Abstract
Glucocorticoids can inhibit solid tumor growth possibly due to an inhibitory effect on angiogenesis. The antitumor effects of the free drugs have only been observed using treatment schedules based on high and frequent dosing for prolonged periods of time. As long-circulating liposomes accumulate at sites of malignancy, we investigated the tumor-inhibiting potential of liposome-encapsulated prednisolone phosphate. Liposomal prednisolone phosphate could inhibit tumor growth dose-dependently, with 80% to 90% tumor growth inhibition of subcutaneous B16.F10 melanoma and C26 colon carcinoma murine tumor models at 20 mg/kg by single or weekly doses. Prednisolone phosphate in the free form was completely ineffective at this low-frequency treatment schedule, even when administered at a dose of 50 mg/kg. In vitro studies did not show an inhibitory effect of prednisolone (phosphate) on tumor cell, nor on endothelial cell proliferation. Histologic evaluation revealed that liposomal prednisolone phosphate-treated tumors contained a center with areas of picnotic/necrotic cells, which were not apparent in untreated tumors or tumors treated with the free drug. In conclusion, the present study shows potent antitumor effects of liposomal formulations of glucocorticoids in a low dose and low-frequency schedule, offering promise for liposomal glucocorticoids as novel antitumor agents.
Keywords: Corticosteroids, liposomes, tumor therapy, drug targeting, angiogenesis
Introduction
Glucocorticoids have a wide spectrum of activities on cell trafficking, cell-cell interactions, and cell communication, leading to pronounced anti-inflammatory and immunosuppressive effects. Glucocorticoids exert their effects by diffusion through the cell membrane and binding to their cytosolic receptors. Subsequently, these receptors become activated and translocate to the nucleus where they directly modulate DNA transcription of a variety of genes. In addition, glucocorticoid receptors may directly or indirectly antagonize the activity of several transcription factors, most notably nuclear factor-κB [1,2]. At high concentrations, glucocorticoids also exert rapid nongenomic effects on cells by interacting nonspecifically with cell membranes, or specifically with membrane-bound glucocorticoid receptors [3].
In tumor therapy, glucocorticoids have been used for their anti-inflammatory and antiemetic effects, and in the treatment of hematologic malignancies based on their efficient cytolytic activity on cells of lymphoid origin [4]. Reports in the last two decades demonstrated that glucocorticoids could also inhibit solid tumor growth in experimental animal models [5–8]. However, these preclinical studies further show that high and frequent dosing of glucocorticoids is a prerequisite to obtain antitumor effects. In mice, doses of 100 to 200 mg/kg per day need to be administered for prolonged periods of time to obtain significant tumor growth inhibition [5–8]. These doses resulted in considerable morbidity and mortality as a result of severe immune suppression [6,7].
Targeted delivery of glucocorticoids to tumor tissues could be an attractive strategy to increase intratumoral drug concentrations, thereby reducing the overall dose and hence decreasing the likelihood of side effects [9]. We investigated the ability of long-circulating liposomes to deliver glucocorticoids selectively to tumor tissues. These liposomes have previously been shown to accumulate at sites of malignancy as a result of the enhanced permeability of the tumor vasculature compared to the healthy endothelium [10]. In the present study, antitumor activity of liposomal prednisolone phosphate was investigated in subcutaneous C26 colon carcinoma and B16.F10 melanoma models, and compared to the antitumor activity of free prednisolone phosphate in different dosing schemes. In addition, to evaluate the importance of targeted delivery, effects of short-circulating liposomes, which predominantly home to the spleen, were compared to that of tumor-targeted long-circulating liposomes [10]. Finally, to establish the possible mechanisms of antitumor activity, histologic analyses of tumors were performed, focusing on neovascularization pattern in relation to tumor cell morphology.
Materials and Methods
Liposome Preparation
Long-circulating liposomes were prepared as described previously [11]. In brief, appropriate amounts of dipalmitoyl-phosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany), cholesterol (Sigma, St. Louis, MO), and poly(ethylene glycol) 2000-distearoylphosphatidylethanolamine (Lipoid GmbH), in a molar ratio of 1.85:1.0:0.15, respectively, were dissolved in chloroform:methanol (2:1 vol/vol) in a round-bottom flask. A lipid film was made under reduced pressure on a rotary evaporator and dried under a stream of nitrogen. Liposomes were formed by addition of an aqueous solution of 100 mg/ml prednisolone phosphate disodium salt (Bufa, Uitgeest, The Netherlands). A water-soluble phosphate derivative of prednisolone was used to ensure stable encapsulation in the liposomes as previous experiments showed that unmodified prednisolone, although showing high initial encapsulation efficiency, was rapidly lost upon intravenous injection. For labeling of the liposomes with 0.5 mCi of 111Inoxine (Mallinckrodt Medical, Petten, The Netherlands), the lipid film was hydrated in 10 mM Hepes/135 mM NaCl buffer, pH 7.4, containing 5 mM DTPA acting as the indium chelator to a final lipid concentration of 10 µmol/ml, according to a procedure described by Boerman et al. [12]. Liposome size was reduced by multiple extrusion steps through polycarbonate membranes (Nuclepore, Pleasanton, CA) with a final pore size of 50 nm.
Short-circulating liposomes were prepared similarly; only poly(ethylene glycol) 2000-distearoylphosphatidylethanolamine was replaced by egg phosphatidylglycerol (Lipoid GmbH). For the preparation of short-circulating liposomes, the poly(ethylene glycol)-conjugated lipid was replaced with the negatively charged phospholipid phosphatidylglycerol. The short-circulating liposomes were formed by the addition of 10 mg/ml prednisolone phosphate in 10 mM Hepes/135 mM NaCl buffer, pH 7.4, to the lipid film, and extrusion took place through polycarbonate membranes of 400 nm. The use of larger membrane pores for the short-circulating liposomes reduces circulation time compared to the long-circulating liposomes, and prevents efficient extravasation at the target site [13,14]. Unencapsulated material for both liposome types was removed by dialysis, with repeated changes of buffer against 10 mM Hepes/135 mM NaCl buffer, pH 7.4, at 4°C.
Mean particle size distribution of the liposomes was determined by dynamic light scattering detected at an angle of 90° to the laser beam on a Malvern 4700 System (Malvern Instruments, Malvern, UK). In addition to the mean particle size, the system reports a polydispersity index with a value between 0 and 1. A polydispersity index of 1 indicates large variations in particle size; a reported value of 0 means that size variation is absent. Mean particle size was found to be 0.1 µm with a polydispersity value of around 0.1, whereas the short-circulating liposomes had a mean particle size of 0.5 µm with a polydispersity value of around 0.3. Thus, the polydispersity values indicate limited variation in particle size.
The ζ-potential of liposomes, as a measure for surface charge, was determined using a zetasizer equipped with PCS v1.35 software (Malvern Instruments). Liposomes were prepared in 5% aqueous Hepes/NaCl buffer and the instrument was calibrated with electrophoresis standard latex particles. Long-circulating liposomes had a near neutral surface charge, whereas short-circulating liposomes displayed a surface charge of -14 mV.
Phospholipid content was determined with a phosphate assay, performed according to Rouser et al. [15], on the organic phase after extraction of liposomal preparations with chloroform. The aqueous phase after extraction was used for determining the prednisolone phosphate content by high-performance liquid chromatography as described previously [11]. The detection limit for the high-performance liquid chromatography setup was 20 ng/ml. The liposomal preparation contained ∼2 mg/ml prednisolone phosphate and ∼60 µmol/ml phospholipid. Preparation methods based on freeze-thawing of the liposomes before extrusion did not improve encapsulation efficiency.
Colloidal gold-labeled SSL were prepared as described previously [16]. Briefly, the lipid film was prepared as described above. A 1.1% (wt/vol) aqueous solution of AuCl2 (Sigma-Aldrich Co., Zwijndrecht, The Netherlands) was fourfold diluted with sodium citrate (28 mM)/potassium carbonate (7 mM) buffer, filtered (0.2 µm), and used to hydrate the lipid film at 4°C. Liposomes were prepared by multiple extrusion. The resulting yellow liposome suspension was placed at 37°C, after which the color of the suspension turned purple. Unencapsulated colloidal gold was removed by gel filtration of the liposomes over a Sephacryl SF S1000 column (Pharmacia, Uppsala, Sweden) using Hepes/NaCl buffer as the eluents. Resulting liposomes were 0.1 µm in size and displayed a near-neutral surface charge.
Cells
B16.F10 murine melanoma and C26 murine colon carcinoma cells were cultured at 37°C in a 5% CO2-containing humidified atmosphere in DMEM medium (Gibco, Breda, The Netherlands) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Gibco), 100 IU/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (Gibco). Human umbilical vein endothelial cells (HUVEC) (Glycotech, Rockville,MD) were cultured in complete EGM endothelial cell growth medium (Cambrex, East Rutherford, NJ).
Murine Tumor Models
Male C57Bl/6 and Balb/c mice (6–8 weeks of age) were obtained from Charles River (Maastricht, The Netherlands), and kept in standard housing with standard rodent chow and water available ad libitum at a 12-hour light/dark cycle. Experiments were performed according to national regulations and were approved by the local animal experiments ethical committee. For tumor induction, 1 x 106 B16.F10 melanoma or C26 colon carcinoma cells were inoculated subcutaneously in the flank of syngeneic C57Bl/6 or Balb/c mice, respectively. B16.F10 tumors became palpable around 7 days and C26 tumors around 10 days after tumor cell inoculation.
Tissue Distribution of 111In-Labeled Liposomes in Tumor-Bearing Mice
At a tumor volume of approximately 1 cm3, mice were injected intravenously with 25 µmol/kg lipid (corresponding to 30 x 106 cpm/mouse) of 111In-labeled liposomes. At 6 and 24 hours after injection, animals were killed by CO2 asphyxiation; a blood sample was taken; the tumor, lungs, liver, spleen, and kidneys were dissected; the tissues were weighed; and radioactivity was counted. Injection standards were included to account for physical decay.
Tumor Growth Inhibition
Effect of dose Mice received a single intravenous injection of an indicated dose of free prednisolone phosphate or liposomal prednisolone phosphate at the time when the tumor became palpable (tumor volume ∼20 mm3). At 7 days after treatment, tumor size was measured and tumor volume was calculated according to the formula: V = 0.52a2b, where a is the smallest and b is the largest superficial diameter.
Dosing schedule Free prednisolone phosphate or liposomal prednisolone phosphate was intravenously administered at a dose of 20 mg/kg on days 1, 7, and 14, or by single injection on day 7 or 14 after tumor cell inoculation. Tumor size was measured regularly, and tumor volume was calculated as described above.
Analysis of Amount of Prednisolone in Tissues
At a tumor volume of approximately 1 cm3, mice were injected intravenously with 20 mg/kg liposomal prednisolone phosphate or free prednisolone phosphate. At 24 hours after injection, animals were killed and tumor was dissected. The tissues were weighed and homogenized. Two micrograms of methylprednisolone was added as an internal standard, after which prednisolone (phosphate) was extracted from the tissue with ethylacetate at pH 2 and evaporated until dryness under a nitrogen flow. Samples were reconstituted in ethanol: water 1:1 vol/vol and analyzed by high-performance liquid chromatography as described previously [11]. Calibration curves were prepared by spiking control organs from untreated mice with known amounts of prednisolone phosphate and prednisolone and analyzing these samples according to the same procedure.
Effect of Prednisolone on Cell Proliferation In Vitro
To determine whether prednisolone (phosphate) had a direct antiproliferative effect on cells, 5 x 103 cells/well HUVEC, C26, and B16.F10 were plated in a 96-well plate. Prednisolone (phosphate) was added and dissolved in ethanol using corresponding concentrations of ethanol as controls, whereas prednisolone phosphate was added in the Hepes/NaCl buffer. Cell viability was determined after 24, 48, and 72 hours of incubation by XTT assay (Sigma) according to the manufacturer's instructions.
Histology
C26 and B16.F10 tumor-bearing mice received a single intravenous injection of either free or liposomal 20 mg/kg prednisolone phosphate when tumor volume reached 20 mm3. Tumors were dissected 72 hours after injection. Tumors were fixed with liquid nitrogen for immunohistochemical staining and with 2% glutaraldehyde for toluidin blue staining. Five-micrometer slides were cut. Sections from frozen tissues were fixed in acetone and air-dried for immunohistochemical analysis [17]. Rat anti-mouse CD31 antibody (BD Pharmingen, Heidelberg, Germany) was used as a primary antibody and incubated for 45 minutes at room temperature. After washing three times with PBS supplemented with 5% fetal calf serum, endogenous peroxidase activity was inhibited by incubating with 0.075% H2O2 for 20 minutes. As a secondary antibody, rabbit anti-rat HRP (DAKO A/S, Glostrup, Denmark) was used with 2% normal mouse serum. Sections were incubated for 45 minutes at room temperature and washed three times with PBS with 5% fetal calf serum. After incubation with the tertiary antibody, goat anti-rabbit HRP (DAKO A/S) with 2% normal mouse serum (for 30 minutes and washing three times) was stained with peroxidase substrate 3-amino, 9-ethylcarbazole. Slides were counterstained in hematoxylin (Sigma-Aldrich Co.) and mounted in Kaiser's glycerol gelatin (Merck, Darmstadt, Germany).
Glutaraldehyde fixed sections were dehydrated and embedded in glycol methacrylate. Slides were stained with toluidin blue (Fluka, Zwijndrecht, The Netherlands).
All slides were evaluated by light microscopy regarding tumor tissue density, location, number and structure of blood vessels, presence of hemorrhagic areas, and presence, size, and location of picnotic/necrotic areas. These observations were related to the cellular localization of liposomes in tumor tissues. For this purpose, liposomes were labeled with colloidal gold. Twenty-four hours after injection of the liposomes, tumors were dissected and snap-frozen in liquid nitrogen, and 5-µm slides were cut. Slides were evaluated by light microscopy after silver enhancement of the colloidal gold and hematoxylin/eosin staining. For transmission electron microscopy, tumor tissue was fixed in 2% glutaraldehyde in PBS, postfixed in 2% glutaraldehyde/0.1 M sodium cacodylate followed by 2% OsO4/0.1 M sodium cacodylate, stained with uranyl acetate, dehydrated in acetone, and embedded in Durcupan plastic. Seventy- to 90-nm sections were cut, collected on copper G200 grids, and examined at an accelerating voltage of 60 kV in a Philips EM 201 transmission electron microscope (Philips Analytical Electron Optics, Eindhoven, The Netherlands).
Statistical Analyses
Data were analyzed by one-way ANOVA with Dunnett's posttest using GraphPad InStat version 3.05 for Windows, GraphPad Software (San Diego, CA). Data were logarithmically transformed to correct for significant differences between the SD values of groups, when appropriate, according to Bartlett's test. Spearman's rank correlation coefficient was calculated to identify dose response.
Results
Tissue Distribution of 111In-Labeled Liposomes
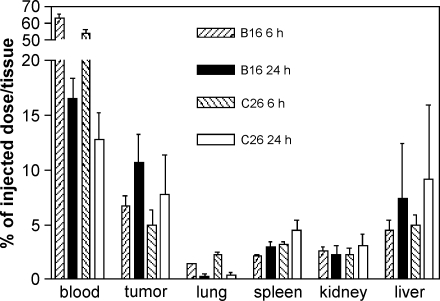
Figure 1 presents the tissue distribution data of the 111In-labeled liposomes at 6 and 24 hours after intravenous injection in C26 or B16.F10 tumor-bearing mice. Approximately 60% of the injected dose (ID) was still present in the circulation at 6 hours after administration in both tumor models, whereas 15% ID was still circulating at 24 hours postinjection. These values correspond to previous data on the circulation kinetics of liposomes [10]. Seven to 10% ID could be recovered from tumor tissues in both the C26 and B16.F10 models at 24 hours after injection, which was about two-fold higher than the levels at 6 hours postinjection. At both time points, approximately the same amount was present in the livers of both strains of tumor-bearing mice. Relatively low amounts of 111In-labeled liposomes were recovered from the spleen, kidney, and lung in both mouse models at 6 and 24 hours after injection.
Figure 1.
Tissue distribution of 25 µmol/kg lipid. 111In-labeled liposomes at 6 and 24 hours after intravenous administration in B16.F10 tumor-bearing C57Bl/6 mice or C26 tumor-bearing Balb/c mice. Mean ± SD; n = 5 animals per experimental group.
Antitumor Activity of Liposomal Prednisolone Phosphate Versus Free Prednisolone Phosphate: Single Dose-Response Relationship
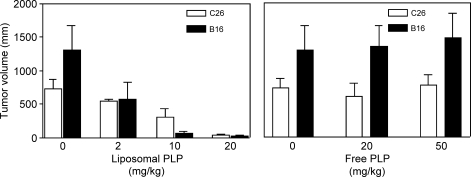
To compare the effects of different doses of liposomal prednisolone phosphate and free prednisolone phosphate on tumor growth, B16.F10 or C26 tumor-bearing mice received a single injection of either formulation at the moment that the tumor became palpable. At 1 week after injection, tumor volumes were smaller with increasing doses of liposomal prednisolone phosphate in both mouse models, as shown in Figure 2 (B16: Spearman correlation coefficient r = 0.92, P < .001; C26 Spearman correlation coefficient r = 0.82, P < .01). These correlation coefficients indicate a positive correlation between the dose of liposomal prednisolone phosphate and antitumor effects. Prednisolone phosphate, 20 mg/kg, was the maximum dose of the liposomal formulation that could be administered in view of the maximal injection volume. Treatment of B16.F10 and C26 tumor-bearing mice with 20 or 50 mg/kg free prednisolone phosphate did not significantly affect tumor volumes compared to vehicle-treated control animals (Figure 2).
Figure 2.
Effects of liposomal (left) and free prednisolone phosphate (right) on tumor growth in B16.F10 or C26 tumor-bearing mice. Mice received a single injection of the indicated dose and formulation of prednisolone phosphate on the day that the tumors became palpable. Tumor volume after 1 week is reported. Mean ± SD; n = 5 animals per experimental group.
Dependence of Antitumor Effect on Treatment Schedule
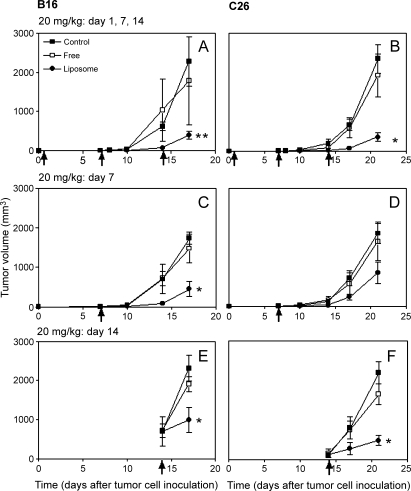
To determine if (and to what extent) the antitumor effects depended on the treatment schedule, liposomal and free prednisolone phosphate were injected at a dose of 20 mg/kg on days 1, 7, and 14, or as a single dose of 20 mg/kg on day 7 or 14 after tumor cell inoculation. The results are shown in Figure 3.
Figure 3.
Effects of different treatment schedules of free and liposomal prednisolone phosphate on tumor growth. The formulations were injected at a dose of 20 mg/kg on days 1, 7, and 14 (A and B), or as a single injection on day 7 (C and D) or day 14 (E and F) in B16.F10 tumor-bearing mice (A, C, and E) or C26 tumor-bearing mice (B, D, and F). Mean ± SD; n = 5 animals per experimental group.
B16 model The tumor volumes of B16.F10 tumor-bearing mice that received treatment on days 1, 7, and 14 are shown in Figure 3A. Tumors became palpable on day 7 in all treatment groups, indicating that none of the treatments delayed tumor growth between days 1 and 7. A second dose of liposomal prednisolone phosphate on day 7, however, resulted in 92 ± 10% tumor growth inhibition between days 7 and 14 compared to controls (P < .05), whereas free prednisolone phosphate did not affect tumor volume. On day 14, mice received a third injection. On day 17, the average tumor volume in the liposomal prednisolone phosphate-treated group was 79 ± 26% smaller (P < .01) than the tumor volume in the mice receiving free prednisolone phosphate and vehicle. At this time point, the experiment was ended as 4 of 10 mice from the latter two groups had large tumor sizes (>2 cm3).
After a single injection of liposomal or free prednisolone phosphate on day 7, a significantly smaller tumor volume was only seen after treatment with liposomal prednisolone phosphate, with average inhibition of tumor growth of 89 ± 24% on day 14 and 67 ± 27% on day 17 compared to controls (P < .05, both time points) (Figure 3C). Furthermore, even a single injection of liposomal prednisolone phosphate on day 14 produced 58 ± 31% tumor growth inhibition on day 17 compared to controls (P < .05) (Figure 3E).
C26 model C26-bearing mice received the first injection on day 1 and a second on day 7 after tumor cell inoculation. As tumors in all treatment groups became palpable around day 10, the effect on tumor growth of the first injections appeared to be minimal, although tumor volume was 89 ± 9% smaller in liposomal prednisolone phosphate-treated animals than in controls on day 14. On day 21, 1 week after the third dose on day 14, average tumor volume in liposomal prednisolone phosphate-treated animals was 89 ± 10% smaller than that in controls (P < .01) (Figure 3B).
Although a single dose of liposomal prednisolone phosphate on day 7 resulted in 66 ± 32% tumor growth inhibition on day 14 and 67 ± 33% inhibition on day 21, these differences were not statistically significant compared to the control and free drug-treated groups (Figure 3D). A single injection of liposomal prednisolone phosphate on day 14 resulted in 78 ± 19% tumor growth inhibition (P < .05) (Figure 3F).
These results indicate that liposomal prednisolone phosphate can effectuate strong antitumor effects, provided that a palpable tumor mass is present at the time of injection. Likely, the degree of liposomal tumor localization is minimal in small—not yet palpable—tumors as vascular integrity is hardly compromised in this time frame and, consequently, the enhanced permeability and retention effect are absent. These results would also suggest that local accumulation of liposomes is critically important for therapeutic efficacy.
Importance of the Long-Circulation Property of Liposomes for Tumor Growth Inhibition
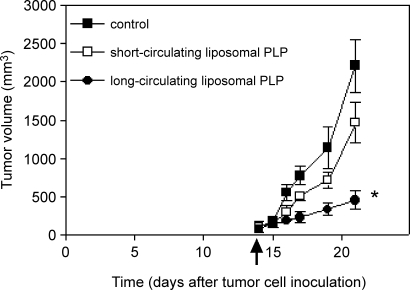
As circulation time of liposomes is positively correlated to target site accumulation [10–14], liposomal circulation time may be a critical factor for achieving antitumor efficacy. We evaluated a prednisolone phosphate-containing short-circulating liposome type and compared the antitumor activity of this formulation to long-circulation liposomal prednisolone phosphate in C26 tumor-bearing mice. Both formulations were injected on day 14 after tumor cell inoculation, as the previous experiment showed the lowest variation in effects of a single injection of long-circulating liposomal prednisolone phosphate in this model at this time point. The tumor volume of short-circulating liposomes-encapsulated prednisolone phosphate-treated animals was not significantly different from saline-treated animals, whereas animals treated with liposomes-encapsulated prednisolone phosphate experienced a significantly reduced tumor growth rate, with tumor volume being 71 ± 7% smaller compared to vehicle-treated animals (P < .05) (Figure 4). These results indicate that the long-circulating property is important for the antitumor effect. As liposomal circulation time is positively correlated to liposome accumulation in the tumor, these results imply the importance of liposomal tumor localization for antitumor efficacy, while making a possible (immunosuppressive) effect brought about peripherally less likely to be the main determinant.
Figure 4.
Effects of short-circulating and long-circulating prednisolone phosphate liposomes on C26 tumor growth. Tumor-bearing mice received a single injection of 20 mg/kg of the indicated formulations of prednisolone phosphate on day 14 after tumor cell inoculation. Mean ± SD; n = 5 animals per experimental group.
Effects of Prednisolone on HUVEC, B16.F10, or C26 Proliferation In Vitro
To evaluate whether the antitumor effect was due to a direct cytotoxic effect of prednisolone or prednisolone phosphate, HUVEC, B16.F10, and C26 cells were incubated in vitro for 24, 48, and 72 hours with increasing concentrations of prednisolone or prednisolone phosphate ranging from 1 ng/ml to 100 µg/ml. No decrease in cell viability was noted up to the maximum concentrations tested for C26 and HUVEC (data not shown). Only B16.F10 cells showed a modest inhibition of proliferation at the highest concentration of 100 µg/ml prednisolone after incubation for 48 or 72 hours with apparent reductions in proliferation of 21 ± 3% and 41 ± 2% (mean ± SD of three measurements), respectively, compared to vehicle-treated control cells. Prednisolone phosphate did not affect cell proliferation at any of the concentrations tested.
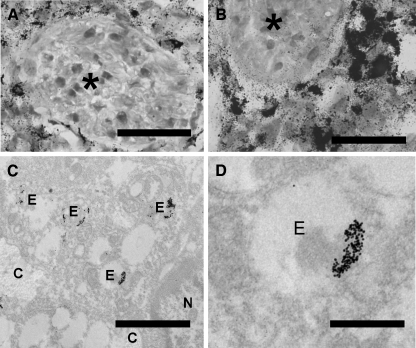
To relate these modest effects to drug levels in the tumor tissues, prednisolone and prednisolone phosphate levels at 24 hours after intravenous injection of liposomal prednisolone phosphate or free prednisolone phosphate were determined by high-performance liquid chromatography analysis. At this time point, total prednisolone (phosphate) levels in the tumor tissue were undetectable after injection of free prednisolone phosphate (n = 3–4). For the liposomal formulation, total prednisolone (phosphate) concentrations of 10 ± 1 µg/g in C26 tumor tissues and 19 ± 6 µg/g in B16.F10 tumor tissues were measured (n = 3–4 mean ± SD), which is approximately 2% to 5% ID. These concentrations would make a direct effect of liposomal prednisolone on tumor cell proliferation unlikely. However, these concentrations represent overall tumor levels and do not account for temporary regional peak concentrations. Intratumoral distribution of colloidal gold-labeled liposomes at 24 hours after injection shows that there are large regional variations in degrees of liposome localization, with liposomes being mainly observed in the immediate vicinity of blood vessels in both tumor models (Figure 5, A and B). Within these areas, liposomes are predominantly taken up by macrophages and recovered in endosomal compartments (Figure 5, C and D). It is conceivable that the high concentrations in these specific areas could have diverse pharmacologic effects on different cell types.
Figure 5.
Intratumoral distribution of liposomes. Colloidal gold-labeled liposomes were intravenously injected in C26 tumor-bearing (A) or B16.F10 tumor-bearing (B) mice. Vascular lumina are indicated by an asterisk (A and B; bar = 25 µm). Macrophages that are filled with silver-enhanced colloidal gold and are located close to the tumor blood vessels can be visualized. Transmission electron microscopy images confirm that these cells are macrophages (C) (n = nucleus; c = collagen; e = endosome; bar = 2 µm) and show that the colloidal gold particles are present in endosomal vesicles (D) (bar = 600 nm).
Histologic Examination of Tumor Tissue
To evaluate the effects of treatment on tumor histology, we compared toluidin blue and CD31-stained sections of B16.F10 and C26 tumor tissues from liposomal prednisolone phosphate-treated and untreated mice. Treatment was initiated when the tumors became palpable and tumors were evaluated 3 days after treatment as this was the time frame in which pronounced neovascularization occurred. It is thought that inhibition of this neovascularization forms the basis of the antitumor effects of glucocorticoids. Tumor sections were evaluated regarding tumor cell density, location, number, and structure of blood vessels to assess possible changes related to angiogenesis; the presence of hemorrhagic areas to determine the vascular integrity of capillaries; and the presence, size, and location of picnotic/necrotic cell areas to investigate tumor viability (Table 1).
Table 1.
Effect of Treatment with Liposomal Prednisolone Phosphate, Free Prednisolone Phosphate, or Vehicle on Tumor Size and Tumor Histology. C26 B16.F10
| C26 | B16.F10 | |||||||
| Start | End | Start | End | |||||
| C | F | L | C | F | L | |||
| Tumor size (mm 3) | 16 | 59 | 48 | 15 | 14 | 163 | 136 | 17 |
| Blood vessels | - | + | + | + | - | + | + | + |
| Hemorrhagic area | - | - | - | - | - | ± | ± | ± |
| Necrosis | - | - | - | +* | - | ±† | ±† | +* |
| High cell density | - | + | + | + | ++ | ++ | ++ | ++ |
Tumor tissue was excised from C26 or B16.F10 tumor-bearing mice at the start of treatment or 3 days after a single intravenous injection with liposomal prednisolonephosphate (L), or free prednisolone phosphate (F) at a dose of 20 mg/kg, or vehicle (C). Tissue slides were evaluated regarding vascularization (CD31 staining), tumorcell density, and occurrence of necrosis and hemorrhagic areas (toluidine bluestaining).
Slides were scored as: (-) (nearly) absent; (+) present; (++) strongly present.
Predominantly in tumor core.
Infrequently observed without preferential localization.
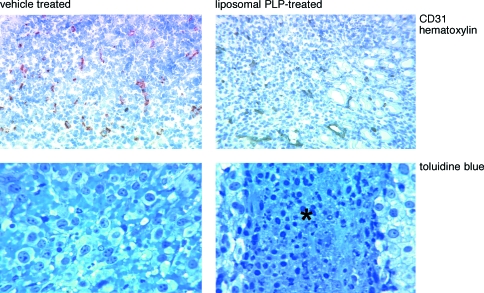
The most striking observation in the antitumor experiments was that tumors from liposomal prednisolone phosphate-treated mice had a size that was comparable to the tumor size at the start of treatment, whereas control tumors showed a sharp increase in tumor volume. Despite this pronounced difference in tumor size, tumor morphology was remarkably similar. More specifically, liposomal prednisolone phosphate appeared to have no influence on the increase in tumor vascularization in both tumor models, the increase in tumor cell density in C26 tumors, or the increase in a number of hemorrhagic areas in B16.F10 tumors that were observed in control or free prednisolone phosphate-treated animals within this time frame. Apparently, liposomal prednisolone phosphate does not affect tumor “maturation” despite a strong inhibition of tumor growth rate.
The only notable difference was that in all of the liposomal prednisolone phosphate-treated tumors, areas of picnotic/necrotic cells in the center of the tumor could be observed. These patterns were not observed in free prednisolone phosphate- or vehicle-treated controls (Table 1, Figure 6). Although we did not directly assess the functionality of blood vessels in this area, the vessels seemed perfused as indicated by the presence of erythrocytes. Therefore, necrosis in these areas did not seem to be the result of lack of vascularization.
Figure 6.
Effect of single liposomal prednisolone phosphate treatment on the morphology of tumor tissues and blood vessel density. Liposome-treated tumors showed similar blood vessel density as vehicle-treated control tumors (upper panels), a pattern resembling that observed for free prednisolone phosphate-treated tumors (not shown). In all liposome-treated tumors, areas of necrosis in the core of the tumor were observed, which were absent in vehicle-treated (lower panels) and free prednisolone phosphate-treated tumors (not shown). Similar observations were made in B16.F10 tumor-bearing mice (not shown).
Discussion
The present study demonstrates for the first time that liposome-encapsulated glucocorticoids exert strong tumor growth-inhibiting effects in vivo. Antitumor effects were observed when liposomal prednisolone phosphate was administered in a low-frequency (single dose or weekly) dosing schedule and at substantially lower doses than required for free glucocorticoids [5–7,9].
Liposomes have previously been used to increase the delivery of a variety of drugs to tumor tissues [18–20]. Probably, the best-known formulation in this respect is liposome-encapsulated doxorubicin, marketed as Doxil or Caelyx [19]. The long-circulating property of these formulations allows liposomes to extravasate as a result of the enhanced vascular permeability in solid tumor tissues, leading to selective accumulation at the site of malignancy [18–20]. Indeed, in both the B16.F10 and C26 tumor models used in this study, 7% to 10% of the ID of liposomes localized in the tumor at 24 hours after injection, which is similar to previously reported data [10,18–20]. At this time point, approximately 15% of the ID was still circulating in the blood stream, which is consistent with a long-circulatory behavior.
Furthermore, liposome encapsulation increased the levels of the drug in the tumor at 24 hours after injection compared to administration of the free drug. When administered in free form, no prednisolone (phosphate) could be detected in the tumor tissues, whereas administration of the liposomal form resulted in 2% to 5% ID of prednisolone in the tumor tissue. The fact that this percentage is substantially lower than the 7% to 10% ID of (radioactively labeled) liposomes that accumulate at the site of the malignancy is likely to be explained by intratumoral conversion of prednisolone phosphate to prednisolone. This subsequently leads to a redistribution of the drug over the body as prednisolone easily passes membranes.
Administration of liposome-encapsulated prednisolone phosphate resulted in a dose-dependent antitumor effect in both the B16.F10 and C26 subcutaneous tumor models. In both tumor models, the maximum dose of 20 mg/kg (determined by the maximal injection volume) resulted in approximately 90% tumor inhibition over a 1-week period when administered as a single dose at the moment that the tumor became palpable. In contrast, free prednisolone phosphate did not affect tumor growth even at a dose of 50 mg/kg.
The in vitro studies indicate that the underlying mechanism of tumor growth inhibition is probably not related to a direct inhibition of tumor cell proliferation. Several other studies have also shown that the pharmacologic effect of glucocorticoids does not occur through a direct action on tumor cells, but is mediated by interference with the tumor neovascularization [21–25]. However, we also did not observe an effect of prednisolone phosphate or prednisolone on the proliferation of HUVEC. This indicates that a direct effect on proliferating endothelial cells is absent. Nevertheless, other angiogenesis-driving processes such as production and/or release of proangiogenic and antiangiogenic factors [24,25] may still be affected in vivo by the prolonged exposure to high levels of the drug. The preferential localization of liposomes in the immediate vicinity of tumor neovasculature may cause extremely high local drug levels, which can mediate such effects. In addition, the hypothesis that inflammatory processes in and around the tumor are important in the angiogenic cascade could mean that glucocorticoids' immunosuppressive action may also be of relevance in this respect [26,27]. It has also been suggested that tumor-associated macrophages may secrete mitogens, growth factors, and enzymes that stimulate both tumor cell survival and growth as well as angiogenesis. In this view, liposomal prednisolone phosphate may disturb the symbiotic relationship between tumor cells and macrophages [28]. The observations of strong macrophage uptake of liposomes in the tumor tissue may support this theory. Additional research is required to address these issues in detail.
Microscopic analysis of tumor tissues indicated that treatment with liposomal prednisolone phosphate has pronounced effects on tumor size and produced necrotic areas in the core of the tumor, but overall has remarkably limited effects on tumor morphology. Liposomal treatment did not affect the increase in tumor vascularization seen in both models, nor the increase in tumor cell density in C26 tumors, nor the increase in the number of hemorrhagic areas in B16.F10 tumors. These observations do not exclude or support an antiangiogenic mechanism of action. In previous studies with angiogenesis inhibitors, it was shown that tumor vessels may grow or remain viable during antiangiogenic treatment. The absolute number of blood vessels, however, determines the number of tumor cells that can be supported, thereby dictating tumor volume without an apparent reduction in tumor blood vessel density [29]. Future studies investigating the effects of liposomal prednisolone phosphate on the molecular level should result in a clearer understanding of the precise mechanism of action of our formulation.
Regardless of the proposed underlying mechanism, various studies have reported a concentration-dependent inhibition of the angiogenic process by glucocorticoids [21–25]. This concentration dependency is further illustrated by a study in rats demonstrating that local administration of glucocorticoids in sponge implants, which act as a slow-release vehicle, was more effective in inhibiting angiogenesis than systemic treatment [30]. Also in our study, the importance of prolonged high local drug levels is supported by the observation that the same dose of liposomal prednisolone phosphate in short-circulating liposomes did not inhibit tumor growth. Short-circulating liposomes were formed by omitting the poly(ethylene glycol)-conjugated lipid. Exchanging this lipid for phosphatidylglycerol introduced a negative charge on the liposome surface, which promotes macrophage uptake and thereby reduces circulation time of the short-circulating liposomes even further [13]. These short-circulating liposomes are rapidly taken up by macrophages mainly in the liver and spleen, and are therefore unable to accumulate at the tumor. The limited localization of short-circulating liposomes in the tumor was paralleled by a decrease in activity, again indicating that local high levels of glucocorticoids in tumors are pivotal for antitumor effects.
In conclusion, the present study shows for the first time that liposome-encapsulated prednisolone phosphate exerts potent antitumor effects when administered in a low-frequency dosing schedule. The liposomal formulation caused high intratumoral levels of prednisolone phosphate for a prolonged period of time. The advantage of the current system for future clinical use is the relatively low-dose and low-frequency schedule with which prednisolone phosphate can be administered to induce antitumor efficacy. Furthermore, the use of glucocorticoids may reduce toxicity in combination therapy as they have a different side effects profile than the traditional oncolytics. Future studies will focus on the molecular effects of liposomal prednisolone phosphate treatment on the various stages of neovascularization representative of clinical conditions.
Footnotes
This study was funded by the Dutch Cancer Society (grant UU 2000-2185).
References
- 1.Almawi WY, Melemedjian OK. Molecular mechanisms of glucocorticoid antiproliferative effects antagonism of transcription factor activity by glucocorticoid receptor. J Leukoc Biol. 2002;71:9–15. [PubMed] [Google Scholar]
- 2.De Bosscher K, Vanden Berghe W, Haegeman G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids negative interference of activated glucocorticoid receptor with transcription factors. J Neuroimmunol. 2000;10:916–922. doi: 10.1016/s0165-5728(00)00297-6. [DOI] [PubMed] [Google Scholar]
- 3.Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids. 2002;67:529–534. doi: 10.1016/s0039-128x(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Glucocorticoids in cancer therapy. Biotherapy. 1992;4:37–44. doi: 10.1007/BF02171708. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Erturk E, Mayer R, Cockett AT. Efficacy of antitumor chemotherapy in C3H mice enhanced by the antiangiogenesis steroid cortisone acetate. Cancer Res. 1987;47:5021–5024. [PubMed] [Google Scholar]
- 7.Penhaligon M, Camplejohn RS. Combination heparin plus cortisone treatment of two transplanted tumors in C3H/He mice. J Natl Cancer Inst. 1985;74:869–873. [PubMed] [Google Scholar]
- 8.Pucci M, Lotti T, Tuci F, Brunetti L, Rindi L, Fibbi G, Pasquali F, Chiarugi VP. Modulation of growth of melanoma. Int J Dermatol. 1988;27:167–169. doi: 10.1111/j.1365-4362.1988.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe PE, Derbyshire EJ, Andrade SP, Press N, Knowles PP, King S, Watson GJ, Yang YC, Rao-Bette M. Heparin-steroid conjugates new angiogenesis inhibitors with antitumor activity in mice. Cancer Res. 1993;53:3000–3007. [PubMed] [Google Scholar]
- 10.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 11.Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 12.Boerman OC, Storm G, Oyen WJ, van Bloois L, van der Meer JW, Claessens RA, Crommelin DJ, Corstens FH. Sterically stabilized liposomes labeled with indium-111 to image focal infection. J Nucl Med. 1995;36:1639–1644. [PubMed] [Google Scholar]
- 13.Schiffelers RM, Bakker-Woudenberg IA, Snijders SV, Storm G. Localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue influence of liposome characteristics. Biochim Biophys Acta. 1999;142:1329–1339. doi: 10.1016/s0005-2736(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 15.Rouser G, Fleisner S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 16.Schiffelers RM, Storm G, Bakker-Woudenberg IA. Host factors influencing the preferential localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue. Pharm Res. 2001;18:780–787. doi: 10.1023/a:1011080211226. [DOI] [PubMed] [Google Scholar]
- 17.Schraa AJ, Kok RJ, Moorlag HE, Bos EJ, Proost JH, Meijer DK, Leijde LF, Molema G. Targeting of RGD-modified proteins to tumor vasculature a pharmacokinetic and cellular distribution study. Int J Cancer. 2002;102:469–475. doi: 10.1002/ijc.10727. [DOI] [PubMed] [Google Scholar]
- 18.Harrington KJ. Liposomal cancer chemotherapy current clinical applications and future prospects. Expert Opin Invest Drugs. 2001;10:1045–1061. doi: 10.1517/13543784.10.6.1045. [DOI] [PubMed] [Google Scholar]
- 19.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54(Suppl 4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 20.Storm G, Crommelin DJ. Colloidal systems for tumor targeting. Hybridoma. 1997;161:19–25. doi: 10.1089/hyb.1997.16.119. [DOI] [PubMed] [Google Scholar]
- 21.Crowley P, Lai NY, DeYoung N, Pearce P, Funder JW, Gill PG. Inhibition of growth of B16F10 melanoma by glucocorticoids does not result directly from receptor-mediated inhibition of tumour cells. Oncology. 1998;45:331–335. doi: 10.1159/000226634. [DOI] [PubMed] [Google Scholar]
- 22.Cariou R, Harousseau JL, Tobelem G. Inhibition of human endothelial cell proliferation by heparin and steroids. Cell Biol Int Rep. 1998;12:1037–1047. doi: 10.1016/0309-1651(88)90028-8. [DOI] [PubMed] [Google Scholar]
- 23.Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids induction of capillary basement membrane dissolution. Endocrinology. 1986;119:1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- 24.Blei F, Wilson EL, Mignatti P, Rifkin DB. Mechanism of action of angiostatic steroids suppression of plasminogen activator activity via stimulation of plasminogen activator inhibitor synthesis. J Cell Physiol. 1993;155:568–578. doi: 10.1002/jcp.1041550315. [DOI] [PubMed] [Google Scholar]
- 25.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Fan TP. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 26.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 27.O'Byrne, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–483. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;96:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 29.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 30.Hori Y, Hu DE, Yasui K, Smither RL, Gresham GA, Fan TP. Differential effects of angiostatic steroids and dexamethasone on angiogenesis and cytokine levels in rat sponge implants. Br J Pharmacol. 1996;118:1584–1591. doi: 10.1111/j.1476-5381.1996.tb15578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]