobutamine could discriminate between viable and nonviable myocardium in patients with ICM.
METHODS
Patient Characteristics. Patients with ICM being considered for coronary artery bypass grafting were screened for enrollment. Fourteen patients (age 61 ± 10 years) with coronary artery disease (CAD), defined as at least one vessel with >70% stenosis and at least one nonviable myocardial segment by conventional clinical assessment were enrolled. The average ejection fraction of ICM patients was 27 ± 9%. Patients with significant valvular abnormalities were excluded. A group of 16 healthy volunteers (age 29 ± 8 years) were enrolled as controls. The study was approved by the Human Studies Committee at Washington University, St. Louis, MO, and all subjects gave written consent. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
A clinical reviewer (J.R.) blinded to the MRI strain data assessed individual segments as viable or nonviable based on extensive review of all available data, including nuclear viability imaging, echocardiography, and cardiac catheterization information. All segments classified as nonviable had at least SPECT or PET nonviability and hypokinesis/akinesis by echocardiography or ventriculography.
Imaging Protocol. Imaging was performed in a 1.5 Tesla MR scanner (Magnetom Vision, Siemens Medical Systems, Iselin, NJ) at rest and with dobutamine (10 μg/kg/min) infusion. A full description of our imaging protocol has been previously published4,5. Briefly, a set of parallel short axis images were obtained at 8mm intervals. An additional set of long axis images oriented radially and intersecting the centroid of the ventricle were also obtained. For each selected imaging plane, a single-slice MR tagged image was collected with a sequence consisting of a SPAMM (Spatial Modulation of Magnetization) radiofrequency tissue-tagging preparation followed by a 2-D FLASH (Fast Low flip Angle Shot) cine image acquisition. Image acquisition was synchronized with real-time electrocardiogram (ECG) at the time of the MRI scanning.
Image Analysis. Five midventricular short axis image sets and four radially oriented long axis image sets were used in this analysis. Analysis of the MR images was carried out using custom software developed in our laboratory. Endocardial and epicardial boundaries were manually identified on each of the short and long axis image sets. An initial spline representation of the tag lines on the end-diastolic images was constructed based on the spacing between adjacent tag lines. Tag lines were located on successive images using a computer-based, automated algorithm that adjusts an initial guess for each tag line based on the local pixel density function6. Figure 1 provides an example of the tag lines from short axis images at end-systole. The tag lines are also located on the long axis images, as seen in Figure 2. Spline curves from corresponding tag lines were used to construct a spline surface representation of the tag surfaces. Three-dimensional systolic displacements were computed along the intersection curves of individual short and long axis tag surfaces using a previously described and validated method4. Analysis of the displacement data was carried out in the finite element software package StressCheck (ESRD Inc., St. Louis, Missouri). A finite element mesh of a 3D mid-ventricular slab of the LV was constructed as follows: Endocardial and epicardial contours from each of the short axis images at end diastole were used to construct a spline surface representation of the endocardial and epicardial surfaces. A mesh for each model was manually created consisting of six hexahedral elements corresponding to the anteroseptal, anterior, anterolateral, posterolateral, posterior and posteroseptal walls. Predicted displacements at any point within the domain of the model were obtained from a least squares fitting of the measured displacement data using finite element basis functions. Average regional values of circumferential strain were computed from the results of this fitting using the Almansi strain tensor7. The results for strain can then be displayed in a three-dimensional color contour map of the finite element model of the ventricle, as demonstrated in Figure 3.
Figure 1.
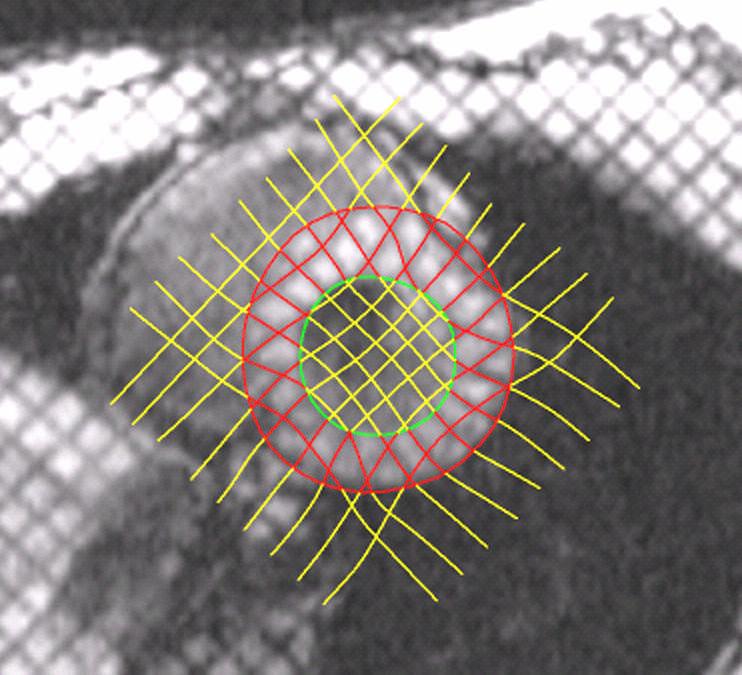
Short axis image at end systole demonstrating myocardial borders and computer-assisted tag line representation.
Figure 2.
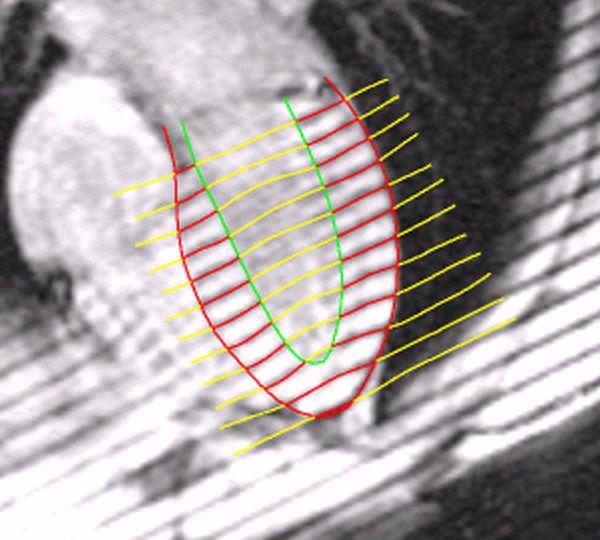
Long axis image at end systole demonstrating myocardial borders and computer-assisted tag line representation. The combination of short and long axis tag lines allows tracking of myocardial points in 3 dimensions.
Figure 3.
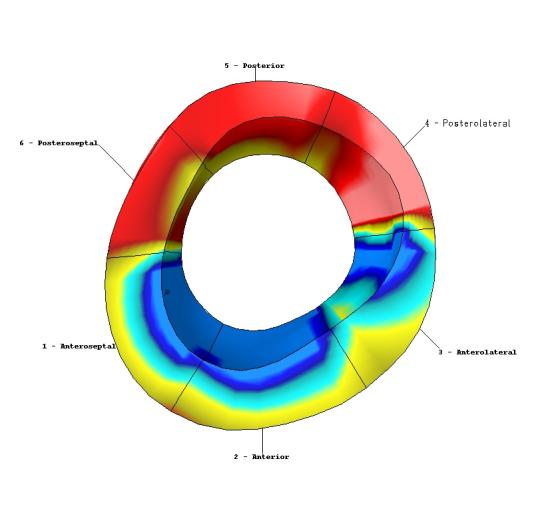
Color contour map of calculated circumferential strain in mid-LV finite element model. Areas in red indicate reduced contraction in this example from a patient who suffered a large inferoposterior infarct.
Statistical Analysis. Comparisons between the same ventricular regions at rest and during Dobutamine infusion were done using a paired Student's t-test. All other comparisons were completed using unpaired Student's t-tests. In all cases, a value of p<0.05 was considered significant. Receiver-operator characteristic (ROC) curves were generated with and without dobutamine administration in both viable and nonviable segments by the SPSS statistical package (SPSS, Inc., Chicago, Illinois).
RESULTS
Average values of circumferential strain (Ecc) were computed for each of the six LV regions at rest and during dobutamine infusion; a global value was obtained by averaging these six values. The global values for Ecc at rest showed significantly increased contraction (more negative strain values) in the healthy volunteers compared to the ICM patients (−0.27 ± 0.03 vs. −0.15 ± 0.06, p<0.001). The global strain values with dobutamine administration likewise showed a significant difference in contraction in the control over the ICM patients (−0.37 ± 0.10 vs. −0.17 ± 0.08, p<0.001). The administration of dobutamine caused a significant change in strain in the control subjects (p<0.001), however no significant change occurred in the ICM group (p=0.33).
For our analysis of regional strain, the 14 ICM patients each had 6 segments assessed for a product of 84 segments included. Blinded clinical review classified 21 (25%) of these segments as nonviable and 63 (75%) as viable. At rest, average Ecc in the nonviable segments was −0.08 ± 0.06, whereas in the viable segments the average Ecc was −0.17 ± 0.08, which was a significant difference (p<0.001). Within the group of viable segments, 38 (60%) of the segments had normal (within 2 standard deviations of control) resting strain values, and 25 (40%) had reduced strain values. With dobutamine, the average Ecc values from nonviable and viable segments maintained significant separation (−0.07 ± 0.06 vs. −0.21 ± 0.11, p<0.001). Ecc in viable segments improved significantly in response to dobutamine (p=0.04) whereas Ecc did not change in nonviable segments (p=0.50). The improvement in strain with dobutamine in the viable segments was similar (p=0.84) between patients with normal and reduced resting strain values.
The results were used to generate ROC curves to determine the accuracy of both resting strain and dobutamine strain in differentiating viable and nonviable segments. The resultant ROC curve for resting strain had an area under the curve of 0.771. A resting Ecc value greater than −0.095 predicted nonviability with 71% sensitivity and 73% specificity. The ROC curve for strains obtained during dobutamine infusion improved upon these results, with an area under the curve of 0.895. Using a cutoff of −0.115, dobutamine strain values correlated with conventional viability assessments with 91% sensitivity and 78% specificity.
DISCUSSION
The clinical management of patients with ICM can be optimized by assessing the extent of viable myocardium that is present. Patients with viable tissue stand to benefit from revascularization. Patients without viability may avoid the risk of revascularization procedures, as benefit in this population is unlikely. Presently employed methods to assess viability rely, at least in part, upon the observer to make subjective judgments when classifying a region as viable or nonviable. In some testing modalities, the definition of viability is somewhat arbitrary. With the goal of limiting interobserver variability and improving accuracy of quantification of regional strain variability, we have developed a computer-based means in which 3D LV strain can be semi-automatically quantified using patient-specific, tissue-tagged MRI-derived displacement data.
Our results have shown that circumferential strain values accurately differentiated normal myocardial contraction from abnormal myocardial contraction, both in global and regional assessments. This was evident in comparisons between healthy volunteers and ICM patients, in addition to comparisons between viable and nonviable segments in the ICM patients. Significant differences in strain between these groups were seen in both resting images and dobutamine images. Furthermore, the change in strain in response to dobutamine also showed significant differences between groups. Viable myocardial segments displayed similar improvements in strain regardless of whether resting strain was normal or reduced.
While the resting strain values were significantly different between viable and nonviable segments, the use of dobutamine in our study clearly improved the accuracy of our analysis. The addition of dobutamine improved the test's sensitivity from 71% to 91% and the specificity from 73% to 78%. Our results compare favorably to published results of other noninvasive viability techniques. A large meta-analysis reported sensitivities of 81%, 81-86%, 93% and specificities of 80%, 59-66%, 58% for dobutamine echocardiography, single photon emission computed tomography, and positron emission tomography, respectively8.
Limitations. Our study used a blinded conventional viability assessment as our standard for comparison, which provided the best available surrogate for what is used in the real-world clinical setting. Long-term follow-up of the ventricles' response to revascularization will provide the best assessment of MRI-derived strain's accuracy and will allow improved comparison to presently-used viability studies. In this study, the strain analysis was conducted of only the mid-ventricular region, thus obviously not providing any information on the clinically important LV apex. This region was chosen for this proof-of-concept study to validate accuracy in determining myocardial viability because it is the most accurately imaged and mathematically modeled region of the left ventricle. As our magnetic resonance imaging capabilities have continued to improve, accuracy in the mobile, geometrically variable base and apex regions has also improved and will soon allow inclusion of full LV data in our analysis.
Conclusions. This study demonstrated a technique in which 3D LV strain comparisons were made from clinically acquired MRI radiofrequency tissue-tagged images utilizing a semi-automated, computer-based algorithm. This analysis of resting strain, dobutamine strain, and dobutamine strain response differentiated viable and nonviable myocardium. Although there are still limitations in this methodology, this study represents one more step in our laboratory's ambitious goal to profoundly impact the accuracy of clinically-applicable regional strain analysis such that the mathematical quantification of myocardial function moves to an unprecedented level. Our goal is ultimately to reduce the influence of subjective observer variability by the extensive implementation of computerized analysis.
Footnotes
Supported by NIH Grant RO1 HL069967
Conflict of Interest Disclosures: None
References
- 1.Baker DW, Jones R, Hodges J, Massie BM, Konstam MA, Rose EA. Management of heart failure: the role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. JAMA. 1994;272:1528–34. doi: 10.1001/jama.272.19.1528. [DOI] [PubMed] [Google Scholar]
- 2.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular systolic dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 3.Ragosta M, Beller GA, Watson DD, Kaul S, Gimple LW. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. Circulation. 1993;87:1630–41. doi: 10.1161/01.cir.87.5.1630. [DOI] [PubMed] [Google Scholar]
- 4.Moulton MJ, Creswell LL, Downing SW, Actis RL, Szabo A, Vannier MW, Pasque MK. Spline surface interpolation for calculating 3-D ventricular strains from MRI tissue tagging. Am J Physiol. 1996;270(1 Pt 2):H281–97. doi: 10.1152/ajpheart.1996.270.1.H281. [DOI] [PubMed] [Google Scholar]
- 5.Moustakidis P, Cupps BP, Pomerantz BJ, Scheri RP, Maniar HS, Kates AM, Gropler RJ, Pasque MK, Sundt TM. Noninvasive, Quantitative Assessment of Left Ventricular Function in Ischemic Cardiomyopathy. J Surg Res. 2004;116(2):187–96. doi: 10.1016/j.jss.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 6.McVeigh ER, Zerhouni EA. Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology. 1991;180:677. doi: 10.1148/radiology.180.3.1871278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung Y. A first course in continuum mechanics. 3rd Prentice Hall; Englewood Cliffs, NJ: 1994. [Google Scholar]
- 8.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, Specificity, and Predictive Accuracies of Various Noninvasive Techniques for Detecting Hibernating Myocardium. Curr Probl Cardiol. 2001;26(2):141–86. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]


