Abstract
To investigate the cellular/molecular basis of the activity of a novel lipophilic camptothecin, gimatecan (ST1481), against slowly proliferating cells, we performed a comparative study of topotecan and gimatecan in human bladder cancer models (HT1376 and MCR). Gimatecan was significantly more effective than topotecan in inhibiting the growth of HT1376 tumor, thus reflecting antiproliferative potency. In both HT1376 and MCR cells, gimatecan caused a persistent S-phase arrest, indicating an efficient DNA damage checkpoint. This response was consistent with a cytostatic effect, because no evidence of apoptosis was detected. In contrast to gimatecan, topotecan at equitoxic concentrations caused an early and persistent downregulation of topoisomerase I. Modulation of protein level could not be solely ascribed to the proteasome-mediated degradation of the enzyme because the proteasome inhibitor PS341 sensitized MCR but not HT1376 cells to camptothecins, suggesting alternative mechanisms of drug-induced topoisomerase I downregulation. Indeed, the two camptothecins caused a differential inhibition of topoisomerase I transcription, which is more marked in topotecan-treated cells. The HT1376 model was more sensitive to this immediate decrease of mRNA level. Our data document a marked antitumor activity of gimatecan against a bladder carcinoma model. A limited downregulation of topoisomerase I by gimatecan provides additional insights into the cellular basis of drug potency.
Keywords: Bladder carcinoma, DNA topoisomerase I, gimatecan, camptothecins, antitumor activity
Introduction
Camptothecins are among the most promising antitumor agents [1,2]. On the basis of their therapeutic interest, intense research efforts have provided insights to understand their mechanism of action and to exploit their antitumor potential [3,4]. Camptothecins are DNA-damaging agents characterized by a unique mechanism of action because they are target-specific inhibitors of DNA topoisomerase I by stabilizing the covalent enzyme-DNA complex (cleavable complex) [5,6].
Due to the specific mechanism of topoisomerase I-mediated cytotoxicity, a characteristic feature of camptothecin action is their preferential or selective toxicity to proliferating cells [7]. Indeed, these agents are considered S-phase-specific because the lethal DNA lesions are formed during DNA synthesis as a consequence of collision between the replication machinery and the enzyme-DNA-cleavable complex [6]. For this reason, tumors with a high fraction of proliferating cells are expected to be more responsive to camptothecins than slowly growing tumors.
We have recently reported that slowly proliferating cells are still sensitive to a novel potent camptothecin analogue, gimatecan (ST1481) [8]. The molecular/cellular basis of this unique feature remains unknown. To elucidate the molecular events responsible for the potency and efficacy of gimatecan against slowly growing tumor cells, we have chosen two bladder carcinoma cell systems. The results of cellular pharmacology and antitumor activity studies support the therapeutic potential of the novel camptothecin against bladder carcinoma and provide additional insights on the molecular events responsible for its antiproliferative and antitumor potency.
Materials and Methods
Cell Lines
The study was performed on two human bladder carcinoma cell lines, including HT1376 and MCR (established in our laboratory), characterized by similar doubling times (38 and 42 hours, respectively). HT1376 cells are known to harbor a p53 mutation, whereas the MCR cells, characterized in our laboratory, harbor two p53 mutations: one in exon 4 (CGC→CCC) and one in exon 9 (CAG→TAG), with the latter producing a stop codon that determines a truncated protein form. Cells were maintained as a monolayer in culture medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine, and were subcultured weekly. All experiments were performed during exponential cell growth.
Drugs
ST1481 (t-butoxyiminomethylcamptothecin, gimatecan; Sigma-Tau, Pomezia, Rome, Italy) was dissolved in dimethylsulfoxide (DMSO) and stored at -20°C until use. Immediately before treatment, ST1481 and topotecan (Glaxo-SmithKline, Brentford, UK) were diluted in culture medium. Because the molecular weights of topotecan and gimatecan were similar (457.96 and 447.5, respectively), the concentrations were usually expressed in micrograms per milliliter, or nanograms per milliliter.
Antitumor Activity Studies
Antitumor activity experiments were carried out using female athymic Swiss nude mice 8 to 10 weeks of age (Charles River, Calco, Italy). Mice were maintained in laminar flow rooms, keeping temperature and humidity constant. Mice had free access to food and water. Experiments were approved by the Ethics Committee for Animal Experimentation of the Istituto Nazionale Tumori (Milan, Italy).
Tumor lines were established in vivo by subcutaneous injection of 107 cells from in vitro cultures. Randomized groups of five mice bearing bilateral subcutaneous tumors were used. The mean tumor doubling time in control mice was 8.6 days. The drugs were delivered by gavage in a volume of 10 ml/kg body weight every fourth day for four times (q4 x d x 4) at their maximum tolerated doses starting when tumors were measurable (around 50 mg). For statistical analysis, tumor volumes of ST1481-treated versus TPT-treated mice were compared by Student's t test.
Cell Sensitivity Studies
Cell sensitivity to drugs was measured by growth inhibition assay after 1, 6, and 24 hours of exposure to ST1481 and 1-hour exposure to topotecan. Cells in the logarithmic growth phase were seeded in triplicate in 50-mm plates. Twenty-four hours after seeding, the drug was added to the medium. Cells were harvested 72 hours after drug exposure and counted with a cell counter. In independent experiments, antiproliferative effects of drug treatment were assessed by the sulforhodamine B (SRB) assay [9]. IC50 is defined as the drug concentration causing a 50% reduction in cell number compared to that of untreated control.
The effects of combination of camptothecins with PS341 on cell growth were assessed by SRB assay. Following a 1-hour exposure to camptothecin, cells were incubated for a further 72 hours with the proteasome inhibitor, PS341. Drug interaction was examined according to the method of Drewinko et al. [10], using the following formula:
where SF indicates the survival fraction observed for each drug (a and b), or for cotreatment (a + b). The nature of the interaction is interpreted on the basis of CI values (i.e., CI > 1, synergism; CI = 1, additivity; CI < 1, antagonism).
Cell cycle perturbation
Control cells and cells exposed to 0.003 and 0.03 µg/ml gimatecan (ST1481) for 1, 6, or 24 hours followed by a 72-hour incubation in drug-free medium were trypsinized, washed twice with PBS, fixed in ice-cold ethanol 70%, and stained in a solution containing RNAase (10 kU/ml; Sigma-Aldrich, St. Louis, MO), NP40 (0.01%; Sigma-Aldrich), and propidium iodide (20 µg/ml; Sigma-Aldrich). Samples were stored for 60 minutes and analyzed by flowcytometry (FACS Vantage flow cytometer; Becton Dickinson, San Jose, CA). For each sample, 10,000 events were collected for subsequent analysis. Data analysis was performed using CELL Quest software (Becton Dickinson). Data were elaborated using Modfit (DNA Modeling System) software (Verity Software House, Inc., Topsham, ME) and expressed as fractions of cells in different cell cycle phases. Samples were run in triplicate, and each experiment was repeated three times. Values of treated samples are expressed as a percentage of controls.
Apoptosis
After a 6-hour exposure to 0.03 µg/ml gimatecan (ST1481) followed by 12, 24, 48, or 72 hours of incubation in drug-free medium, cells were trypsinized, washed twice with PBS, and fixed in 1% paraformaldehyde in PBS on ice for 15 minutes. After two washes in PBS, cells were resuspended in ice-cold 70% ethanol, stored overnight at -20°C, then washed in PBS and incubated in 50 µl of solution containing terminal deoxynucleotidyltransferase and FITC-conjugated dUTP deoxynucleotides (1:1) in reaction buffer (Boehringer Mannheim, Mannheim, Germany) for 60 minutes at 37°C in the dark. After washing in PBS containing 0.1% Triton X-100, cells were stained with 5 µg of propidium iodide (Sigma-Aldrich) and 10 kU of RNAase (Sigma-Aldrich) in 1 ml of PBS overnight at 4°C in the dark. Flow cytometric analysis was performed on a FACS Vantage flow cytometer (Becton Dickinson). Data acquisition and analysis were performed using CELLQuest software (Becton Dickinson). For each sample, 10,000 events were recorded.
Preparation of Nuclear Extract
After 1-hour exposure to ST1481 or topotecan at IC50 concentrations followed by 4, 24, or 72 hours of incubation in drug-free medium, cells (5 x 106) were harvested by trypsinization, washed with PBS, and resuspended in 5 ml of nuclear buffer [100 mM NaCl, 1 mM KH2PO4, 5 mM MgCl2, 1 mM EGTA, 10 mM β-Me, 10% (vol/vol) glycerol, pH 6.4]. After addition of 45 ml of nuclear buffer containing 0.35% (vol/vol) Triton X-100 and 0.5 mM PMSF, the cell suspension was kept on ice for 30 minutes and the nuclei, collected by centrifugation at 1000g for 10 minutes, were washed once with Triton X-100-free nuclear buffer, then incubated for 1 hour at 4°C in lysis buffer [100 mM NaCl, 5 mM KH2PO4, 0.5 mM EGTA, 10 mM β-Me, 10% (vol/vol) glycerol, 10 mM NaHSO3, pH 7.0] containing 0.35 M NaCl in gentle rotation. The lysate was centrifuged at 12,000g for 15 minutes and the protein concentration was determined using the BioRad Protein Concentration Assay (Bio-Rad Laboratories, Hercules, CA). The nuclear protein extract was immediately assayed for topoisomerase I activity and Western blot analysis.
Topoisomerase I Activity Assay
The activity of DNA topoisomerase I was determined by measuring the relaxation of supercoiled pBR322 DNA (Invitrogen Corporation, Carlsbad, CA). The reaction mixture (final volume, 20 µl) containing 20 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 0.1 mM EDTA, 0.5 µg/ml bovine serum albumin (BSA), 150 mM NaCl, and 0.375 of µg pBR322, and different dilutions of nuclear extract was incubated at 37°C for 30 minutes. The reaction was stopped by adding 1% sodium dodecyl sulfate (SDS) and 0.3 mg/ml proteinase K. The samples were loaded on 1% agarose gel in TBE buffer (0.089 M Tris base, 0.089 M boric acid, and 0.002 M EDTA) and were run for 6 hours at 40 V. After staining with 0.5 µg/ml ethidium bromide, the gels were photographed under UV light.
Western Blot Assay
For the determination of topoisomerase I expression, nuclear protein extracts (3 µg) were fractionated onto 8% SDS polyacrylamide gel and transferred onto nitrocellulose membrane. For the analysis of 20S proteasome subunit α2 expression, a total cell lysate (80 µg of protein) was used. Filters were probed overnight with purified mouse antihuman DNA topoisomerase I monoclonal antibody (BD PharMingen, San Diego, CA) or mouse monoclonal antibody against 20S proteasome subunit α2 (Affiniti, Devon, UK). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). A rabbit antiactin antibody was used as control for loading. Antibody binding to the nitrocellulose blots was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech Italia, Cologno Monzese, Italy).
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis of Topoisomerase I Gene
Expression of topoisomerase I gene was analyzed by RT-PCR. Total cellular RNA was isolated by using a commercially available kit (Talent, Trieste, Italy). Two micrograms of RNA was reversed-transcribed into cDNA with the use of oligo(dt) primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen Corporation). To amplify the topoisomerase I transcript, the 5′-atgagtggggaccacctccacaa-3′/5′-ttcattagtcatttcctttctccagt-3′ primers were used. The amplified fragment was 866 bp. PCR conditions were as follows: 95°C, 9 minutes for one cycle; 95°C, 1 minute, 52°C, 1 minute, 72°C, 1 minute for 30 cycles followed by 10-minute extension at 72°C. In the case of β-actin, which was used as a control (actin primers: 5′-gaaactaccttcaactccatc-3′ and 5′-ggcggctccatcctggcctcg-3′), the annealing temperature was 62°C and the amplified product was 300 bp. The amplification products were separated on a 1.5% agarose gel containing ethidium bromide and UV-visualized by using a VDS Image Master (Amersham Pharmacia Biotech Italy). The amplified products were quantified by Imagequant program.
Results
Antitumor Activity Studies
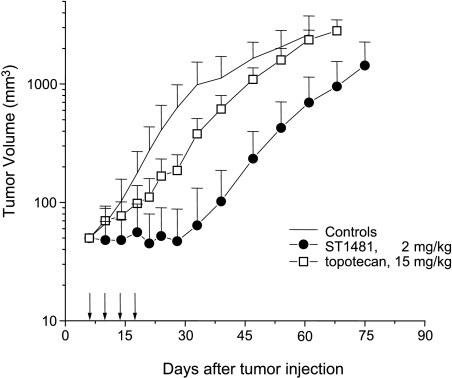
The antitumor activity of the two camptothecins, gimatecan (ST1481) and topotecan, was investigated only in the model HT1376 growing subcutaneously in athymic Swiss nude mice because the MCR cells were tumorigenic in not all mice (Figure 1). This comparative study was performed with the schedule q4d x 4, using optimal doses of each agent (i.e., maximum tolerated doses), chosen on the basis of previous studies [11]. Topotecan exhibited only a moderate efficacy at the optimal oral dose of 15 mg/kg. Using the same schedule, the optimal dose of gimatecan (2 mg/kg) was substantially more effective, causing a marked tumor growth inhibition during treatment. The antitumor effects of the two camptothecins were significantly different (P < .01, 1 week after the end of treatment). The increased inhibition of tumor growth by gimatecan was also reflected in log cell kill values (0.6 vs 1.6, for topotecan versus gimatecan).
Figure 1.
Antitumor activity of topotecan and gimatecan against the human bladder carcinoma xenograft. Treatment per os, every fourth day for four times. Arrows indicated the days of treatment.
Cellular Sensitivity Studies
Table 1 shows the antiproliferative activity of gimatecan determined after different exposure times. Dose-response curves in the range of 3 to 300 ng/ml (not shown) and IC50 values (Table 1) indicated that the growth-inhibitory effect of the drug was dose-dependent and time-dependent. HT1376 cells were more sensitive than MCR cells at least following a short-term exposure. The marginal difference in growth rate did not account for the differential sensitivity. The antiproliferative potency of the drug was substantially higher than that of topotecan used as a reference compound in both cell lines (Table 1). This finding was consistent with the increased antitumor activity of gimatecan.
Table 1.
Cellular Sensitivity to Gimatecan (ST1481) and Topotecan.
| Drug | Exposure Time | IC50 (ng/ml)* | |
| MCR | HT1376 | ||
| Topotecan | 1 | 900 ± 40 | 800 ± 20 |
| ST1481 | 1 | 90 ± 3 | 9.0 ± 0.4 |
| 1† | 81 ± 20† | 20 ± 9† | |
| 24 | 5.0 ± 0.2 | 2.8 ± 0.1 | |
IC50 values were calculated from the dose-response curve.
Cells were exposed to the drug for the indicated times and the antiproliferative effects were determined after incubation in drug-free medium for 72 hours, with the cell counting method or SRB method.
Cell Cycle Analysis and Apoptosis
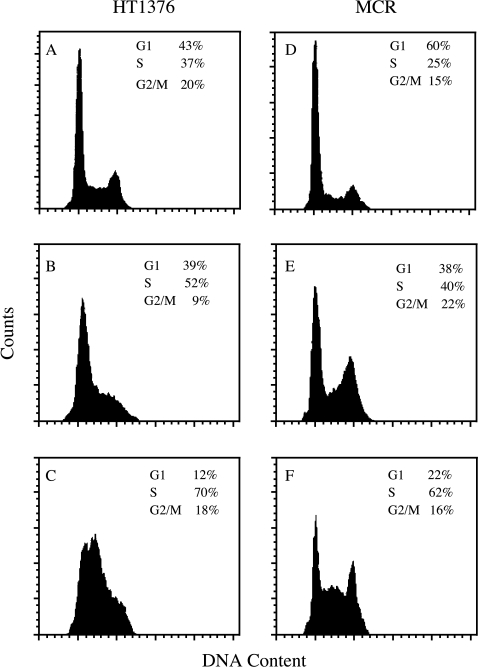
The effects of gimatecan (ST1481) on cell cycle progression were examined in cells treated for 24 hours at two drug levels in the range of antiproliferative concentrations, and then incubated in drug-free medium for 72 hours. At 0.003 µg/ml (approximately IC50), a substantial fraction of cells was found in S-phase, and the number of S-phase cells increased after treatment with a higher concentration (0.03 µg/ml; i.e., IC80) (Figure 2). The cytofluorimetric analysis of TUNEL-positive cells revealed no appreciable amount of apoptotic cells in both cell lines at 72 hours after a 6-hour exposure to 0.03 µg/ml ST1481 (not shown).
Figure 2.
Cell cycle analysis of HT1376 and MCR cells after exposure to gimatecan (ST1481). Cells were exposed to 0.003 µg/ml (approximately IC50) and 0.03 µg/ml (approximately IC80) of ST1481 for 24 hours and processed for cytofluorimetric analysis after 72-hour incubation in drug-free medium. (A and D) Untreated control. (B and E) Cells treated with 0.003 µg/ml. (C and F) Cells treated with 0.03 µg/ml.
Effect on Topoisomerase I Activity and Expression
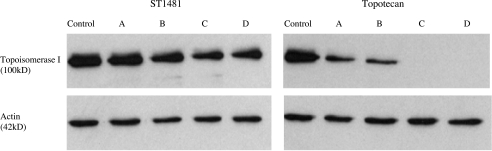
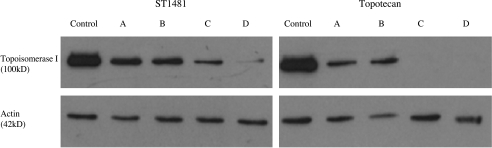
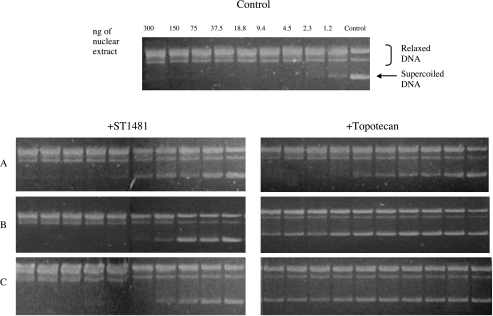
The effect of the exposure to gimatecan (ST1481) and topotecan on topoisomerase I protein level and enzymatic activity was investigated. HT1376 and MCR cells were incubated with equitoxic concentrations (approximately IC50) of ST1481 or topotecan for 1 hour. The drugs were then removed from the culture medium and, after 4, 24, and 72 hours, Western blot and topoisomerase I activity assays of nuclear extracts were performed. The immunoblots displayed a decrease in topoisomerase I protein level after drug treatment. As shown in Figures 3 and 4, ST1481-treated and topotecan-treated cells revealed a different pattern of modulation of topoisomerase I level. Topoisomerase I reduction was more marked after exposure to topotecan in both cell lines. A 1-hour treatment with topotecan strongly reduced the protein level both in HT1376 and MCR cells, with the reduction being evident also after 4 hours of incubation of drug-free medium. A complete downregulation of topoisomerase I was observed 24 and 72 hours after drug removal. In contrast, when cells were treated with ST1481, the disappearance of topoisomerase I was slower. Indeed, the protein was still detected in the nuclear extracts of HT1376 cells after 72 hours of incubation in drug-free medium. We therefore evaluated the effect of ST1481 and topotecan on topoisomerase I enzyme activity (Figures 5 and 6). The decreased topoisomerase I level in topotecan-treated cells resulted in a dramatic reduction of enzyme activity. Indeed, no activity was found in the nuclear extracts of HT1376 and MCR cells treated with topotecan after 24 and 72 hours of incubation in drug-free medium. The reduction of the enzymatic activity was greater in topotecan-treated than in ST1481-treated cells. The nuclear extract from MCR cells revealed the presence of enzymatic activity 4 and 24 hours after drug removal, whereas nuclear extract from HT1376 cells was still active after 72 hours of incubation in ST1481-free medium. Thus, the results of the enzyme activity assays revealed a good correlation with the analysis of protein levels.
Figure 3.
Effect of gimatecan (ST1481) or topotecan treatment on topoisomerase I expression of HT1376 cell line. Nuclear protein extract was obtained from cells treated with ST1481 (0.01 µg/ml) or topotecan (1 µg/ml) for 1 hour, immediately after treatment (A) or after 4 hours of washout (B), or after 24 hours of washout (C), or after 72 hours of washout (D). Equal amounts of nuclear protein (3 µg) were resolved on 8% SDS polyacrylamide gel, blotted onto nitrocellulose membrane, and probed with antihuman DNA topoisomerase I monoclonal antibody. The bands of topoisomerase I were visualized using chemiluminescence system. Actin was used as a control of protein loading.
Figure 4.
Effect of gimatecan (ST1481) or topotecan treatment on topoisomerase I expression in MCR cell line. Nuclear protein extract was obtained from cells treated with ST1481 (0.1 µg/ml) or topotecan (1 µg/ml) for 1 hour, immediately after treatment (A), or after 4 hours of washout (B), or after 24 hours of washout (C), or after 72 hours of washout (D). Equal amounts of nuclear protein (3 µg) were resolved on 8% SDS polyacrylamide gel, blotted onto nitrocellulose membrane, and probed with antihuman DNA topoisomerase I monoclonal antibody. The bands of topoisomerase I were visualized using chemiluminescence system. Actin was used as a control of protein loading.
Figure 5.
Modulation of topoisomerase I activity by gimatecan (ST1481) or topotecan in HT1376 cell line. Cells were treated with ST1481 (0.01 µg/ml) or topotecan (1 µg/ml) for 1 hour. After 4 hours (A), 24 hours (B), and 72 hours (C) of incubation in drug-free medium, nuclear protein was extracted from 5 x 106 cells as indicated in Material and Methods section. Supercoiled plasmid DNA (375 ng) was unwinded by 30-minute incubation at 37°C with adequately diluted nuclear protein extract and resolved on 1% agarose gel. Arrow: Bands of supercoiled plasmid DNA. Bracket: Bands of plasmid DNA unwinded by topoisomerase I. Figures are representatives of at least three experiments.
Figure 6.
Modulation of topoisomerase I activity by gimatecan (ST1481) or topotecan in MCR cell line. Cells were treated with ST1481 (0.1 µg/ml) or topotecan (1 µg/ml) for 1 hour. After 4 hours (A), 24 hours (B), and 72 hours (C) of incubation in drug-free medium cells, nuclear protein was extracted from 5 x 106 cells as indicated in Materials and Methods section. Supercoiled plasmid DNA (375 ng) was unwinded by 30-minute incubation at 37°C with adequately diluted nuclear protein extract and resolved on 1% agarose gel. Arrow: Bands of supercoiled plasmid DNA. Bracket: Bands of plasmid DNA unwinded by topoisomerase I. Figures are representatives of at least three experiments.
Expression of the 20S Proteasome Subunit α2 and Effect of a Proteasome Inhibitor
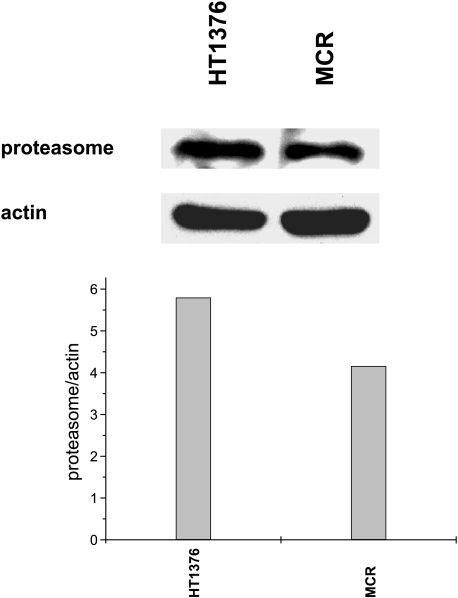
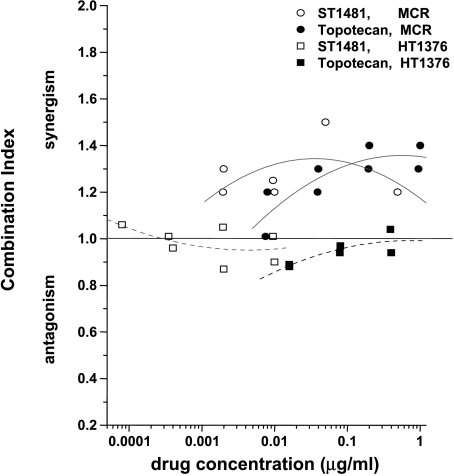
Camptothecin-induced downregulation of topoisomerase I has been ascribed to degradation of the enzyme by a ubiquitin/26S proteasome pathway [12]. The different responses of HT1376 and MCR cells to ST1481 did not reflect a different expression of the proteasome itself (Figure 7). Indeed, in spite of an increased downregulation of topoisomerase I in treated MCR cells, the level of proteasome was somewhat lower than in HT1376 cells. In an attempt to provide indirect evidence that, in bladder carcinoma cells, the downregulation of topoisomerase I reflects degradation of the enzyme by a ubiquitin/26S proteasome pathway, we studied the effect of the proteasome inhibitor, PS341, on the antiproliferative activity of both camptothecins (Figure 8). Only a moderate potentiation of camptothecin activity was observed in MCR cells, when cells were exposed to subtoxic concentration of PS341 for 72 hours after 1-hour exposure to the camptothecin. No potentiation was observed in HT1376 cells. The lack of synergistic effects of camptothecins and proteasome inhibitors suggests that the downregulation of topoisomerase I is not directly related to downstream events involving the proteasome-mediated degradation of the enzyme.
Figure 7.
Western blot analysis of the proteasome in HT1376 and MCR cells. The relative expression level of the proteasome subunit α2 was normalized with respect to actin.
Figure 8.
Interaction between gimatecan (ST1481) or topotecan and PS341. Antiproliferative activity of the combination was determined after 1-hour exposure to the camptothecin and 72-hour exposure to PS341 using SRB assay. Dose-response curves for each camptothecin were determined in the presence of subtoxic concentrations of PS341 (i.e., 0.001 µg/ml; i.e., under conditions that did not produce antiproliferative effects of the proteasome inhibitor). The combination index was calculated according to the method of Skehan et al. [9].
Effect on Topoisomerase I Transcription
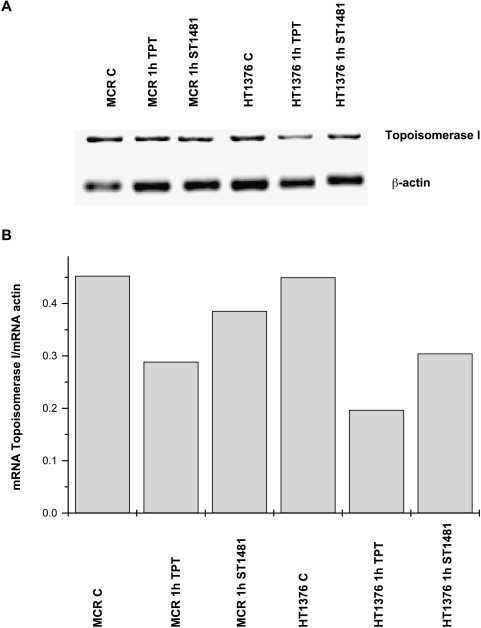
The effect of exposure to ST1481 and topotecan on topoisomerase I mRNA level was investigated in HT1376 and MCR cells incubated with 0.01 and 0.1 µg/ml ST1481, respectively, and with 1 µg/ml topotecan for 1 hour. After RNA extraction, RT-PCR analysis was performed by using specific primers. As shown in Figure 9, a similar pattern of drug-induced topoisomerase I mRNA decrease was observed in both cell lines. However, HT1376 cells were more sensitive and topotecan induced a greater mRNA reduction in both cell lines.
Figure 9.
RT-PCR analysis of topoisomerase I in MCR and HT1376 cell lines treated with gimatecan (ST1481) and topotecan. (A) PCR products with primers for topoisomerase I and β-actin are shown. After PCR, the amplification products were separated on a 1.5% agarose gel. (B) Expression levels of topoisomerase I after 1-hour exposure to ST1481 (0.1 µg/ml for MCR and 0.01 µg/ml for HT1376) and topotecan (1 µg/ml for both cell lines). The amplified products were quantified and the gene expression was normalized with respect to actin.
Discussion
The bladder carcinoma models used in the present study are characterized by a slow proliferation rate. Slowly growing tumors are expected to be relatively resistant to camptothecin treatment. Indeed, HT1376 tumor xenografts exhibited a marginal responsiveness to topotecan. In contrast, the effect of gimatecan was remarkable because, using the same intermittent treatment schedule, it produced a significant tumor growth inhibition (93%). This level of activity was achieved in spite of an apparent cytostatic response and lack of apoptosis of the HT1376 observed in vitro. A lack of correlation between an early apoptotic response and in vivo efficacy of gimatecan has been already observed in the human prostate carcinoma model PC3 and has been ascribed to a delayed apoptosis following a persistent arrest in G2 [13]. In other cell lines, we have found that S-phase arrest is associated with a relative resistance to gimatecan [8]. It is conceivable that the activation of the S-phase checkpoint reflects a protective mechanism to prevent the generation of double-strand breaks rather than a manifestation of the presence of lethal lesions. Indeed agents that perturb cell progression through S-phase reduce the effectiveness of camptothecins [14]. The results of the present work and previous observations in other cells support our interpretation and could account for a lack of apoptosis. However, because multiple signals regulate apoptosis and cell cycle, the cell propensity to undergo apoptosis may be cell type-specific [15].
The present study also provides evidence of a marked increase of the antiproliferative and antitumor potency of gimatecan over topotecan. Previous studies have indicated a peculiar behavior of gimatecan at cellular and molecular levels [16]. The lipophilic nature of gimatecan accounts for the substantially increased cellular accumulation compared to topotecan [13,17]. In addition, the preferential subcellular localization of gimatecan in lysosomes could allow the release of an active lactone form of the drug because the acidic environment of lysosomes may favor the stabilization of the closed lactone [18]. In addition to the favorable cellular pharmacokinetics, gimatecan is a potent inhibitor of topoisomerase I and produces a persistent stabilization of the DNA-enzyme-cleavable complex [11,19]. The present study may suggest an additional mechanism involved in antitumor potency and efficacy. Indeed, the available results obtained in both bladder carcinoma models indicated that topotecan induced a more marked downregulation of topoisomerase I compared to gimatecan. The gimatecan-induced downregulation was more pronounced in MCR cells than in HT1376 cells, and may be consistent with the increased sensitivity of HT1376 to gimatecan. This event is regarded as an important determinant of sensitivity/resistance of tumor cells to camptothecins and has been ascribed to a process downstream from the topoisomerase I-DNA-cleavable complex mediated by ubiquitin/26S proteasome [12]. Because 26S proteasome inhibitors are expected to sensitize tumor cells to camptothecins [12], we studied the effect of PS341 as a specific inhibitor in combination with both camptothecins. The results indicated a moderate potentiation of both agents by PS341 only in MCR cells, thus suggesting the contribution of alternative mechanisms of topoisomerase I downregulation. The downregulation of topoisomerase I, following treatment with topoisomerase I inhibitors, appears to be a heterogeneous phenomenon involving upstream and downregulation processes [12,20,21]. Indeed, we have found an early inhibition of topoisomerase I transcription induced by both camptothecins. This effect was more pronounced in topotecan-treated cells. The effects of topotecan and gimatecan on downregulation of the enzyme protein in HT1376 paralleled the differential effect of the two agents at mRNA levels. This finding and the lack of sensitization by the proteasome inhibitor PS341 suggest that, in HT1376 cells, the topoisomerase I downregulation is primarily the result of the inhibition of transcription. In contrast, in MCR cells, the downregulation of topoisomerase I was likely the contribution of drug effects at both mRNA level and downstream level. Gimatecan produced a limited effect in both events, resulting in a reduced downregulation of the target. A plausible the differential effect of the two camptothecins could be related to the increased specificity of gimatecan for the target enzyme. Indeed, as a consequence of the inhibitory potency [19], a lower number of drug molecules could be required to produce topoisomerase I-mediated DNA damage. Because the inhibition of transcription is expected to involve several genes, the increased potency and specificity of gimatecan for the target enzyme could spare aspecific cellular effects caused by arrest of transcription. In addition, the stability of the covalent DNA-enzyme complex [19] could result in a slower 26S proteasome-mediated degradation of topoisomerase I. Regardless of the mechanisms involved, the evidence of a limited impact of gimatecan on the topoisomerase I levels provides novel insights into the cellular basis of the drug efficacy because the modulation of the target by camptothecins may affect the drug sensitivity/resistance of tumor cells. Indeed, camptothecin-induced downregulation of topoisomerase I has been proposed as a resistance mechanism [12]. The differential effect of gimatecan and topotecan on topoisomerase I level represents a potential therapeutic advantage because optimal treatment with camptothecins requires protracted administration.
In conclusion, this study provides evidence that gimatecan was effective also against a slowly growing tumor and very potent both in vitro and in vivo, in spite of an apparent cytostatic effect and lack of apoptosis. Multiple events, including antiangiogenic effects [22], and the persistence of gimatecan effects may contribute to the potency and antitumor efficacy of the drug [16]. The present study, showing a mild downregulation of topoisomerase I level and a mild inhibition of transcription by gimatecan, may suggest that these events could contribute to drug antitumor specificity because the inhibition of gene expression is expected to cause severe toxicity [21].
Acknowledgement
The authors are grateful to Rosella Silvestrini for the helpful discussion.
Footnotes
This work was partially supported by the Associazione Italiana Ricerca sul Cancro, Milan; the Ministero della Salute, Rome; and the CNR-MIUR, Rome, Italy.
References
- 1.Zunino F, Pratesi G. Camptothecins in clinical development. Exp Opin Invest Drugs. 2004;13:269–284. doi: 10.1517/13543784.13.3.269. [DOI] [PubMed] [Google Scholar]
- 2.Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003;361:2235–2242. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 3.Zunino F, Dallavalle S, Laccabue D, Beretta GL, Merlini L, Pratesi G. Current status and perspectives in the development of camptothecins. Curr Pharm Des. 2003;8:2505–2520. doi: 10.2174/1381612023392801. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: current perspectives. Bioorg Med Chem. 2004;12:1585–1604. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Pommier Y, Pourquier P, Fan Y, Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:83–106. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP. Mechanism of action of camptothecin. In: Liehr JG, Giovanella BC, Verschraegen CF, editors. The Camptothecins. Unfolding Their Anticancer Potential. Vol. 922. New York Academy of Sciences; 2000. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Darzynkiewicz Z, Bruno S, Del Bino G, Traganos F. The cells cycle effects of camptothecin. In: Pantazis P, Giovanella BC, Rothenberg ML, editors. The Camptothecins. From Discovery to the Patients. Vol. 803. New York Academy of Sciences; 1996. pp. 93–110. [DOI] [PubMed] [Google Scholar]
- 8.Pratesi G, De Cesare M, Carenini N, Perego P, Righetti SC, Cucco C, Merlini L, Penco S, Carminati C, et al. Pattern of antitumor activity of a novel camptothecin, ST1481, in a large panel of human tumor xenografts. Clin Cancer Res. 2002;8:3904–3909. [PubMed] [Google Scholar]
- 9.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 10.Drewinko B, Loo TL, Brown JA, Gottlieb JA, Freireich EJ. Combination chemotherapy in vitro with adriamycin. Observations of additive, antagonistic, and synergistic effects when used in two-drug combination on cultured human lymphoma cells. . Cancer Biochem Biophys. 1976;1:187–195. [PubMed] [Google Scholar]
- 11.De Cesare M, Pratesi G, Perego P, Carenini N, Tinelli S, Merlini L, Penco S, Pisano C, Bucci F, Vesci L, et al. Potent antitumor activity and improved pharmacological profile of ST1481, a novel 7-substituted camptothecin. Cancer Res. 2001;61:7189–7195. [PubMed] [Google Scholar]
- 12.Desai SD, Li T-K, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61:5926–5932. [PubMed] [Google Scholar]
- 13.Zuco V, Supino R, De Cesare M, Carenini N, Perego P, Gatti L, Pratesi G, Pisano C, Martinelli R, Bucci F, et al. Cellular bases of the antitumor activity of a 7-substituted camptothecin in hormone-refractory human prostate carcinoma models. Biochem Pharmacol. 2003;65:1281–1294. doi: 10.1016/s0006-2952(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand R, O'Connor PM, Kerrigan D, Pommier Y. Sequential administration of camptothecin and etoposide circumvents the antagonistic cytotoxicity of simultaneous drug administration in slowly growing human colon carcinoma Ht-29 cells. Eur J Cancer. 1992;28:743–748. doi: 10.1016/0959-8049(92)90107-d. [DOI] [PubMed] [Google Scholar]
- 15.Miele L. Regulation of cell cycle and apoptosis. In: Giordano A, Soprano KJ, editors. Cancer Drug Discovery and Development: Cell Cycle Inhibitors in Cancer Therapy: Current Strategies. Totowa, NJ: Humana Press Inc.; 2003. pp. 83–118. [Google Scholar]
- 16.Pratesi G, Beretta GL, Zunino F, Gimatecan F. A novel camptothecin with a promising preclinical profile. Anticancer Drugs. 2004;15:545–552. doi: 10.1097/01.cad.0000131687.08175.14. [DOI] [PubMed] [Google Scholar]
- 17.Perego P, De Cesare M, De Isabella P, Carenini N, Beggiolin G, Pezzoni G, Palumbo M, Tartaglia L, Pratesi G, Pisano C, et al. A novel 7-modified camptothecin analog overcomes breast cancer resistance protein-associated resistance in a mitoxantrone-selected colon carcinoma cell line. Cancer Res. 2001;61:6034–6037. [PubMed] [Google Scholar]
- 18.Croce AC, Bottiroli G, Supino R, Favini E, Zuco V, Zunino F. Subcellular localization of the camptothecin analogues, topotecan and gimatecan. Biochem Pharmacol. 2004;67:1035–1045. doi: 10.1016/j.bcp.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Dallavalle S, Ferrari A, Biasotti B, Merlini L, Penco S, Gallo G, Marzi M, Tinti MO, Martinelli R, Pisano C, et al. Novel 7-oxyiminomethyl derivatives of camptothecin with potent in vitro and in vivo antitumor activity. J Med Chem. 2001;44:3264–3274. doi: 10.1021/jm0108092. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi K, Kohno K, Kawanami K, Wada M, Kanematsu T, Kuwano M. Drug-induced down-regulation of topoisomerase I in human epidermoid cancer cells resistant to saintopin and camptothecins. Cancer Res. 1996;56:2348–2354. [PubMed] [Google Scholar]
- 21.Desai SD, Zhang H, Rodriguez-Bauman A, Yang JM, Wu X, Gounde MK, Rubin EH, Liu LF. Transcription-dependent degradation of topoisomerase I-DNA covalent complexes. Mol Cell Biol. 2003;23:2341–2350. doi: 10.1128/MCB.23.7.2341-2350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrangolini G, Pratesi G, De Cesare M, Supino R, Pisano C, Marcellini M, Giordano V, Laccabue D, Lanzi C, Zunino F. Antiangiogenic effects of the novel camptothecin ST1481 (Gimatecan) in human tumor xenografts. Mol Cancer Res. 2003;1:863–870. [PubMed] [Google Scholar]