Abstract
Indoleamine 2,3-dioxygenase (IDO), a catabolizing enzyme of tryptophan, is supposed to play a role in tumor immune escape. Its expression in solid tumors has not yet been well elucidated: IDO can be expressed by the tumor cells themselves, or by ill-defined infiltrating cells, possibly depending on tumor type. We have investigated IDO expression in 25 cases of non-small cell lung cancer (NSCLC). Using histochemistry and immunohistochemistry, we found that IDO was expressed not by tumor cells, but by normal cells infiltrating the peritumoral stroma. These cells were neither macrophages nor dendritic cells, and were identified as eosinophil granulocytes. The amount of IDO-positive eosinophils varied in different cases, ranging from a few cells to more than 50 per field at x200 magnification. IDO protein in NSCLC was enzymatically active. Therefore, at least in NSCLC cases displaying a large amount of these cells in the inflammatory infiltrate, IDO-positive eosinophils could exert an effective immunosuppressive action. On analyzing the 17 patients with adequate follow-up, a significant relationship was found between the amount of IDO-positive infiltrate and overall survival. This finding suggests that the degree of IDO-positive infiltrate could be a prognostic marker in NSCLC.
Keywords: Non-small cell lung cancer; indoleamine 2,3-dioxygenase; eosinophil granulocytes; immune escape; prognostic marker
Introduction
A fundamental question in tumor pathogenesis is why the immune system is unable to eradicate transformed cells and why, instead, it becomes tolerant of tumor growth and metastasis. A possible mechanism is the exploitation, by the tumor itself, of the mechanisms normally used by the immune system for preventing self-tissue destruction and autoimmune diseases. This mechanism is likely to be involved in downregulating tumor recognition. The occurrence of regulatory T cells suppressing immune response in human tumors has long since been described [1].
However, an entirely new immunosuppressive mechanism displayed by cells of the myeloid lineage has been described recently. The enzyme, indoleamine 2,3-dioxygenase (IDO), by virtue of its catabolization of the essential amino acid tryptophan, is endowed with a very powerful immunosuppressive activity. In pregnant mice, IDO expression in the placenta is fundamental in preventing the mother's immune system from attacking the semiallogenic fetus: inhibition of IDO activity results in the loss of all allogenic fetuses [2]. It has also been demonstrated in vitro that IDO expression can be induced in both macrophages and dendritic cells (DCs) [3–5], which thereafter acquire the capacity to inhibit cell proliferation. As result of IDO activity, both T and NK cells reverse their commitment to cell cycle progression and enter an arrested state [6,7], with both adaptive and innate immunity being consequently affected.
It has been suggested that IDO may be a microenvironmental factor that could play a role in tumor evasion from a T cell-mediated rejection [8]. Recent evidence suggests that IDO expression occurs in tumor-draining lymph nodes and in tumor tissues as well. In the first case, accumulation of IDO-positive DCs was found in draining nodes from patients with melanoma, breast, colon, lung, and pancreatic cancers [5]. Less clear, instead, is the pattern of IDO expression in solid tumor tissues. Uyttenhove et al. [9] have found IDO expression in tumor cells in a number of human solid tumors, even though the percentage of IDO-positive specimens and IDO-positive tumor cells varied greatly among the different types of tumors. More recently, other researchers demonstrated that, in hepatocarcinoma, IDO expression is restricted to tumor-infiltrating cells, not to tumor cells [10]. The precise identity of the former cells could not be determined, and the cells were considered to likely be macrophages or DCs.
Non-small cell lung cancer (NSCLC) is not only a frequent tumor but also an aggressive one: median survival is severely reduced in advanced tumors, and the different chemotherapy and radiotherapy approaches result in poor enhancement of survival. Tumor-infiltrating lymphocytes in NSCLC are anergic and do not proliferate, but they can be expanded after purification and restimulation [11]. For this reason, NSCLC could be a tumor where IDO-dependent immune suppression could take place.
Materials and Methods
Patients
Archival formalin-fixed paraffin-embedded tumor samples were obtained from 21 patients who underwent surgical therapy at the Surgery Clinic, Policlinico S. Matteo, University of Pavia (Pavia, Italy), and from patients who underwent surgical therapy at the Thoracic Surgery Department, Ospedale S. Croce e Carle (Cuneo, Italy). The fresh tumor samples used for the evaluation of IDO activity, as well as the cryostat section used in immunohistochemistry, were from the latter center.
All the patients had NSCLC, and none of them had received therapy before surgery.
Antibodies
A rabbit antihuman IDO antiserum, raised against a synthetic peptide [12], and a mouse monoclonal antibody raised against a GST fusion protein from human IDO (both from Chemicon, Temecula, CA) were used to detect IDO on paraffin and frozen sections, respectively. To detect macrophages and DCs, an anti-CD68 (Dako, Carpinteria, CA) MoAb and an anti-S100 (Dako) MoAb were used. To detect NSCLC cells, the AE1/AE3 biclonal antibody (Progen Biotechnik, Heidelberg, Germany) and the MNF116 (Dako) MoAb were used. These reagents react with cytokeratins expressed in NSCLC [13,14]. To detect eosinophils, we used a mouse MoAb against human Major Basic Protein (MBP; Cymbus Biotechnology, Hants, UK).
Western Blotting Analysis of Anti-IDO Antiserum Specificity
For protein extraction, cells were lysed for 30 minutes on ice in RIPA buffer [10 mM Tris, pH 7.5, 150 mM NaCl, 1% sodium deoxycholate, 1% Nonidet NP-40, 01% sodium dodecyl sulfate (SDS), 1 mM EDTA, 10 mM KCl, 20 µg/ml leupeptin, PMSF, and aprotinin]. Protein concentration was determined using the protein assay reagent (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Equal amounts of proteins (25 µg) were fractionated on a 10% SDS polyacrylamide minigel and transferred to nitro-cellulose filters (Hybond-C; Amersham Biosciences, Little Chalfont, UK). After staining with Ponceau Red, blots were saturated overnight in 5% dried milk in TTBS (20 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20). The primary antibody, a rabbit anti-IDO, was diluted 1:1500 in blocking buffer. As a secondary antibody, we used a horseradish peroxidase (HRP)-conjugated antirabbit IgG (Amersham Biosciences). The ECL Western blotting method (Amersham Biosciences) was used for detection. As a control, we used purified rabbit IDO, obtained as previously described [6].
Immunohistochemistry and Histochemistry
Sections of formalin-fixed paraffin-embedded tumor samples were stained with the rabbit anti-IDO as follows. Slides were hydrated through a graded series of ethanol and treated for 10 minutes at room temperature with 0.5 µg/ml proteinase K in PBS, as recommended by Chemicon. When required, endogenous peroxidases were blocked with the peroxidase block from Dako. Slides were then incubated for 30 minutes with the rabbit anti-IDO antibody (1:300) in PBS containing 0.05% Tween 20 and 10% normal goat serum. Secondary antibody was either a HRP-conjugated or an alkaline phosphatase (AP)-conjugated antirabbit (EnVision+ System; Dako), revealed with AEC (Dako) or Fast Blue (Sigma-Aldrich, St. Louis, MO), respectively. The latter staining was done in the presence of Levamisole to block endogenous phosphatases. Counterstaining was performed with hematoxylin.
The same protocol was used for immunocytochemistry on activated macrophages or purified eosinophils. Air-dried smears were fixed for 2 minutes with 10% formalin, digested for 5 minutes with proteinase K, and processed as above.
In the case of double staining, the rabbit anti-IDO was developed with the AEC system described above, then a second reaction with a mouse antibody (either anticytokeratins, anti-S100, or anti-CD68) was performed and revealed with Fast Blue.
In order to verify the specificity of the rabbit antiserum, we also used an anti-IDO mouse monoclonal on frozen sections. In this case, sections were fixed for 10 minutes in cold methanol/acetone (1:1), and stained with the mouse anti-IDO diluted 1:300 in PBS containing 0.05% Tween and 10% normal goat serum. Secondary antibody was a biotin-conjugated antimouse (BioSpA, Milan, Italy), revealed with AP-conjugated streptavidin (BioSpa) and Fast Red (LabVision Corporation, Fremont, CA). The same section was then destained and stained with the MoAb anti-MBP, revealed with HRP-conjugated streptavidin (BioSpA) and DAB substrate (LabVision).
The specificity of the immunoreactions was confirmed by the negative results obtained on adjacent sections by replacing the primary antibody with rabbit or mouse serum.
Peroxidase-positive cells were identified by a histochemistry reaction with diaminobenzidine (DAB). Slides, either unstained or previously stained with IDO and Fast Blue, were incubated 20 for minutes at room temperature with DAB, a peroxidase substrate that stains peroxisomes brownish red [15].
To identify eosinophils by histochemistry, the slides stained with IDO were first photographed in specific fields by recording the microscope coordinates. Afterward, slides were stained with a standard hematoxylin-eosin stain, a standard May Grunwald-Giemsa stain, or Luna's stain [16]. The latter technique produces a distinctive red stain and is specific for eosinophils because it stains only eosinophil granules, Charcott-Leyden crystals, and erythrocytes. The previously photographed cells were then identified through the coordinates and rephotographed. Images were taken with an Olympus (Nagano, Japan) C3030 zoom digital camera attached to an Olympus BX51 microscope, with Olympus Camedia Master software.
In order to evaluate IDO-positive infiltrate, each sample was stained for IDO, then seven fields at x200 magnification were randomly chosen by each of two investigators (S.A. and G.F.), and IDO-positive cells were counted.
Separation of Cells from Peripheral Blood
To obtain eosinophils, heparinized venous blood was obtained (after informed consent) from healthy individuals with eosinophil counts in the upper normal range. A standard two-step separation, dextran sedimentation followed by Fycoll, was performed and contaminant erythrocytes were removed by osmotic lysis. Eosinophils were then purified by removing neutrophils with CD16 MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) by following exactly the manufacturer's instructions.
To obtain neutrophils and macrophages, heparinized venous blood was obtained (after informed consent) from healthy volunteers.
To obtain IDO-positive macrophages, PBMCs were isolated by centrifugation on a discontinuous density gradient of Fycoll-Hypaque (Biochrom KG, Berlin, Germany). Afterward, monocytes were enriched from adherent PBMCs by means of immunomagnetic beads (IO Beads; Immunotech-Coulter, Marseille, France), precisely following the manufacturer's instructions. MCSF (Vinci-Biochem, Florence, Italy) was then added at a concentration of 200 U/ml, and cells were grown for 5 days. IFN-γ (100 U/ml) was then added and, after 48 hours, cells were collected for Western blot analysis.
To obtain neutrophils, a standard two-step separation (dextran and Fycoll) was performed, followed by osmotic lysis of contaminant erythrocytes.
Purity of the enriched populations was checked by Luna's staining for eosinophils, by flow cytometry analysis of CD14 membrane expression for monocytes, and by May Grunwald-Giemsa staining for neutrophils. Only samples above 95% purity were taken into account.
Separation of Cells from Tumor Tissue
At the time of surgery a specimen of neoplastic tissue was collected. The procedure for selecting these samples has been previously described [17]. Samples were checked by the anatomopathologist. Tissue samples were then processed as previously described [18]. Briefly, after removal of necrotic areas, tissues were dissociated by sterile mechanical dissection, and then incubated in tissue culture containing 500 U/ml collagenase and 300 U/ml hyaluronidase. After stirring for 2 hours, the suspension was filtered through a wire grid. Cells were then separated on a Percoll discontinuous gradient with densities of 1.090, 1.085, 1.080, 1.075, 1.070, and 1.065. Cells on top of each layer and at the bottom of the 1.090 layer were collected separately. The amount of eosinophils in the different cell fractions was evaluated by May Grunwald-Giemsa staining.
Given the marked difference in IDO-positive infiltrate, evaluation of IDO activity was performed in the three tumor samples displaying the highest amount of infiltrating eosinophils, out of the six that were analyzed.
Enzymatic Assay for IDO Activity
IDO activity was assayed by the colorimetric method [19], with minor modifications. Briefly, 2 x 106 cells were disrupted by freezing and thawing, the lysate (250 µl) was cleared by centrifugation, and an equal amount of 2x IDO buffer (100 mM PBS, pH 6.5, with 40 mM ascorbate, 20 µM methylene blue, 200 µg/ml catalase, and 800 µM l-tryptophan, all reagents from Sigma) was added. After 30 minutes at 37°C, 100 µl of 30% trichloroacetic acid was added to stop the reaction, and a further incubation for 30 minutes at 52°C was performed. After centrifugation, the supernatant was mixed with an equal amount of Ehrlich's reagent (0.8% p-dimethylaminobenzaldehyde in acetic acid), the color was allowed to develop for 10 minutes, and then absorbance was read at 490 nm in a spectro-photometer. Serial dilutions of l-kynurenine were used as standards. One unit of IDO activity was defined as the amount of enzyme producing 1 nmol/hr kynurenine. The amount of protein in the samples was assayed by the Bradford method, using the Bio-Rad Protein Assay with BSA as standard.
Results and Discussion
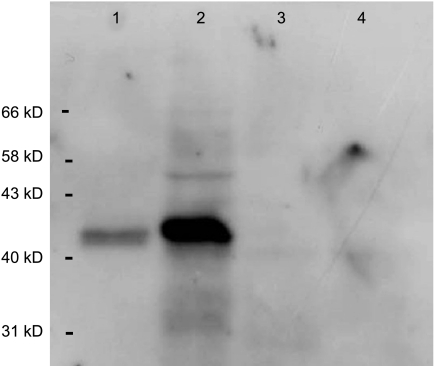
In order to assess IDO expression, an IDO-specific rabbit antiserum was used [12]. This antiserum has been raised against the same synthetic peptide used in Ref. [5]. The specificity of the antiserum was further checked by Western blot analysis on granulocyte neutrophils, which do not express IDO, on IFN-γ-treated macrophages, which do express IDO, and on purified IDO from rabbit small intestines. As shown in Figure 1, the antiserum reacted with macrophages but not with neutrophils, and recognized purified rabbit IDO.
Figure 1.
Evaluation of the specificity of the anti-IDO antiserum. Western blot analysis was performed on IDO protein, purified from rabbit small intestines as described in Ref. [6] (lane 1), on monocyte-derived macrophages treated with IFN-γ (lane 2), neutrophil PMNs (lane 3), and PMNs treated with IFN-γ (lane 4).
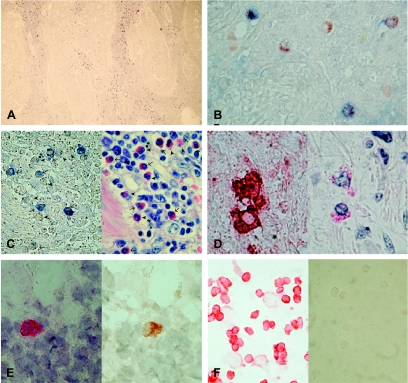
Using this antiserum, we found, in all 25 NSCLC tested, that IDO-positive cells were scattered within the peritumoral stroma, and only rarely within the tumor parenchyma (Figure 2A). This suggests that IDO expression in these tumors occurs in inflammatory cells rather than in neoplastic ones. Indeed, double staining with antibodies reacting with cytokeratins expressed by NSCLC cells, namely, AE1/AE3 and MNF116 [13,14], did not show colocalization with IDO (data not shown). Instead, IDO-positive cells were found to be endowed with peroxisomes, although not all cells with peroxisomes expressed IDO (Figure 2B), indicating that they could have a myeloid origin. However, neither the CD68 marker nor the S100 marker showed colocalization with IDO (data not shown), ruling out the possibility that IDO-positive cells were DCs or macrophages. In order to better identify the cell type(s) expressing IDO, IDO-stained NSCLC tissues were photographed, destained, and restained with different cytochemical techniques. Restaining with the hematoxylin-eosin technique indicated that IDO-positive cells had granules in the cytoplasm (data not shown). This finding suggested the possibility that these cells could be granulocytes. For this reason, restaining with a standard May Grunwald-Giemsa method was performed. In fact, the IDO-positive cells did appear to be granulocytes and frequently showed a bilobated nucleus; furthermore, the eosinophilic granules in their cytoplasm indicated that they could be eosinophil granulocytes (Figure 2C). To check this hypothesis, a restaining with Luna's technique, specific for eosinophils, was performed. IDO-positive cells were also positive for Luna's staining (Figure 2D). In order to corroborate this finding, a mouse anti-IDO monoclonal antibody was used on frozen sections, and the slides, stained for IDO, were then restained with a MoAb reacting with MBP, a marker specific for eosinophils at all stages of activation. IDO and MBP colocalized (Figure 2E), enabling us to confirm that IDO-positive cells were eosinophil granulocytes. No positivity on tumor cells was detected. The pattern of IDO expression was therefore identical with both antibodies.
Figure 2.
Cell expression of IDO protein in NSCLC. (A) IDO-positive cells accumulate in the reactive stroma. Immunohistochemistry was performed on formalin-fixed paraffin-embedded sections using the anti-IDO antiserum, and staining IDO in blue. Original magnification, x25. (B) IDO-positive cells are endowed with peroxysomes: IDO was stained in blue and peroxysomes in brown. Original magnification, x250. (C) Morphologic evaluation of IDO-positive cells with May Grunwald-Giemsa staining. The same sample was stained in blue for IDO (left panel) then destained and restained with May Grunwald-Giemsa (right panel). Arrows indicate IDO-positive eosinophils, whereas arrowheads indicate IDO-negative eosinophils. Original magnification, x250. (D) Morphologic evaluation of IDO-positive cells with Luna's staining. The same sample was stained in red for IDO (left panel) then destained and restained with Luna's staining, a staining technique specific for eosinophils (right panel). Original magnification, x500. (E) Colocalization of IDO and MBP. In this case, immunohistochemistry was performed on cryostat sections using a mouse anti-IDO revealed with Fast Red (left panel). After destaining, the sample was restained for MBP, using a specific MoAb and DAB substrate (right panel). Original magnification, x500. (F) Peripheral blood eosinophils do not express IDO. Eosinophils purified from peripheral blood (right panel) and monocyte-derived macrophages treated with IFN-γ (left panel) were stained in red for IDO. Original magnification, x100.
This is the first evidence supplied so far that cells of the granulocytic lineage can express IDO and potentially exert an immunosuppressive role. The mechanisms that induce IDO expression in eosinophils remain to be elucidated, but macrophages in the same samples do not express IDO, implying that IDO induction in the two cells follows different pathways.
In order to check whether eosinophils constitutively express IDO or not, eosinophils from peripheral blood were checked for IDO expression, using IFN-γ-treated macrophages as positive control. The latter cells were positive, but peripheral blood eosinophils did not show any IDO expression (Figure 2F). This finding indicates that eosinophils do not constitutively express IDO, but rather IDO expression is acquired as a result of signals received from the tumoral environment. In a few cases, such as the one displayed in Figure 2C, a minor but significant amount of the tumor-infiltrating eosinophils does not express IDO, substantiating the finding that IDO expression is induced.
It has to be noted that both the large majority of IDO-positive eosinophils and all of the immune system cells potentially endowed with antitumor cytolytic activity are located in the peritumoral stroma. In this area, the IDO-positive eosinophils are, in fact, in close contact with tumor-infiltrating lymphocytes, as it can be seen in Figure 2C; thus, the enzyme could exert its immunosuppressive activity at its best.
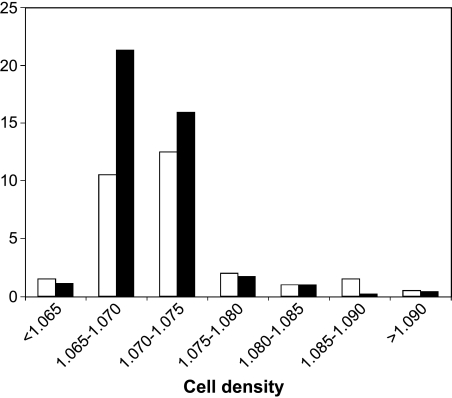
However, this event can occur only if the enzyme is active. In order to investigate whether or not IDO is enzymatically active, cell fractions of different density were isolated from NSCLC tissue, and IDO activity was assayed in each. IDO activity was predominantly found in the two cell fractions with a density between 1.065 and 1.070, and with a density between 1.070 and 1.075 (Figure 3). This finding indicates that the enzyme in IDO-positive cells is active, and has the potential to exert its immunosuppressive activity. Furthermore, the relationship between IDO activity and the percentage of eosinophils in the different cell fractions corroborates our conclusion that IDO-positive cells in NSCLC are indeed eosinophils. Although we did not succeed in demonstrating a direct immunosuppressive activity of NSCLC-infiltrating eosinophils on T-cell proliferation, due to the great difficulty in isolating the former cells, the very existence of an enzymatically active IDO strongly supports the hypothesis of an IDO-mediated immune suppression.
Figure 3.
IDO activity and amount of eosinophils in the cell fractions isolated by density from NSCLC. Cell suspensions were obtained from fresh tumor samples by mechanical dissection and enzymatic digestion. Seven cell subpopulations, having different densities, were obtained by separation on a Percoll discontinuous gradient. Each subpopulation was assayed for IDO activity (U/mg protein; black columns) and percentage of eosinophils (white columns). A single representative experiment, out of the three that were performed, gives similar results.
The occurrence of non-neoplastic cells expressing IDO within the tumor was recently described by Ishio et al. [10] in human hepatocellular carcinoma. However, they were not able to identify the cells responsible for IDO expression and referred to them as tumor-infiltrating cells, which were likely to be macrophages or DCs. However, Uyttenhove et al. [9] found IDO expression by tumor cells in a number of human solid tumors. However, in both hepatocarcinomas and NSCLC, the proportion of IDO-positive tumor cells was very low, and positivity in the latter was weak. Furthermore, they also found non-neoplastic, albeit unidentified, IDO-positive cells at the periphery of many tumors.
It is possible that in tumors such as prostatic, pancreatic, or colorectal carcinomas, IDO is expressed mainly by tumor cells, whereas in other tumors such as NSCLC or hepatocarcinomas, IDO is expressed mainly by tumor-infiltrating inflammatory cells. More generally, different mechanisms at different levels contribute to IDO expression in tumors: IDO can be expressed, even concomitantly, in the tumor and draining lymph nodes, by tumor cells, tumor-associated eosinophils, DCs, and macrophages. This fact seems to indicate that IDO expression can confer a growth advantage to tumors, at least for those tumors where an immune-mediated rejection could take place, such as NSCLC. In these cases, effective IDO-mediated immune suppression could foster tumor growth. Because tumor-infiltrating eosinophils are solely responsible for the occurrence of an IDO-dependent immunosuppression in NSCLC, tumor progression could be accelerated in those cases displaying a large amount of these cells in the inflammatory infiltrate.
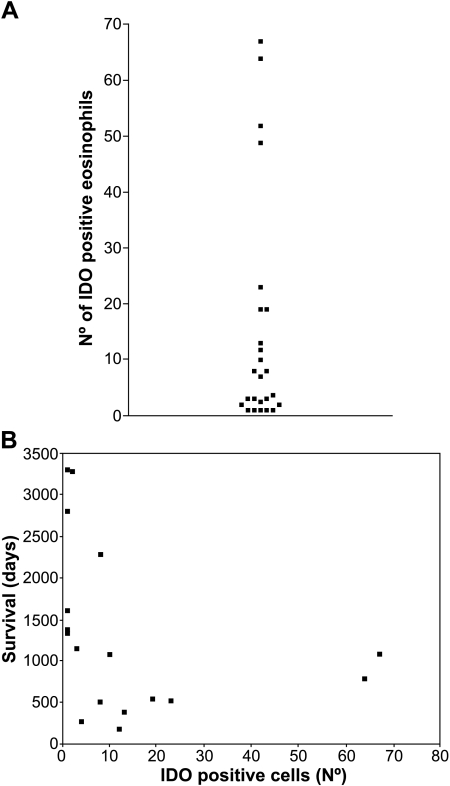
In this regard, it has to be noted that the amount of IDO-positive eosinophils greatly changes from one case to another. In Figure 4A, the frequency of IDO-positive cells in the 25 different samples examined is displayed. The amount of IDO-positive cells ranges from a few scattered cells to more than 50 cells per field at x200 magnification.
Figure 4.
Amount of IDO-positive infiltrate in the different patients, and its relationship with survival. (A) The frequency of IDO-positive cells differs in the different NSCLC. Each sample was stained for IDO, seven fields at x200 magnification were randomly chosen by each of two investigators, and then IDO-positive cells were counted; the mean value is shown. Each dot represents a single patient. (B) For each patient, the amount of IDO-positive infiltrate was related to the overall survival.
In order to investigate whether or not a relationship between IDO-positive infiltration and disease outcome could be found in NSCLC, patients with complete staging and adequate follow-up were selected. In these 17 patients, the amount of IDO-positive infiltrate was compared to survival, and an inverse correlation was found (Figure 4B; Spearman correlation coefficient: r = -0.65, P = .005). The patients were then divided into two groups: the group of patients surviving less than 3 years, and the group of patients surviving more than 3 years. A summary of the distribution of clinical data and of IDO-positive infiltrate between the two groups is provided in Table 1. A marked difference in IDO-positive infiltrates between the two groups was found. We are well aware of the uneven distribution between the two groups of parameters, such as stage and grading, that affect survival. We cannot conclude whether a large IDO-positive infiltrate is the cause or the result of a poor prognosis. Further studies and a much larger cohort of patients are required to confirm the prognostic role of IDO-positive infiltrate.
Table 1.
Relationship between IDO Expression and Clinical Data.
| Survival <3 Years (n = 9) | Survival >3 Years (n = 8) | |
| Age (mean ± 1 SD; years) | 32–70 (53.7 ± 12.4) | 35–67 (54.2 ± 12.6) |
| Sex (M/F) | 8/1 | 7/1 |
| Histotype | ||
| Squamous cell carcinoma | 2 | - |
| Adenocarcinoma | 7 | 8 |
| Tumor | ||
| T1 | 1 | 5 |
| T2 | 7 | 3 |
| T3 | 1 | - |
| Grading | ||
| G1 | - | 2 |
| G2 | 5 | 5 |
| G3 | 4 | 1 |
| Lymph nodes | ||
| N0 | 4 | 6 |
| N1 | 3 | 2 |
| N2 | 2 | - |
| Stage | ||
| IA | 1 | 5 |
| IB | 2 | 1 |
| IIB | 4 | 2 |
| IIIA | 2 | - |
| Survival (mean ± 1 SD; days) | 174–1080 | 1145–3300 |
| (592 ± 307) | (2163 ± 821) | |
| Deceased* | 9 | 4 |
| IDO-positive cells per field† | 4–67 | 1–8 |
| (mean ± 1 SD) | (23.2 ± 23.5) | (2.1 ± 2.3) |
All patients died due to systemic relapse.
The amount of IDO-positive cells was evaluated as indicated in the Materials and Methods section.
It has to be noted that immunotherapy has been taken into account in NSCLC [11,20–22]. If the physio-pathologic relevance of IDO-dependent immune suppression is confirmed, adoptive immunotherapy should be reconsidered. Either a way to overcome this suppression should be pursued, or adoptive immunotherapy should be restricted to patients displaying low levels of IDO-positive infiltrate.
The relevance of IDO expression in eosinophils should also be investigated in certain nontumoral diseases, such as asthma, where an eosinophil infiltrate plays a patho-physiological role. Indeed, we do not know whether the conditions inducing IDO expression in eosinophils infiltrating the inflammatory peritumoral stroma are peculiar to the tumor environment, or can be found in other inflammatory sites.
Acknowledgements
We thank Guido Ferlazzo for helpful discussions, and we are grateful to Adriana Morando and Giovanni Pittaluga for their help with immunohistochemistry.
Footnotes
This work was supported by Progetti Finalizzati Ministero della Salute 2002 and 2003 and MIUR-PRIN 2002.
References
- 1.North RJ. Cyclophosphamide-facilitated adoptive immuno-therapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogenic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;1899:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Lee JR, Jhaver KG, Jhonson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 6.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and NK cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 9.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Parmentier DN, Boon T, Van den Eynde BJ. Evidence for a tumoral resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 10.Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactive role of indoleamine 2,3-dioxygenase in human hepato-cellular carcinoma. J Gastroenterol Hepatol. 2004;19:319–326. doi: 10.1111/j.1440-1746.2003.03259.x. [DOI] [PubMed] [Google Scholar]
- 11.Ratto GB, Zino P, Mirabelli S, Minuti P, Aquilina R, Fantino G, Spessa E, Ponte M, Bruzzi P, Melioli G. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected non-small cell lung carcinoma. Cancer. 1996;78:244–251. doi: 10.1002/(SICI)1097-0142(19960715)78:2<244::AID-CNCR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Honig A, Rieger L, Kapp M, Sutterlin M, Dietl J, Kammerer U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2004;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson AG, Graham AN, Pezzella F, Agneta G, Goldstraw P, Pastorino U. Does the use of immunohistochemistry to identify micrometastases provide useful information in the staging of node-negative non-small cell lung cancer? Lung Cancer. 1997;18:231–240. doi: 10.1016/s0169-5002(97)00065-2. [DOI] [PubMed] [Google Scholar]
- 14.Osaki T, Oyama T, Gu C, Yamashita T, So T, Takenoyama M, Sugio K, Yasumoto K. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected Stage I non-small cell lung cancer. J Clin Oncol. 2002;20:2930–2936. doi: 10.1200/JCO.2002.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak AM, Weller PF, Monahan-Earley RA, Letourneau L, Ackerman SJ. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab Invest. 1990;62:590–607. [PubMed] [Google Scholar]
- 16.Luna LG. Manual of Histologic Staining Methods for the AFIP. 3rd ed. New York: Graw-Hill; 1968. pp. 162–163. [Google Scholar]
- 17.Semino C, Ferlazzo G, Ratto GB, Meliloli G. Analysis of HLA class-I specific natural killer cell receptors expressed on T lymphocytes infiltrating non-small-cell lung cancer. Lung Cancer. 2001;34:395–405. doi: 10.1016/s0169-5002(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 18.Melioli G, Ratto GB, Guastella M, Meta M, Biassoni R, Semino C, Casartelli G, Pasquetti W, Catrullo A, Moretta L. Isolation and in vitro expansion of lymphocytes infiltrating non-small cell lung carcinoma: functional and molecular characterization for their use in adoptive immunotherapy. Eur J Cancer. 1994;30A:97–102. doi: 10.1016/s0959-8049(05)80027-9. [DOI] [PubMed] [Google Scholar]
- 19.Daubener W, Waganat K, Pilz S, Seghrouchni S, Fisher HG, Hadding U. A new, simple, bioassay for human IFN-gamma. J Immunol Methods. 1994;168:39–47. doi: 10.1016/0022-1759(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt W, Bildat S, Korfee S. Combined modality therapy in NSCLC. Ann Oncol. 2000;11:85–95. doi: 10.1093/annonc/11.suppl_3.85. [DOI] [PubMed] [Google Scholar]
- 21.Korst RJ, Crystal RG. Active, specific immunotherapy for lung cancer: hurdles and strategies using genetic modification. Ann Thorac Surg. 2003;76:1319–1326. doi: 10.1016/s0003-4975(03)00651-9. [DOI] [PubMed] [Google Scholar]
- 22.Engelman EG. Dendritic cell-based cancer immunotherapy. Semin Oncol. 2003;30(Suppl 8):23–29. doi: 10.1016/s0093-7754(03)00229-x. [DOI] [PubMed] [Google Scholar]