Abstract
Resveratrol, a grape polyphenol, is thought to be a cancer preventive, yet its effects on metastatic breast cancer are relatively unknown. Since cancer cell invasion is dependent on cell migration, the chemotactic response of MDA-MB-231 metastatic human breast cancer cells to resveratrol, estradiol (E2), or epidermal growth factor (EGF) was investigated. Resveratrol decreased while E2 and EGF increased directed cell migration. Resveratrol may inhibit cell migration by altering the cytoskeleton. Resveratrol induced a rapid global array of filopodia and decreased focal adhesions and focal adhesion kinase (FAK) activity. E2 or EGF treatment did not affect filopodia extension but increased lamellipodia and associated focal adhesions that are integral for cell migration. Combined resveratrol and E2 treatment resulted in a filopodia and focal adhesion response similar to resveratrol alone. Combined resveratrol and EGF resulted in a lamellipodia and focal adhesion response similar to EGF alone. E2 and to a lesser extent resveratrol increased EGFR activity. The cytoskeletal changes and EGFR activity in response to E2 were blocked by EGFR1 inhibitor indicating that E2 may increase cell migration via crosstalk with EGFR signaling. These data suggest a promotional role for E2 in breast cancer cell migration but an antiestrogenic, preventative role for resveratrol.
Keywords: Resveratrol, estradiol, filopodia, focal adhesions, cell migration
Introduction
Estrogen (E2) acts by regulating gene transcription through two major intracellular estrogen receptors (ERs), ERα and ERβ, to play a critical role in the establishment and maintenance of female reproductive function as well as in the initiation and progression of breast and gynecologic cancers [1,2]. Consequently, inhibition of ERα has become a major strategy for the prevention and treatment of breast cancer [3]. However, this approach is limited to the treatment of metastatic breast cancer because ERα expression is often lost during breast cancer progression to the metastatic state [4]. These ERα(-) cancers may still retain the more recently identified ERβ as well as membrane-bound forms of ER, and more studies are necessary to understand the role of these ER isoforms in breast cancer malignancy. In addition to the well-established long-term (genomic) effects of E2 on gene transcription [5], E2 also induces short-term (nongenomic) effects. Such nonclassic effects of E2 have been reported from a variety of cell types including breast cancer cells and are thought to be modulated by plasmamembrane ERs that can cross-activate a variety of signaling cascades [6,7].
Recent reports on the rapid, nongenomic action of E2 from a variety of cell types and tissues have demonstrated novel roles for E2 in the regulation of a variety of cell functions relevant to cancer progression [8–11]. E2 cross-activates heterotrimeric G proteins to stimulate adenylate cyclase and phospholipase C, thus inducing protein kinase A (PKA), protein kinase C (PKC), and intracellular Ca2+ fluxes [12,13]. Moreover, E2-bound ERα has been shown to associate with Src tyrosine kinase as well as the regulatory subunit of phosphoinositide 3-kinase (PI3-K) to regulate signaling pathways implicated in cell proliferation, survival, and migration [14,15]. Activation of membrane ERs by E2 has been shown to transactivate epidermal growth factor receptors (EGFRs) potentially through a G protein-coupled pathway [11,16]. EGFRs are tyrosine kinase-type integral membrane receptors that regulate signaling relevant to both genomic effects on cell proliferation and survival as well as nongenomic signaling to affect migration and invasion [17,18]. Interestingly, loss of ERα in breast cancer is associated with overexpression of EGFRs that contribute to tumor malignancy and poor prognosis [19]. Therefore, there is a pressing need to investigate the nongenomic aspects of E2 signaling and how it relates to metastatic breast cancer.
Phytoestrogens are naturally occurring estrogen-like plant compounds that act as agonists or antagonists of E2 and may have protective action against some cancers as well as prevent the undesirable symptoms of menopause [20]. Resveratrol (trans-3,4′,5 trihydroxystilbene), a phytoestrogen present in grape skin and red wine, is known to have cancer-preventive and cardioprotective properties [21,22]. Resveratrol binds to and activates ERs (α and β) to exert both estrogenic and antiestrogenic effects [23,24]. Resveratrol acts as a cancer-preventive agent due to its antioxidant, proapoptotic, and antigrowth properties [21,25,26]. Resveratrol may also be important for breast cancer prevention because it inhibits breast cancer cell growth in ERα(+) and ERα(-) cells [23,27,28]. We have previously demonstrated that in ER(+) breast cancer cells, resveratrol reduces the activity of Akt, a regulator of cell survival, and increases Akt activity in ERα(-) ERβ(+) breast cancer cells [29]. A recent report demonstrated that resveratrol could directly inhibit Akt activity of ER(+) breast cancer cells through an ERα-associated PI3-K pathway [30].
Resveratrol has also been shown to prevent angiogenesis and wound healing of endothelial cells, and such antiangiogenic properties of resveratrol make it a good candidate for the prevention of cancer progression [31–34]. Resveratrol has been demonstrated to reduce hepatoma cell invasion in response to hepatocyte growth factor in vitro and hepatoma and Lewis lung carcinoma invasion in mice [31,35]. Resveratrol was recently shown to inhibit phorbol myristate acetate-induced cervical cancer cell invasion [36]. Although the role of resveratrol in the inhibition of cancer cell growth is well established, the role and mechanisms by which resveratrol may act to prevent cancer metastasis remain to be investigated.
Directed cell migration is an integral component of cancer cell invasion during metastasis. Metastatic cancer cells break cell-cell adhesions and initiate movement out of the primary tumor into surrounding tissues and blood vessels [37]. Cancer cell invasion is regulated by growth factors that can rapidly activate cell surface receptors to induce actin polymerization and reorganization into actin-based extensions such as filopodia (thin needle-shaped structures with parallel actin bundles) and lamellipodia (flat cell surface protrusions with cross-linked actin). Extension of lamellipodia and dynamic turnover of focal adhesions at the leading edge are thought to drive forward migration [37–40]. Filopodia are not essential for cell migration and are considered to function as environmental sensors [39].
Focal adhesions are multimolecular complexes formed by the interaction of integrin receptors with the extracellular matrix (ECM). Focal adhesions contain both structural and signaling components with numerous tyrosine-phosphorylated proteins such as focal adhesion kinase (FAK) and Src as well as actin-binding proteins that anchor focal adhesions to the actin cytoskeleton. FAK is recruited to the membrane in response to integrin as well as growth factor receptor activation. FAK is activated by autophosphorylation at multiple sites that in turn interact with adapter and structural proteins facilitating the modulation of cell proliferation, survival, migration, and cancer cell invasion [41].
Although ERα is commonly lost in metastatic breast cancer [4], these cells still retain the ERβ isoform, which has been shown to interact with resveratrol [42]. Therefore, as a first step toward investigating a role for resveratrol in breast cancer metastasis, we monitored directed cell migration and accompanying changes in the cytoskeleton in response to resveratrol or E2 in the ERα(-) ERβ(+) MDA-MB-231 [43] human metastatic breast cancer cell line. For the first time, the present data demonstrate that resveratrol may inhibit breast cancer cell migration by modulating the actin cytoskeleton to form a global array of filopodia and by decreasing focal adhesion assembly and FAK activity. Conversely, E2 increases cell migration and accompanying lamellipodia extension and focal adhesion assembly. Thus, these data indicate that resveratrol may prevent, whereas E2 may advance, metastatic breast cancer in ERα(-) ERβ(+) tumors.
Materials and Methods
Reagents
All culture media components were from Life Technologies/Gibco (Rockville, MD). EGF was obtained from Upstate Biotechnology, Inc. (Charlottesville, VA). 17β-Estradiol (E2) was obtained from Sigma (St. Louis, MO). trans-Resveratrol was from LKT Laboratories (St. Paul, MN). All chemoattractants were dissolved in DMSO. Rhodamine phalloidin was purchased from Molecular Probes (Eugene, OR). Antiphosphotyrosine and anti-ERα antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ERβ, anti-EGFR, and anti-phosphoEGFR antibodies were from Upstate Biotechnology, Inc. FITC-conjugated goat antimouse antibody was from Cappel (West Chester, PA). Tyrphostin AG1478 was purchased from Calbiochem (San Diego, CA).
Cell Culture
Human breast cancer cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2.
Migration Assay
Migration assays were conducted according to Ref. [44]. MDA-MB-231 cells were serum-starved in phenol red-free DMEM for 24 hours. Cells were then trypsinized, recovered with trypsin inhibitor (0.5 mg/ml), and seeded at 1 x 105 cells per chamber in the upper well of Costar wells (VWR, Suwanee, GA) containing membranes with 8-µm-diameter pores. DMSO (control), E2 (0.1 µM), EGF (50 ng/ml), or resveratrol (50 µM) was added as a chemoattractant to the bottom wells for 8 hours. For experiments where the effect of resveratrol was analyzed in combination with E2 or EGF, resveratrol was added to the bottom well for 10 minutes followed by E2 or EGF for the duration of the experiment. Cells on the upper surface of the membrane were removed, and cells that had moved through to the other side of the membrane were stained with propidium iodide and quantified. For statistical purposes, the total number of cells migrated in 10 microscopic fields per well were counted for at least three separate experiments.
Immunofluorescence Microscopy
Cells were seeded at 1.5 x 105 cells per cover slip and grown overnight in DMEM in six-well plates. Cells were serum-starved in phenol red-free DMEM for 24 hours. Cells were then treated for 10 minutes with DMSO (control), E2 (0.1 µM), EGF (50 ng/ml), or resveratrol (10, 50, or 100 µM). For experiments where the effect of resveratrol was analyzed in combination with E2 or EGF, cells were preincubated in resveratrol for 10 minutes and incubated with E2 or EGF for a further 10 minutes. For experiments using tyrphostin AG1478 to inhibit EGFR activity, cells were preincubated in 50 µM tyrphostin AG1478 for 15 minutes as described in Ref. [15]. Cells were fixed with 3.7% formaldehyde in PBS for 15 minutes, permeabilized with 0.2% Triton X-100 for 20 minutes, and blocked with 5% bovine serum albumin (BSA). Cells were then probed with rhodamine phalloidin to visualize F-actin and anti-phosphotyrosine followed by FITC-conjugated secondary antibody to visualize focal adhesions, as described in Ref. [45]. Micrographs at x600 magnification were digitally captured using a SpotII digital camera and software (Diagnostic Instruments, Inc., Sterling Heights, MI). Cells in 10 microscopic fields per treatment were counted for three separate experiments.
Analysis of ER Expression and FAK and EGFR Activity
Cells were disrupted in lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 50 mM sodium fluoride, 10% glycerol, 1% NP-40, 1 mM DTT, 0.5% deoxycholate, and protease inhibitors] on ice. Lysates were centrifuged at 14,000 rpm, and the supernatant mixed with Laemmli sample buffer, equally loaded, and separated on 10% SDS-PAGE gels. Proteins were transferred to PVDF membranes, blocked with 5% BSA, and probed with specific primary antibodies. Positive bands were detected using a horseradish peroxidase (HRP)-conjugated secondary antibody and developed with Super Signal West Fempto Substrate (Pierce Biotechnology, Rockford, IL). For analysis of ER isoform or EGFR expression, cell lysates containing equal amounts of protein, as determined by total protein assays (Bio-Rad, Hercules, CA), were loaded and specific ER isoforms detected using monoclonal antibodies to ERα, ERβ, EGFR, or phosphoEGFR.
For analysis of FAK activity, anti-FAK (against the N-terminus) and anti-phosphoFAK (Tyr-397) antibodies were used as probes. The densities of positive bands were quantified using Scion Image software. The relative FAK activity was calculated as the ratio of the density of phosphoFAK in stimulated cell lysates to the density of FAK in stimulated cell lysates divided by the ratio of the density of phosphoFAK in unstimulated cell lysates to the density of FAK in unstimulated cell lysates, as described in Ref. [29]. In our previously published results, FAK activity was quantified using a C-terminal anti-FAK antibody to detect total FAK levels. Herein, this assay has been improved by the use of a total anti-FAK antibody that detects the N-terminus of FAK, thus including all of the proteins detected by an antiphosphoFAK tyr-397 antibody. The results are representative of three separate experiments.
For the analysis of EGFR activity, cells were pretreated with tyrphostin AG1478 (50 µM) for 15 minutes as described in Ref. [15]. Cells were then treated with 50 ng/ml EGF or vehicle for 10 minutes, lysed, and Western-blotted as described above using an antibody against phosphoEGFR (Y1173).
Statistical Analysis
Data are expressed as mean ± S.E.M. P values were calculated from unpaired t-tests using Microsoft Excel and considered significant at values less than .05.
Results
To determine the effect of resveratrol on cell functions relevant to cancer cell invasion, we investigated the changes in cell migration, cell surface actin structures, focal adhesion assembly, and FAK and EGFR activity induced by 10-minute exposure to resveratrol (50 µM) compared to DMSO (control), E2 (0.1 µM), or EGF (50 ng/ml). The concentration of resveratrol used is comparable to the range of concentrations used to demonstrate interactions with ER [24] and signal transduction through modulation of gene expression [36]. The concentration for resveratrol used is well within the published range for resveratrol action, where different cell types, including breast cancer cells, were incubated in concentrations of resveratrol ranging from 1 to 100 µM for over 24 hours [30,34,46–48]. E2 concentration is in the range used for the demonstration of nongenomic effects in breast cancer cells [15,49]. The concentration of EGF is in the range used to activate EGFR and elicit effects on the actin cytoskeleton and invasion of breast cancer cells [50,51].
Resveratrol Decreases Migration Whereas E2 Increases Migration of MDA-MB-231 Cells
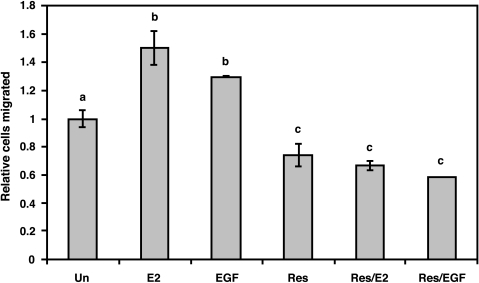
As shown in Figure 1, the role of E2 and resveratrol as chemoattractants in directed cell migration was analyzed in ERα(-) β(+) MDA-MB-231 metastatic human breast cancer cells. E2 and EGF treatments both significantly increased cell migration by 50% and 30%, respectively, compared to control. Resveratrol treatment resulted in significantly decreased cell migration by 26% compared to control. To investigate the ability of resveratrol to inhibit E2 or EGF action, cells were treated with resveratrol prior to E2 (Res/E2) or EGF (Res/EGF). Combined treatment of Res/E2 or Res/EGF was significantly decreased from unstimulated control by 33% and 41%, respectively, but were not significantly different from resveratrol-treated cells alone. In Res/E2 treatments, the number of cells that migrated was significantly reduced by 55% when compared to E2 alone. In Res/EGF treatments, the number of cells that migrated was also significantly reduced by 55% when compared to EGF alone. Thus, resveratrol effectively inhibited cell migration even in the presence of E2 or EGF, both stimulators of directed cell migration.
Figure 1.
Effects of E2, EGF, and trans-resveratrol on directed cell migration of MDA-MB-231 cells. Cells were serum-starved in phenol red-free media for 24 hours and migration assays were conducted using the following as chemoattractants for 8 hours: DMSO as control (Un); 0.1 µM E2 (E2); 50 ng/ml EGF (EGF); 50 µM resveratrol (Res), pretreated with Res for 10 minutes followed by E2 for 10 minutes (Res/E2), or pretreated with Res for 10 minutes followed by EGF for 10 minutes (Res/EGF). The number of cells that migrated through the upper chamber of Costar wells was quantified and made relative to DMSO control. Data are expressed as mean cells migrated ± SEM of at least three independent experiments. Treatments denoted by the same letter indicate no significant difference between those treatments. Treatments denoted by different letters indicate a significant difference between those treatments at P < .05.
Resveratrol, But Not E2, Induces Filopodia Extension in ER(+) Breast Cancer Cells
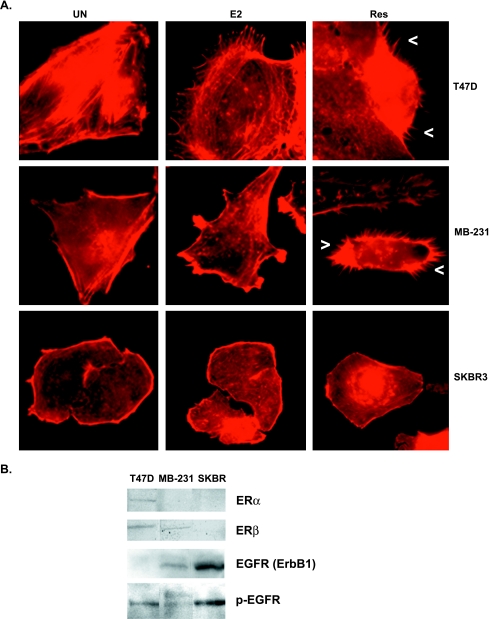
To investigate a structural mechanism for the migratory response of ERα(-) β(+) MDA-MB-231 to resveratrol and E2, we monitored the effect of these compounds on the actin cytoskeleton of ERα(+) T47D, ERα(-) β(+) MDA-MB-231, and ERα(-) β(-) SKBR3 human breast cancer cell lines. The ER α and β protein expression status of all three cell lines was confirmed by Western blot analysis with monospecific antibodies (Figure 2B). As shown in Figure 2A, addition of resveratrol or E2 to quiescent T47D and MDA-MB-231 human breast cancer cell lines resulted in rapid reorganization of the actin cytoskeleton. However, the actin cytoskeletal response to resveratrol compared to E2 was structurally very different. Treatment with resveratrol resulted in a dynamic global extension of filopodia, which contain bundles of actin filaments; whereas treatment with E2 resulted in extension of lamellipodia, which contain cross-linked networks of actin filaments. In the ERα(-) β(+) MDA-MB-231 cells, we have observed significant increases in filopodia extension from 5 to 30 minutes following resveratrol treatment at concentrations ranging from 10 to 100 µM with saturation at 50 µM resveratrol (data not shown). Resveratrol-induced increases in filopodia were also observed for the ERαβ(+) T47D cells (Figure 2A). However, E2 or resveratrol did not affect the actin cytoskeleton of the ERαβ(-) metastatic breast cancer cell line, SKBR3 (Figure 2A). As confirmed in Figure 2B, the SKBR3 cell line is known to be deficient in ER mRNA expression [52]. Therefore, these effects of E2 and resveratrol on the actin cytoskeleton may be ER-dependent but not specific to ERα.
Figure 2.
Effect of E2 and trans-resveratrol on the actin cytoskeleton of ER (+) and (-) cells. (A) Micrographs of T47D, MDA-MB-231, and SKBR3 cells at x600 magnification. Cells were serum-starved in phenol red-free media for 24 hours and stimulated for 10 minutes with DMSO as control (Un), E2 (0.1 µM), or 50 µM resveratrol (Res). Cells were stained with rhodamine phalloidin to visualize F-actin. Arrowheads (<) indicate a filopodium. (B) Western blot of whole cell lysates of T47D, MDA-MB-231, and SKBR3. Equal amounts of proteins from cell lysates were loaded on SDS-PAGE and Western-blotted using anti-ERα (65 kDa band) or anti-ERβ (53 kDa band). Equal amounts of cell lysates were also probed with anti-EGFR1 (185 kDa) or anti-phosphoEGFR (Y1173) (185 kDa).
Because we investigate a role for E2 and resveratrol in crosstalk with EGFR signaling, expression of EGFR was also confirmed in these breast cancer cell lines (Figure 2B). Although the EGFR antibody was not sensitive enough to detect the low expression of EGFR in T47D cells, Western blot analysis of lysates from cells grown in serum with a phospho-specific EGFR antibody detected activated EGFR in T47D cells as well as in MDA-MB-231 and SKBR3 cells. Therefore, the observed lack of an actin cytoskeletal response following E2 or resveratrol in SKBR3 cells is not due to lack of EGFR function but probably due to their ER(-) status.
Stimulation of ERα(-) β(+) MDA-MB-231 cells with EGF resulted in lamellipodia formation, as has been reported in other breast cancer cell lines [37]. Interestingly, E2 exerted a similar, but not as pronounced, effect as EGF by inducing lamellipodia. This has previously been shown in ERαβ(+) MCF-7 breast cancer cells [38]. The number of filopodia extended in cells treated with E2 or EGF did not differ significantly from controls (Figures 2–4). These parallel cytoskeletal responses to E2 or EGF suggest that E2 may exert effects on the actin cytoskeleton through EGFR.
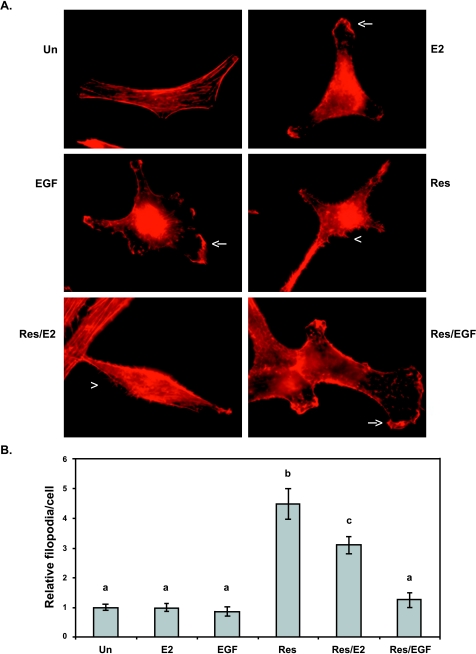
Figure 3.
Effect of E2, EGF, or trans-resveratrol on the actin cytoskeleton of MDA-MB-231 cells. Cells were serum-starved in phenol red-free media for 24 hours and stimulated for 10 minutes with DMSO as control (Un); 0.1 µM E2 (E2); 50 ng/ml EGF (EGF); and 50 µM resveratrol (Res), pretreated with Res for 10 minutes followed by E2 for 10 minutes (Res/E2), or pretreated with Res for 10 minutes followed by EGF for 10 minutes (Res/EGF). Cells were stained with rhodamine phalloidin to visualize F-actin. (A) Micrographs at x600 magnification. Arrowheads (<) indicate a filopodium; arrows (←) indicate a lamellipodium. (B) Filopodia number was quantified for at least 10 microscopic fields per treatment per experiment and made relative to DMSO control (Un). Data expressed as mean filopodia ± SEM of three independent experiments. Treatments denoted by the same letter indicate no significant difference between those treatments. Treatments denoted by different letters indicate a significant difference between those treatments at P < .05.
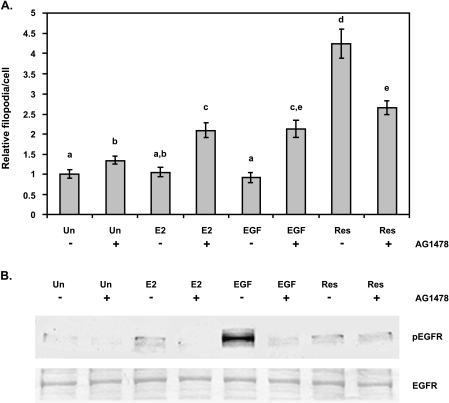
Figure 4.
Effect of tyrphostin AG1478 on filopodia formation in MDA-MB-231 cells. (A) Cells were serum-starved in phenol red-free media for 24 hours and pretreated with vehicle (-AG1478) or tyrphostin AG1478 (+AG1478) for 15 minutes then treated with DMSO as control (Un), 0.1 µM E2 (E2), 50 ng/ml EGF (EGF), or 50 µM resveratrol (Res) for 10 minutes. Cells were stained with rhodamine phalloidin to visualize F-actin. Filopodia number was quantified for at least 10 microscopic fields per treatment per experiment and made relative to DMSO control (Un). Data expressed as mean filopodia ± SEM of three independent experiments. Treatments denoted by the same letter indicate no significant difference between those treatments. Treatments denoted by different letters indicate a significant difference between those treatments at P < .05. (B) Quiescent cells were pretreated with DMSO (-AG1478) or tyrphostin AG1478 (+AG1478) for 15 minutes then treated with DMSO as control (Un), 0.1 µM E2 (E2), 50 ng/ml EGF (EGF), or 50 µM resveratrol (Res) for 10 minutes. Cells were immediately lysed and equal amounts of protein were separated on SDS-PAGE and Western-blotted for activated EGFR using an anti-phosphoEGFR (Y1173) antibody. The result is representative of two separate experiments.
As shown in Figure 3, the number of filopodia extended in response to 50 µM resveratrol was significantly increased by ∼4.5-fold compared to control, E2, or EGF. To evaluate the role of resveratrol as an inhibitor of E2 or EGF action on the cytoskeleton, the number of filopodia was quantified in cells preincubated with resveratrol and subsequently stimulated with E2(Res/E2) or EGF (Res/EGF). In Res/E2 treatments, the number of filopodia was significantly greater than unstimulated control or E2 alone by ∼3-fold. However, the number of filopodia extended in this Res/E2 combined treatment was still significantly less (31%) when compared to resveratrol alone. Resveratrol pretreatment followed by EGF (Res/EGF) did not significantly increase filopodia number when compared to EGF alone. Thus, these results demonstrate that resveratrol may counteract the effect of E2 on the actin cytoskeleton. In the presence of EGF, resveratrol did not affect direct EGF signaling to the actin cytoskeleton, indicating that resveratrol may not directly compete with EGF to alter EGFR signaling.
Resveratrol and E2 Effects on the Actin Cytoskeleton of MDA-MB-231 Cells Are Modulated by EGFR Inhibitor
Plasmamembrane ERs have been implicated in the cross-activation of tyrosine kinase-type growth factor receptors, such as EGFR [7,11]. To further evaluate the role of EGFR on resveratrol-mediated effects on the actin cytoskeleton, we investigated the effect of tyrphostin AG1478, an EGFR1 specific inhibitor. As shown in Figure 4A, treatment of unstimulated cells with AG1478 (Un+) resulted in a significant increase in the number of filopodia when compared to unstimulated control (Un-). This increase may be due to a nonspecific effect of AG1478 treatment or inhibition of intrinsic EGFR activity.
When E2- or EGF-treated cells were pretreated with AG1478 ( or EGF+, respectively), the filopodia number increased significantly compared to unstimulated plus AG1478 (Un+) treatment. There was a significant ∼2-fold increase of filopodia in cells treated with E2 in the presence of AG1478 () compared to those treated with E2 in the absence of AG1478 (), or in cells treated with EGF in the presence of AG1478 (EGF+) compared to those treated with EGF in the absence of AG1478 (EGF-). These increases may also be a direct result of inhibition of EGFR activity or a nonspecific effect of AG1478.
Conversely, AG1478 treatment partially reduced the number of filopodia extended in response to resveratrol (Res+) by ∼1.4-fold when compared to resveratrol alone (Res-). The number of filopodia in resveratrol-treated cells in the presence of AG1478 (Res+) was still significantly higher than the number of filopodia in unstimulated cells in the presence of AG1478 (Un+) (∼2-fold) and in E2-stimulated cells in the presence of AG1478 () (27%), and nearly significant for EGF-stimulated cells in the presence of AG1478 (EGF+) (24%; P = .06). Thus, EGFR signaling appears to play a partial role in resveratrol signaling to the actin cytoskeleton.
To determine the effect of E2 or resveratrol on EGFR activation, EGFR activity was detected by a monospecific antibody to the phosphotyrosine residue 1173 of EGFR, which is autophosphorylated upon receptor occupation. Our results are limited by the sensitivity of the phosphoEGFR (Y1173) antibody and by the fact that EGFR is phosphorylated on several other phosphotyrosine residues upon activation [19]. As shown in Figure 4B, there was a very low intrinsic EGFR activity in quiescent MDA-MB-231 cells. As expected, EGF stimulation resulted in a marked increase in phosphoEGFR levels, which was abolished by AG1478 treatment. Similarly, E2 induced EGFR activity, but to a lesser degree than EGF, and was inhibited by AG1478. Interestingly, resveratrol also increased EGFR activity but to a lesser degree than E2. This activity did not appear to be attenuated by AG1478. These results reveal a potential resveratrol-induced AG1478-insensitive fraction of EGFR signaling.
Resveratrol Decreases, Whereas E2 Increases, Focal Adhesion Number in MDA-MB-231 Cells
Because focal adhesions are commonly associated with cell surface actin structures such as filopodia and lamellipodia [38], cells stimulated with E2, EGF, or resveratrol were examined for focal adhesions. As shown in Figure 5b, EGF increased the number of focal adhesions per cell compared to unstimulated controls in a statistically significant manner by ∼2-fold. Increased focal adhesion assembly in response to E2 was more moderate at 20% but still statistically significant when compared to controls, again demonstrating a similar but not as pronounced effect on the cytoskeleton as EGF. Moreover, the focal adhesions extended in response to E2 or EGF were observed to be smaller and distributed in lamellipodia, and thus characteristic of motile cells.
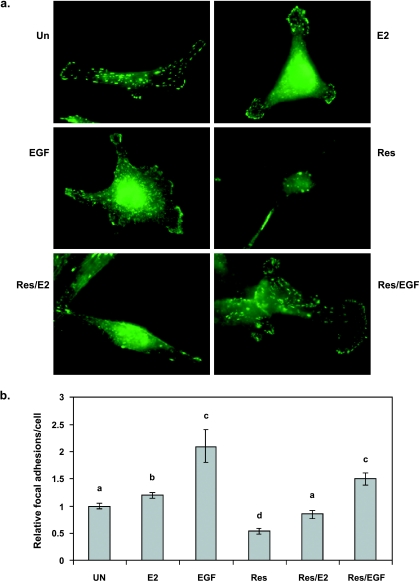
Figure 5.
Effect of E2, EGF, or trans-resveratrol on focal adhesion assembly in MDA-MB-231 cells. Cells were serum-starved in phenol red-free media for 24 hours and stimulated for 10 minutes with DMSO as control (Un); 0.1 µM E2 (E2); 50 ng/ml EGF (EGF); 50 µM resveratrol (Res), pretreated with Res for 10 minutes followed by E2 for 10 minutes (Res/E2), or pretreated with Res for 10 minutes followed by EGF for 10 minutes (Res/EGF). Cells were probed with antiphosphotyrosine primary antibody and FITC-conjugated secondary antibody to visualize focal adhesions. (a) Micrographs at x600 magnification. (b) Focal adhesion number per cell was quantified for at least 10 microscopic fields per treatment per experiment and made relative to DMSO control (Un). Data expressed as mean focal adhesions ± SEM of three independent experiments. Treatments denoted by the same letter indicate no significant difference between those treatments. Treatments denoted by different letters indicate a significant difference between those treatments at P < .05.
Resveratrol treatment resulted in significantly reduced focal adhesion assembly by 36% compared to control (Figure 5). Resveratrol-induced filopodia did not appear to be associated with focal adhesions. To investigate the ability of resveratrol to inhibit E2 or EGF action, focal adhesion number was quantitated in E2- or EGF-treated cells after resveratrol treatment. When cells were treated with resveratrol prior to E2 (Res/E2), focal adhesions per cell were significantly reduced by 29% when compared to E2 alone. There was also a 29% reduction in focal adhesion number observed for cells treated with resveratrol prior to EGF (Res/EGF) when compared to EGF alone (P = .07). However, focal adhesions in Res/E2 and Res/EGF treatments were still significantly higher than resveratrol alone by ∼1.6- and ∼2.8-fold, respectively. These results indicate at least a partial role for ER in resveratrol-mediated inhibition of focal complex assembly.
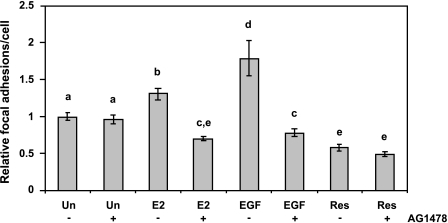
As shown in Figure 6, AG1478 treatment did not significantly alter the amount of focal adhesions in unstimulated cells. Compared to E2 alone (), treatment with AG1478 () reduced the number of focal adhesions significantly by 41%. The number of focal adhesions per cell in response to EGF (EGF-) also decreased significantly in the presence of tyrphostin AG1478 (EGF+) by 56%. The decrease in focal adhesion number in the presence of AG1478 in E2-treated () or EGF-treated (EGF+) cells was significantly lower than that of unstimulated cells in the presence of AG1478 (Un+) by 13% and 9%, respectively. We did not observe a significant reduction in focal adhesion number in the presence of tyrphostin AG1478 (Res+) in resveratrol-treated cells (Res-). This result demonstrates that the increased focal adhesions in both E2- and EGF-treated cells are probably due to EGFR activity.
Figure 6.
Effect of tyrphostin AG1478 on focal adhesion assembly in MDA-MB-231 cells. Cells were serum-starved in phenol red-free media for 24 hours and pretreated with vehicle (-AG1478) or tyrphostin AG1478 (+AG1478) for 15 minutes then treated with DMSO as control (Un), 0.1 µM E2 (E2), 50 ng/ml EGF (EGF), or 50 µM trans-resveratrol (Res) for 10 minutes. Cells were probed with an anti-phosphotyrosine antibody followed by FITC secondary antibody to visualize focal adhesions. Focal adhesion number was quantified for at least 10 microscopic fields per treatment per experiment and made relative to DMSO control (Un). Data expressed as mean focal adhesions ± SEM of three independent experiments. Treatments denoted by the same letter indicate no significant difference between those treatments. Treatments denoted by different letters indicate a significant difference between those treatments at P < .05.
Resveratrol Decreases FAK Activity in MDA-MB-231 Cells
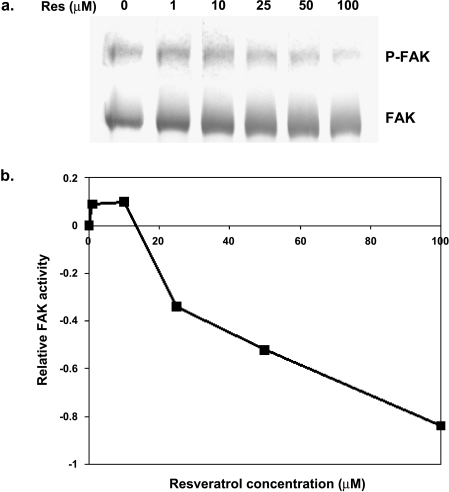
Because FAK is a signaling intermediate that is recruited to focal adhesions immediately following integrin activation and is also regulated by growth factor receptor stimulation, we determined FAK activity in response to resveratrol. FAK activity in response to resveratrol was determined by analysis of autophosphorylation of FAK following a range of resveratrol concentrations as described in Ref. [29]. In MDA-MB-231 cells, resveratrol at 10 minutes slightly increased FAK activity at 1 and 10 µM. Endogenous FAK activity decreased when treated with 25 µM resveratrol by 34%, at 50 µM by 52%, and at 100 µM by 84% (Figure 7). Thus, the decreased FAK activity in response to resveratrol corresponds to the decreased focal adhesion assembly by resveratrol.
Figure 7.
Effect of resveratrol on FAK activity in MDA-MB-231 cells. Cells were starved for 24 hours in phenol red- and serum-free media and treated with DMSO as vehicle (0) or resveratrol (1, 10, 25, 50, or 100 µM) for 10 minutes. (a) Equal amounts of protein were run on SDS-PAGE and Western-blotted using FAK (N-terminus) or phosphoFAK (tyr-397) antibodies. (b) The integrated density of phosphoFAK and FAK bands from Western blots was quantified. Relative activity is the difference between the ratio of phosphoFAK to total FAK with stimulation, and the ratio of phosphoFAK to FAK without stimulation. The result is representative of three separate experiments.
Discussion
Overall, the data presented demonstrate that although E2 and EGF increase directed cell migration, lamellipodia extension, and focal adhesion assembly, resveratrol exerts an opposite effect by inhibiting cell migration, increasing filopodia formation, and decreasing the number of focal adhesions and FAK activity. Thus, rapid resveratrol signaling to the cytoskeleton of ERα(-) ERβ(+) MDA-MB-231 breast cancer cells appears to function in an antiestrogenic manner.
Cell migration is crucial for cancer cell invasion and metastasis. Herein, we report that resveratrol inhibits cell migration, whereas E2 acts similar to EGF, a known promoter of cell migration. This resveratrol-induced inhibitory effect on cell migration was still evident when cells were pretreated with resveratrol followed by EGF or E2. The demonstrated inhibitory effect of resveratrol on directed cell migration is also substantiated by previous studies, which reported that resveratrol blocked the wound healing response of epithelial cells [33,34] and invasion of phorbol myristate acetate-induced cervical cancer cell invasion [36]. This inhibitory effect of resveratrol on directed cell migration could be accounted for by its reported antiproliferative and proapoptotic properties. However, the short exposure times of our experiments (10 minutes for detection of filopodia and focal adhesion assembly and 8 hours for migration) makes this possibility highly unlikely. We have examined the cells (by propidium iodide staining of nuclei) at the end of our experiments and they appear to be viable. Previous reports on the role of resveratrol in apoptosis, where the authors incubated cells for over 24 hours in micromolar concentrations of resveratrol, support our inference that we are monitoring rapid signaling effects and not effects of resveratrol on cell growth [31,53–56].
In fibroblast-like cells such as MDA-MB-231 breast cancer cells, directed motility in response to a chemoattractant is driven by cell polarization, polymerization of actin, and incorporation of cross-linked actin filaments into leading-edge lamellipodia and filopodia that are stabilized by making focal adhesions with the ECM [39,57]. Lamellipodia are considered to be essential for directed cell migration, whereas filopodia are not essential for cell migration but considered to serve as environmental sensors. Filopodia are often reorganized to form leading-edge lamellipodia during cell migration [39]. Thus, the observed cytoskeletal response to resveratrol—where cells appear relatively unpolarized and extend large numbers of unorganized peripheral filopodia that are not associated with focal adhesions and do not get converted to lamellipodia—is hypothesized to be directly responsible for the inhibition of cell migration by resveratrol.
FAK is one of the first signaling intermediates recruited to nascent focal adhesions. FAK-mediated increases in cell proliferation, motility, and invasion have been correlated with tumor malignancy [41,58]. Therefore, the observed reduced FAK activity in response to resveratrol at concentrations of 25 µM and above may also contribute to the inhibitory effect of resveratrol on cell migration. This result coincides with our reported decrease of focal adhesion number with resveratrol at 50 µM. Reduction in focal contacts with the ECM is known to activate apoptosis [58]; thus, the resveratrol-mediated decreased FAK activity and focal adhesion number may represent another pathway by which resveratrol can induce apoptosis.
Rapid E2 effects have been shown to be mediated through EGFR signaling [11]. EGFR activity is known to regulate pathways leading to actin reorganization by nongenomic mechanisms [50]. EGFR signaling activates the Rho family GTPases, Rac and Cdc42, that regulate extension of cell surface actin structures such as filopodia and lamellipodia [38,59]. Our results show that E2 acts similarly to EGF and activates EGFR, increases directed cell migration, lamellipodia extension, and focal adhesion assembly. Thus, E2 and EGF may promote directed cell migration by similar mechanisms that involve extension of lamellipodia with multiple dynamic focal adhesions that support protrusion and traction at the cell front during motility. Recent data have demonstrated that EGF-induced filopodia are always associated with basal focal adhesions and the filopodia that contain shaft adhesions (focal adhesions inside the filopodia) are useful for directed motility by conversion into lamellipodia [60]. Interestingly, the filopodia extended in response to resveratrol were not associated with basal or shaft adhesions. Thus, the filopodia and focal adhesions assembled in resveratrol-treated cells may be structurally and functionally different from those assembled in response to E2 or EGF.
The effects of E2 and resveratrol on the cytoskeleton were only evident in cells that expressed a functional ER isoform and not in the ERαβ(-) SKRB3 cell line. When ERβ(+) MDA-MB-231 breast cancer cells were preincubated in resveratrol and subsequently treated with E2, the cytoskeletal response of increased filopodia and decreased focal adhesions was similar to that of resveratrol alone, indicating that resveratrol probably exerts its effects on the actin cytoskeleton through ER. The slightly reduced cytoskeletal response of resveratrol combined with E2, when compared to resveratrol alone, may be due to the lower binding affinity of resveratrol for ERβ compared to E2 [61]. However, resveratrol has been shown to exert potentially ER-independent effects on modulation of enzymes such as cyclooxygenases and PKC isoforms [25,26]. Thus, some of the effects of resveratrol on the actin cytoskeleton may indicate an ER-independent alternate pathway that blocks or overpasses ER action.
To investigate the potential role of EGFR signaling on resveratrol and E2 effects on the cytoskeleton, we evaluated the effect of inhibiting the kinase activity of EGFR1 with tyrphostin AG1478. As expected, we observed an AG1478-sensitive EGFR phosphorylation response to both E2 and EGF. We also observed a reduction in lamellipodia and the number of focal adhesions assembled in response to EGF or E2 in the presence of AG1478. According to recent reports, E2 acts through G proteins to activate EGFR-mediated signaling to MAPK and Akt activity pathways that regulate cell proliferation and survival [11,15,62]. The present study has elucidated a novel role for E2 in rapid signaling to the actin cytoskeleton to promote directed migration that may represent another relevant E2/EGFR-mediated signaling pathway.
When cells were treated with resveratrol prior to EGF, resveratrol did not directly interfere with EGF for signaling to EGFR to exert effects on the cytoskeleton. However, addition of AG1478 inhibited a portion of the filopodia extended in response to resveratrol. Thus, at least some of the resveratrol-mediated effects on the actin cytoskeleton are regulated by EGFR signaling. It is also possible that the filopodia extended by resveratrol in the presence of AG1478 may still be under EGFR regulation because resveratrol-induced autophosphorylation of EGFR could not be inhibited by AG1478. This interesting result may be due to an AG1478-insensitive resveratrol-mediated EGFR activation. This possibility is substantiated by a recent report that demonstrates an AG1478-independent activation of EGFR phosphorylation through Src activity [63].
Interestingly, treatment of unstimulated cells with AG1478 (Un+) resulted in a significant increase in the number of filopodia when compared to unstimulated control (Un-). This implies that EGFR activity was necessary to prevent filopodia formation on unstimulated cells (Un-). Moreover, when E2- or EGF-treated cells were pretreated with AG1478, there was also a significant increase in filopodia formation compared to E2 or EGF alone. These perplexing results may be explained if EGFR activity is important for the conversion of filopodia into lamellipodia, as previously reported [64]. In our microscopy studies, we have observed that when MDA-MB-231 cells were stimulated with EGF, they responded initially (at 5 minutes) by filopodia extension. These filopodia then merged into lamellipodia, which were completely formed by 10 minutes. Based on this observation, it is possible that AG1478-sensitive EGFR signaling is important for the conversion of filopodia to lamellipodia, which may be regulated by EGF or E2 through EGFR1 signaling. Thus, in the presence of AG1478, the fraction of filopodia induced by AG1478-insensitive alternate EGFR signaling may remain as filopodia instead of reorganizing into lamellipodia.
The present study makes a significant contribution to the field of nongenomic signaling of E2 and related compounds by investigation of a novel role for E2 and resveratrol in cancer cell migration through rapid reorganization of the actin cytoskeleton. For the first time, we demonstrate that resveratrol inhibits cell migration in response to E2 or EGF. This inhibitory effect of resveratrol on cell migration may be due to rapid filopodia formation without accompanying cell polarization or attachment to the ECM. Taken together, these findings indicate that resveratrol may have the potential to play a preventive role in the progression of ERα(-) ERβ(+) metastatic breast cancers that express EGFR. A growing body of literature suggests that moderate red wine consumption may have several health benefits, including a lowered risk of breast cancer. The data presented implicate an additional beneficial role for resveratrol in the prevention of breast cancer cell invasion and metastasis.
Acknowledgements
We wish to thank Kristen Wall and Drs. Mona Mehdy and Michelle Lane for editorial assistance and Adi Dubash and Lakshmi Krishnamoorthy for technical assistance. We also thank Dr. Delia Brownson for her technical and intellectual contributions to the conception of this project.
Abbreviations
- E2
17β-estradiol
- EGF
epidermal growth factor
- ER
estrogen receptor
- FAK
focal adhesion kinase
Footnotes
This investigation was supported by NIH/NCI grants CA83957-01A1 and DAMD17-02-1-0582 (to S.D.) and the Hispanic Scholarship Fund and Sigma Xi (to N.A.).
References
- 1.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 2.Cavalieri EL, Rogan EG. A unified mechanism in the initiation of cancer. Ann NY Acad Sci. 2002;959:341–354. doi: 10.1111/j.1749-6632.2002.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 3.Salih AK, Fentiman IS. Breast cancer prevention: present and future. Cancer Treat Rev. 2001;27:261–273. doi: 10.1053/ctrv.2001.0235. [DOI] [PubMed] [Google Scholar]
- 4.Shen Q, Brown PH. Novel agents for the prevention of breast cancer: targeting transcription factors and signal transduction pathways. J Mammary Gland Biol Neoplasia. 2003;8:45–73. doi: 10.1023/a:1025783221557. [DOI] [PubMed] [Google Scholar]
- 5.Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM. Modulation of epidermal growth factor receptor in endocrine-resistant, estrogen-receptor-positive breast cancer. Ann NY Acad Sci. 2002;963:104–115. doi: 10.1111/j.1749-6632.2002.tb04101.x. [DOI] [PubMed] [Google Scholar]
- 7.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 8.Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther. 2001;299:408–414. [PubMed] [Google Scholar]
- 9.Gray GA, Sharif I, Webb DJ, Seckl JR. Oestrogen and the cardiovascular system: the good, the bad and the puzzling. Trends Pharmacol Sci. 2001;22:152–156. doi: 10.1016/s0165-6147(00)01640-0. [DOI] [PubMed] [Google Scholar]
- 10.Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–D1391. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- 11.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 12.Winter DC, Taylor C, O'S C, Harvey BJ. Mitogenic effects of oestrogen mediated by a non-genomic receptor in human colon. Br J Surg. 2000;87:1684–1689. doi: 10.1046/j.1365-2168.2000.01584.x. [DOI] [PubMed] [Google Scholar]
- 13.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways: Part II. The role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 16.Pietras RJ. Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J. 2003;9:361–373. doi: 10.1046/j.1524-4741.2003.09510.x. [DOI] [PubMed] [Google Scholar]
- 17.Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. 2004;30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Wells A, Kassis J, Solava J, Turner T, Lauffenburger DA. Growth factor-induced cell motility in tumor invasion. Acta Oncol. 2002;41:124–130. doi: 10.1080/028418602753669481. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 20.Herrington D. Role of estrogens, selective estrogen receptor modulators and phytoestrogens in cardiovascular protection. Can J Cardiol. 2000;16:5E–9E. [PubMed] [Google Scholar]
- 21.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 22.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 23.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- 24.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann NY Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 26.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 27.Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179:297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh TC, Burfeind P, Laud K, Backer JM, Traganos F, Darzynkiewicz Z, Wu JM. Cell cycle effects and control of gene expression by resveratrol in human breast carcinoma cell lines with different metastatic potentials. Int J Oncol. 1999;15:245–252. doi: 10.3892/ijo.15.2.245. [DOI] [PubMed] [Google Scholar]
- 29.Brownson DM, Azios NG, Fuqua BK, Dharmawardhane SF, Mabry TJ. Flavonoid effects relevant to cancer. J Nutr. 2002;132:3482S–3489S. doi: 10.1093/jn/132.11.3482S. [DOI] [PubMed] [Google Scholar]
- 30.Pozo-Guisado E, Lorenzo-Benayas MJ, Fernandez-Salguero PM. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int J Cancer. 2004;109:167–173. doi: 10.1002/ijc.11720. [DOI] [PubMed] [Google Scholar]
- 31.Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001;167:151–156. doi: 10.1016/s0304-3835(01)00476-1. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Cao R, Brakenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/s0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 33.Brakenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 34.Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- 35.De LV, Monvoisin A, Neaud V, Krisa S, Payrastre B, Bedin C, Desmouliere A, Bioulac-Sage P, Rosenbaum J. trans-Resveratrol, a grapevine-derived polyphenol, blocks hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells. Int J Oncol. 2001;19:83–88. [PubMed] [Google Scholar]
- 36.Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW, Kwon TK. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 37.Condeelis JS, Wyckoff JB, Bailly M, Pestell R, Lawrence D, Backer J, Segall JE. Lamellipodia in invasion. Semin Cancer Biol. 2001;11:119–128. doi: 10.1006/scbi.2000.0363. [DOI] [PubMed] [Google Scholar]
- 38.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc Natl Acad Sci. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 40.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 41.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 43.Tong D, Schuster E, Seifert M, Czerwenka K, Leodolte S, Zeillinger R. Expression of estrogen receptor beta isoforms in human breast cancer tissues and cell lines. Breast Cancer Res Treat. 2002;71:249–255. doi: 10.1023/a:1014465916473. [DOI] [PubMed] [Google Scholar]
- 44.Leng J, Klemke RL, Reddy AC, Cheresh DA. Potentiation of cell migration by adhesion-dependent cooperative signals from the GTPase Rac and Raf kinase. J Biol Chem. 1999;274:37855–37861. doi: 10.1074/jbc.274.53.37855. [DOI] [PubMed] [Google Scholar]
- 45.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneuchi M, Sasaki M, Tanaka Y, Yamamoto R, Sakuragi N, Dahiya R. Resveratrol suppresses growth of Ishikawa cells through down-regulation of EGF. Int J Oncol. 2003;23:1167–1172. [PubMed] [Google Scholar]
- 47.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–163. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 48.Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002;64:1375–1386. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- 49.Tsai EM, Wang SC, Lee JN, Hung MC. Akt activation by estrogen in estrogen receptor-negative breast cancer cells. Cancer Res. 2001;61:8390–8392. [PubMed] [Google Scholar]
- 50.Chan AY, Raft S, Bailly M, Wyckoff JB, Segall JE, Condeelis JS. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J Cell Sci. 1998;111(Part 2):199–211. doi: 10.1242/jcs.111.2.199. [DOI] [PubMed] [Google Scholar]
- 51.Lotz M, Wang HH, Cance W, Matthews J, Pories S. Epidermal growth factor stimulation can substitute for c-Src overexpression in promoting breast carcinoma invasion. J Surg Res. 2003;109:123–129. doi: 10.1016/s0022-4804(02)00100-2. [DOI] [PubMed] [Google Scholar]
- 52.Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, Lansdown MR, Parkes AT, Hanby AM, Markham AF, Speirs V. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 53.Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223–1232. doi: 10.1210/jcem.87.3.8345. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh CT, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 55.Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–1005. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- 56.Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–441. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 57.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 58.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 59.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 60.Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhat KP, Pezzuto JM. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res. 2001;61:6137–6144. [PubMed] [Google Scholar]
- 62.Stoica GE, Franke TF, Moroni M, Mueller S, Morgan E, Iann MC, Winder AD, Reiter R, Wellstein A, Martin MB, Stoica A. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI3-K/Akt pathway. Oncogene. 2003;22:6054–6067. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 63.Sato K, Nagao T, Iwasaki T, Nishihira Y, Fukami Y. Src-dependent phosphorylation of the EGF receptor Tyr-845 mediates Stat-p21waf1 pathway in A431 cells. Genes Cells. 2003;8:995–1003. doi: 10.1046/j.1356-9597.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 64.Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]