Abstract
We investigated the antitumoral efficacy, endocrine consequences, and molecular mechanisms underlying cell death induced by the Hecate-chorionic gonadotropin (CG)β conjugate, a fusion protein of a 23-amino acid lytic peptide Hecate with a 15-amino acid (81–95) fragment of the human CGβ chain. Transgenic (TG) mice expressing the inhibin α-subunit promoter (inhα)/Simian Virus 40 T-antigen (Tag) transgene, developing luteinizing hormone (LH) receptor (R) expressing Leydig and granulosa cell tumors, and wild-type control littermates were treated either with vehicle, Hecate, or Hecate-CGβ conjugate for 3 weeks. Hecate-CGβ conjugate treatment reduced the testicular and ovarian tumor burden (P < .05), whereas a concomitant increase (testis; P < .05) or no change (ovary) in tumor volumes occured with Hectate treatment. A drop in serum progesterone, produced by the tumors, and an increase in LH levels occured in Hecate-CGβ treated mice, in comparison with vehicle and Hecate groups, providing further support for the positive treatment response. Hecate-CGβ conjugate induced a rapid and cell-specific membrane permeabilization of LHR-expressing cells in vitro, suggesting a necrotic mode of cell death without activation of apoptosis. These results prove the principle that the Hecate-CGβ conjugate provides a novel specific lead into gonadal somatic cell cancer therapy by targeted destruction of LHR-expressing tumor cells.
Keywords: Lytic peptide, Leydig and granulosa cell tumors, Hecate, LHR, necrosis
Introduction
Early diagnosis and prevention of human gonadal tumors remain difficult, which makes them a fatal group of malignancies [1]. Although testicular tumors account for only 1% of all tumors in males, they are the most common malignancy in men between 15 and 34 years of age [2]. Leydig cell tumors are generally benign and account for about 2% of all testicular tumors, with malignancy occurring in about 10% of the cases. If metastases are found, chemotherapy is the treatment of choice because Leydig cell tumors are not radiosensitive [1]. Ovarian carcinoma is often called the “silent killer” because the disease remains usually undetectable until the advanced stage (II or IV) due to lack of diagnostic tests and absence of solid symptoms [3,4]. Among ovarian tumors, those in granulosa cells are rare, accounting for 3.0% to 7.6% of primary ovarian tumors. However, prospects of their treatment, in comparison to other ovarian cancers, are still poor [5,6]; tumorrelated mortality rate is 37.3% [3,7], and approximately 80% of patients die of recurrent disease [7,8].
The major problems of cancer chemotherapies are related to required therapeutic concentrations of drugs, exerting undesirable effects on normal cells and causing severe side effects [9]. The development of targeted antitumor drug delivery systems has a great potential in enhancing chemotherapeutical efficacy and specificity. Most of the lytic peptides produced by insects, amphibians, and mammals have an amphipathic structure, and they preferentially bind and insert into negatively charged cell membranes [9]. In contrast to normal eukaryotic cells with low membrane potential, the prokaryotic and cancer cell membranes maintain a large membrane potential, and therefore many lytic peptides preferentially disrupt prokaryotic and cancer cell membranes rather than those of healthy eukaryotic cells [10].
To eradicate cancer cells and to reduce the toxicity of treatment toward normal tissues, we developed a novel approach for the treatment of endocrine tumors possessing luteinizing hormone receptors (LHRs). We synthesized the Hecate-chorionic gonadotropin β (Hecate-CGβ) conjugate, fusion protein of the 23-amino acid membrane lytic peptide (Hecate) [11] and the 15-amino acid fragment of the human CGβ chain. The 81- to 95-amino acid region of CGβ composes the most potent region of the full-size CG protein with important [12] roles in receptor binding [13]. We hypothesized that the Hecate-CGβ conjugate could serve as an effective drug for the LHR expressed in gonadal tumors and possibly for some nongonadal tumors [14,15]. The affinity of the Hecate-CGβ conjugate to LHR by targeted destruction of LHR-expressing prostate, breast, ovarian, and testicular cancer cell lines [16,17], and in prostate PC-3, ovarian OVCAR-3, and breast MDA-MB-435S tumor xenografts implanted in nude mice in vivo has been demonstrated [16,18,19]. There are no existing reports wherein the therapeutic efficacy, endocrine, physiological, and pathophysiological consequences of Hecate or Hecate-CGβ conjugate treatment been evaluated, with the use of a transgenic (TG) preclinical model of tumorigenesis in a heritable immunocompetent model of disease.
In the present study, we investigated the therapeutic efficacy of the Hecate-CGβ conjugate to eradicate LHR-expressing tumor cells in vivo in a TG mouse model expressing the inhibin α-subunit promoter (inhα)/Simian Virus 40 T-antigen (Tag) transgene and developing gonadal tumors [20,21], as well as monitored the subsequent endocrine effects of the treatment as an indication of the therapeutic effect. We also investigated in vitro the susceptibility of LHR-expressing tumor cells to Hecate-CGβ conjugate treatment and addressed the mechanisms underlying induced cell death, which has not yet been studied or reported.
Materials and methods
Cell Lines and Cultures
The murine Leydig tumor cell line (mLTC-1) (LHR-positive) [37] was cultured in Waymouth medium (Sigma Chemical Co., St. Louis, MO), supplemented with 9% heat-inactivated horse serum (Life Technologies, Paisley, Scotland, UK) and 4.5% heat-inactivated fetal calf serum (iFCS; Bioclear, Wokingham, Berks, UK) containing 0.1 g/l gentamicin (Gibco BRL, Gaithersburg, MD). Murine Leydig tumor BLT-1 (LHR-positive) [20], murine granulose KK-1 (LHR-positive) [21], prostate cancer PC-3 (LHR-positive) [38], and colon carcinoma HT-29 (LHR-negative) [39] cells were maintained in DMEM/Ham's F-12 1:1 medium (Sigma), supplemented with 10% iFCS, 50 IU/l penicillin, and 0.5 g/l streptomycin (Sigma). The cells were allowed to grow on 9.6-cm-diameter Petri dishes (Greiner Labortechnik, Frickenhausen, Germany), or on 6- or 24-well plates (Greiner) to 70% to 80% confluency under a humidified atmosphere of 95% and 5% CO2 at 37°C.
Preparation of Drugs
Hecate and the Hecate-CGβ conjugate were synthesized and purified in the Peptide and Protein Laboratory, Department of Virology, Hartman Institute, University of Helsinki (Helsinki, Finland) as described earlier [29].
Experimental Animals
TG mice of 5.5 months age, expressing the inhα/SV40 Tag transgene [40,22], all possessing macroscopic gonadal tumors, were selected for the experiment (7–10 mice per group). Wild-type (WT) littermate mice (C57Bl) were used as controls (n = 4–7 per group). For routine genotyping, PCR analyses were carried out using DNA extracts from tail biopsies, as previously described [21]. Mice were housed two to four per cage, after weaning at the age of 21 days, in a room with controlled light (12 hours of light, 12 hours of darkness) and temperature (21 ± 1°C). The mice were specific pathogen-free and they were routinely screened for common mouse pathogens. Avertin anesthesia was used in surgical operations and postoperative analgesia (buprenorphine, 3 µg/mouse, by intraperitoneal injections) was administered on a routine basis. The University of Turku Ethical Committee on Use and Care of Animals approved the animal experiments.
Hecate and Hecate-CGβ Treatments
At the beginning of the experiment (at the age of 5.5 months), the gonadal size was assessed during laparotomy under general anesthesia, by measuring their length, depth, and width. The product of these three measurements (expressed in mm3) was taken as approximation of the total gonadal volume, and the tumor volume burden was assessed as total gonadal volume per gram of body weight. Animals were treated with either vehicle or Hecate-CGβ conjugate (12 mg/kg) or Hecate (12 mg/kg) by intraperitoneal injections. The mice were injected once per week for three consecutive weeks, according to the earlier treatment protocols for in vivo treatment for nude mice xenografts [16,19,17]. Seven days following the last treatment, the mice were sacrificed by cervical dislocation, and blood was collected by cardiac puncture. Body, tumor, and different organ weights were recorded. Tissues were either snap-frozen in liquid nitrogen or fixed in Bouin's solution or 4% paraformal-dehyde, dehydrated, and embedded in paraffin. Paraffin sections of 5 µm thickness were stained with hematoxylin/eosin for histologic analysis.
Northern Hybridization Analysis
Total RNA was isolated from WT and TG mice whole testis or ovary (C; Hecate- and Hecate-CGβ conjugate-treated groups) using the single-step guanidinium thiocyanate-phenol-chloroform extraction method [41]. Twenty micrograms of RNA per lane was resolved on 1.2% denaturing agarose gel and transferred onto Hybond-XL nylon membranes (Amersham International plc, Aylesbury, Bucks, UK). Membranes were prehybridized overnight at 65°C in a solution containing 5x SSC, 5x Denhardt's solution, 0.5% SDS, 50% formamide, and 5 g/l denatured calf thymus DNA. A complementary RNA probe for the rat LHR generated from a fragment of the LHR cDNA, spanning nucleotides 441 to 849 of its extracellular domain subcloned into the pGEM-4Z plasmid, was used for hybridization [12]. The [32P]dUTP-labeled (800 Ci/mmol; Amersham International) probe was generated using a Riboprobe system II kit (Promega, Madison, WI). The probes were purified with NickColumns (Pharmacia Biotech, Uppsala, Sweden). Hybridization was carried out at 65°C for 20 hours in the same prehybridization solution after the addition of labeled probe. After hybridization, the membranes were washed twice in 2x SSC and 0.1% SDS at room temperature for 10 minutes, followed by two washes in 0.1x SSC and 0.1% SDS at 65°C to remove most of the background. Finally, the membranes were exposed to Kodak X-ray films (Kodak XAR-5; Eastman Kodak, Rochester, NY) at -70°C for 4 to 7 days, or to a phosphor imager (Fujifilm BAS-5000; Fujifilm IaI, Tokyo, Japan) for 4 to 24 hours. The intensities of specific bands were quantified using the Tina software (Raytest, Staubenhardt, Germany) and related to those of the 28S ribosomal RNA in the gel stained with ethidium bromide. The molecular sizes of the mRNA species were estimated by comparison with mobilities of the 18S and 28S ribosomal RNA.
Hormone Measurements
LH concentration in sera was measured by a supersensitive immunofluorometric assay for rat LH (Delfia; Wallac Oy, Turku, Finland) as described earlier [40]. Progesterone was measured from diethyl ether extracts of the sera by RIA as described earlier [42,43]. We took additional serum samples (n = 7–8) from 6.5-month-old tumor-bearing inhα/Tag male and female mice treated with vehicle as controls for hormone analyses.
Fluorescent Microscopy for PI Staining
To prove the preferential destruction of LHR-expressing cells and to determine the mechanisms involved in cell death caused by the Hecate-CGβ conjugate, we cocultured two cell lines, either murine Leydig mLTC-1 (LHR-positive) and human colon carcinoma HT-29 cells (LHR-negative) or murine granulosa KK-1 (LHR-positive) and human colon carcinoma HT-29 cells (LHR-negative) (data shown only for mLTC-1 and HT-29 cells), on the same glass well slides. Because the cells differed markedly in their shape and size, they were easy to distinguish. The cells were incubated for 24 hours in complete Waymouth medium, followed by a wash with 1x PBS, and a further incubation in the presence or absence of 0.5 µM Hecate or Hecate-CGβ conjugate for 15 minutes. Thereafter, the medium was removed and replaced with fresh medium containing PI, which does not enter cells with intact membranes, and thus differentiates between lysed and intact cells when studied by fluorescence microscopy. In the second set of experiments, we treated mLTC-1 and HT-29 cells for 30 minutes with concentrations of 0.5, 1, and 5 µM Hecate. In the third set of experiments, we treated mLTC-1 cells with 0.5 µM concentration of the Hecate-CGβ conjugate for 0, 15, and 30 minutes.
FACS Analysis
Cells from two Leydig tumor cell lines, mLTC-1 and BLT-1 [20], and a prostate cancer cell line, PC-3, were seeded into six-well plates in complete medium and incubated at 37°C in 5% CO2 for 24 hours. Cells were washed once with PBS and then incubated for the next 4 hours at increasing concentrations (0.1, 0.5, and 1 µM) of Hecate or Hecate-CGβ conjugate. As an apoptotic positive control, we used 0.1% of hydrogen peroxide added to the culture media. Both adherent and floating cells were collected and stained with PI, and cellular DNA content was analyzed by FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) as previously described [44,45].
Immunoblotting Analysis for Caspase-3 Activation
Western blotting analysis was used to determine whether caspase-3 was activated with an antibody recognizing the precursor and the active subunits. One day before stimulation, mLTC-1 cells were plated on six-well plates at a density of 1.2 x 105 cells/well in 0.5 ml of complete Waymouth medium. The cells were washed once with PBS and then incubated for 60 minutes at 0.5, 1, and 5 µM Hecate or Hecate-CGβ conjugate. Total cell lysates were prepared as described previously [46] and subjected to electrophoresis through a 12.5% SDS-PAGE gel (60 µg protein/well). After electrophoresis, proteins were electrotransferred to nitrocellulose membranes. After blocking the membrane, it was incubated with the primary rabbit monoclonal antibody (Cell Signaling, Beverly, MA). Signals were visualized using ECL Plus Western blotting detection reagents (Amersham Pharmacia Biotech, Buckinghamshire, UK) and finally exposed to X-ray film.
Caspase Inhibitor Z-VAD.fmk
Cells were seeded into six-well plates at a density of 2 x 105 cells/well. Twenty-four hours later, the medium was replaced with new medium containing 20 µM pan-caspase inhibitor Z-VAD.fmk (Calbiochem, Nottingham, UK) and incubated for 1 hour. Hecate or Hecate-CGβ conjugate was then added at concentrations of 0.1, 0.5, and 1 µM and incubated overnight. Incubation with the conjugate was stopped after 16 hours. A colorimetric MTT assay was then performed to measure cell survival [47]. We used 0.1% H2O2 as the positive control for apoptosis. Viability in the treated cells was expressed as a percentage of controls. The untreated controls were assigned a value of 100%.
Statistical Analysis
Statistical ANOVA paired t test analyses of variance were carried out using a StatView program for Windows (version 5.0.1) (SAS Institute, Inc., Cary, NC). All data are presented as the mean ± SEM.
Results
Hecate-CGβ Treatment Reduced Tumor Volume
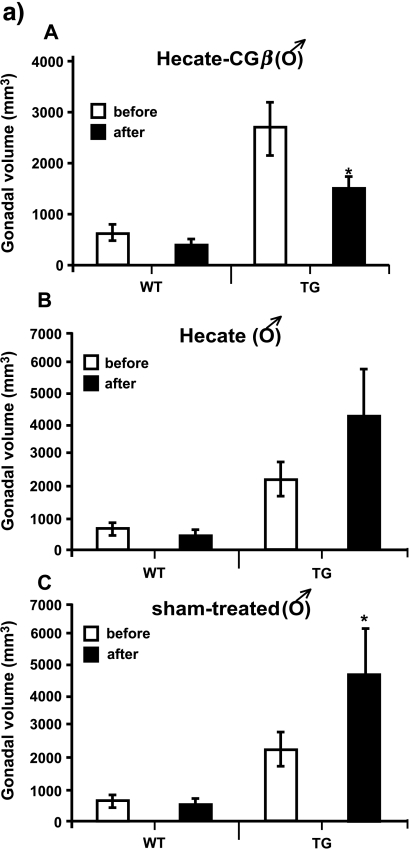
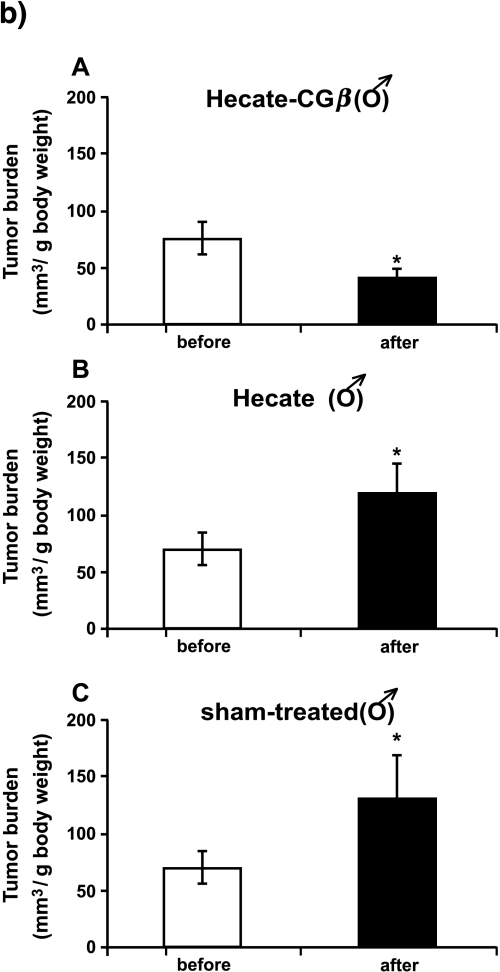
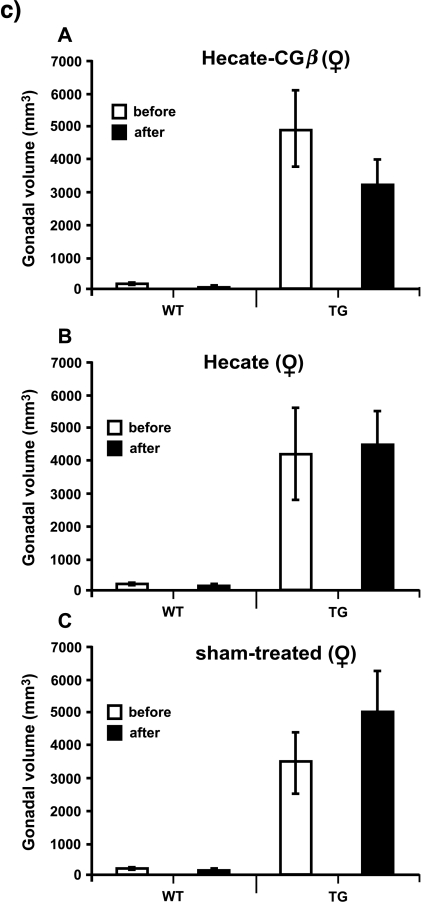
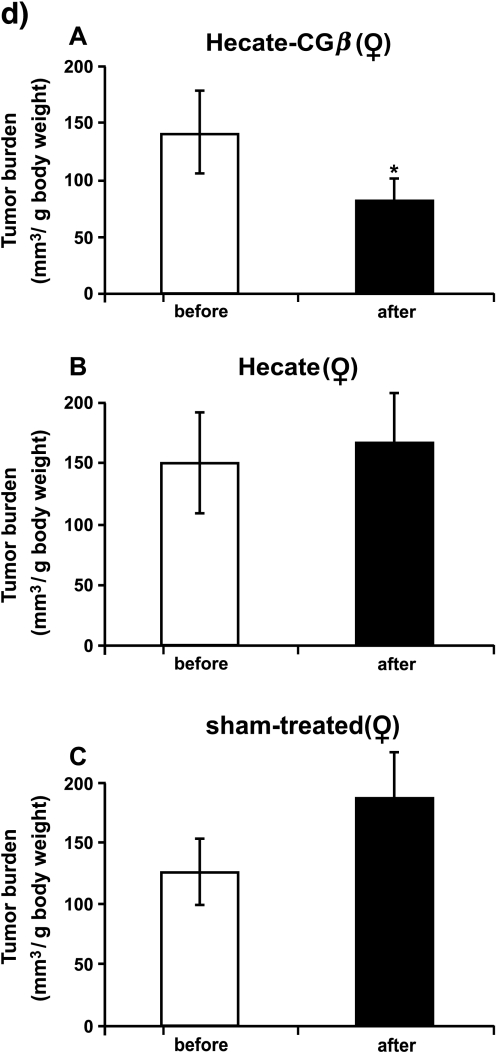
We were able to achieve an antineoplastic effect of the Hecate-CGβ conjugate on the inhα/Tag TG mice. Here we have used the same treatment strategy protocols used earlier and established for in vivo xenograft treatments [16,18,19]. Following 1-month treatment, total testicular tumor volume decreased by an average of 58% (Figure 1a; P < .05; compared to the volume before treatment) and that of the ovaries by 36% (Figure 1c). As the age-related tumor progression rate was variable between animals, which has been observed in the inhα/Tag and Tag mice before [22,20,21,23], we decided to show the tumor burden, too. The gonadal tumor burden (i.e., tumor volume/g body weight) after Hecate-CGβ conjugate treatment in both sexes decreased significantly in comparison with that analyzed before treatment (Figure 1, b and d; P < .05). In the Hecate-treated males, tumors grew steadily as in the sham-treated control groups (Figure 1, a and b); but in females, the ovarian tumor volumes did not change significantly (Figure 1, c and d). Hecate-CGβ conjugate and Hecate treatments showed no significant influence on gonadal volume in the WT mice group (Figure 1, a and c). There were no statistically significant differences between the body weights among comparable groups (like Hecate- or conjugate-treated, before and after the WT and TG groups).
Figure 1.
Total gonadal volumes of male (a) and female (c) wild-type (WT) control littermate mice and inhα/Tag transgenic (TG) mice, before and after 3 weeks of Hecate-CGβ conjugate (Panels A), Hecate (Panels B), or sham treatment (Panel C). (b and d) Tumor burden volumes (tumor volume/g body weight). The values are mean ± SEM of both gonads. *P < .05, Hecate, Hecate-CGβ conjugate, or sham-treated gonadal volume after treatment versus before treatment. WT, wildtype control littermate mice; TG, transgenic mice expressing the inhibinα Simian Virus 40 T-antigen (inhα/Tag).
Hecate-CGβ Treatment Was Specific Toward LHR-Possessing Cells
To assess whether the Hecate-CGβ treatment reduced the LHR mRNA levels in comparison to Hecate- and sham-treated TG control groups, we made a quantitative evaluation from RNA extracts of the total testes and ovaries of TG mice. Northern hybridization revealed that there was a concomitant decrease in LHR mRNA expression in the Hecate-CGβ-treated testes and ovaries (P < .01 and P < .05; versus Hecate-treated groups and/or the sham-treated TG control group) (Figure 2), which confirmed the LHR-specific destruction effects of Hecate-CGβ and was in line with the reduced tumor volume data (Figure 1).
Figure 2.
Northern hybridization analysis of LHR mRNA expression in Hecate and Hecate-CGβ conjugate-treated or sham-treated testicular and ovarian tissues of inhα/Tag transgenic (TG) males and females. Each lane contains 20 mg of total RNA. The migration of the 28S and 18S rRNA are shown on the left side of the LHR panel. The sizes of the different LHR mRNA splice variants (in kb) are presented on the right. Two lanes for each type of sample are depicted. One lane of WT male and female gonad mRNA expression is shown on the right as a positive control for LHR mRNA. The upper panel shows on the top the ethidium bromide (EtBr) staining of the 28S rRNA for RNA loading control. The middle panel shows Northern hybridization for LHR mRNA. The lower panel shows the densitometric quantification of the longest (7.0 kb) LHR mRNA splice variant (open bar for Hecate and filled bars for sham-treated and Hecate-CGβ-treated) in arbitrary densitometric units (mean of TG sham-treated testis and ovaries regarded as 100%) corrected for intensity of the 28S rRNA band. Each bar represents the mean ± SEM of three independent experiments in duplicates. **P < .01, *P < .05 (P < .01 and P < .05; Hecate-CGβ-treated versus Hecate-treated and/or sham-treated TG control group). TG, transgenic mice expressing the inhibinα Simian Virus 40 T-antigen (inhα/Tag); TG-C, shamtreated TG mice.
Endocrine Consequences of Treatment
We have earlier shown that the serum progesterone levels rise significantly and those of gonadotropins drop along with tumorigenesis (6-month-old TG mice) in comparison with WT control littermates [24,25]. After 3 weeks of treatment with the Hecate-CGβ conjugate, serum progesterone concentration decreased significantly both in females and males (Figure 3A; P < .05; versus Hecate-treated groups and the sham-treated TG control group) and serum LH concentration increased in the Hecate-CGβ conjugate-treated groups (Figure 3B; P < .01 to P < .05; versus Hecate-treated groups and the sham-treated TG control group). The hormonal values thus allowed us to monitor the positive treatment effects of the Hecate-CGβ conjugate.
Figure 3.
Serum progesterone (A) and LH (B) concentrations in inhα/Tag transgenic female and male mice after the 3-week treatment with either Hecate, Hecate-CGβ conjugate, or sham (TG-C). **P < .01, *P < .05 (P < .01 and P < .05; Hecate-CGβ-treated versus Hecate-treated and/or shamtreated TG-C control group). TG, transgenic mice expressing the inhibin; Simian Virus 40 T-antigen (inhα/Tag); TG-C, sham-treated TG mice.
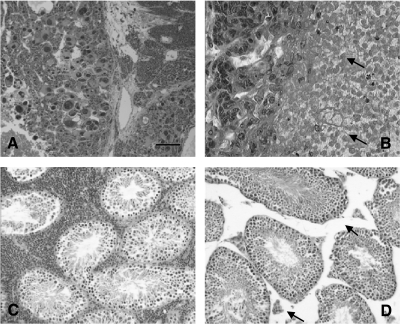
Histopathologic Analysis Demonstrates Antitumoral Effect of Hecate-CGβ Conjugate Treatment
Histopathologic analysis showed that Hecate-CGβ conjugate treatment of TG mice induced a definite degradation of testicular and ovarian tumor mass (more marked in testis). In many parts of the histologic tumor sections, we found clear changes in tumor tissues, apparently induced by the treatment. Especially in testicular sections, a high number of neoplastic Leydig cells were destroyed, and the tumor tissues apparently contained necrotic cells (Figure 4, B and D). In contrast, in tissue sections collected from TG mice treated with Hecate as well as in sham-treated TG mice (data not shown), cell death appeared quite sporadically and multiple mitoses were seen in the tumors (Figure 4, A and C). The histologic results supported the data of reduced tumor volumes in Figure 1. We studied the cross-sections of several other organs (lungs, pancreas, liver, spleen, adrenals, and uterus) for posttreatment side effects or metastases. Only in two males and one female treated with Hecate or vehicle were lung metastases found, but never in Hecate-CGβ conjugate-treated groups. Histologic study revealed no apparent effects of the treatments in extragonadal organs (data not shown).
Figure 4.
Histologic pictures of hematoxylin/eosin-stained ovarian granulosa (A and B) and testicular Leydig cell (C and D) tumors of inhα/Tag mice treated with Hecate (A and C) or Hecate-CGβ conjugate for three consecutive weeks. Arrows indicate that the interstitial tissue (B and D) has dramatically shrunken after Hecate-CGβ conjugate treatment (the bar in panel A is 40 µm; panels B-D have the same magnification as panel A).
Mode of Antitumor Effect and Specificity of Selective Cell Death
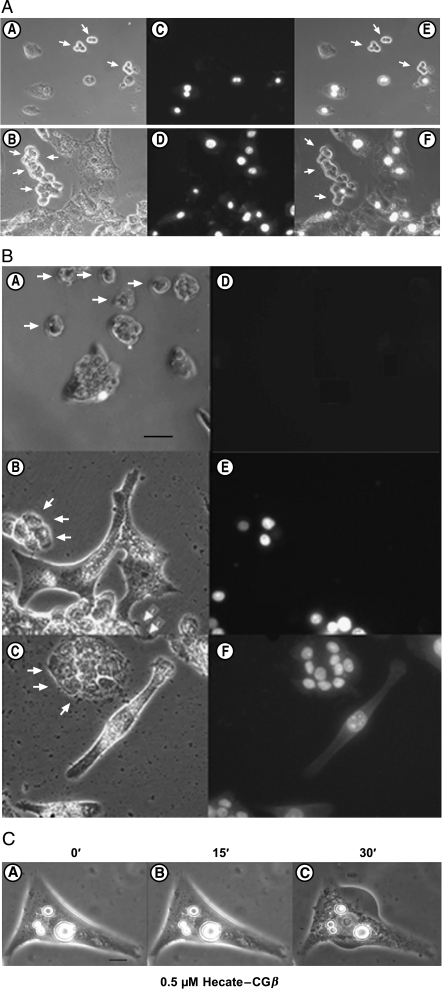
The preferential killing of LHR-expressing mLTC-1 Leydig cells, but not of LHR-negative colon carcinoma HT-29 cells, was clearly shown with the propidium iodide (PI) exclusion experiment (Figure 5A). Nuclear PI staining showed that 15 minutes of 0.5 µM Hecate-CGβ conjugate treatment induced rapid permeabilization of the cell membrane and killing of LHR-positive mLTC-1 cells, but not of HT-29 cells (Figure 5A). Hecate treatment for 30 minutes at 1 µM concentration killed HT-29 cells only; but at 5 µM concentration, it killed both HT-29 and mLTC-1 cells (vs 0.5 µM Hecate-CGβ conjugate, killing mLTC-1 cells; Figure 5A) with similar efficacy, regardless of the LHR expression (Figure 5B). At 0.5 µM concentration, Hecate did not have any effects on either cell line (Figure 5B). It has never been shown earlier in such a simple but trivial way that the Hecate-CGβ conjugate selectively kills cells possessing LHR but spares cells without LHR.
Figure 5.
Fluorescent and light microscopic analysis of cocultured Leydig tumor mLTC-1 (LHR-positive, bigger cells) and colon carcinoma HT-29 (LHR-negative, smaller cells) (marked with arrows). (a) Pretreated with 0.5 µM Hecate-CGβ conjugate for 15 minutes in the presence of nonpermeable PI in the media. The left panels (A and B) showed the light microscopic pictures of two different cell mixtures. The upper and lower middle darker field panels (C and D) showed PI fluorescent microscopy. The cell membranes of the mLTC-1 cells were preferentially perturbed by the Hecate-CGβ conjugate. Panels E and F are merged pictures of light and PI fluorescent microscopy (the bar in panel A is 40 µm; panels B–F have the same magnification as panel A). PI, propidium iodide. (b) Pretreated for 30 minutes with an increasing dose of Hecate: (A and D) 0.5 µM, (B and E) 1 µM, and (C and F) 5 µM Hecate in the presence of nonpermeable PI in the media. The left panels (A–C) show the light microscopy pictures. The right darker panels (D–F) show PI fluorescent microscopy. Hecate killed both mLTC-1 cells and HT-29 cells with the highest (5 µM) concentration used not depending on their LHR content. A concentration of 1 µM Hecate killed only HT-29 cells (the bar in panel A is 40 µm; panels B–F have the same magnification as panel A). PI, propidium iodide. (c) Light microscopic picture of a single mLTC-1 Leydig tumor cell incubated in 0.5 µM Hecate-CGβ conjugate for 0, 15, and 30 minutes. Cell swelling could be observed at 30 minutes (C), which strongly suggests necrosis as the mode of cell death (the bar in the panel A is 40 µm; panels B–C have the same magnification as panel A).
We analyzed further whether the molecular mode of cell death induced by the Hecate-CGβ conjugate could be by necrosis. Light microscopic analysis revealed that the moderate dose of 0.5 µM Hecate-CGβ conjugate for 30 minutes induced swelling of the mLTC-1 cells (Figure 5C), indicating that the cells died as a result of acute injury, swelling, and bursting, which strongly suggest necrosis [26,27].
Further Studies on Molecular Mechanisms of the Selective Death of LHR-Expressing Cells Induced by the Hecate-CGβ Conjugate
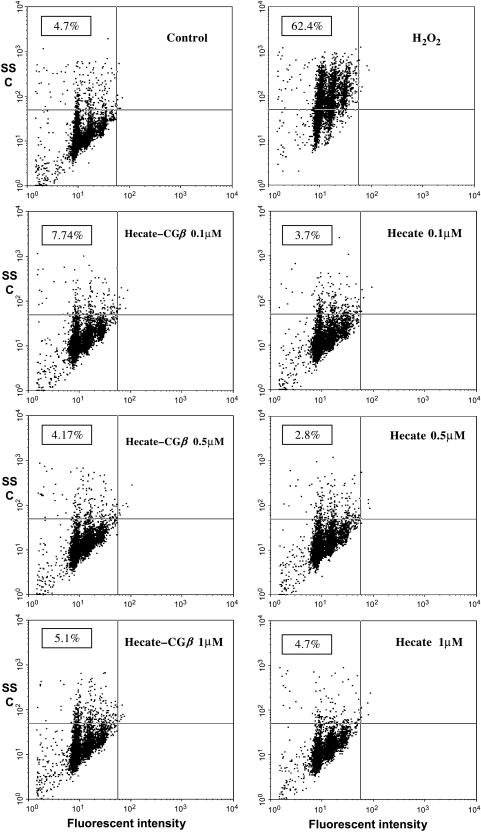
Flow cytometric analysis. The cells from all the three cell lines (data shown only for mLTC-1 cells) treated with Hecate or Hecate-CGβ conjugate were lysed, cell nuclei were stained with PI, and the percentage of cells undergoing apoptosis by nuclear fragmentation was determined. The percentage of cells undergoing apoptosis in the presence of H2O2 was 62.4%, 53.6%, and 88.8% for mLTC-1, BLT-1, and PC-3 cells, respectively (Figure 6). All three cell lines challenged with increasing concentrations of Hecate-CGβ conjugate or Hecate (0.1, 0.5, and 1 µM) for 4 hours showed lack of nuclear fragmentation (Figure 6).
Figure 6.
Flow cytometric analysis of mLTC-1 cells treated for 4 hours with concentrations of 0.1, 0.5, and 1 µM Hecate-CGβ conjugate or Hecate. Cells treated with 0.1% H2O2 for 4 hours were used as positive apoptotic controls. After incubation, cells were lysed in a hypotonic solution containing nonpermeable PI and analyzed by FACS (FACSCalibur flow cytometer; Becton Dickinson). Neither Hecate-CGβ conjugate nor Hecate treatment induced nuclear fragmentation in mLTC-1 Leydig cells. The numbers on the top left quadrant of the panels represent the percentage of apoptotic cells. The data are representative of three separate experiments with similar results.
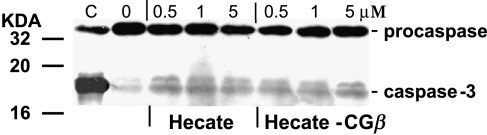
Analysis of caspase-3 activation. Incubation of mLTC-1 cells with Hecate-CGβ conjugate or Hecate at 0.5 µM concentration for 90 minutes did not induce the activation of caspase-3 when analyzed by Western blot analysis (Figure 7). This treatment caused a decrease in cell viability but no active form (17 kDa) of caspase-3 could be detected (Figure 7). These data provide strong evidence that the cell death occurred without the proteolysis of procaspase.
Figure 7.
Western blot analysis of caspase-3. mLTC-1 cells were treated with 0.5, 1, and 5 µM of either Hecate or Hecate-CGβ conjugate for 60 minutes. Adherent and detached cells were assayed for cleaved caspase-3. No detectable active caspase-3 activity in Hecate- and Hecate-CGβ-treated Leydig tumor mLTC-1 cells could be observed. “C”—mLTC-1 cells treated with 0.1% H2O2 showing cleaved caspase-3, positive control for apoptosis; on the left side, the molecular marker sizes are shown in kilodaltons.
Next, to check whether any activation of the apoptotic pathways had taken place while causing cell death by Hecate or Hecate-CGβ conjugate, we used the pan-caspase inhibitor to pretreat the BLT-1 and mLTC-1 Leydig tumor cells. Both BLT-1 and mLTC-1 cell viability showed similar decreased levels after treatment with the Hecate-CGβ conjugate, as it was caused without the inhibitor pretreatment levels (results not shown). The minimum concentration of the Hecate-CGβ conjugate leading to 50% cell death (EC50) was 0.1 µM for mLTC-1 cells. The same concentration of the conjugate decreased the viability of BLT-1 cells until 40%. In the presence of 0.5 to 1 µM concentration of the conjugate and pretreatment with the Z-VAD.fmk inhibitor, cell viability oscillated between 50% and 40% for mLTC-1 cells and between 35% and 30% for BLT-1 cells (results not shown). The presence or absence of the Z-VAD.fmk inhibitor did not significantly change Leydig tumor cell viability after the treatment of the Hecate-CGβ conjugate, but in the positive control for apoptosis with 0.1% of H2O2, the presence of Z-VAD.fmk was able to block apoptosis significantly (50%) (results not shown). These results further proved that the mode of cell death caused by the Hecate or Hecate-CGβ conjugate was not apoptosis, but necrosis, as shown in Figure 5C.
Discussion
In the present study, we took advantage of the established inhα/Tag TG mouse model of gonadal somatic cell tumorigenesis [20,28,21,24,23], where the pathophysiological and endocrine responses induced by tumorigenesis are well-known. This information allowed us to gain insight into the specific endocrine and paracrine consequences of the Hecate and Hecate-CGβ conjugate treatments. Here, we were able to demonstrate in TG mice that the targeted treatment with the Hecate-CGβ conjugate, apparently through binding to LHR, brought about effective blockage and/or reduction of tumor growth. Hecate-CGβ conjugate treatment was able to reduce the total gonadal tumor volumes of inhα/Tag mice, without causing any noticeable side effects.
It has been previously shown that the Hecate-CGβ conjugate is able to bind specifically to target cell membrane LHR [29], including LHR-expressing tumor cells [16,17]. The higher killing efficiency of the Hecate-CGβ conjugate of Leydig versus granulosa cells may be due to a higher level of LHR expression in the former cell types. In accordance, the granulosa (KK-1) and Leydig (BLT-1) cell lines derived from the inhα/Tag mouse gonadal tumors expressed 9870 vs 36,000 binding sites per cell, respectively [20,21]. The Leydig cell tumors with a higher number of LHR per cell were more susceptible to the destructive action of the Hecate-CGβ conjugate, as shown by histopathologic analysis. We did not observe any drug-related alterations in other organs like the liver, kidney, or uterus. Besides the LHR expression of the tumor cells, electrostatic interactions between negatively charged tumor cell membranes and nontumor cells may explain the specificity of the action [30]. Some in vivo studies have shown that the specific anticancer activities of some native and synthetic cationic lytic peptides (e.g., Hecate) occurred only when they were conjugated to homing domains and were quite limited [30,31]. Hecate might be cytotoxic to prokaryotic [11] and eukaryotic [17] cells, but only at much higher concentrations, and then to cell types regardless of their LHR expression [29], as also shown in this study.
In the inhα/Tag TG mice, the onset of tumor formation paralleled increased serum progesterone and decreased gonadotropin concentrations, with the former apparently originating from an increased number of progesterone-secreting tumor cells in the ovary [28,24,25], indicating that the tumors were endocrinologically active and able to exert enhanced negative feedback effects on pituitary function [24,25,23]. The steroidogenesis of Leydig BLT-1 and mLTC-1 cells was partly impaired because it did not proceed beyond progesterone to testosterone [20]. However, progesterone was also able to exert negative feedback actions on gonadotropin secretion [24,25], which explains the suppression of their secretion in male mice. Hence, if the targeted antitumor therapy with the Hecate-CGβ conjugate kills Leydig and granulosa tumor cells, then the elevated level of serum progesterone would decrease and serum LH concentration would increase. Indeed, after 3 weeks of treatment with the Hecate-CGβ conjugate, the progesterone concentration was significantly decreased in the inhα/Tag mice and serum LH concentrations were significantly elevated, as compared with the respective levels of untreated tumor mice and those receiving Hecate. This was considered a distinct sign of endocrine recovery from the hormonally active tumorigenesis under the Hecate-CGβ conjugate treatment.
We finally wanted to investigate the molecular mechanisms underlying the mode of action of the Hecate-CGβ conjugate, namely the mechanisms involved in targeted cell death. The cytotoxic effect of most antitumor agents depends on the induction of apoptosis in susceptible tumor cells [32]. Apoptotic cell death is characterized by a series of specific events including the activation of the caspase cascade, cell shrinkage, cell membrane blebbing, and condensation and fragmentation of chromatin [33]. There is evidence that the sensitivity of various tumor types to current therapeutic methods depends on the activation of multiple apoptosis-regulatory proteins [33,31,34]. There is a preliminary report showing the involvement of phospholipase PKC rather than PKA (PKC increased the sensitivity to the drug) in breast cancer cell lysis by lytic peptide hecate [19], although it has been also shown that PKC activation can be antiapoptotic, too [35,36]. In this study, two types of cocultured cell lines with or without endogenous LHR treated with Hecate-CGβ proved the high specificity for the LHR-expressing cells only, which has not been shown earlier so precisely. The chain of events showed that the binding of the Hecate-CGβ conjugate to the LHR and the rapid (within 15 minutes of PI administration) permeabilization and perturbation of cell membrane were the cause of cell death. It seems that once the membrane layer integrity is disturbed, which happens rapidly within minutes, the transmembrane electrochemical potential collapses and cell death occurs. Cell viability measurements and fluorescence microscopic observations verified that the cells died as a result of injury, swelling, and bursting, suggesting signs of mechanisms of induction of necrosis, which also has never been shown before. All attempts to demonstrate apoptosis as a result of the treatments yielded negative results.
Taken together, the present data provided strong evidence for a rapid and specific killing mechanism by the Hecate-CGβ conjugate toward LHR-expressing gonadal tumor cells through necrotic plasma membrane disruption. Cancer cells commonly have impaired/deleterious or mutated genes involved in apoptosis, making them often apoptosis-resistant. Therefore, the specific targeted necrosis induced by the Hecate-CGβ conjugate for cancer cells may offer an advantage and assure that the neoplastic cells would not develop drug resistance to destructive mechanism. Our in vivo results demonstrate that the targeted therapy of gonadal somatic cell tumors expressing LHR by the Hecate-CGβ conjugate is potentially useful and worthy of further testing, with potential for future human trials.
Acknowledgements
We thank Jorma Toppari and Bill Hansel for fruitful discussions, and Tarja Laiho and Johanna Lahtinen for technical assistance.
Footnotes
This project was supported by the Committee of Scientific Research in Poland (grant no. 5P06K00917), the Finnish Cancer Society, and the Academy of Finland.
References
- 1.Freeman DA. Steroid hormone-producing tumors in man. Endocr Rev. 1986;7:204–220. doi: 10.1210/edrv-7-2-204. [DOI] [PubMed] [Google Scholar]
- 2.Kinkade S. Testicular cancer. Am Fam Physician. 1999;59:2539–2544. 2549–2550. [PubMed] [Google Scholar]
- 3.Crayford TJ, Campbell S, Bourne TH, Rawson HJ, Collins WP. Benign ovarian cysts and ovarian cancer: a cohort study with implications for screening. Lancet. 2000;355:1060–1063. doi: 10.1016/S0140-6736(00)02038-9. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89:2068–2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Malmstrom H, Hogberg T, Risberg B, Simonsen E. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994;52:50–55. doi: 10.1006/gyno.1994.1010. [DOI] [PubMed] [Google Scholar]
- 6.Savage P, Constenla D, Fisher C, Shepherd JH, Barton DP, Blake P, Gore ME. Granulosa cell tumours of the ovary: demographics, survival and the management of advanced disease. Clin Oncol (R Coll Radiol) 1998;10:242–245. doi: 10.1016/s0936-6555(98)80008-3. [DOI] [PubMed] [Google Scholar]
- 7.Cronje HS, Niemand I, Bam RH, Woodruff JD. Review of the granulosa-theca cell tumors from the Emil Novak Ovarian Tumor Registry. Am J Obstet Gynecol. 1999;180:323–327. doi: 10.1016/s0002-9378(99)70207-3. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984;8:575–596. doi: 10.1097/00000478-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 10.Chan SC, Yau WL, Wang W, Smith DK, Sheu FS, Chen HM. Microscopic observations of the different morphological changes caused by anti-bacterial peptides on Klebsiella pneumoniae and HL-60 leukemia cells. J Pept Sci. 1998;4:413–425. doi: 10.1002/(SICI)1099-1387(199811)4:7%3C413::AID-PSC160%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Henk WG, Todd WJ, Enright FM, Mitchell PS. The morphological effects of two antimicrobial peptides, hecate-1 and melittin, on Escherichia coli. Scanning Microsc. 1995;9:501–507. [PubMed] [Google Scholar]
- 12.LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–3279. doi: 10.1210/endo-126-6-3277. [DOI] [PubMed] [Google Scholar]
- 13.Morbeck DE, Roche PC, Keutmann HT, McCormick DJ. A receptor binding site identified in the region 81-95 of the beta-subunit of human luteinizing hormone (LH) and chorionic gonadotropin (hCG) Mol Cell Endocrinol. 1993;97:173–181. doi: 10.1016/0303-7207(93)90225-9. [DOI] [PubMed] [Google Scholar]
- 14.Rao CV. The beginning of a new era in reproductive biology and medicine: expression of low levels of functional luteinizing hormone/human chorionic gonadotropin receptors in nongonadal tissues. J Physiol Pharmacol. 1996;47:41–53. [Google Scholar]
- 15.Ziecik AJ, Derecka K, Gawronska B, Stepien A, Bodek G. Nongonadal LH/hCG receptors in pig: functional importance and parallels to human. Semin Reprod Med. 2001;19:19–30. doi: 10.1055/s-2001-13907. [DOI] [PubMed] [Google Scholar]
- 16.Gawronska B, Leuschner C, Enright FM, Hansel W. Effects of a lytic peptide conjugated to beta HCG on ovarian cancer: studies in vitro and in vivo. Gynecol Oncol. 2002;85:45–52. doi: 10.1006/gyno.2001.6558. [DOI] [PubMed] [Google Scholar]
- 17.Leuschner C, Enright FM, Melrose PA, Hansel W. Targeted destruction of androgen-sensitive and -insensitive prostate cancer cells and xenografts through luteinizing hormone receptors. Prostate. 2001;46:116–125. doi: 10.1002/1097-0045(20010201)46:2<116::aid-pros1015>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Hansel W, Leuschner C, Gawronska B, Enright F. Targeted destruction of prostate cancer cells and xenografts by lytic peptide-betaLH conjugates. Reprod Biol. 2001;1:20–32. [PubMed] [Google Scholar]
- 19.Leuschner C, Enright FM, Gawronska B, Hansel W. Membrane disrupting lytic peptide conjugates destroy hormone dependent and independent breast cancer cells in vitro and in vivo. Breast Cancer Res Treat. 2003;78:17–27. doi: 10.1023/a:1022169525521. [DOI] [PubMed] [Google Scholar]
- 20.Kananen K, Markkula M, El-Hefnawy T, Zhang FP, Paukku T, Su JG, Hsueh AJ, Huhtaniemi I. The mouse inhibin alpha-subunit promoter directs SV40 T-antigen to Leydig cells in transgenic mice. Mol Cell Endocrinol. 1996;119:135–146. doi: 10.1016/0303-7207(96)03802-6. [DOI] [PubMed] [Google Scholar]
- 21.Kananen K, Markkula M, Rainio E, Su JG, Hsueh AJ, Huhtaniemi IT. Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoter/Simian Virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol. 1995;9:616–627. doi: 10.1210/mend.9.5.7565808. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D. Transgenic mice as probes into complex systems. Science. 1989;246:1265–1275. doi: 10.1126/science.2686032. [DOI] [PubMed] [Google Scholar]
- 23.Rahman NA, Kananen Rilianawati K, Paukku T, Mikola M, Markkula M, Hamalainen T, Huhtaniemi IT. Transgenic mouse models for gonadal tumorigenesis. Mol Cell Endocrinol. 1998;145:167–174. doi: 10.1016/s0303-7207(98)00184-1. [DOI] [PubMed] [Google Scholar]
- 24.Kananen K, Rilianawati, Paukku T, Markkula M, Rainio EM, Huhtanemi I. Suppression of gonadotropins inhibits gonadal tumorigenesis in mice transgenic for the mouse inhibin alpha-subunit promoter/Simian Virus 40 T-antigen fusion gene. Endocrinology. 1997;138:3521–3531. doi: 10.1210/endo.138.8.5316. [DOI] [PubMed] [Google Scholar]
- 25.Rahman NA, Huhtaniemi IT. Ovarian tumorigenesis in mice transgenic for murine inhibin alpha subunit promoter-driven Simian Virus 40 T-antigen: ontogeny, functional characteristics, and endocrine effects. Biol Reprod. 2001;64:1122–1130. doi: 10.1095/biolreprod64.4.1122. [DOI] [PubMed] [Google Scholar]
- 26.Papo N, Shahar M, Eisenbach L, Shai Y. A novel lytic peptide composed of dl-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem. 2003;278:21018–21023. doi: 10.1074/jbc.M211204200. [DOI] [PubMed] [Google Scholar]
- 27.Papo N, Shai Y. New lytic peptides based on the d,l-amphipathic helix motif preferentially kill tumor cells compared to normal cells. Biochemistry. 2003;42:9346–9354. doi: 10.1021/bi027212o. [DOI] [PubMed] [Google Scholar]
- 28.Kananen K, Markkula M, Mikola M, Rainio EM, McNeilly A, Huhtaniemi I. Gonadectomy permits adrenocortical tumorigenesis in mice transgenic for the mouse inhibin alpha-subunit promoter/Simian Virus 40 T-antigen fusion gene: evidence for negative autoregulation of the inhibin alpha-subunit gene. Mol Endocrinol. 1996;10:1667–1677. doi: 10.1210/mend.10.12.8961275. [DOI] [PubMed] [Google Scholar]
- 29.Bodek G, Rahman NA, Zaleska M, Soliymani R, Lankinen H, Hansel W, Huhtaniemi I, Ziecik AJ. A novel approach of targeted ablation of mammary carcinoma cells through luteinizing hormone receptors using Hecate-CGbeta conjugate. Breast Cancer Res Treat. 2003;79:1–10. doi: 10.1023/a:1023351819956. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Xu X, Hong S, Chen J, Liu N, Underhill CB, Creswell K, Zhang L. RGD-tachyplesin inhibits tumor growth. Cancer Res. 2001;61:2434–2438. [PubMed] [Google Scholar]
- 31.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 32.Hannun YA. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89:1845–1853. [PubMed] [Google Scholar]
- 33.Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lorenzo MJ, Gamen S, Etxeberria J, Lasierra P, Larrad L, Pineiro A, Anel A, Naval J, Alava MA. Resistance to apoptosis correlates with a highly proliferative phenotype and loss of Fas and CPP32 (caspase-3) expression in human leukemia cells. Int J Cancer. 1998;75:473–481. doi: 10.1002/(sici)1097-0215(19980130)75:3<473::aid-ijc23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Blagosklonny MV, Prabhu NS, El-Deiry WS. Defects in p21WAF1/CIP1, Rb, and c-myc signaling in phorbol ester-resistant cancer cells. Cancer Res. 1997;57:320–325. [PubMed] [Google Scholar]
- 36.Emons G, Muller V, Ortmann O, Schulz KD. Effects of LHRH-analogues on mitogenic signal transduction in cancer cells. J Steroid Biochem Mol Biol. 1998;65:199–206. doi: 10.1016/s0960-0760(97)00189-1. [DOI] [PubMed] [Google Scholar]
- 37.Rebois RV. Establishment of gonadotropin-responsive murine Leydig tumor cell line. J Cell Biol. 1982;94:70–76. doi: 10.1083/jcb.94.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 39.Thomas DR, Philpott GW, Jaffe BM. Prostaglandin E (PGE) control of cell proliferation in vitro: characteristics of HT-29. J Surg Res. 1974;16:463–465. doi: 10.1016/0022-4804(74)90070-5. [DOI] [PubMed] [Google Scholar]
- 40.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 41.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 42.Huhtaniemi I, Nikula H, Rannikko S. Treatment of prostatic cancer with a gonadotropin-releasing hormone agonist analog: acute and long term effects on endocrine functions of testis tissue. J Clin Endocrinol Metab. 1985;61:698–704. doi: 10.1210/jcem-61-4-698. [DOI] [PubMed] [Google Scholar]
- 43.Vuorento T, Lahti A, Hovatta O, Huhtaniemi I. Daily measurements of salivary progesterone reveal a high rate of anovulation in healthy students. Scand J Clin Lab Invest. 1989;49:395–401. doi: 10.3109/00365518909089113. [DOI] [PubMed] [Google Scholar]
- 44.Fried J, Perez AG, Clarkson BD. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures. Some pitfalls in staining and data analysis. J Histochem Cytochem. 1978;26:921–933. doi: 10.1177/26.11.82573. [DOI] [PubMed] [Google Scholar]
- 45.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 46.Yang XH, Sladek TL. Overexpression of the E2F-1 transcription factor gene mediates cell transformation. Gene Expr. 1995;4:195–204. [PMC free article] [PubMed] [Google Scholar]
- 47.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]