Abstract
The main objectives of this study were (i) to determine if gut wall-associated microorganisms are responsible for the capacity of earthworms to emit nitrous oxide (N2O) and (ii) to characterize the N2O-producing bacteria of the earthworm gut. The production of N2O in the gut of garden soil earthworms (Aporrectodea caliginosa) was mostly associated with the gut contents rather than the gut wall. Under anoxic conditions, nitrite and N2O were transient products when supplemental nitrate was reduced to N2 by gut content homogenates. In contrast, nitrite and N2O were essentially not produced by nitrate-supplemented soil homogenates. The most probable numbers of fermentative anaerobes and microbes that used nitrate as a terminal electron acceptor were approximately 2 orders of magnitude higher in the earthworm gut than in the soil from which the earthworms originated. The fermentative anaerobes in the gut and soil displayed similar physiological functionalities. A total of 136 N2O-producing isolates that reduced either nitrate or nitrite were obtained from high serial dilutions of gut homogenates. Of the 25 representative N2O-producing isolates that were chosen for characterization, 22 isolates exhibited >99% 16S rRNA gene sequence similarity with their closest cultured relatives, which in most cases was a soil bacterium, most isolates were affiliated with the gamma subclass of the class Proteobacteria or with the gram-positive bacteria with low DNA G+C contents, and 5 isolates were denitrifiers and reduced nitrate to N2O or N2. The initial N2O production rates of denitrifiers were 1 to 2 orders of magnitude greater than those of the nondenitrifying isolates. However, most nondenitrifying nitrate dissimilators produced nitrite and might therefore indirectly stimulate the production of N2O via nitrite-utilizing denitrifiers in the gut. The results of this study suggest that most of the N2O emitted by earthworms is due to the activation of ingested denitrifiers and other nitrate-dissimilating bacteria in the gut lumen.
Living earthworms from both forest and garden soils emit the greenhouse gas nitrous oxide (N2O) and may account for up to 30% of the total N2O emitted from soils that are inhabited by earthworms (27, 29). A recent study with earthworms in limed and unlimed soil columns confirmed the capacity of earthworms to emit N2O (7). The emission of N2O by earthworms is stimulated by nitrate and nitrite, and denitrifying bacteria in the gut of the earthworm appear to be involved in this emission (27, 29). N2O is an intermediate in denitrification (i.e., the reduction of nitrate to N2) (45). Nitrification and the dissimilative reduction of nitrate to nitrite or ammonium can also result in significant production of N2O (3). During the dissimilative reduction of nitrate, N2O is produced via the nonspecific reaction of nitrate reductase with nitrite (38). During nitrification, N2O can be produced as a side product when ammonia is oxidized to nitrite via the intermediate hydroxylamine (6, 34, 44).
It is not yet known if the emission of N2O by earthworms originates from an autochthonous, gut wall-attached microbial population or is due to the enhanced activity of ingested soil-derived microorganisms in the special environment of the earthworm gut. The goals of the present study were (i) to determine if gut wall-associated microorganisms are responsible for the capacity of garden soil earthworms to emit N2O, (ii) to enumerate different physiological groups of bacteria that might produce N2O in the earthworm gut, and (iii) to isolate and characterize representative N2O-producing bacteria from the earthworm gut.
MATERIALS AND METHODS
Field sites and sampling.
All earthworms used in this study were Aporrectodea caliginosa Savigny and were identified by standard protocols (8). Earthworms and soil samples from the uppermost 10 cm of the soil were obtained from a regional garden in Heinersreuth, Germany, that has been described previously (29) and also from a regional garden in Unternschreez, Germany. Soil from the latter site had a C/N ratio of 12.9 and a pH (H2O) of 6.8 and contained (per kg [dry weight] of soil) 50.3 g of total C, 41.6 g of organic C, 3.9 g of total N, 1.4 mg of NH4+, 26.6 mg of NO3−, and 1.2 mg of NO2−. Earthworms and soil samples were collected in April through November 1999, in May 2000, and in December 2001. Earthworms collected in March 2002 were from a regional meadow (Hofmanns Wiese); this site is described elsewhere (23). Samples were transported in aseptic beakers and were used immediately or stored at 10°C in the dark for no more than 24 h before processing. Randomly chosen worms and soil samples from both sites were tested for in vivo emission of N2O (29); on a fresh weight basis, all worms emitted larger amounts of N2O than the soil from which they originated emitted.
Microcosms with gut and soil homogenates.
Homogenates were prepared in an O2-free chamber (Mecaplex, Grenchen, Switzerland) as described previously (26); sterile anoxic sodium phosphate buffer (15 mM, pH 7) or sterile anoxic sodium phosphate buffer (10 mM, pH 7) plus saline (NaCl, 130 mM) were used as homogenization buffers. For anatomical localization of N2O production, the gut of a worm was opened, and the contents were removed for homogenization. The remaining gut walls were washed twice with sterile phosphate-buffered saline, removed from the earthworm, and washed three additional times in petri dishes with sterile phosphate-buffered saline. Gut or soil homogenates (0.1 to 0.5 g [fresh weight] per 10 ml of buffer) and gut walls (0.05 to 0.5 g [fresh weight] per 10 ml of buffer) were anoxically transferred to gas-tight, argon-flushed serum vials (10 or 30 ml) or tubes (27 ml). Substrates were added from sterile anoxic stock solutions. The headspace and the liquid phase of microcosms were sampled with sterile syringes. Gases were analyzed immediately; liquid samples were stored at −20°C until they were analyzed. Microcosms were prepared in triplicate and were incubated upright at 15°C in the dark.
Cultivation media.
Anoxic media were prepared by using modified Hungate techniques (24). The gas phases were 100% argon for anoxic media and air for oxic media; the pH was adjusted to 7.0. Solidified media contained 15 g of agar per liter. The following media were used: (i) TSB100, which contained 0.275 g of tryptic soy broth without dextrose (TSB) (Difco Laboratories, Detroit, Mich.) per liter; (ii) TSB10, which contained 2.75 g of TSB per liter; (iii) TSB+G, which contained 27.5 g of TSB per liter plus 5 mM glucose; (iv) TSB10+G, which was TSB10 containing 5 mM glucose; (v) TSB10+G+NO3−, which was TSB10 containing 2 mM glucose and 5 mM KNO3 (27); (vi) NB, which contained 0.8 g of nutrient broth (Difco) per liter; (vii) NB+AS+NO3−, which was NB containing 3 mM acetate, 6 mM succinate, and 5 mM KNO3 (10, 40); (viii) Luria-Bertani medium containing 10 g of tryptone (Difco) per liter, 5 g of yeast extract (Sigma, Deisenhofen, Germany) per liter, and 5 g of NaCl per liter; (ix) TY, which contained 2 g of tryptone per liter and 2 g of yeast extract per liter; (x) IS1, which contained 2.75 g of TSB per liter, 1.01 g of KNO3 per liter, 6.52 g of Na2HPO4 · H2O per liter, 3.17 g of NaH2PO4 · 2H2O per liter, 0.0017 g of CuCl2 per liter, and 2 mM glucose; (xi) IS2, which was IS1 without glucose but with 5 mM butyrate; (xii) IS3, which was IS1 without TSB but with 0.8 g of NB per liter; and (xiii) IS4, which contained 20 g of beef extract (Difco) per liter, 30 g of Casamino Acids (Difco) per liter, 5 g of yeast extract per liter, and 5 g of K2HPO4 per liter. Mineral medium containing 5 mM ammonium (MMA) and mineral medium containing 10 mM nitrite (MMN) were prepared by using a previously described protocol (35).
Enumeration of microorganisms.
Earthworms and soil samples were collected in April 1999. Gut sections of five worms were pooled for preparation of gut homogenates. The homogenates contained 17 mg (fresh weight) of gut sections per ml of buffer or 118 mg (fresh weight) of soil per ml of buffer and were serially diluted (1:10) in saline (8.5 g of NaCl liter−1). Most-probable-number (MPN) (11, 12) tubes were incubated horizontally (oxic tubes) or vertically (anoxic tubes) at 15°C in the dark for 6 months and were checked periodically for growth by measuring the optical density at 660 nm.
Denitrifiers and other nitrate dissimilators were enumerated in TSB10+G+NO3− and NB+AS+NO3− (10, 27, 40). Growth-positive tubes were scored positive for denitrification if more than 20% of the reduced nitrate N was recovered in N2 or N2O (40) and were scored positive for nitrate dissimilation if more than 20% of the initial concentration of nitrate (5 mM) was consumed. Ammonia oxidizers were enumerated in MMA; tubes were scored positive if nitrite accumulated and the pH indicator bromothymol blue changed color (35). Nitrite oxidizers were enumerated in MMN; tubes were scored positive if nitrite was completely utilized (35). General aerobes and general fermentative anaerobes were enumerated in TSB10; tubes that showed an increase in optical density at 660 nm of ≥0.1 were scored positive (26). Methanogens were enumerated in TSB10; tubes were scored positive if >3 nmol of methane per tube was produced.
Isolation of N2O-producing bacteria from the earthworm gut.
Streak plating was done in an O2-free chamber, and several isolation methods were utilized to maximize the likelihood of obtaining a highly diverse collection of N2O-producing isolates. In method A, 0.1-ml aliquots from the highest denitrification-positive MPN tubes were streaked onto solidified anoxic NB+AS+NO3− or TSB10+G+NO3−. In method B, 0.1-ml aliquots of 10−3 and 10−4 saline serial dilutions from gut homogenates were streaked onto anoxic solidified TSB10+G+NO3− and anoxic NB+AS+NO3−. In method C, gut homogenates were serially diluted in anoxic TSB10 supplemented with KNO3 (1 mM) and either cellobiose (5 mM), raffinose (1 mM), acetate (1 mM), succinate (1 mM), citrate (1 mM), or pectin (0.01%). In method D, gut homogenates were serially diluted in TSB10, TSB100, NB, Luria-Bertani medium, or TY (all anoxic) supplemented with KNO2 (1 mM) and either N-acetylglucosamine (2.5 mM), lactate (2.5 mM), gluconate (2.5 mM), or galactose (2.5 mM). For methods C and D, tubes were incubated for 14 days at 15°C in the dark, and 0.1-ml aliquots from the highest N2O-positive dilutions (10−4 to 10−6) were streaked onto solidified anoxic medium (the medium was the same as that used for the dilutions). Streak plates were incubated in an O2-free jar for 7 to 10 days at 15°C. Randomly picked colonies having differing morphologies were transferred to liquid medium, and the resulting cultures were checked for the production of N2 and N2O. N2- and N2O-producing cultures were restreaked twice and then transferred to liquid media. Isolates ED2, ED3, ED4, ED5, EN1, and EN2 were maintained in anoxic IS1. Isolates ED1 and EN3 were maintained in anoxic IS2 and anoxic IS3, respectively. Isolates MH5, MH8, MH47, and AG24 were maintained in anoxic IS4. Isolates AG15, AG23, MH14, and MH21 were maintained in oxic TSB+G. All other isolates were maintained in oxic TSB10+G. The isolates were routinely cultivated at 15°C and are available upon request.
Initial N2O production rates of isolates.
Isolates were grown to the late stationary phase and diluted 10-fold into anoxic media without nitrate; tubes were flushed with 100% argon to remove carryover N2O. Nitrate (final concentration, 10 mM) or nitrite (final concentration, 2 mM) was added with sterile syringes. Tubes were incubated vertically in the dark at 15°C. Initial N2O production rates were determined when the production of N2O was linear; this period occurred during the first 24 h of incubation. Cell numbers were determined microscopically.
Analytical methods.
Denaturing gradient gel electrophoresis (DGGE) of amplified 16S rRNA gene fragments (approximately 550 bp) was performed as previously described (37). Partial 16S rRNA gene sequences were determined for either DGGE fragments or PCR products that were obtained with the GM3-GM4 bacterial primer pair (Escherichia coli positions 8 to 23 and 1492 to 1477) (31). Sequencing was done by MWG Biotech AG (Ebersberg, Germany). Public databases (GenBank, EMBL) were searched with the program BLAST (1). Alignments of sequences and distance matrix calculations were performed with the program ARB (Department of Microbiology, Technical University of Munich, Munich, Germany) (http://www.arb-home.de). Gases and organic compounds were analyzed as described previously (43). Nitrate, nitrite, and ammonium contents were determined colorimetrically (9, 19, 22).
RESULTS
Anatomical location of N2O production.
The production of N2O by gut contents accounted for 87 to 99% of the total combined N2O produced by gut contents and gut wall homogenates (Table 1), indicating that the production of N2O in earthworms occurs primarily in the gut contents. The production of N2O was greater with nitrite than with nitrate.
TABLE 1.
Initial production of N2O by gut contents and gut walls of the earthworm A. caliginosaa
| Expt | Supplement (2 mM) | N2O production (nmol h−1 g [fresh wt]−1)b
|
|
|---|---|---|---|
| Gut contents | Gut walls | ||
| Ac | Nitrite | 1.92 ± 0.66 | 0.29 ± 0.13 |
| Nitrate | 0.73 ± 0.52 | 0.08 ± 0.01 | |
| Bd | Nitrite | 1.48 ± 0.10 | 0.03 ± 0.01 |
| Nitrate | 0.23 ± 0.05 | 0.00 ± 0.03 | |
Gut content and gut wall microcosms were anoxic and contained 2.75 g of TSB liter−1 and 1.7 mM glucose.
The values are the means ± standard deviations for triplicate analyses obtained during the first 10 h of incubation.
Worms were collected in December 2001.
Worms were collected in March 2002.
Denitrification by earthworm gut contents versus denitrification by soil.
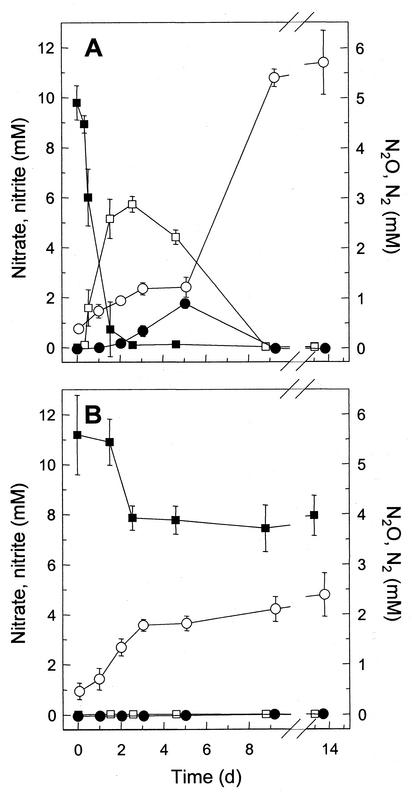
N2 was the sole nitrate-derived end product of both gut content and soil microcosms (Fig. 1). However, denitrification was more rapid and more complete in gut content microcosms (Fig. 1A) than in soil microcosms (Fig. 1B). Furthermore, nitrite and N2O were transient products in gut content microcosms but were essentially not detected in soil microcosms. This general pattern was observed for worms collected in both 1999 and 2000. Addition of electron donors (cellobiose, glucose, syringate, or acetate) did not affect the production of N2O by nitrate-supplemented gut content homogenates during incubation for 10 days (data not shown).
FIG. 1.
Utilization of supplemental nitrate by gut content (A) and soil (B) homogenates under anoxic conditions at 15°C. The homogenates contained 24 and 161 mg (dry weight) of gut contents and soil, respectively, per ml of sodium phosphate buffer (15 mM, pH 7). The organic carbon contents were 1.3 and 5.9 mg per ml of gut contents and soil homogenate, respectively. Symbols: ▪, nitrate; □, nitrite; •, N2O; ○, N2. Worms and soil were collected in August 1999; experiments were performed in triplicate. The error bars indicate standard deviations.
Enumeration of cultured N2O-producing and other gut microbes.
Approximately 6 × 106 denitrifiers per g (dry weight) were found in gut sections (Table 2); this value was equivalent to approximately 60% of the general fermentative anaerobes and 60% of the nitrate dissimilators. In contrast, the MPN values for denitrifiers in soil were approximately 2 orders of magnitude lower than those obtained for gut sections and equivalent to only 1% of the general fermentative anaerobes in the soil or 10% of the nitrate dissimilators in the soil.
TABLE 2.
Comparative enumeration of cultured microorganisms from gut sections of the earthworm A. caliginosa and from garden soil
| Physiological group | Medium | Incubation conditions | MPN (g [dry wt]−1)a
|
|
|---|---|---|---|---|
| Gut sections | Soil | |||
| Denitrifiersb | TSB10+G+NO3− | Anoxic | 6 × 106 (a) | 2.1 × 104 (b) |
| Nitrate dissimilatorsb | TSB10+G+NO3− | Anoxic | 1 × 107 (a) | 4 × 105 (b) |
| Ammonium oxidizers | MMA | Oxic | 2 × 105 (c) | 2 × 104 (c) |
| Nitrite oxidizers | MMN | Oxic | 3 × 105 (a) | 2 × 104 (b) |
| General aerobes | TSB10 | Oxic | 3 × 108 (a) | 9 × 106 (b) |
| General fermenters | TSB10 | Anoxic | 1 × 107 (a) | 2 × 105 (b) |
| Methanogens | TSB10 | Anoxic | 1 × 102 (c) | 2 × 101 (c) |
Statistically significant differences (at P = 0.05 [10, 12]) between values obtained for gut sections and soil are indicated by different letters. Values followed by the same letter were not statistically different for comparisons between gut sections and soil.
Similar values were obtained with NB-based medium.
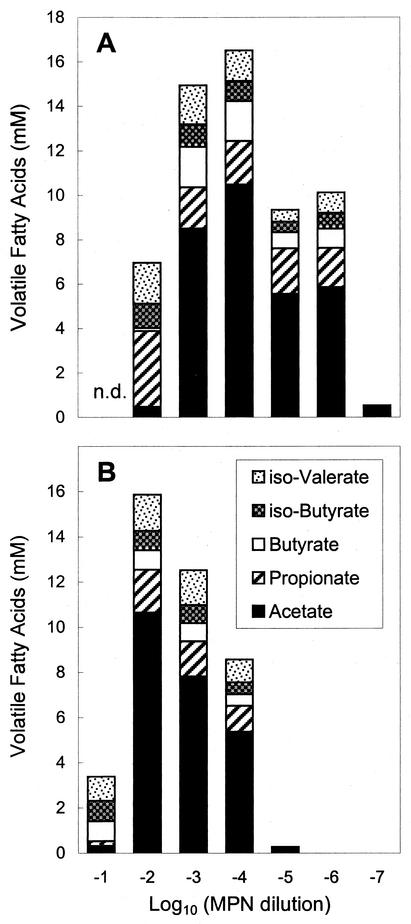
The product profiles of anoxic TSB10 MPN cultures were evaluated to assess the physiological diversity of the cultured fermentative anaerobes (Fig. 2). Acetate was the predominant soluble product in all growth-positive high dilutions of gut and soil homogenates. The hydrogen concentrations never exceeded 1.2 mM (based on the total amount of hydrogen in both the liquid and gas phases). Methane was detected only in the 10−1 and 10−2 dilutions. The product profiles of gut and soil MPN dilutions did not differ significantly, suggesting that the fermentative anaerobes in the gut and in the soil were functionally similar.
FIG. 2.
Fermentation product profiles for anoxic TSB10 MPN dilutions of earthworm gut sections (A) and soil (B). n.d., not determined.
Isolation and phylogenetic assessment of N2O-producing bacteria from the earthworm gut.
A total of 136 isolates that produced either N2O or N2 were obtained from high-dilution tubes (mostly 10−5 to 10−6) of gut homogenates by utilizing cultivation conditions that simulated some of the in situ conditions of the earthworm gut (i.e., high concentrations of organic substrates, anoxia, 1 mM nitrite, and 1 to 5 mM nitrate [23]). Twenty-five distinct groups were identified by combining genetic screening (DGGE) and an analysis of the morphologies and growth characteristics of the isolates. A representative isolate of each group was characterized (Table 3). Of the 25 selected isolates, 21 were affiliated with either the gamma subclass of the class Proteobacteria (gamma-proteobacteria) or the gram-positive bacteria with low DNA G+C contents. Two isolates (ED5 and MH21) exhibited less than 97% 16S rRNA gene sequence similarity with their closest cultured relatives and might be new species.
TABLE 3.
Phylogenetic groups, closest relatives (inferred from a partial 16S rRNA gene sequence comparison), and N2O production for nitrate- or nitrite-reducing bacteria isolated from the gut of the earthworm A. caliginosa
| Isolate (accession no.) | Closest relative (accession no.) | % Sequence similarity | Length of sequence (bp) | Source of closest relative | Reduction of nitrate | Reduction of nitrite | Production of N2 | % of N from nitrate or nitrite recovered in N2Oa | Initial N2O production rate (pmol h−1 107 cells−1)b |
|---|---|---|---|---|---|---|---|---|---|
| Alpha-proteobacteria | |||||||||
| MH37 (AJ318921) | Sinorhizobium sp. strain BK10 (AJ012211) | 99.5 | 584 | Soil | +c | 0 | − | 2 | 232 (22) |
| Beta-proteobacteria | |||||||||
| ED4 (AJ318915) | Ralstonia pickettii LMG7160 (AJ270260) | 100 | 650 | Rhizoplane, soil | + | + | − | 111 | 3,280 |
| ED1 (AJ318917) | Dechlorimonas sp. strain SIUL (AF170356) | 99.4 | 1,487 | Contaminated soil | + | + | + | 0 | 3,130 (300) |
| Gamma-proteobacteria | |||||||||
| ED2 (AJ318918) | Pseudomonas sp. strain Ant5 (AF184220) | 99.9 | 711 | Soil | + | + | + | 0 | 6,700 (760) |
| ED3 (AJ318919) | Pseudomonas sp. strain KA2 (AF195876) | 100 | 750 | Contaminated soil | + | + | + | 0 | 60 (20) |
| EN1 (AJ318914) | Pseudomonas putida ATCC 17472 (AB016428) | 99.9 | 801 | Soil | + | 0 | − | 1 | 540 (60)d |
| EN2 (AJ318913) | Pseudomonas brassicacearum 520-1 (AJ292381) | 99.8 | 650 | Soil | + | 0 | − | 1 | 160 (60)d |
| AG18 (AJ318898) | Aeromonas hydrophila ATCC 7966T (X74677) | 100 | 549 | Tin of milk | + | 0 | − | <0.5 | 39 (11) |
| AG26 (AJ318899) | Aeromonas media ATCC 33907T (X74679) | 100 | 548 | River water | + | 0 | − | 1.5 | 120 (4) |
| AG15 (AJ318902) | Buttiauxella warmboldiae DSM 9404 (AJ233406) | 100 | 549 | Snail | + | + | − | 5 | 203 (11) |
| AG23 (AJ318908) | Enterobacter asburiae JCM 6051 (AB004744) | 99.4 | 549 | Aphid gut | + | 0 | − | 3 | 28 (2) |
| Cytophaga-Flavobacterium group | |||||||||
| ED5 (AJ318907) | Flavobacterium johnsoniae DSM 2064 (M59051) | 95 | 1,447 | Soil | + | + | + | 0 | 4,900 |
| Gram-positive bacteria with low DNA G+C contents | |||||||||
| MH5 (AJ318903) | Clostridium glycolicum DSM 1288T (X74750) | 99.1 | 642 | Soil | − | + | − | 20 | 7 (2) |
| MH47 (AJ318906) | Clostridium putrefaciens DSM 1291 (AF127024) | 100 | 609 | Ham | − | + | − | 43 | 118 (19) |
| AG24 (AJ318904) | Clostridium mangenotii ATCC 25761 | 99.8 | 523 | Soil | + | 0 | − | 5 | 89 (3) |
| MH8 (AJ318905) | C. mangenotii ATCC 25761 (M59098) | 99.2 | 639 | Soil | − | + | − | 100 | 54 (12) |
| EN3 (AJ318900) | Bacillus mycoides ATTC 6462 | 100 | 750 | Soil | + | 0 | − | <0.5 | 290 (10)d |
| MH48 (AJ318901) | B. mycoides ATTC 6462 (AB021192) | 100 | 621 | Soil | + | 0 | − | 7 | 24 (1) |
| AG5 (AJ318916) | Paenibacillus sp. strain P51-3 (AJ297715) | 100 | 550 | Vegetable purees | + | + | − | 6 | 142 (6) |
| MH13 (AJ318920) | Paenibacillus sp. strain P51-3 (AJ297715) | 97.8 | 276 | Vegetable purees | + | + | − | 3 | 78 (13) |
| MH72 (AJ318910) | Paenibacillus sp. strain P51-3 (AJ297715) | 99 | 1,461 | Vegetable purees | + | 0 | − | 3 | 35 (3) |
| MH44 (AJ318922) | Paenibacillus sp. strain 61724 (AF227827) | 99.5 | 437 | Soil | + | + | − | 11 | 12 (1) |
| MH14 (AJ318912) | Paenibacillus burgondia B2 (AJ011687) | 99.1 | 699 | Mycorhizosphere | + | + | − | 8 | 9 (1) |
| MH21 (AJ318909) | Paenibacillus borealis KK19 (AJ011322) | 95 | 1,512 | Soil | − | + | − | 10 | 314 (42) |
| MH70 (AJ318911) | Paenibacillus amylolyticus NRRL B-290T (D85396) | 100 | 171 | Soil | − | + | − | <0.5 | 31 (25) |
Determined at the end of incubation.
The values are the means of triplicate experiments or the averages of duplicate experiments. The values in parentheses are the standard deviations of the initial N2O production rates for triplicate experiments.
+, positive reaction; −, negative reaction; 0, accumulation of nitrite.
The initial N2O production rates and N balances were determined in separate experiments.
Reduction of nitrate and production of N2O by gut isolates.
Four of the five denitrifying isolates (ED1, ED2, ED3, and ED5) reduced nitrate completely to N2 with sequential formation of the intermediates nitrite and N2O, while the remaining denitrifier (ED4) formed only N2O (Table 3 and data not shown). Most known denitrifiers reduce nitrate completely to N2 (41, 45). Fifteen of the 25 isolates did not reduce nitrate to N2 but produced nitrite and small amounts of N2O; typically, less than 6% of the nitrate consumed was recovered in N2O. These 15 isolates were therefore classified as nondenitrifying nitrate-dissimilating bacteria. Most of them were also capable of growing by fermentation (data not shown). Five isolates (MH5, MH47, MH8, MH21, and MH70) could not utilize nitrate but reduced nitrite to NH4+ and/or N2O. Regardless of their end product profiles, all isolates formed N2O during the first 24 h of incubation. The initial N2O production rates of the denitrifying strains ED1, ED2, ED4, and ED5 were 1 to 2 orders of magnitude higher than those of the other isolates (Table 3).
DISCUSSION
Activation hypothesis for the production of N2O in earthworms.
Production of N2O by earthworms occurs primarily in the gut and is stimulated by nitrate and nitrite but not by ammonium (27, 29). The highest concentrations of N2O occur in the gut lumen (23), and the capacity of gut contents to produce N2O was greater than that of gut walls (Table 1). Although gut wall-attached microorganisms have been observed in earthworms (25), such microorganisms do not appear to be the primary source of the N2O that is emitted by earthworms. This conclusion suggests that the N2O-producing microorganisms of the earthworm gut are primarily microorganisms that have been ingested, which in the case of A. caliginosa consist of microorganisms found in bulk soil or on organic material in soil (e.g., decaying plant roots [15]).
Nitrate was rapidly consumed and sequentially reduced to nitrite, N2O, and N2 in gut content microcosms; in contrast, denitrification in soil microcosms occurred at lower rates without the formation of intermediates and ceased after 2 days (Fig. 1). These results indicate that the amount of electron donors in the earthworm gut is very large and is in marked contrast to the limited amount of electron donors in soil (39). Indeed, the amount of readily utilizable organic substrates in earthworm gut contents is significantly greater than that in soil (26, 42), and addition of organic substrates to gut microcosms did not enhance denitrification or the production of N2O. The initial dynamics for the reduction of nitrate in gut microcosms suggest that the activities (or de novo synthesis) of nitrite reductase and N2O reductase in the gut are delayed compared to the activity of nitrate reductase. Similar dynamics for denitrification by pure cultures of denitrifiers (5) and certain soils (13) have been observed previously. The results obtained from microcosm experiments indicate that the emission of N2O by earthworms is due to the initiation of denitrification or other nitrate-dissimilating activities by ingested bacteria that are activated by the anoxic, substrate-rich conditions of the earthworm gut (23).
Consistent with results obtained for earthworms from forest soil (27), the MPN values for aerobes and anaerobes obtained from the gut contents of A. caliginosa from garden soil were approximately 2 orders of magnitude higher than those obtained for the soil from which the worms were obtained (Table 2). Since the gut passage time is less than 20 h (4), multiplication of microorganisms in the gut and the selective feeding habits of earthworms (14, 26, 32) are unlikely to be reasons for the large difference between the numbers of cultured microorganisms found in the earthworm gut and the numbers of cultured microorganisms found in the soil. Indeed, the total cell counts in the gut of the earthworm Lumbricus terrestris are only one- to sevenfold higher than the total cell counts in the surrounding soil (36). After comparing the numbers obtained with the cultivation methods used in the present study with the total cell counts in the earthworm gut and soil (16, 36), one can speculate that the microorganisms cultured from the earthworm gut accounted for 1 to 6% of the total microorganisms in the gut, whereas the microorganisms cultured from the soil accounted for only 0.1% of the total microorganisms in the soil. While similar numbers have been reported for soils (2), even higher percentages (28 to 82%) have been obtained for earthworms (32). In addition, the percentage of the total microbial cells in soils that are detected by fluorescence in situ hybridization with the bacterial probe EUB338 increases 3- to 12-fold during passage through the gut of L. terrestris, presumably because the ribosome contents of the bacteria increase after activation in the gut (16). Production of intestinal mucus by the earthworm may be an important factor for such activation, and it has been proposed that the digestive system of the earthworm is mutualistic (42).
N2O-producing bacteria in the earthworm gut.
The highest MPN values for N2O producers were observed for nitrate-dissimilating bacteria, a finding that is in agreement with previous reports for soil microbial communities (10, 20). However, the numerical difference between denitrifiers and nitrate dissimilators was marginal (Table 2). The number of denitrifying bacteria obtained from the gut of A. caliginosa was on the same order of magnitude as the number reported for Octolasion lacteum (9.3 × 106 cells g [dry weight]−1) (27), an earthworm with a feeding behavior similar to that of A. caliginosa (28). In contrast to the cultured numbers of methanogens in the gastrointestinal tracts of many other animals (21, 30), the cultured numbers of methanogens in the gut of A. caliginosa were insignificant. Thus, it is not surprising that earthworms and anoxic earthworm gut homogenates do not emit methane (27).
The phylogenetic and functional similarities between the N2O-producing gut isolates and previously characterized soil bacteria support the hypothesis that the N2O-producing microorganisms of the gut are primarily ingested with the food of the earthworm. Most isolates were affiliated with the gamma-proteobacteria or with the low-G+C-content gram-positive bacteria. These phylogenetic groups have been found in fresh casts of Lumbricus rubellus (18), and high numbers of gamma-proteobacteria were detected in the gut of L. terrestris (16).
Nitrite and N2O were intermediates during the reduction of nitrate to N2 by four of the denitrifying isolates, a pattern similar to that observed with gut content microcosms. The N2O production rates for whole worms (normalized by using a fresh weight contribution of gut sections to whole-worm weight of 27% ± 9% [n = 5]) were approximately 1,000 and 2,300 pmol g (fresh weight)−1 h−1 for unsupplemented and nitrate-supplemented worms, respectively (29). If the MPN analyses underestimate the number of denitrifiers (e.g., 6 × 106 cells g [dry weight]−1 [Table 2]) by at least 10-fold, the initial N2O production rates of the denitrifying isolates (e.g., for ED5, 4,900 pmol of N2O h−1107 cells−1 [Table 3]) are within the range of the estimated N2O production rates of whole worms. Thus, N2O production by ingested soil denitrifiers could indeed account for in vivo emission of N2O by earthworms.
The high numbers obtained for nitrate-dissimilating and nitrifying bacteria in the gut suggest that the nondenitrifying, N2O-producing bacterial groups might also contribute to the overall emission of N2O by earthworms. Nitrifiers that might be attached to or spatially near the gut wall could theoretically consume oxygen that might pass through the gut epithelial cells and provide nitrite and/or nitrate for organisms capable of reducing nitrite and/or nitrate to N2O in the gut contents. Nitrifiers might also be a direct source of N2O during nitrifier denitrification (17, 33) or oxidation of ammonia (34) at the gut wall. Likewise, nitrate-dissimilating bacteria can produce N2O from nitrite (38), a reaction that could theoretically be enhanced by the relatively high concentrations of nitrite in the gut (23). Conditions in the gut (i.e., high concentrations of organic substrates and nitrogen [23]) should theoretically stimulate the dissimilation of nitrate (41). However, in gut microcosms, all supplemental nitrate was eventually recovered as N2 (Fig. 1A), indicating that the dissimilation of nitrate to ammonium is not the primary N2O-producing process in the gut of the earthworm. Nonetheless, nitrite is transiently accumulated in cultures of many nitrate-dissimilating bacteria (40), including 10 of the N2O-producing isolates characterized in the present study. Thus, nitrate-dissimilating bacteria might contribute to (i) the high nitrite concentrations in the earthworm gut (23) and (ii) the in vivo emission of N2O by earthworms via nitrite-utilizing denitrifiers in the gut.
Conclusion.
Microcosm experiments, MPN analyses, and the phylogenetic and physiological properties of N2O-producing bacteria from the gut of earthworms suggest that the emission of N2O by earthworms is primarily due to the activation of ingested denitrifiers and other nitrate-dissimilating bacteria by the in situ conditions of the earthworm gut. This hypothesis is evaluated in the companion paper (23).
Acknowledgments
Julian Ihssen and Marcus Horn contributed equally to this study.
Support for this study was provided by the Deutsche Forschungsgemeinschaft (grant DFG DR310/2-1), by the German Ministry of Education, Research, and Technology (grant PT BEO 51-0339476C), and by an Otto-Hahn Award from the Max Planck Society to Andreas Schramm.
We thank Ralf Mertel and Matthias Kador for their excellent technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, I. C., and J. S. Levine. 1986. Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl. Environ. Microbiol. 51:938-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barley, K. P. 1961. The abundance of earthworms in agricultural land and their possible significance in agriculture. Adv. Agron. 13:249-268. [Google Scholar]
- 5.Baumann, B., M. Snozzi, A. J. B. Zehnder, and J. R. van der Meer. 1996. Dynamics of denitrification activity of Paracoccus denitrificans in continous culture during aerobic-anaerobic changes. J. Bacteriol. 178:4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollmann, A., and R. Conrad. 1998. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biol. 4:387-396. [Google Scholar]
- 7.Borken, W., S. Gründel, and F. Beese. 2000. Potential contribution of Lumbricus terrestris L. to carbon dioxide, methane and nitrous oxide fluxes from a forest soil. Biol. Fertil. Soils 32:142-148. [Google Scholar]
- 8.Brohmer, P. 1984. Fauna von Deutschland, 16 Auflage. Quelle und Meyer, Heidelberg, Germany.
- 9.Cataldo, D. A., M. Haroon, L. E. Schrader, and V. L. Young. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Soil Sci. Plant Nutr. 6:71-80. [Google Scholar]
- 10.Chèneby, D., A. Hartmann, C. Hénault, E. Topp, and J. C. Germon. 1998. Diversity of denitrifying microflora and ability to reduce N2O in two soils. Biol. Fertil. Soils 28:19-26. [Google Scholar]
- 11.Cochran, W. G. 1950. Estimation of bacterial densities by means of the “most probable number.” Biometrics 6:105-116. [PubMed]
- 12.De Man, J. C. 1975. The probability of most probable numbers. Eur. J. Appl. Microbiol. 1:67-78. [Google Scholar]
- 13.Dendooven, L., and J. M. Anderson. 1994. Dynamics of reduction enzymes involved in the denitrification process in pasture soil. Soil Biol. Biochem. 26:1501-1506. [Google Scholar]
- 14.Doube, B. M., and G. G. Brown. 1998. Life in a complex community: functional interactions between earthworms, organic matter, microorganisms, and plants, p. 179-211. In C. A. Edwards (ed.), Earthworm ecology. St. Lucie Press, Boca Raton, Fla.
- 15.Edwards, C. A., and P. J. Bohlen. 1996. Biology and ecology of earthworms, 3rd ed. Chapman & Hall, London, United Kingdom.
- 16.Fischer, K., D. Hahn, R. I. Amann, O. Daniel, and J. Zeyer. 1994. In situ analysis of the bacterial community in the gut of the earthworm Lumbricus terrestris L. by whole-cell hybridization. J. Can. Microbiol. 41:666-673. [Google Scholar]
- 17.Freitag, A., M. Rudert, and E. Bock. 1987. Growth of Nitrobacter by dissimilatoric nitrate reduction. FEMS Microbiol. Lett. 48:105-109. [Google Scholar]
- 18.Furlong, M. A., D. R. Singleton, D. C. Coleman, and W. B. Whitman. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadkari, D. 1984. Influence of herbicides Goltix and Sencor on nitrification. Zentralbl. Mikrobiol. 139:623-631. [PubMed] [Google Scholar]
- 20.Gamble, T. N., M. R. Betlach, and J. M. Tiedje. 1977. Numerically dominant denitrifying bacteria from world soils. Appl. Environ. Microbiol. 33:926-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackstein, J. H. P., and C. K. Stumm. 1994. Methane production in terrestrial arthropods. Proc. Natl. Acad. Sci. USA 91:5441-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrigan, W. F., and M. E. McCance. 1966. Laboratory methods in microbiology. Academic Press, London, United Kingdom.
- 23.Horn, M. A., A. Schramm, and H. L. Drake. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ. Microbiol. 69:1662-1669. [DOI] [PMC free article] [PubMed]
- 24.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3B:117-132.
- 25.Jolly, J. M. 1993. Scanning electron microscopy of the gut microflora of two earthworms: Lumbricus terrestris and Octolasion cyaneum. Microb. Ecol. 26:235-245. [DOI] [PubMed] [Google Scholar]
- 26.Karsten, G., and H. L. Drake. 1995. Comparative assessment of the aerobic and anaerobic microfloras of earthworm guts and forest soils. Appl. Environ. Microbiol. 61:1039-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karsten, G. R., and H. L. Drake. 1997. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl. Environ. Microbiol. 63:1878-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, K. E. 1985. Earthworms. Academic Press, Sydney, Australia.
- 29.Matthies, C., A. Grießhammer, M. Schmittroth, and H. L. Drake. 1999. Evidence for involvement of gut-associated denitrifying bacteria in emission of nitrous oxide (N2O) by earthworms obtained from garden and forest soils. Appl. Environ. Microbiol. 65:3599-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, T. L., and M. J. Wolin. 1986. Methanogens in human and animal intestinal tracts. Syst. Appl. Microbiol. 7:223-229. [Google Scholar]
- 31.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen, J. C., and N. B. Hendriksen. 1993. Effect of passage through the intestinal-tract of detritivore earthworms (Lumbricus spp.) on the number of selected gram-negative and total bacteria. Biol. Fertil. Soils 16:227-232. [Google Scholar]
- 33.Poth, M., and D. D. Focht. 1985. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl. Environ. Microbiol. 49:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie, G. A. F., and D. J. D. Nicholas. 1972. Identification of the sources of nitrous oxide produced by oxidation and reductive processes in Nitrosomonas europaea. Biochem. J. 126:1181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt, E. L., and L. W. Belser. 1994. Autotrophic nitrifying bacteria, vol. 5. Soil Science Society of America Book Series. Soil Science Society of America, Madison, Wis.
- 36.Schönholzer, F., D. Hahn, and J. Zeyer. 1999. Origins and fate of fungi and bacteria in the gut of Lumbricus terrestris L. studied by image analysis. FEMS Microbiol. Ecol. 28:235-248. [Google Scholar]
- 37.Schramm, A., D. De Beer, M. Wagner, and R. Amann. 1998. Identification and activity in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor. Appl. Environ. Microbiol. 64:3480-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, S. M. 1983. Nitrous oxide production by Escherichia coli is correlated with nitrate reductase activity. Appl. Environ. Microbiol. 45:1545-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens, R. J., R. J. Laughlin, L. C. Burns, J. R. M. Arah, and R. C. Hood. 1997. Measuring the contributions of nitrification and denitrification to the flux of nitrous oxide from soil. Soil Biol. Bichem. 29:139-151. [Google Scholar]
- 40.Tiedje, J. M. 1994. Denitrifiers, p. 245-267. In Methods of soil analysis, part 2. Microbiological and biochemical properties, vol. 5. Soil Science Society of America Book Series. Soil Science Society of America, Madison, Wis.
- 41.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-243. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, New York, N.Y.
- 42.Trigo, D., and P. Lavelle. 1993. Changes in respiration rate and some physicochemical properties of soil during gut transit through Allolobophora molleri (Lumbricidae, Oligochaeta). Biol. Fertil. Soils 15:185-188. [Google Scholar]
- 43.Wagner, C., A. Griesshammer, and H. L. Drake. 1996. Acetogenic capacities and the anaerobic turnover of carbon in a Kansas prairie soil. Appl. Environ. Microbiol. 62:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webster, E. A., and D. W. Hopkins. 1996. Contributions of different microbial processes to N2O emission from soil under different moisture regimes. Biol. Fertil. Soils 22:331-335. [Google Scholar]
- 45.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]