Abstract
Gastrin-releasing peptide receptor (GRPR) and the epidermal growth factor receptor (EGFR) are expressed in several cancers including non-small cell lung cancer (NSCLC). Here we demonstrate the activation of EGFR by the GRPR ligand, gastrin-releasing peptide (GRP), in NSCLC cells. GRP induced rapid activation of p44/42 MAPK in lung cancer cells through EGFR. GRP-mediated activation of MAPK in NSCLC cells was abrogated by pretreatment with the anti-EGFR-neutralizing antibody, C225. Pretreatment of NSCLC cells with neutralizing antibodies to the EGFR ligands, TGF-α or HB-EGF, also decreased GRP-mediated MAPK activation. On matrix metalloproteinase (MMP) inhibition, GRP failed to activate MAPK in NSCLC cells. EGF and GRP both stimulated NSCLC proliferation, and inhibition of either EGFR or GRPR resulted in cell death. Combining a GRPR antagonist with the EGFR tyrosine kinase inhibitor, gefitinib, resulted in additive cytotoxic effects. Additive effects were seen at gefitinib concentrations from 1 to 18 µM, encompassing the ID50 values of both gefitinib-sensitive and gefitinib-resistant NSCLC cell lines. Because a major effect of GRPR appears to be promoting the release of EGFR ligand, this study suggests that a greater inhibition of cell proliferation may occur by abrogating EGFR ligand release in consort with inhibition of EGFR.
Keywords: EGFR, GRPR, MAPK, signal transduction, non-small cell lung cancer
Introduction
Although lung cancer is the most commonly occurring cancer worldwide, available treatment options are often ineffective. The 5-year survival rates of patients suffering from lung cancer remain low at less than 15% [1]. Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancer cases. NSCLC tumors are known to express high levels of the epidermal growth factor receptor (EGFR) [2], and agents targeting EGFR have shown promise for NSCLC treatment.
Overexpression of EGFR increases cellular proliferation and is associated with poor prognosis in a number of tumor types, including NSCLC [3]. Several signaling pathways lie downstream of EGFR including PI3-kinase, MAPK, signal transducers and activators of transcription (STAT) proteins, and PLCγ-1. A number of clinical trials in NSCLC have been carried out using novel drugs targeting EGFR. Although a correlation between levels of EGFR expression and treatment response was not observed, some late-stage patients with adenocarcinoma of the lung have responded favorably to EGFR inhibition with EGFR tyrosine kinase inhibitor, ZD1839 (gefitinib) [4]. Response rates of lung cancer patients to EGFR inhibition in clinical trials were less than 15%, despite an almost universal expression of the EGFR protein. This has prompted questions about the mechanisms of response to EGFR inhibitors. Recently, EGFR mutations have been described in patients who are associated with increased susceptibility to ZD1839; however, some patients with partial response did not have a mutated EGFR [5,6]. The sensitivity of NSCLC cell lines to gefitinib varies over a 100-fold range; cell lines with EGFR mutations generally display ID50 values in the 0.1 to 1.0 µM range, whereas cell lines lacking mutations generally display ID50 values in the 10 to 20 µM range [5,6].
G-protein-coupled receptors (GPCRs) are seven-transmembrane spanning proteins that are involved in a number of cellular processes. We previously demonstrated expression of the GPCR gastrin-releasing peptide receptor (GRPR) in NSCLC [7]. Neutralizing antibody (2A11) to the GRPR ligand, gastrin-releasing peptide (GRP), resulted in the cell death of head and neck squamous cell cancer (HNSCC) [8]. Several GPCRs including GRPR have been reported to trigger signaling pathways through EGFR [9] in a number of cell systems [10].
Both EGFR and GRPR are activated in NSCLC by their corresponding ligands, TGF-α and GRP, in an autocrine manner. In this study, we examined the interactions between GRPR and EGFR in NSCLC cell lines because both receptors are expressed in NSCLC and are potential targets for therapy. Stimulation by GRP results in rapid activation of EGFR and signaling through MAPK downstream of EGFR, which is dependent on EGFR activation. We also demonstrated that matrix metalloproteases (MMPs) play a role in GRP-mediated activation of EGFR, and that combining inhibitors of these pathways increases the antiproliferative activity observed against lung cancer cells.
Materials and Methods
Cells and Reagents
Lung adenocarcinoma cell line 201T was maintained in BME supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) [7]. Growth factors, EGF and GRP, were obtained from Sigma-Aldrich Corporation (St. Louis, MO). The EGFR-specific small molecule tyrosine kinase inhibitors, PD153035 (Calbiochem-Novabiochem Corporation, San Diego, CA) and ZD1839 (gefitinib, or Iressa), obtained from AstraZeneca Pharmaceuticals, LP (Wilmington, DE), were used to inhibit EGFR. In addition, an anti-EGFR antibody C225 (ImClone Systems Incorporated, New York, NY) specific to the extracellular domain of EGFR was used to block EGFR ligand binding. The GRP antagonist, PD176252, was obtained from Pfizer, Inc. (New York, NY) [11]. Broad-spectrum inhibitors, Marimastat (British Biotech, Oxford, UK) and GM6001 (Calbiochem-Novabiochem Corporation), were used to inhibit cellular MMPs. Antibodies to p42/44 phospho-MAPK and MAPK were obtained from Cell Signaling Technologies (Beverly, MA). Anti-EGFR antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-phospho-tyrosine antibody PY99 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was obtained from Oncogene Research Products (Boston, MA).
In Vitro Assay
Approximately 2 x 105 cells/ml were plated in 100-mm dishes in BME supplemented with 10% heat-inactivated fetal bovine serum. Cells were allowed to adhere overnight and serum-starved for 48 hours with daily changes in BME media. Cells were pretreated with inhibitors or antibodies for 2 hours followed by stimulation with either the vehicle (water) or growth factors, EGF (10 ng/ml) or GRP (400 nM), for 10 minutes in the absence or presence of inhibitors. Cells were harvested in cold lysis buffer (10 mM Tris-HCl, pH 7.6, 50 mM Na4P2O7, 50 mM NaF, 1 mM NaV3O4, 1% Triton X-100, and 1x protease inhibitor cocktail tablet that included a broad-spectrum potent inhibitor of protein tyrosine phosphatases; Roche, Mannheim, Germany) after two washes in ice-cold PBS.
Immunoprecipitation and Immunoblotting
Cell lysates were briefly sonicated on ice and cleared by centrifugation at 4°C for 5 minutes at 14,000 rpm. Protein quantification was carried out using the Protein Assay Solution (BioRad Laboratories, Hercules, CA). For immunoprecipitations, 100 µg of protein was mixed with 3 µg of anti-EGFR antibody (Upstate Biotechnology) and 40 µl of Protein G-agarose beads (Invitrogen). Lysates were placed on an orbital shaker overnight at 4°C. The beads were precipitated by centrifugation, washed thrice in ice-cold lysis buffer, and resuspended in 4x sample buffer. Beads were boiled for 5 minutes. The proteins were resolved on an 8% SDS-PAGE gel and transferred to a nitrocellulose membrane in a semidry transfer apparatus. Membranes were blocked in 5% milk followed by immunoblotting with the appropriate antibodies.
In Vitro Proliferation and Cytotoxicity Assays
NSCLC cells 201T were plated at a density of 2 x 104 cells/ml in 24-well plates in BME supplemented with 10% heat-inactivated fetal calf serum and treated after 24 hours. For proliferation assays, cells were serum-starved for 24 hours followed by stimulation with either EGF (10 ng/ml) or GRP (400 nM) either in the presence or absence of C225 (6 µg/ml). For cytotoxicity assays, cells were treated with either vehicle control (DMSO), ZD1839 (Iressa), or PD176252 in serum-containing media for 72 hours. Cell viability was assessed by measuring the number of metabolically active cells using the MTT assay. IC50 dose was calculated using PRISM Version 3 (Graphpad Software, San Diego, CA).
Results
Lung Cancer Cells Express EGFR
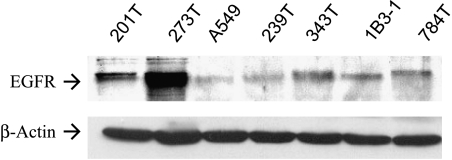
A panel of six human NSCLC cell lines and the immortalized human bronchial epithelial cell line IB3-1 were examined for EGFR expression by immunoblotting. All cell lines expressed the EGFR receptor (Figure 1). An adenocarcinoma line (201T) was demonstrated to express moderate levels of the EGFR receptor. In addition, this line was found to express GRPR by immunoblotting (data not shown). On the basis of the simultaneous expression of both receptors, we chose this line for further studies.
Figure 1.
NSCLC cells express EGFR. Western blot analysis demonstrating levels of EGFR in NSCLC cells including adenocarcinoma (201T). Immortalized epithelial cells (IB31) also expressed EGFR. The same membrane was probed for β-actin to demonstrate an equal loading of protein in all lanes.
GRP Stimulation Increases EGFR and MAPK Phosphorylation
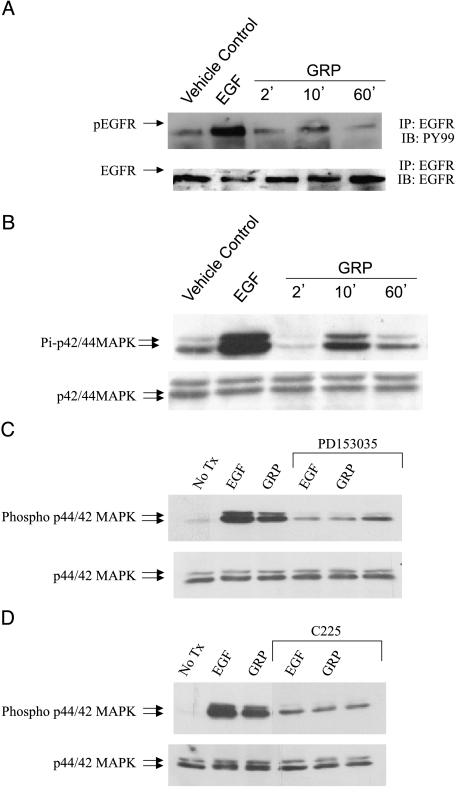
Adenocarcinoma cells 201T were serum-starved for 72 hours prior to growth factor stimulation. Treatment with GRP resulted in phosphorylation of EGFR at 10 minutes (Figure 2A). EGF at a concentration that gives maximal EGFR stimulation was used as a positive control, and is higher than what can be achieved by autocrine release of ligand. GRP stimulation for 10 minutes also resulted in activation of MAPK in 201T cells in a time-dependant manner (Figure 2B). Maximal phosphorylation of MAPK was achieved at 10 minutes. A squamous cell cancer line (273T) also demonstrated EGFR and MAPK activation on GRP stimulation (data not shown).
Figure 2.
GRP stimulation activates EGFR and downstream MAPK. NSCLC cells 201T were serum-starved for 72 hours followed by stimulation with either EGF (10 ng/ml) or GRP (400 nM) for various time points. (A) Cell lysates were immunoprecipitated with EGFR and subjected to immunoblotting with phospho-tyrosine antibody PY99. The membrane was stripped and immunoblotted for total EGFR to demonstrate an equal loading of protein in all lanes. (B) Cell lysates were analyzed for activated and total p42/44 MAPK levels by immunoblotting. NSCLC cells were serum-starved for 72 hours followed by pretreatment with EGFR-specific inhibitors, (C) PD153035 or (D) C225, for 2 hours. Untreated cells or inhibitor-treated cells were then stimulated with EGF (10 ng/ml) or GRP (400 nM) for 10 minutes. Cell lysates were analyzed for phospho-p42/44 MAPK and total p42/44 MAPK levels by immunoblotting.
To investigate the mechanism whereby GRP activates MAPK in NSCLC cells, we pretreated 201T cells with EGFR-specific tyrosine kinase inhibitors, PD153035 (100 nM) (Figure 2C) or C225 (6 µg/ml) (Figure 2D). Again, EGF, alone and with EGFR inhibitors at a concentration that gives maximal stimulation of EGFR, was used as a positive and negative control, respectively. After 2 hours of preincubation, the cells were stimulated with GRP in the presence or absence of inhibitors for 10 minutes. Activated and total levels of MAPK were determined by immunoblotting. Both EGF and GRP failed to activate MAPK when EGFR was inhibited in NSCLC cells, whereas both were able to activate MAPK in the absence of EGFR inhibition. This indicates that GRP activates MAPK primarily through EGFR in NSCLC cells.
TGF-α and HB-EGF Are Involved in GRP-Mediated EGFR Activation
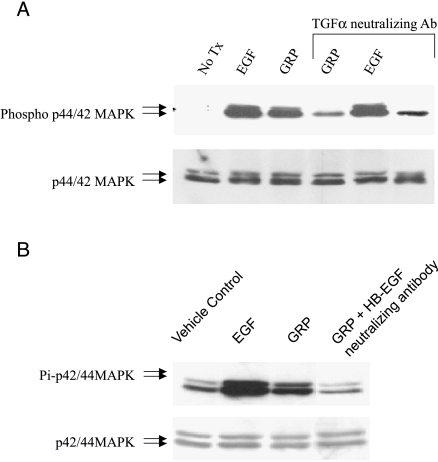
We have previously demonstrated that GRP-mediated EGFR activation in head and neck cells occurs through extracellular ligand binding to the receptor. EGFR ligands, TGF-α and heparin binding-EGF (HB-EGF), are present on the membrane surface as proligands. Cleavage of these proligands releases activated ligands into the extracellular milieu. We also previously investigated the role of both these ligands in GRP-mediated EGFR activation in HNSCC. Neutralizing antibodies to TGF-α and HB-EGF were preincubated with 201T cells for 2 hours prior to GRP stimulation. Cell lysates were analyzed by immunoblotting for activated and total MAPK. Both TGF-α and HB-EGF-neutralizing antibodies abrogated GRP-mediated MAPK activation in NSCLC cells (Figure 3, A and B, respectively). TGF-α-neutralizing antibody had no effect on EGF stimulation of MAPK, as expected. These results imply that on GRP stimulation, both EGFR proligands, TGF-α and HB-EGF, are cleaved from the membrane surface and released into the extracellular milieu. Once released from the membrane surface, the ligands bind to the extracellular domain of EGFR in an autocrine fashion, activating the receptor.
Figure 3.
GRP stimulation results in EGFR activation through extracellular ligand binding to the receptor. Serum-starved NSCLC cells were pretreated for 2 hours with (A) TGF-α-neutralizing antibody (6 µg/ml) or (B) HB-EGF-neutralizing antibody (6 µg/ml) followed by EGF (10 ng/ml) or GRP (400 nM) stimulation for 10 minutes. Immunoblotting was carried out for phospho-p42/44 MAPK and total p42/44 MAPK.
MMPs Are Required for GRP-Mediated EGFR Activation
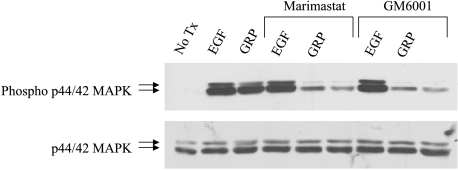
MMPs are involved in the processing of EGFR proligands bound to the membrane surface. Blocking MMP activity with broad-spectrum MMP inhibitors abrogated autocrine activation of EGFR in head and neck cancer cells [9,12]. To investigate the role of MMPs in GRP-mediated EGFR activation, 201T cells were pretreated with either of two MMP inhibitors, Marimastat (20 nM) or GM6001 (200 nM). After 2 hours of pretreatment with the MMP inhibitors, cells were stimulated with GRP in the presence or absence of the MMP inhibitors. Cell lysates were then harvested and analyzed by immunoblotting for activated and total MAPK. Inhibition of MMPs abrogated GRP-mediated, but not EGF-mediated, MAPK activation in NSCLC cells (Figure 4). This suggests that on GRP stimulation of NSCLC cells, MMPs are activated and cleave the EGFR proligands from the membrane surface to facilitate EGFR activation.
Figure 4.
MMPs are involved in GRP-mediated EGFR activation. Serum-starved NSCLC cells were pretreated with MMP inhibitors, Marimastat or GM6001, for 2 hours prior to GRP (400 nM) stimulation. Immunoblotting was carried out with phospho-p42/44 MAPK and total p42/44 MAPK.
GRP Stimulates Proliferation of NSCLC Cells through EGFR
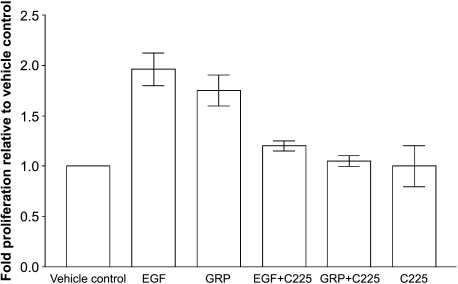
Serum-starved NSCLC cells 201T were stimulated with either EGF or GRP for 48 hours in the presence or absence of C225 (6 µg/ml) followed by MTT assay. Both EGF and GRP stimulated 201T cell proliferation (Figure 5). GRP stimulated 201T cell proliferation significantly compared to the vehicle control (P = .004) as determined by the Wilcoxon-Mann-Whitney test using STATXACT (Version 6; Cytel Software Cooperation, Cambridge, MA). Both EGF an GRP-mediated 201T proliferation were abrogated by EGFR inhibition with antibody C225. This demonstrates that EGFR mediates 201T cell proliferation that is initiated by GRP.
Figure 5.
GRP stimulates NSCLC cell proliferation through EGFR. NSCLC cells were serum-starved for 24 hours followed by stimulation with EGF (10 ng/ml) or GRP (400 nM) either in the presence or absence of anti-EGFR antibody C225 (6 µg/ml) for 48 hours. Metabolically active cells were estimated by MTT assay.
EGFR and GRPR Can Be Effectively Targeted in NSCLC Cells
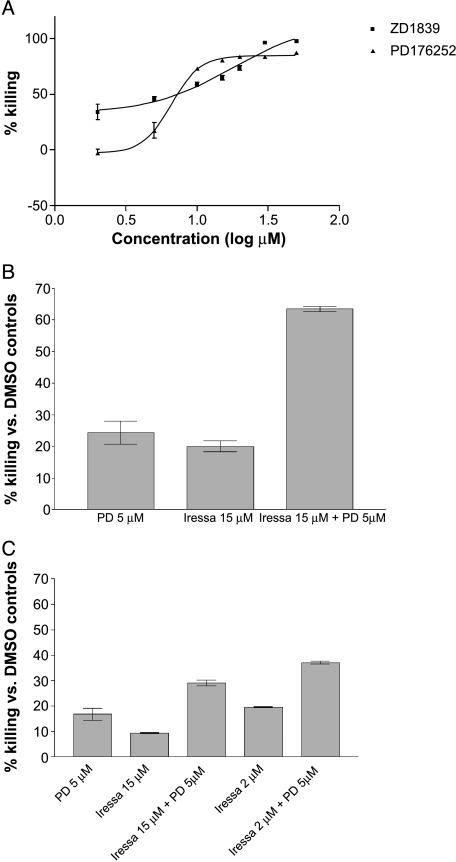
The antitumor efficacy of targeting EGFR and GRPR with specific small molecule inhibitors was tested in vitro. NSCLC cells 201T were treated with varying concentrations of either EGFR-specific tyrosine kinase inhibitor ZD1839 or GRPR antagonist PD176252 for 72 hours followed by MTT assay. The IC50 for the inhibitors was determined. ZD1839 was cytotoxic to 50% of the cells at a concentration of 18 µM, whereas PD176252 had an IC50 of 8 µM (Figure 6A). We then combined both inhibitors at sub-IC50 concentrations to investigate the antitumor effects of dual receptor blockade in vitro. NSCLC cells were treated with a combination of ZD1839 and PD176252 for 72 hours followed by the MTT assay. Both compounds inhibited NSCLC cell growth additively at concentrations lower that the IC50 for either compound alone (15 µM ZD1839 and 5 µM PD176252), achieving more than 60% cell kill with the combination (Figure 6B). Combining lower, more clinically relevant concentrations (1 and 2 µM) of ZD1839/gefitinib with PD176252 also results in additive cytotoxic effects (30–40% cell kill for the combination compared to 10% to 18% with either drug alone; Figure 6C). Combining the two compounds at the IC50 concentrations resulted in 99% cell kill (data not shown). This indicates that combined targeting of EGFR and GRPR using small molecule inhibitors is more cytotoxic compared to inhibition of either receptor alone in NSCLC cells, throughout the concentration-response range.
Figure 6.
Targeting EGFR and GRPR has antitumor efficacy against NSCLC in vitro. (A) NSCLC cells 201T were treated with increasing concentrations of either EGFR inhibitor, ZD1839 (0–50 µM), or GRPR antagonist, PD176252 (0–50 µM), for 72 h followed by the MTT assay. The IC50 for ZD1839 (18 µM) and PD 176252 (8 µM) was calculated using nonlinear regression analysis. (B) NSCLC cells were treated with ZD1839 (15 µM), PD176252 (5 µM), or a combination of both inhibitors for 72 hours prior to MTT assay. (C) NSCLC cells were treated with ZD1839 (1 or 2 µM), PD176252 (5 µM), or a combination of both inhibitors for 72 hours prior to MTT assay.
Discussion
Increased expression levels of EGFR have been reported in over 70% of NSCLC [13]. EGFR overexpression in NSCLC cells increases cellular proliferation and correlates with poor outcome [14,15]. In addition, NSCLC cells grown in serum-free medium express an autocrine loop for GRPR and GRP [16]. We have also demonstrated that GRP is mitogenic in many NSCLC cell lines and that autocrine loops for GRPR and its ligands are present [7]. Here we demonstrate for the first time the activation of EGFR and one of its downstream pathways by GRP in NSCLC cells.
Several signaling pathways are triggered following the activation of EGFR including MAPK and PI3K, contributing to cell proliferation and survival. We and others have demonstrated that GPCRs mediate the activation of EGFR in HNSCC [9]. In the present study, we demonstrate that GRP stimulation of NSCLC cells results in activation of EGFR and the MAPK pathway downstream of EGFR. Moreover, abrogation of EGFR by EGFR-specific tyrosine kinase inhibitors also blocks GRP-mediated MAPK activation. This result corroborates our previous findings in HNSCC where GRP activated MAPK primarily through EGFR, and suggests that this pathway cross-talk is a general effect found in many tumor types [9]. GRP has been reported to have a low affinity for the neuromedin B receptor [17]. However, GRP stimulation fails to activate MAPK in GRPR-deficient murine fibroblasts, indicating that GRP activates MAPK primarily through GRPR, not NMBR (data not shown). In another report, GRPR and NMBR have been demonstrated to have distinct functions with only a partial overlap [18]. We have previously demonstrated that in EGFR-deficient cells, GRP fails to activate MAPK [9]. Our results indicate that through GRPR, GRP activates MAPK primarily through EGFR in NSCLC cells because EGFR abrogation reduces GRP-mediated MAPK activation to baseline levels.
GRP could activate EGFR either through an extracellular or an intracellular mechanism. We and others have previously demonstrated that EGFR activation by GPCRs involves extracellular ligand binding to EGFR in HNSCC [9,19] and colon cancer cells [20]. Interestingly, the specific ligands released from the membrane surface on GPCR activation vary among different cell types. We found that in NSCLC cells, both TGF-α and HB-EGF are involved in GRP-mediated EGFR activation; whereas in HNSCC cells, HB-EGF is not implicated [9]. TGF-α is commonly reported to be involved in EGFR activation on GPCR stimulation in different cell types, including colon cancer and HNSCC [20,19].
EGFR ligands exist on the membrane surface as inactive proligands. These proligands are activated by proteolytic cleavage from the membrane surface into active extracellular ligands that can bind to EGFR. MMPs have been previously implicated in EGFR proligand processing [21]. Blocking MMP activity with broad-spectrum MMP inhibitors such as Marimastat and GM6001 in NSCLC cells abrogated MAPK activation by GRP, implying that MMPs play a role in EGFR ligand release from the membrane surface. This finding in NSCLC cells is similar to the reported mechanism of EGFR activation by GRP in HNSCC cells [9].
Our data suggest that GRPR contributes to NSCLC cell proliferation, most likely by triggering the release of EGFR ligands from the cell surface. Targeting GRPR with the GRPR antagonist, PD176252, was cytotoxic to NSCLC cells and corroborates previously reported effects in lung cancer cells with a comparable IC50 dose [11]. Inhibition of GRPR may severely limit the autocrine release of EGFR ligands in both NSCLC and HNSCC, acting as a brake on EGFR signaling. In addition, we demonstrated that 201T NSCLC cells are susceptible to EGFR blockade using ZD1839 (gefitinib), with an IC50 of 18 µM, near the reported IC50 for other gefitinib-resistant NSCLC cells that lack EGFR mutations [5,6]. Gefitinib combined with a GRPR antagonist showed additive cytotoxic effects against NSCLC. Combination of the GRPR antagonist with gefitinib at 1 to 2 µM (concentrations that are similar to those currently achievable in serum with daily gefitinib dosing in patients) resulted in up to 40% cell kill, compared to 10% to 20% with gefitinib alone, whereas over 90% cell kill was achieved with high-concentration gefitinib (18 µM). Cytotoxicity in NSCLC with ZD1839/gefitinib at high concentrations may be the result of achieving the ID50 for gefitinib-resistant cells that lack EGFR mutation and may also result in inhibition of other tyrosine kinases such as erbB2 and PDGFR, increasing nonspecific killing. However, using the current daily oral dose of gefitinib of 250 to 500 mg, such high levels of drug cannot be achieved in patients [5]. There are new clinical trials in progress that will test intermittent high-dose gefitinib, however, in an attempt to produce transient serum levels high enough to inhibit the EGFR of gefitinib-resistant cells. Agents such as GRPR antagonists that could produce additive effects with EGFR inhibitors could be useful in reducing the serum level of gefitinib required for activity, or in prolonging or enhancing responses to gefitinib in both sensitive and resistant cells.
Our results demonstrate that in NSCLC cells, GRPR stimulation results in EGFR activation, triggering signal transduction through the MAPK pathway. GRPR stimulation also triggers cell proliferation, and combined targeting of EGFR and GRPR may have additive antitumor effects in NSCLC cells. Combined targeting may result in total EGFR pathway blockade by inhibiting both the release of ligand and the receptor tyrosine kinase. This might improve the efficacy of EFGR tyrosine kinase inhibitors, especially in tumors that lack activating EGFR mutations. This is the first report in NSCLC cells demonstrating that GRPR can trigger signal transduction pathways through EGFR, identifying targets for combined antitumor therapy in NSCLC cells [22].
Footnotes
This work was supported by a grant (U01 CA84968) from the Early Detection Research Network (J.R.G. and J.M.S.), a grant (P50 CA90440) from the Specialized Program of Research Excellence (J.M.S.), and a National Institutes of Health grant (1 R01 CA 098372-01). S.M.T. and V.Y.W.L. were supported by Career Development Awards from the University of Pittsburgh Lung Cancer SPORE.
References
- 1.Carney DN. Lung cancer—time to move on from chemotherapy. N Engl J Med. 2002;346:126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 2.Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- 3.Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, Mossetti C, Ardissone F, Lausi P, Scagliotti GV. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller VA, Kris MG. “Targeting” the epidermal growth factor receptor tyrosine kinase with gefitinib (Iressa) in non-small cell lung cancer (NSCLC) Semin Cancer Biol. 2004;14:33–40. doi: 10.1016/j.semcancer.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Siegfried JM, Krishnamachary N, Gaither Davis A, Gubish C, Hunt JD, Shriver SP. Evidence for autocrine actions of neuromedin B and gastrin-releasing peptide in non-small cell lung cancer. Pulm Pharmacol Ther. 1999;12:291–302. doi: 10.1006/pupt.1999.0210. [DOI] [PubMed] [Google Scholar]
- 8.Lango MN, Dyer KF, Lui VW, Gooding WE, Gubish C, Siegfried JM, Grandis JR. Gastrin-releasing peptide receptor-mediated autocrine growth in squamous cell cancer of the head and neck. J Natl Cancer Inst. 2002;94:375–383. doi: 10.1093/jnci/94.5.375. [DOI] [PubMed] [Google Scholar]
- 9.Lui VW, Thomas SM, Zhang Q, Wentzel AL, Siegfried JM, Li JY, Grandis JR. Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor. Oncogene. 2003;22:6183–6193. doi: 10.1038/sj.onc.1206720. [DOI] [PubMed] [Google Scholar]
- 10.Santiskulvong C, Sinnett-Smith J, Rozengurt E. EGF receptor function is required in late G(1) for cell cycle progression induced by bombesin and bradykinin. Am J Physiol Cell Physiol. 2001;281:C886–C898. doi: 10.1152/ajpcell.2001.281.3.C886. [DOI] [PubMed] [Google Scholar]
- 11.Moody TW, Leyton J, Garcia-Marin L, Jensen RT. Nonpeptide gastrin releasing peptide receptor antagonists inhibit the proliferation of lung cancer cells. Eur J Pharmacol. 2003;474:21–29. doi: 10.1016/s0014-2999(03)01996-4. [DOI] [PubMed] [Google Scholar]
- 12.P OC, author. Rhys-Evans P, Eccles S. A synthetic matrix metalloproteinase inhibitor prevents squamous cancer cell proliferation by interfering with epidermal growth factor receptor autocrine loops. Int J Cancer. 2002;100:527–533. doi: 10.1002/ijc.10531. [DOI] [PubMed] [Google Scholar]
- 13.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Zhang X, Bai CX, Chen J, Wei MQ. Inhibition of epidermal growth factor receptor expression by RNA interference in A549 cells. Acta Pharmacol Sin. 2004;25:61–67. [PubMed] [Google Scholar]
- 15.Onn A, Correa AM, Gilcrease M, Isobe T, Massarelli E, Bucana CD, O'Reilly MS, Hong WK, Fidler IJ, Putnam JB, et al. Synchronous overexpression of epidermal growth factor receptor and HER2-neu protein is a predictor of poor outcome in patients with stage I non-small cell lung cancer. Clin Cancer Res. 2004;10:136–143. doi: 10.1158/1078-0432.ccr-0373-3. [DOI] [PubMed] [Google Scholar]
- 16.Siegfried JM, Han YH, DeMichele MA, Hunt JD, Gaither AL, Cuttitta F. Production of gastrin-releasing peptide by a non-small cell lung cancer cell line adapted to serum-free and growth factor-free conditions. J Biol Chem. 1994;269:8596–8603. [PubMed] [Google Scholar]
- 17.Tokita K, Hocart SJ, Coy DH, Jensen RT. Molecular basis of the selectivity of gastrin-releasing peptide receptor for gastrin-releasing peptide. Mol Pharmacol. 2002;61:1435–1443. doi: 10.1124/mol.61.6.1435. [DOI] [PubMed] [Google Scholar]
- 18.Ohki-Hamazaki H, Sakai Y, Kamata K, Ogura H, Okuyama S, Watase K, Yamada K, Wada K. Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor. J Neurosci. 1999;19:948–954. doi: 10.1523/JNEUROSCI.19-03-00948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell cancer cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–6336. [PubMed] [Google Scholar]
- 20.McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J Biol Chem. 2002;277(45):42603–42612. doi: 10.1074/jbc.M206487200. [DOI] [PubMed] [Google Scholar]
- 21.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 22.Culy CR, Faulds D. Gefitinib. Drugs. 2002;62:2237–2248. doi: 10.2165/00003495-200262150-00008. discussion 2249–2250. [DOI] [PubMed] [Google Scholar]