Abstract
An unselected series of 310 colorectal carcinomas, stratified according to microsatellite instability (MSI) and DNA ploidy, was examined for mutations and/or promoter hypermethylation of five components of the WNT signaling cascade [APC, CTNNB1 (encoding β-catenin), AXIN2, TCF4, and WISP3] and three genes indirectly affecting this pathway [CDH1 (encoding E-cadherin), PTEN, and TP53]. APC and TP53 mutations were each present more often in microsatellite-stable (MSS) tumors than in those with MSI (P < .001 for both). We confirmed that the aneuploid MSS tumors frequently contained TP53 mutations (P < .001), whereas tumors with APC mutations and/or promoter hypermethylation revealed no associations to ploidy. Mutations in APC upstream of codons 1020 to 1169, encoding the β-catenin binding site, were found in 15/144 mutated tumors and these patients seemed to have poor clinical outcome (P = .096). Frameshift mutations in AXIN2, PTEN, TCF4, and WISP3 were found in 20%, 17%, 46%, and 28% of the MSI tumors, respectively. More than half of the tumors with heterozygote mutations in AXIN2 were concurrently mutated in APC. The present study showed that more than 90% of all samples had alteration in one or more of the genes investigated, adding further evidence to the vital importance of activated WNT signaling in colorectal carcinogenesis.
Keywords: WNT signaling, colorectal cancer, genomic instability, mutation, hypermethylation
Introduction
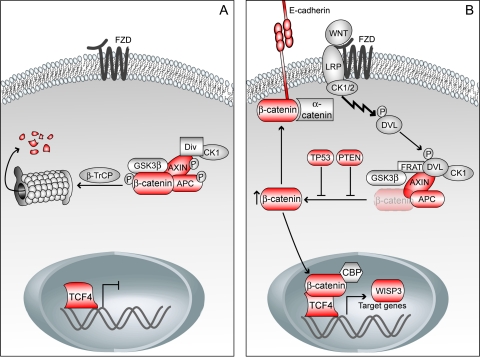
The Wingless-type MMTV integration site family (WNT) signaling cascade plays a vital role in embryogenesis, and its deregulation is also implicated in carcinogenesis. This pathway has mainly been uncovered through studies of Drosophila melanogaster, but is highly conserved among several organisms including mammalians [1]. β-Catenin is the key component of the canonical WNT pathway and is regulated by a multiprotein complex consisting of, among other proteins, adenomatous polyposis coli (APC), AXIN, and glycogen synthase kinase-3β (GSK-3β). In the absence of a WNT signal, β-catenin is initially primed, followed by further phosphorylation, leading to proteasomal degradation [2] (Figure 1A). In the presence of WNT stimulation, proper assembly of the multiprotein complex is inhibited, causing accumulation of free cytosolic β-catenin, which translocates into the nucleus, leading to transcription of downstream target genes [3] (Figure 1B). Additionally, free cytosolic β-catenin might form a complex with E-cadherin and α-catenin and participate in calcium-dependent cell-cell adhesion [4].
Figure 1.
(A) In the absence of a WNT signal, a multiprotein complex consisting of β-catenin, APC, AXIN, GSK-3β, diversin (Div), and CK1 is formed, leading to phosphorylation and subsequent proteosomal degradation of β-catenin. (B) In the presence of a WNT signal, the multiprotein complex is not formed properly and β-catenin remains unphosphorylated and accumulates in the cytoplasm. Free cytosolic β-catenin translocates into the nucleus, leading to transcription of downstream target genes. It might also form a complex with E-cadherin and α-catenin and participate in cell-cell adhesion. The tumor-suppressor proteins TP53 and PTEN indirectly inhibit activation of the WNT signaling pathway. Components with red color are investigated in the present study. β-TrCP, β-transducin repeat-containing protein; CBP, CREB-binding protein; DVL, dishevelled; FRAT, frequently rearranged in advanced T-cell lymphomas; FZD, frizzled; LRP, low-density lipoprotein receptor-related protein.
Inactivation of the tumor-suppressor gene APC, a key regulator of the WNT signaling pathway, is one of the earliest transforming events observed in colorectal tumorigenesis [5]. APC is altered, by DNA sequence changes and/or by promoter hypermethylation, in most colorectal carcinomas [6,7]. As well as being part of the multiprotein complex targeting β-catenin for proteolysis, APC interacts with nuclear β-catenin, reducing its transcriptional activity and enhancing its nuclear export [8]. Furthermore, it was recently shown that APC could bind the microtubule-associated protein EB1, suggesting a potential effect of APC also on chromosome segregation [9,10]. A subgroup of colorectal tumors with wild-type APC shows oncogenic mutations in CTNNB1, the gene encoding β-catenin [11]. These mutations mainly affect specific serine and threonine residues within exon 3 of CTNNB1, the phosphorylation sites for the kinases casein kinase 1(CK1) and GSK-3β, and thereby impede its degradation [11]. As mentioned, β-catenin binds to E-cadherin and thereby influences the cell-cell contact. Hypermethylation of the promoter region of CDH1, the gene encoding E-cadherin, has been observed in a subgroup of colorectal carcinomas [12], giving rise to increased level of free cytosolic β-catenin. However, this change alone is not enough to activate the WNT signaling pathway [13,14].
Two AXIN gene family members have been identified in humans, AXIN1 and AXIN2, and both genes show mutations in some colorectal carcinomas with wild-type APC and CTNNB1 [15,16]. Recently, it was observed that activation of the WNT signaling pathway elevated the level of AXIN2, implicating AXIN2 in a negative feedback loop controlling WNT signaling [17,18].
Accumulation of β-catenin induces stabilization of the tumor-suppressor protein TP53. TP53 enhances degradation of β-catenin through a negative feedback mechanism [19]. This process was recently shown to require both CK1 and GSK-3β, indicating that TP53 induces phosphorylation of β-catenin prior to degradation [19]. Thus, in this manner, the mutation status of TP53 is important for the activity along the WNT cascade.
Similarly, PTEN influences the WNT pathway by hindering the activation of integrin-linked kinase (ILK), which inhibits GSK-3β and thereby causes accumulation of β-catenin [20].
Two types of genomic instability have been observed in colorectal carcinogenesis, microsatellite instability (MSI; also known as MIN) and chromosome instability (CIN) [21]. MSI is seen as a phenotypic trait in tumors of hereditary nonpolyposis cases and in a subgroup of sporadic carcinomas, both with defect mismatch repair (MMR). However, most colorectal carcinomas show losses or gains of whole, or parts of, chromosomes and thus exhibit CIN. However, whether an underlying instability process drives the tumor development or not is a subject for ongoing debate [22]. Distinct pathologic features characterize these two tumor groups (MSI and CIN). The MSI tumors, which account for 10% to 15% of all colorectal carcinomas, are associated with mucinous histology, lymphocytic infiltration, diploidy, poor differentiation, and location in the proximal colon [23]. The CIN tumors are typically microsatellite-stable (MSS), aneuploid, and located in the distal colon or in the rectum [21]. Furthermore, epigenetic changes are observed in subgroups of both MSI and CIN colorectal carcinomas [24].
Tumors with defect MMR preferentially accumulate changes in repetitive sequences. In the WNT pathway, genes such as TCF4, WISP3, and PTEN all harbor repetitive sequences within their coding region, and are reported mutated in MSI colorectal tumors [25–27].
Several genes, being part of or indirectly affecting the WNT signaling pathway, have previously been analyzed in colorectal tumors. However, the series has been small and only a few components have been examined within the same sample set. Therefore, we addressed this issue further by mutation and methylation analyses of eight relevant genes in a series of more than 300 Norwegian colorectal cancer (CRC) patients. The individual gene status as well as their combinations were evaluated in the MSI and MSS tumors and related to clinicopathologic data including long-term survival data.
Materials and Methods
Materials and MSI Status
A consecutive series of primary tumors from 310 CRC patients, 158 males and 151 females, collected from seven hospitals in the Oslo and Akershus region between 1987 and 1989 was included. The median age at diagnosis was 68.3 years (range 24–92 years). From one patient, clinical data were missing. Surgery was given as the curative treatment for all patients, except for a few patients with rectal tumors who also received postoperative radiation therapy. The survival time was recorded from the date of surgery until death or until the last update (July 1, 1999). Patients who died within 30 days after resection of the tumor were censored.
Determination of the content of tumor cells in our series has previously been described [28], ranging from 62% to 97% with a mean of 84%.
The MSI status was determined by analyzing 19 dinucleotide markers as well as two mononucleotide markers (BAT25 and BAT26) [26,29,30]. Thirty-eight tumors, from 36 patients, were characterized as MSI-high (H), 33 tumors were MSI-low (L), and 237 tumors were MSS. From four tumors, two biopsies were analyzed and showed discordant MSI status. Both in the present study and in previously published studies [31], it has been shown that the MSI-L and MSS tumors are comparable when it comes to genetic changes as well as clinicopathologic parameters, and are, therefore, throughout the rest of the paper, considered as one group—the MSS group. The majority of the MSI tumors was diploid (89%, 32/36) and located in the proximal colon (cecum, ascendens, and right flexure) (69%, 25/36), whereas the MSS tumors were aneuploid (68%, 134/269) and located in the distal colon (left flexure, descendens, and sigmoideum) or rectum (75%, 203/269). For two of the MSI tumors and one of the MSS tumors, clinicopathologic data were not available.
Mutation Screening of APC, TP53, and CTNNB1
We have previously investigated APC exon 15 by using the protein truncation test (PTT) and 144/218 tumors showed mutation in exon 15 of APC [38]. In the present study, six additional tumors (10 biopsies) were analyzed using the same method. Shortly, genomic DNA was amplified by polymerase chain reaction (PCR), and a T7 promoter sequence and a mammalian initiation sequence in-frame with a unique APC sequence were introduced. The PCR product was subjected to coupled transcription and translation in an in vitro assay. Mutations causing premature termination in the protein product showed altered mobility during gel electrophoresis.
Mutations in TP53 exons 5 to 8 were previously searched for by constant denaturant gradient electrophoresis (CDGE). By this protocol, we found 105 mutations in 222 tumor samples [39]. In the present study, an additional 46 cases were examined by temporal temperature gradient electrophoresis (TTGE), a method similar to CDGE but with some modifications. The procedure and primers have been described [40].
All aberrant migration bands detected by PTT or CDGE/TTGE were submitted to direct sequencing on an Applied Biosystems 373 or 377 DNA sequencer (Perkin Elmer, Foster City, CA) (Refs. [38,39] and the present study).
CTNNB1 exons 3, 5, 6, 7, and 8 have previously been analyzed by single-strand conformation polymorphism and sequencing in 218 of the tumors included in this study [38].
Sequence Alterations of AXIN2
Exon 7 of AXIN2, containing four mononucleotide repeats (A)6, (G)7, (C)5, and (C)6, was sequenced in the MSI tumors. The following primer set was used: forward 5′-AAC CCA GTT TCT TTC CTT CT-3′ and reverse 5′-ATC CCT GCC TCA ACC TA-3′ (Prof. Wanguo Liu, personal communication) (DNA Technology AS, Aarhus, Denmark). Standard PCR conditions and direct sequencing of purified PCR products (Microspin S300-HR columns; Amersham Pharmacia Biotech) using the Thermo-Sequenase cycle sequencing kit (Amersham Pharmacia Biotech) were performed according to the manufacturer's protocol on an ABI PRISM 310 Genetic Analyzer (Perkin Elmer).
Frameshift Mutation Analysis of PTEN, TCF4, and WISP3
Two (A)6 repeats within the coding region of PTEN were analyzed in the MSI tumors and the following primer sets were applied: forward 5′-CCT GTG AAA TAA TAC TGG TAT G-3′ and reverse 5′-GTT TCT TCT CCC AAT GAA AGT AAA GTA CA-3′ (exon 7) and forward 5′-GTG CAG ATA ATG ACA AGG AAT A-3′ and reverse 5′-GTT TCT TAC ACA TCA CAT ACA TAC AAG TC-3′ (exon 8) [32] (DNA Technology AS). A seven-base tail (GTT TCT T) was added to each reverse primer to avoid a plus A artifact [33]. Both forward primers were end-labeled with a fluorochrome and the two (A)6 repeats were amplified in multiplex PCR and analyzed on an ABI PRISM 310 Genetic Analyzer under conditions previously described [26]. Abnormal PCR products were confirmed with a new PCR and the mutations were verified by sequencing.
TCF4 with an (A)9 repeat in exon 17 and WISP3 with an (A)9 repeat in exon 4 have formerly been analyzed [26]. However, five novel cases were included in the present study.
Methylation-Specific PCR (MSP) of APC and CDH1
Fifty-three colorectal tumor samples, consisting of 28 MSI and 25 MSS tumors, were analyzed for promoter hypermethylation of APC and CDH1 by MSP [34]. Shortly, MSP distinguishes unmethylated from methylated alleles of a given gene based on sequence alterations produced by bisulfite treatment of DNA, which converts unmethylated but not methylated cytosine to uracil. Subsequent PCR using primers specific to either methylated or unmethylated DNA was then performed. Previously reported primer sets were used for amplification of both the APC fragments [35] and the CDH1 fragments [36] (Medprobe AS, Oslo, Norway). Tumor DNA was treated with bisulfite and amplified as previously described [37]. Methylation-positive samples were scored visually by two of the authors (L.T., G.E.L.) as “+” (weakly methylated) or “++” (heavily methylated). All samples interpreted as methylated were confirmed by an additional PCR. In the association studies, only tumors showing heavily methylation were included as positive samples.
Statistical Analyses
Pearson's chi-square analysis or Fisher's exact test was used in the comparison among different groups. Cause-specific survival analyses (death by CRC) were accomplished using the Kaplan-Meier method and the long rank test was applied to determine the differences in survival. P < .05 was considered as statistically significant. SPSS software (SPSS, Chicago, IL) was used for all the analyses. For hierarchical cluster analysis, the average linkage method was used with Pearson's correlation similarity measure. The cluster analysis and drawing of the dendogram were performed with J-Express Pro [41].
Results
APC, CTNNB1, and TP53 Mutations in MSS and MSI Tumors
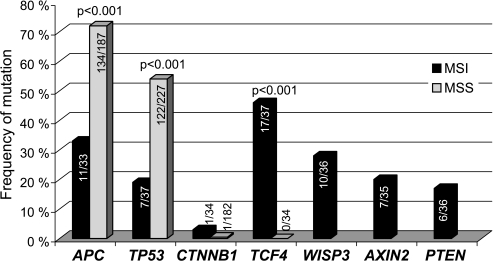
The mutation frequencies of the genes investigated are presented in Figure 2. APC and TP53 mutations were each present more often in the MSS tumors than in the MSI tumors (P < .001 for both) (Table 1). All but one of the 11 MSI tumors with mutation in APC were wild-type TP53, whereas 54% (72/134) of the MSS tumors harbored mutations in both APC and TP53 (P = .005). Interestingly, the majority of APC mutations in the MSI tumors (75%) occurred outside the mutation cluster region (MCR; codons 1286–1514); in contrast, 84% of the mutations in the MSS tumor group were within the MCR (P < .001). Sixteen of 145 (11%) of the MSI and MSS tumors with APC mutation showed two mutations within this gene.
Figure 2.
The mutation frequencies of the seven genes investigated in colorectal carcinomas with different MSI status. WISP3, AXIN2, and PTEN were only analyzed in the MSI tumors. MSI, microsatellite-instabile; MSS, microsatellite-stable.
Table 1.
APC and TP53 Status in Correlation with MSI Status.
| APC and TP53 Status | MSI (n = 38) | MSS (n = 269) | P |
| APC* | |||
| mut (n = 145) | 11 | 134 | <.001 |
| wt (n = 75) | 22 | 53 | |
| TP53† | |||
| mut (n = 129) | 7 | 122 | <.001 |
| wt (n = 135) | 30 | 105 | |
| APC and TP53‡ | |||
| mut and mut (n = 73) | 1 | 72 | .005 |
| mut and wt (n = 72) | 10 | 62 | |
MSI, microsatellite-instable; MSS, microsatellite-stable; mut, mutation; wt, wild type.
APC mutation status was not known in 4 MSI and 82 MSS tumors; from one MSI tumor, a different APC status was detected in the three biopsies analyzed.
TP53 mutation status was not determined in 42 MSS tumors; in one MSI tumor two, biopsies showed discordant TP53 status.
Only tumors with mutation in APC were included in the table.
CTNNB1 was altered in one MSI and one MSS tumor [38]. This MSI tumor was simultaneously altered in TCF4 and WISP3, whereas the MSS tumor additionally showed mutation in APC and TP53.
Neither APC nor TP53 mutations were associated with CIN in the MSI tumor group (Table 2). This was also true when the methylated APC tumors were included (P = .447). However, a correlation between TP53 mutations and CIN was observed in the MSS tumors (P < .001).
Table 2.
APC and TP53 Mutations in Relation to MSI Status and DNA Content.
| MSI Status and DNA Content | APC mut* | APC wt | P | TP53 mut† | TP53 wt | P |
| MSI (n = 36) | ||||||
| CS‡ | 9 | 21 | ns§ | 5 | 28 | ns |
| CIN§ | 1 | 0 | 0 | 2 | ||
| MSS (n = 269) | ||||||
| CS | 59 | 26 | ns | 37 | 57 | <.001 |
| CIN | 75 | 27 | 85 | 48 | ||
MSI, microsatellite-instable; MSS, microsatellite-stable; mut, mutation; wt, wild type; ns, not significant.
APC mutation status was not known in 4 MSI and 82 MSS tumors; from one MSI tumor, a different APC status was detected in the three biopsies analyzed.
TP53 mutation status was not determined in 42 MSS tumors; in one MSI tumor, two biopsies showed discordant TP53 status.
CS, chromosome-stable (2n [flow cytometry; <1.1] and 4n [flow cytometry; 1.9–2.1]).
CIN, chromosome-instable (all other DNA content).
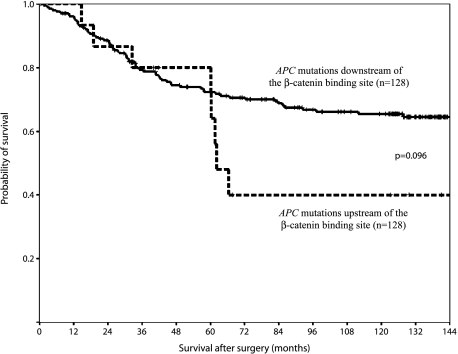
APC mutations residing at the 5′ end of codons 1020 to 1169, encoding the β-catenin binding site (the three 15 amino acid repeats), were seen in 17 tumors, including nine frameshift, six nonsense, and two missense mutations (detected because these two tumors also contained a frameshift mutation located downstream of codons 1020–1169). An early truncation in the APC protein was thus observed in 15 of the patients, and they showed a tendency to shorter cancer-related survival than the ones with APC mutations downstream of the codons encoding the β-catenin binding site (n = 128) (P = .096) (Figure 3). The same was seen when the wild-type tumors (n = 78) were grouped together with the ones with mutations downstream of codons 1020 to 1169 (P = .13).
Figure 3.
Patients with tumors containing APC mutation upstream of codons 1020 to 1169, encoding the β-catenin binding site (the three 15 amino acid repeats), showed a shorter cancer-related survival than those with tumors harboring mutations downstream of codons 1020 to 1169.
We confirmed that patients with mutations affecting the L3 domain of TP53 (codons 236–251) had a significantly shorter cancer-related survival versus patients with wild-type TP53 or other mutations (P = .02). Patients with mutations upstream of codons 1020 to 1169 in APC and/or affecting the L3 domain of TP53 showed a tendency toward shorter cancer-related survival than the patients with other mutations or no mutations in these genes (P = .088). A synergistic effect of early APC mutation and those affecting the zinc binding domain (L3) of TP53 was not observed; however, only four tumors contained both mutations.
Frameshift Mutations of “WNT Genes” in MSI Tumors
AXIN2, PTEN, TCF4, and WISP3 all contain mononucleotide repeats within their coding region and are thus prone for mutations in the MSI tumors. Frameshift mutations within AXIN2, PTEN, TCF4, and WISP3 were found in 7/35 (20%), 6/36 (17%), 17/37 (46%), and 10/36 (28%) among the MSI tumors, respectively. TCF4 was exclusively changed in the MSI tumors (P < .001).
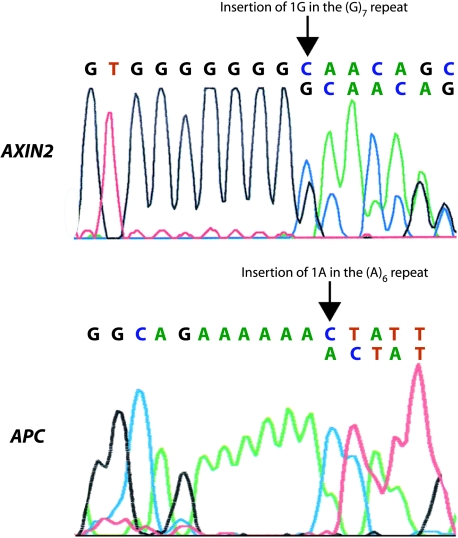
Mutation in exon 7 of AXIN2 was observed in seven MSI tumors, and all were heterozygous mutations. Three tumors contained an insertion of one G in the (G)7 repeat, two tumors showed deletion of one G in the (G)7 repeat, one tumor harbored deletion of one C in the (C)6 repeat, and, finally, one tumor had deletion of one A in the (A)6 repeat. In one primary tumor, two biopsies were analyzed, but a different AXIN2 status was determined, and this tumor was therefore excluded from Figure 2. Interestingly, all three tumors with insertion of one G and one tumor with deletion of one G in the (G)7 repeat were concurrently mutated in APC (Figure 4). Three of these tumors showed the same APC mutation, insertion of one A (GAA-GAAA) at codon 1554, whereas in the last tumor, the APC mutation could not be verified by sequencing.
Figure 4.
AXIN2 and APC mutations within the same MSI colorectal carcinoma (sample 988). Direct sequencing identified an insertion of one G in the (G)7 repeat of AXIN2. PTT and sequencing were used to recognize an insertion of one A at codon 1554 of APC.
Six MSI tumors were mutated in PTEN. The frameshift mutations were evenly distributed between exons 7 and 8. One primary tumor was excluded because three biopsies were analyzed and discordant PTEN status was found. Biallelic mutations were seen in one of the tumors, whereas the others showed monoallelic mutations. In the tumor with homozygous mutation of PTEN, none of the other analyzed genes was changed.
Hypermethylation of APC and CDH1 in MSS and MSI Tumors
Heavy methylation of the promoter region of APC was found in 10 MSI tumors (10/28, 36%) and two were concurrently mutated in APC. On the contrary, in the MSS group, 7/25 (28%) showed hypermethylation of APC and five of these were simultaneously mutated in APC (P = .06). The promoter region of CDH1 was hypermethylated in 39% (11/28) and 42% (10/24) of the MSI and MSS tumors, respectively, and within both groups, alteration of APC (mutation and/or methylation) was observed in more than 60% of the cases.
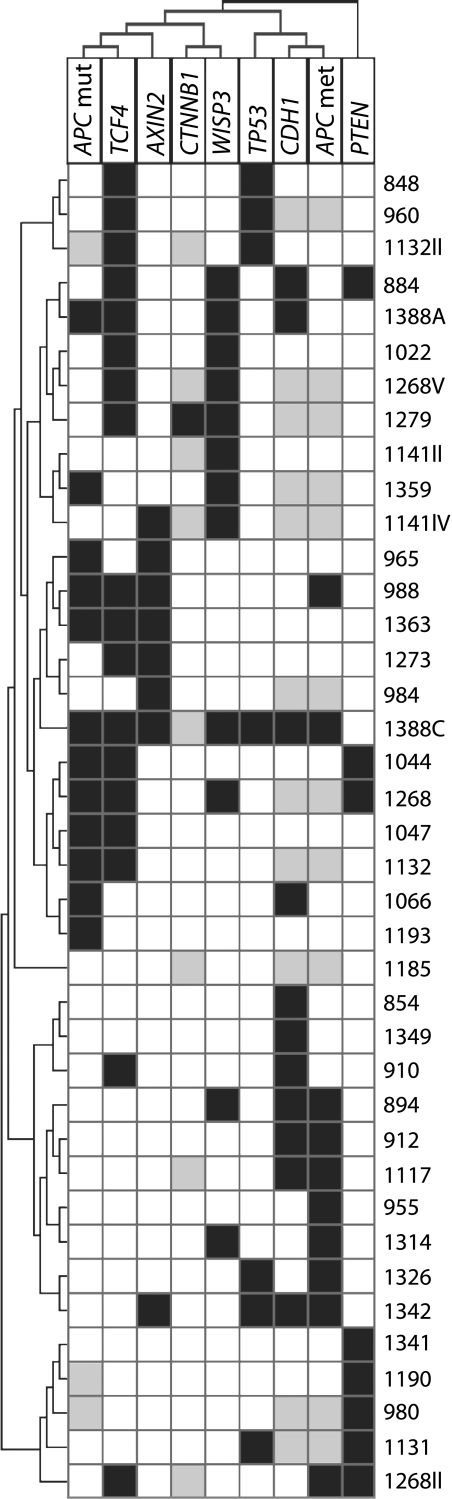
Cluster Analysis of WNT Components in MSI Tumors
Hierarchical cluster analysis was performed on the MSI tumors (Figure 5). Only tumors informative in at least six of the nine loci investigated were included in the analysis (n = 34). Mutations in APC occurred most frequently together with TCF4 mutations, and a subset of these tumors also contained mutations in AXIN2. APC was the sole WNT component changed in one MSI tumor (1193), as was AXIN2 in another tumor (984). Tumors showing hypermethylation of APC additionally harbored epigenetic changes of CDH1. The genes directly involved in the WNT signaling pathway (APC, TCF4, AXIN2, CTNNB1, and WISP3) clustered together, whereas PTEN was most different from all the other components. From three primary tumors (1132, 1141, and 1268), two or more biopsies were analyzed and they showed deviating molecular results and therefore did not cluster together. This was also observed for one patient, in which two primary tumors were included (1388A and 1388C).
Figure 5.
Hierarchical cluster analysis of the MSI tumors using average linkage and Pearson's correlation. The samples are given in the right dendogram, and each analyzed gene is depicted at the top of the dendogram. Each column represents the alteration in one gene over all tumor samples, whereas each row represents the changes in each tumor. Only MSI tumors (n = 34) informative in six of the nine loci investigated were included. From three primary tumors, two or more biopsies were analyzed. Black box, mutation; white box, wild-type; grey box, not determined. Mut, mutation; met, methylation.
We did not observe any differences in cancer-related survival between patients with MSI tumors harboring alterations in three or more of the seven WNT components analyzed (APC, CTNNB1, AXIN2, TCF4, WISP3, PTEN, and TP53) (n= 6) compared to patients with MSI tumors containing alterations in less than three of the components (n = 28) (P = .66).
As many as 90% (279/310) of the tumors included in the present study showed alteration in one or more of the eight WNT genes investigated.
Discussion
The canonical WNT signaling pathway is activated in the vast majority of colorectal tumors. APC is one of the key components of this pathway and dysfunction of APC is observed in familial polyposis coli (FAP) patients and in most cases of sporadic colorectal tumors [6]. Inactivation of APC leads to accumulation of β-catenin in the cytoplasm and nucleus, and thereby activation of WNT signaling. Although several of the WNT components have been reported altered in CRC as well as in other malignancies, to our knowledge, none has studied the interaction of multiple components within the same tumor series.
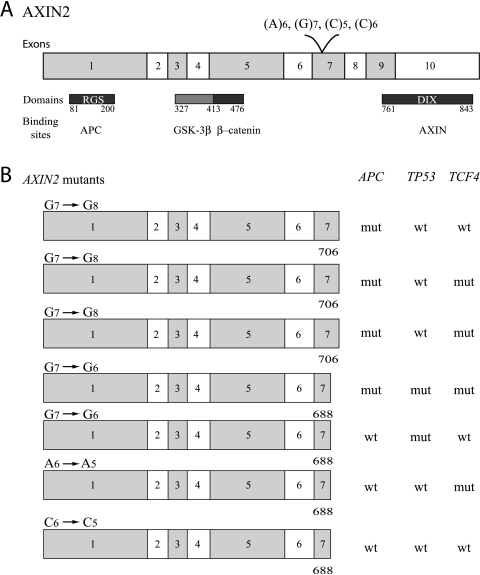
The frequency and distribution of mutations in AXIN2 were comparable with two previously published studies [16,42]. Insertion of one G in the (G)7 repeat predicted a stop at amino acid 706, whereas all the other mutations predicted stop at amino acid 688 (http://us.expasy.org/tools/dna.html). Although the mutations gave rise to stop at different places in the AXIN2 protein, they all led to elimination of the DIX (dishevelled and AXIN) domain (amino acids 761–843), a domain necessary for homo-oligomerization and thus essential for the inhibitory effect of AXIN2 in the WNT pathway [43] (Figure 6). In a previously published study, tumors harboring mutations in AXIN2 were wild-type APC and CTNNB1 [16]. However, in our study, 4/7 (57%) tumors with AXIN2 mutation also harbored mutation in APC. The same was observed for one of the tumors analyzed by Domingo et al. [42]. Among the remaining three tumors with mutation in AXIN2 in the present study, two harbored mutations in either TP53 or TCF4. This suggests that heterozygous mutation of AXIN2 alone is not sufficient to activate the WNT signaling pathway. Tumors with heterozygous mutation of AXIN2 express one wild-type allele that might retain the inhibitory effect of AXIN2; or AXIN1, the homologue of AXIN2, might substitute for the functions of mutated AXIN2. Therefore, other components of the WNT signaling pathway need to be altered in order to fully activate this pathway. Neither of the two previous studies of AXIN2 analyzed several components of the WNT signaling pathway in the same tumor series [16,42].
Figure 6.
(A) The coding region of AXIN2 and corresponding domains and binding sites. (B) The consequence of the different AXIN2 mutations in relation to mutations in APC, TP53, and TCF4. Insertion of one G in the (G)7 repeat of AXIN2 predicted stop at amino acid 706, whereas all the other mutations predicted stop at amino acid 688. In both situations, loss of the DIX domain was observed. Four tumors with AXIN2 mutations were concurrently mutated in APC.
Germline mutations in PTEN have been found in Cowden's syndrome and in a few patients with juvenile polyposis syndrome—both syndromes associated with an increased risk of CRC [6]. Somatic mutations have also been reported in about 15% to 20% of colorectal carcinomas [27,44–46]. PTEN inhibits the activities of phosphatidylinositol-3 kinase (PI-3K) and thus the PI-3K-dependent signaling pathway regulating cell survival and growth. Recently, PTEN and PI-3K were shown to influence the WNT signaling pathway [20]. In PTEN-null prostate cancer, cell lines activated PI-3K-phosphorylated ILK, which subsequently inhibited GSK-3β and led to accumulation of nuclear β-catenin and increased expression of cyclin D1. Reexpression of PTEN increased phosphorylation and degradation of β-catenin [20]. PTEN thus works as a negative regulator of WNT signaling [44,45]. Recently, Nassif et al. [46] showed that PTEN mutations were also present in a subgroup of sporadic MSS colorectal tumors and that biallelic inactivation (two mutations or mutation and loss of heterozygosity) occurred in more than half of these cases, leading to absence of PTEN expression. Epigenetic inactivation of PTEN has also been suggested as a contributing mechanism in colorectal carcinogenesis [44,46]. Overall, these findings indicate that inactivation of PTEN is important during development of both MSI and MSS colorectal tumors, wherein activation of the WNT signaling pathway seems to be one of the central mechanisms. The frequency and distribution of PTEN mutations in the present series are in line with previous reports [27,44,45]. Interestingly, in the tumor with homozygous mutation of PTEN, none of the other WNT components was altered, indicating that this change alone might be sufficient to activate this pathway.
In contrast to the MSS tumors, the MSI tumors with APC mutation were wild-type TP53; however, the majority of these tumors had changes in other WNT components that might partly substitute for the effect of a mutation in TP53. Interestingly, all but one of the MSI tumors with APC mutation outside the MCR showed mutation downstream of the MCR (usually in the hot spot codon 1554), whereas in the subgroup of MSS tumors harboring mutation outside the MCR, the majority of these mutations occurred upstream of the MCR. All MSI tumors with mutation downstream of MCR also had mutations in other WNT components, most frequently within TCF4 and AXIN2, whereas the MSI tumor with APC mutation upstream of the MCR did not show changes in any of the other WNT components analyzed. This might indicate that tumors with mutation downstream of the MCR have kept more of the β-catenin binding and degradation site (codons 1342–2075) and therefore require additional mutations in order to activate the WNT pathway. On the other hand, all except two of the tumors (both MSI and MSS) with mutation upstream of the MCR had lost all β-catenin binding (codons 1020–1169) and downregulation sites (codons 1342–2075) [6], suggesting accumulation of β-catenin in the nucleus and transcription of WNT target genes. APC mutations upstream of codons 1020 to 1169, although heterozygous, might thus alone be sufficient to activate the WNT cascade. The significance of this early mutation is further strengthened by the worse clinical outcome observed in these patients compared to the patients with other APC mutations or the ones with wild-type APC. Even distribution of TP53 L3 domain mutations, which by itself is associated with shorter cancer-related survival, was present in the two APC mutation groups and therefore did not influence the result. The same was true for MSI, which occurred in approximately 7% of each of these tumor groups.
APC has been suggested to control proper chromosome segregation in mouse embryonic stem cells, and APC mutations in these cells have led to karyotypic alterations termed CIN [9,10]. However, by more thorough examination of these mouse cells, polyploidization of the whole genome, rather than losses and gains of one or a few chromosomes, was observed [9]. Furthermore, well-characterized human colon cancer cell lines harboring APC mutations exhibit a stable karyotype after more than thousands of cell divisions [47]. Within our tumor material, no association between APC mutation and CIN was observed, supporting the latter observation; however, an association between APC mutation and tetraploidy (1.9–2.1 by flow cytometry) was not found. On the other hand, an association between TP53 mutations and CIN was seen, in line with our previous reports as well as those of others [30,39,48].
The presence of APC mutation was equally distributed between the MSS tumors and the MSI tumors without hypermethylation of APC. However, among the hypermethylated tumors, a skewed distribution of APC mutations within these two groups was present. One explanation is that both alleles are inactivated by hypermethylation in the MSI tumors and therefore an additional mutation in APC is not required to fully inactivate the protein; whereas only one allele is inactivated by epigenetic mechanisms in the MSS tumors and therefore an additional mutation is necessary.
It has been suggested that lost or reduced expression of E-cadherin alone is not sufficient to activate the WNT signaling pathway [13,14]. The increased level of free cytosolic β-catenin will rapidly be removed by an intact degradation system. However, if components of the degradation complex are also affected, β-catenin will accumulate and the WNT pathway will be activated. In our study, hypermethylation of CDH1 was associated with alteration of APC, thus supporting the latter view.
The majority of the mutations detected in the WNT genes was monoallelic, and usually two or more components were mutated within the same tumor, thereby affecting the WNT pathway at different levels and jointly contributing to the induction of its activation. For instance, frameshift mutations in TCF4, leading to a truncated protein with reduced capability to bind a transcriptional repressor [49], occurred in tumors with an impaired β-catenin degradation system (mutation in APC and AXIN2). PTEN and TP53 influence the WNT signaling pathway in a more indirect manner, and this may explain why they do not cluster together with the mutated WNT components. These two proteins are both involved in several different signaling pathways [50,51] and mutations within these genes may lead to cellular transformation without complete activation of the WNT signaling pathway.
Molecular studies have shown that nearly all colorectal tumors contain an activating mutation of the WNT signaling pathway [5], and this is in agreement with our findings. In the few cases in our series without any changes in the examined genes, other WNT components may be altered. One candidate is AXIN1, which has been reported mutated in a subgroup of MSI colorectal tumors [15]. Another candidate is the PP2R1B gene, encoding the β-isoform of the A subunit of the holoenzyme PP2A, reported mutated in 15% of colorectal tumors [52]. In Xenopus, PP2A is present in the β-catenin degradation complex, where it might dephosphorylate and activate GSK-3β, leading to degradation of β-catenin and inhibition of the WNT signaling pathway [53]. WNT components upstream of the β-catenin degradation complex may also harbor alterations so far not described, however.
Knowledge about molecular mechanisms leading to activation of the WNT signaling pathway is increasing, as well as the information on how this activated pathway contributes to the initiation of colorectal carcinogenesis [5]. We and others clearly demonstrate that this pathway is turned on in nearly all colorectal carcinomas, thus pinpointing the necessity of WNT activation during development of a colorectal tumor.
Acknowledgement
The authors are grateful for the skillful technical assistance of Marianne Berg.
Abbreviations
- APC
adenomatous polyposis coli
- CIN
chromosome instability
- CRC
colorectal cancer
- GSK-3β
glycogen synthase kinase-3β
- MCR
mutation cluster region
- MSI
microsatellite instability
- MSP
methylation-specific PCR
- MSS
microsatellite-stable
- PTEN
phosphatase and tensin homolog
- WNT
Wingless-type MMTV integration site family
Footnotes
This work was supported by the Norwegian Cancer Society (NCS) grant A95068, Ragnhild A. Lothe Lin Thorstensen and Guro E. Lind are postdoctoral and PhD students at the NCS, respectively. Chieu B. Diep and Tone Løvig are postdoctoral students at the Norwegian Foundation for Health and Rehabilitation.
References
- 1.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 2.Uthoff SM, Eichenberger MR, McAuliffe TL, Hamilton CJ, Galandiuk S. Wingless-type frizzled protein receptor signaling and its putative role in human colon cancer. Mol Carcinog. 2001;31:56–62. doi: 10.1002/mc.1039. [DOI] [PubMed] [Google Scholar]
- 3.Nusse R. The Wnt Gene Homepage. ( http://www.stanford.edu/~rnusse/wntwindow.html)
- 4.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255–268. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 5.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 6.Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol Cancer. 2003;2:41. doi: 10.1186/1476-4598-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiltunen MO, Alhonen L, Koistinaho J, Myohanen S, Paakkonen M, Marin S, Kosma VM, Janne J. Hypermethylation of the APC (adenomatous polyposis coli) gene promoter region in human colorectal carcinoma. Int J Cancer. 1997;70:644–648. doi: 10.1002/(sici)1097-0215(19970317)70:6<644::aid-ijc3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Neufeld KL, Zhang F, Cullen BR, White RL. APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 2000;1:519–523. doi: 10.1093/embo-reports/kvd117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 11.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 12.Wheeler JM, Kim HC, Efstathiou JA, Ilyas M, Mortensen NJ, Bodmer WF. Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut. 2001;48:367–371. doi: 10.1136/gut.48.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon J. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 1999;10:369–376. [PubMed] [Google Scholar]
- 14.van de Wetering M, Barker N, Harkes IC, van der HM, Dijk NJ, Hollestelle JG, Klijn JG, Clevers H, Schutte M. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res. 2001;61:278–284. [PubMed] [Google Scholar]
- 15.Shimizu Y, Ikeda S, Fujimori M, Kodama S, Nakahara M, Okajima M, Asahara T. Frequent alterations in the Wnt signaling pathway in colorectal cancer with microsatellite instability. Genes Chromosomes Cancer. 2002;33:73–81. doi: 10.1002/gcc.1226. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling JM, Cunningham JM, Boardman LA, Qian C, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 17.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 18.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de WM, Clevers WM, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/AXIN2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levina E, Oren M, Ben Ze'ev A. Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and Axin dynamics. Oncogene. 2004;23:4444–4453. doi: 10.1038/sj.onc.1207587. [DOI] [PubMed] [Google Scholar]
- 20.Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol. 2001;153:1161–1174. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003;3:695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- 23.Ilyas M, Straub J, Tomlinson IP, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:335–351. doi: 10.1016/s0959-8049(98)00431-6. [DOI] [PubMed] [Google Scholar]
- 24.Toyota M, Ohe-Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA. 2000;97:710–715. doi: 10.1073/pnas.97.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62:2447–2454. [PubMed] [Google Scholar]
- 26.Thorstensen L, Diep CB, Meling GI, Aagesen TH, Ahrens CH, Rognum TO, Lothe TO. WNT1-inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275–1280. doi: 10.1053/gast.2001.29570. [DOI] [PubMed] [Google Scholar]
- 27.Guanti G, Resta N, Simone C, Cariola F, Demma I, Fiorente P, Gentile M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum Mol Genet. 2000;9:283–287. doi: 10.1093/hmg/9.2.283. [DOI] [PubMed] [Google Scholar]
- 28.Meling GI, Lothe RA, Borresen AL, Hauge S, Graue C, Clausen OP, Rognum TO. Genetic alterations within the retinoblastoma locus in colorectal carcinomas. Relation to DNA ploidy pattern studied by flow cytometric analysis. Br J Cancer. 1991;64:475–480. doi: 10.1038/bjc.1991.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Andersen TI, Moller P, Rognum TO. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 30.Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes' stages B and C colorectal cancer patients. J Clin Oncol. 2003;21:820–829. doi: 10.1200/JCO.2003.05.190. [DOI] [PubMed] [Google Scholar]
- 31.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 32.Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–4738. [PubMed] [Google Scholar]
- 33.Brownstein MJ, Carpten JD, Smith JR. Modulation of nontemplated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20:1004–1010. doi: 10.2144/96206st01. [DOI] [PubMed] [Google Scholar]
- 34.Lind GE, Thorstensen L, Meling GI, Løvig T, Hamelin R, Esteller M, Lothe RA. A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Cancer. doi: 10.1186/1476-4598-3-28. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ, et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366–4371. [PubMed] [Google Scholar]
- 36.Graff JR, Herman JG, Myohanen S, Baylin SB, Vertino PM. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Sorensen B, Lind GE, Skotheim RI, Fossa SD, Fodstad O, Stenwig AE, Jakobsen KS, Lothe RA. Frequent promoter hypermethylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene in testicular cancer. Oncogene. 2002;21:8878–8884. doi: 10.1038/sj.onc.1205978. [DOI] [PubMed] [Google Scholar]
- 38.Lovig T, Meling GI, Diep CB, Thorstensen L, Norheim AS, Lothe RA, Rognum TO. APC and CTNNB1 mutations in a large series of sporadic colorectal carcinomas stratified by the microsatellite instability status. Scand J Gastroenterol. 2002;37:1184–1193. doi: 10.1080/003655202760373407. [DOI] [PubMed] [Google Scholar]
- 39.Borresen-Dale AL, Lothe RA, Meling GI, Hainaut P, Rognum TO, Skovlund E. TP53 and long-term prognosis in colorectal cancer: mutations in the L3 zinc-binding domain predict poor survival. Clin Cancer Res. 1998;4:203–210. [PubMed] [Google Scholar]
- 40.Lothe RA, Smith-Sorensen B, Hektoen M, Stenwig AE, Mandahl N, Saeter G, Mertens F. Biallelic inactivation of TP53 rarely contributes to the development of malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer. 2001;30:202–206. [PubMed] [Google Scholar]
- 41.Dysvik B, Jonassen I. J-Express: exploring gene expression data using Java. Bioinformatics. 2001;17:369–370. doi: 10.1093/bioinformatics/17.4.369. [DOI] [PubMed] [Google Scholar]
- 42.Domingo E, Espin E, Armengol M, Oliveira C, Pinto M, Duval A, Brennetot C, Seruca R, Hamelin R, Yamamoto H, et al. Activated BRAF targets proximal colon tumors with mismatch repair deficiency and MLH1 inactivation. Genes Chromosomes Cancer. 2004;39:138–142. doi: 10.1002/gcc.10310. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999;10:255–265. doi: 10.1016/s1359-6101(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhou XP, Loukola A, Salovaara R, Nystrom-Lahti M, Peltomaki P, de la CA, Aaltonen LA. Eng, PTEN mutational spectra, expression levels, and subcellular localization in microsatellite-stable and -unstable colorectal cancers. Am J Pathol. 2002;161:439–447. doi: 10.1016/S0002-9440(10)64200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin KH, Park YJ, Park JG. PTEN gene mutations in colorectal cancers displaying microsatellite instability. Cancer Lett. 2001;174:189–194. doi: 10.1016/s0304-3835(01)00691-7. [DOI] [PubMed] [Google Scholar]
- 46.Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite-stable colorectal cancer. Oncogene. 2004;23:617–628. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]
- 47.Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih I, Vogelstein B, Lengauer B. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci USA. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JK, Chang SC, Yang YC, Li AF. Loss of heterozygosity and DNA aneuploidy in colorectal adenocarcinoma. Ann Surg Oncol. 2003;10:1086–1094. doi: 10.1245/aso.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Duval A, Rolland S, Tubacher E, Bui H, Thomas G, Hamelin R. The human T-cell transcription factor-4 gene: structure, extensive characterization of alternative splicings, and mutational analysis in colorectal cancer cell lines. Cancer Res. 2000;60:3872–3879. [PubMed] [Google Scholar]
- 50.Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–167. [PubMed] [Google Scholar]
- 51.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 52.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]