Abstract
Glioblastoma multiforme is extraordinarily aggressive due to the propensity of cells to migrate away from the tumor core into the surrounding normal brain. In this report, we investigated the role of proline-rich tyrosine kinase 2 (Pyk2) and FAK with regard to influencing glioma cell phenotypes. Expression of Pyk2 stimulated glioma cell migration, whereas expression of FAK inhibited glioma cell migration and stimulated cell cycle progression. Pyk2 autophosphorylation was necessary, but not sufficient, to stimulate cellular migration. The N-terminal domain of Pyk2 is required for stimulation of migration as an N-terminally deleted variant of Pyk2 failed to stimulate migration, whereas expression of an autonomous Pyk2 N-terminal domain inhibited cell migration. Substitution of the C-terminal domain of Pyk2 with the corresponding domain of FAK stimulated cell migration as effectively as wild-type Pyk2; however, substitution of the N-terminal domain of Pyk2 with that of FAK inhibited cell migration, substantiating that the N-terminal domain of Pyk2 was required to stimulate migration. Silencing of Pyk2 expression by RNA interference significantly inhibited glioma migration. Cell migration was restored on reexpression of Pyk2, but expression of FAK in Pyk2 knockdown cells failed to restore migration. We conclude that Pyk2 plays a central role in the migratory behavior of glioblastomas.
Keywords: Glioma, focal adhesion kinase, migration, tyrosine kinase, invasion
Introduction
Treatment of glioblastoma multiforme has been largely unsuccessful due to the predisposition of individual tumor cells to invade the surrounding normal brain. These invasive cells substantially reduce the treatment efficacy of surgical resection, radiation, and chemotherapy [1–5]. Although not one of the more common tumors afflicting patients in the United States (2% of all yearly deaths due to cancer, or ∼13,000 deaths/year), glioblastomas are very aggressive and are associated with a median survival of less than 1 year from initial diagnosis [6,7]. The molecular mechanisms that regulate migration of gliomas from primary tumor sites remain poorly understood. Invasion is a complex process involving both receptor-mediated adhesion to components of the extracellular matrix (ECM) and proteolytic modification of the ECM. Integrin-mediated adhesive interactions play a critical mechanical role in cellular migration by linking the ECM to the intracellular cytoskeleton. Moreover, integrin ligation and receptor clustering lead to assembly of the focal adhesion complex, a complex protein scaffold enriched in signaling molecules, leading to activation of intracellular signaling pathways crucial to cell proliferation and survival [8–10]. Therefore, integrin-mediated adhesion and signaling are uniquely situated to play a central role in the behavior of invasive gliomas. Indeed, the importance of integrin interactions with ECM proteins on glioma adhesion and migration has been demonstrated in previous reports [11–14].
The related nonreceptor tyrosine kinases, FAK and proline-rich tyrosine kinase 2 (Pyk2), are important signaling effectors linking integrin and growth factor receptor signaling to cell proliferation, migration, survival, and apoptosis in many cell types. FAK is the prototypical signaling effector coupling integrin-matrix interactions to intracellular signaling events. FAK is activated rapidly following integrin engagement and exerts downstream effects by serving both as an effector kinase as well as a scaffold protein for various molecules including Src, paxillin, talin, p130cas, Grb2, Grb7, Graf, and PI3-K [15]. Considerable evidence supports the role of FAK in cell migration both in vitro and in vivo [16–18]. Furthermore, increased FAK activity has been correlated with increased cell proliferation and cell cycle progression, which play integral roles in tumor progression [19–21]. Indeed, overexpression of FAK promoted malignant astrocytoma cell proliferation in vivo [22]. In contrast, it has been reported that although FAK was localized to the membrane of nonmigratory astrocytoma cells, it was largely absent in migrating cells [23]. High levels of endogenous FAK also have been correlated with reduced migratory rates of cultured glioma cells in vitro [24]. Furthermore, dephosphorylation and downregulation of FAK activity correlated positively with EGF-induced tumor cell invasion [25].
The related focal adhesion kinase Pyk2 has a conserved domain structure and significant sequence identity to FAK [26,27]. Although Pyk2 can interact with many of the same proteins as FAK, it has a different primary means of activation and a more limited tissue distribution. Notably, Pyk2 is highly expressed in the central nervous system [28]. In contrast to the more rigorously studied FAK, the cellular functions of Pyk2 continue to be defined. Although FAK activity has been readily linked to cell motility, a role for Pyk2 in cell migration has only recently begun to be appreciated. In cell types that naturally express Pyk2 but little or no FAK, such as NK cells, monocytes, or osteoclasts, a critical role for Pyk2 in the regulation of cytoskeletal organization and cell motility has been established [29–32]. Therefore, in cells such as gliomas that express both kinases, the temporal balance between Pyk2 and FAK activity may ultimately regulate cell growth and cell migration. The recent demonstration [33] of a clear correlation between expression of FAK and Pyk2 and increasing malignancy suggests that the differential activity of these two kinases may be particularly relevant to the regulation of the proliferative or migratory behavior of these cells.
The current study investigated the functional contributions of FAK and Pyk2 in determining in vitro glioma cell migration and proliferation. Expression of Pyk2 stimulated glioma cell migration in a dose-dependent manner. Autophosphorylation of Pyk2 Y402 was necessary but insufficient for this phenotypic effect. The N-terminal FERM domain of Pyk2 was required to stimulate migration and was capable of inhibiting migration when expressed as an autonomous fragment. In contrast, expression of FAK inhibited glioma cell migration and stimulated cell cycle progression in a dose-dependent manner. Knockdown of Pyk2 expression by RNAi inhibited glioma cell migration in vitro and in organotypic brain slices. Reconstitution of Pyk2 expression in these cells, but not FAK expression, restored glioma cell migration. These data further indicate that Pyk2 and FAK function in the temporal development of the proliferative or migratory behavior of glioma cells and may be useful therapeutic targets in malignant glioblastoma.
Materials and Methods
Antibodies
Affinity-purified polyclonal antibodies to the C-terminal portion of FAK were obtained from Upstate Biotechnology (Lake Placid, NY). Monoclonal antibody to the C-terminal portion of Pyk2 was obtained from Transduction Laboratories (Lexington, KY). Anti-FLAG M2 monoclonal antibody was obtained from Sigma (St. Louis, MO). Affinity-purified antibody to the HA epitope tag was obtained from Upstate Biotechnology.
Adenoviral Expression Constructs
Recombinant adenoviruses encoding HA epitope-tagged wild-type FAK was previously described [24]. To generate FLAG epitope-tagged wild-type Pyk2, the coding sequence or Pyk2 was cloned in-frame downstream of a 3x FLAG epitope in pcDNA3. The Pyk2 phosphorylation-deficient mutant Y402F was generated using the Quickchange site directed mutagenesis system (Stratagene, La Jolla, CA). To generate the Pyk2 N-terminal deletion mutant Pyk2Δ376, a fragment encoding Pyk2 residue S376-E1009 was amplified by polymerase chain reaction (PCR) and cloned in-frame downstream of the 3x FLAG epitope in pcDNA3. The FLAG epitope-tagged chimeric proteins designated Pyk/FAK and FAK/Pyk were generated by splice overlap extension PCR [34]. Pyk/FAK contains Pyk2 N-terminal residue M1-D665 fused to FAK residue P663-H1052. FAK/Pyk contains FAK N-terminal residue M1-D662 fused to Pyk2 residue P666-E1009. The Pyk2 FERM domain, encoding Pyk2 residue R39-A367, was amplified by PCR and cloned in-frame downstream of a 3x HA epitope in pcDNA3. A fragment encoding Pyk2 residues 1 to 367 was amplified by PCR and cloned downstream of a 3x HA epitope to generate a construct designated Pyk NT. All sequences were verified by DNA sequencing. Recombinant E1-deleted adenovirus for each construct was prepared using the Ad-Easy system as described [35].
Retroviral Constructs
The retroviral transfer vector pQGFP is a derivative of the pQCXIX self-inactivating retroviral vector (Clontech, Palo Alto, CA). The coding sequence for humanized Renilla green fluorescent protein (GFP) was excised from plasmid phrGFP-1 (Stratagene) and cloned into the Not-EcoRV sites of pQCXIX to produce pQGFP. A transcriptional cassette containing an oligonucleotide duplex encoding the human Pyk2 small interfering RNA (siRNA) or FAK siRNA was cloned downstream of the human U6 promoter or cloned into the unique SalI site in the 3′ LTR of pQGFP to produce pGFP.U6Pyki or pGFP.U6FAKi, respectively. The correct sequence and orientation of the U6-siRNA or U6-siFAKi cassette were verified by direct DNA sequencing. Viral stocks were prepared by cotransfecting the GP2-293 packaging cell line (Clontech) with pGFP.U6Pyki or pGFP.U6FAKi and pVSV-G (Clontech). Forty-eight hours after transfection, the supernatant was removed, filtered through a 0.45-µm filter, and spun at 50,000g for 1.5 hours. The pelleted virus was resuspended in 400 µl of Dulbecco's modified Eagle's medium (DMEM) and added to target cells with 5 µg/ml polybrene. Forty-eight hours after infection, cells were harvested and GFP-positive cells were collected by mass sorting on a FACS Vantage flow cytometer (BD Biosciences, San Jose, CA). The coding sequence of Discosoma red fluorescent protein (RFP) from pCMV-DsRed-Express (Clontech) was ligated into pQCXIX to produce the retroviral transfer vector, pQDsRed. SF767 cells stably transduced with RFP were produced as described above.
Cell Culture
Established human malignant glioblastoma cell lines SF767 and G112 were routinely passaged in DMEM containing 10% fetal bovine serum, 1% nonessential amino acids, 2 mM glutamine, 100 U/ml penicillin, and 10 µg/ml streptomycin. For adenoviral infections, 2.5 x 105 cells were added to each well of a six-well plate and cultured for 24 hours before infection.
Immunoblotting
Cells were washed in cold phosphate-buffered saline (PBS) and lysed by addition of 1 µl of ice-cold RIPA buffer: 150 mM NaCl, 10 mM Tris, pH 7.2, 1 mM EGTA, 1 mM orthovanadate, 1% Triton X-100, 1% Na deoxycholate, 1% SDS, 100 µM leupeptin, 5 IU/ml aprotinin, 10 µg/ml soybean trypsin inhibitor, 10 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride, and incubated on ice for 30 minutes. Lysates were clarified by centrifugation at 16,000g for 10 minutes at 4°C. The protein content of the lysate was determined using the BCA assay (Pierce, Rockford, IL). For immunoblotting, equal amounts of protein (10–20 µg) were electrophoresed on 8% to 16% gradient SDS-PAGE gels (Novex, Carlsbad, CA) and transferred to nitrocellulose. Immunoblotting of transferred proteins was performed with the appropriate antibodies at the indicated concentrations for 1 hour at room temperature and was visualized by enhanced chemiluminescence (Perkin Elmer Life Sciences, Boston, MA). Signals were quantified by densitometry utilizing Kodak 1D Image Analysis Software. Efficiency of transfer was routinely monitored by immunoblotting for actin. No significant differences in the amount of actin in the transfers were observed.
Radial Migration Assay
A monolayer radial migration assay was used as previously described [36]. Briefly, slides containing 10 individual 7-mm circular seeding areas surrounded by a hydrophobic template mask (Creative Scientific Methods, Inc., Phoenix, AZ) were coated with laminin at either 1 or 10 µg/ml. A coating concentration of 1 µg/ml was used to minimize the intrinsic migratory rate, thus increasing the positive effect size relative to control. Assays assessing FAK dose response utilized a higher laminin coating concentration (10 µg/ml) to maximize the intrinsic migratory rate and to accentuate the evaluation of the inhibitory effect. Cells were routinely infected with 2, 5, 10, 20, and 40 multiplicities of infection (MOI) of recombinant adenoviruses and cultured for 16 hours before harvest. Infected cells were resuspended in DMEM containing 10% serum and seeded at a density of 2500 cells/well (internal diameter of 1 µm) of a Cell Sedimentation Manifold (Creative Scientific Methods, Inc.). After overnight incubation (16 hours), the manifold was removed and an initial measurement (t0) of the diameter of the cell colony was made using an inverted microscope (Axiovert; Carl Zeiss, Thornwood, NY) and image analysis equipment (Scion Image, Frederick, MD). The change in the diameter of the cell population over time was determined at 24 hours following the initial measurement. Slopes of the lines derived from the measurements (radius versus time) were used to calculate the migration rate of the cells. Linear migration from the initial seeded area at t0 was determined for at least 10 replicate samples for each infected construct. Specific migration rates were calculated by normalizing the measurements to nonspecific migration on BSA. The absolute migration and migratory rates were calculated and group means were determined.
Organotypic Brain Slice Invasion Assay
Slice cultures of whole brains were produced from adult rats essentially as described [37]. Four- to 6-week-old male Wistar rats were sacrificed by isofluorane inhalation, then rat brains were removed from the skull and immediately placed into sterile ice-cold PBS. The cold brains were coronally sectioned with a vibratome (OTS-4000; Electron Microscopy Sciences, Hatfield, PA) into 400 µm slices at +1.75 to +1.0 bregma and the slices placed aseptically onto transwell (8 µm pore size) membranes in six-well dishes. Culture media (DMEM, 10% FCS, 6.5 µg/ml glucose, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2.5 µg/ml amphotericin B) were added into each well to a point just below that which would cover the brain slice. Wild-type SF767 cells (stably expressing RFP) or SFPyk2i cells (expressing GFP) were grown to subconfluency and 1 x 105 cells (in 0.5 µl) were deposited onto each caudoputamen of the brain slice. Cells are allowed to attach to the brain surface (∼1 hour) before the media were added to just the surface of the slice. The slices were incubated at 37°C for 5 days, rinsed with PBS, and fixed in 4% paraformaldehyde. The extent of glioma cell invasion into the brain slice was quantitated by optical sectioning with a LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Cell Cycle Progression Assay
SF767 cells and G112 cells were seeded into six-well plates and infected with virus after 24 hours. Twenty-four hours postinfection, cells were washed once with PBS, removed from the plate with trypsin, and washed in complete media. The cells were again washed in PBS, centrifuged, and resuspended in 1 ml of PBS. Cells were then fixed by adding cold ethanol dropwise while vortexing, followed by incubation on ice for 1 hour. Cells were washed in PBS and resuspended in 100 µl of RNase A (100 µg/ml) for 5 minutes at room temperature. The cells were then stained in 400 µl of propidium iodide (50 µg/ml) and detected on a Becton Dickinson FACScan (BD Biosciences) flow cytometer using a 488-nm laser. The percentage of cells in S-phase was calculated using the Modfit program (Verity Software House, Inc., Topsham, ME).
Statistics
Migration and proliferation data were imported to SAS (Cary, NC), checked, and cleaned. Preliminarily, distributional assumptions were tested using the Kolmogorov-Smirnov test. No data set, within cell line or adhesion kinase, deviated significantly from normality. Therefore, means and standard deviations were chosen for statistical tests. Independent-sample t tests were used for analyses involving two samples, and ANOVA, with post-hoc Scheffe tests, for tests involving Functional Investigation of Pyk2 and FAK Lipinski et al. 437 more than two samples. All tests are two-tailed (criterion level, P < .05).
Results
Glioma Cell Migration Is Stimulated by Pyk2 Expression and Inhibited by FAK Expression
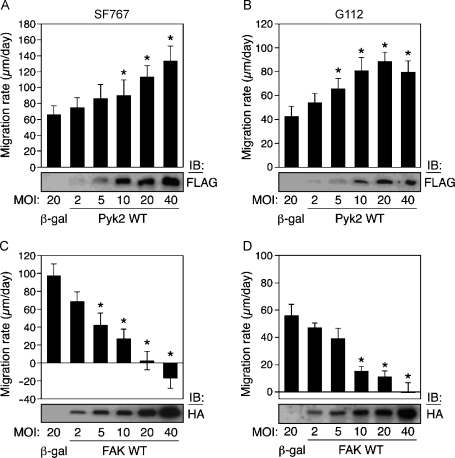
The effect of expression of Pyk2 or FAK on glioma cell migration was assessed in the SF767 and G112 glioma cell lines. These two cell lines were chosen as they exhibit high and low intrinsic migration rates, respectively, that correlate with opposite expression/activity patterns with regard to Pyk2 and FAK [24]. Thus, SF767 cells express significantly higher levels of Pyk2 relative to FAK and a high intrinsic migration rate. Conversely, G112 cells express higher levels of FAK relative to Pyk2 and exhibit a lower intrinsic migration rate. To further investigate the role of Pyk2 and FAK in glioma cell migration, dose response experiments were performed in which glioma cells were infected with increasing MOI of recombinant adenoviruses encoding Pyk2 or FAK. The effects of increasing Pyk2 or FAK expression were examined with a monolayer migration assay.
To investigate the effect of Pyk2 on cell migration, infected cells were plated on a low laminin coating concentration (1 µg/ml) to allow better discrimination of gain of function. Increasing expression of wild-type Pyk2 resulted in a significant dose-dependent increase in migration rate (Figure 1, A and B). This effect was observed in both the highly migratory SF767 and the slower migrating G112 cell lines. SF767 and G112 cells infected with 20 MOI of the control adenovirus encoding β-galactosidase migrated at a rate of 66 ± 11 µm/day (mean ± SD) and 42 ± 7 µm/day, respectively. In contrast, the migration rate of SF767 cells and G112 cells infected with 20 MOI of adenovirus encoding Pyk2 increased to 113.6 ± 14.1 and 88.8 ± 7.7 µm/day, respectively.
Figure 1.
Glioma cell migration is stimulated by Pyk2 expression and inhibited by FAK expression. SF767 (A and C) or G112 (B and D) glioma cell lines were infected with recombinant adenoviruses encoding Pyk2 or FAK at the indicated MOI and migration rate on laminin measured with a monolayer migration assay. Migration rates of infected cells were derived from slopes of lines from measurements of radii of cell population versus time. Cells were plated on 1 µg/ml laminin (A and B) or on 10 µg/ml laminin (C and D). Values represent mean ± SD of 10 replicate samples. Expression of Pyk2 or FAK was detected by immunoblotting of cell lysates with anti-FLAG or anti-HA antibodies, respectively. *P < .05.
To investigate the role of FAK in glioma cell migration, SF767 and G112 cells were infected with wild-type FAK at varying MOI and plated on a high laminin substrate concentration (10 µg/ml) to better observe any loss of migratory function. Expression of FAK significantly inhibited the migration of both cell lines (Figure 1, C and D). SF767 cells expressing β-galactosidase (20 MOI) migrated at 98 ± 12 µm/day, whereas expression of FAK (20 MOI) substantially reduced the migration rate to 2.5 ± 10.2 µm/day. At the highest level of FAK expression (40 MOI), a negative migration rate was derived as the cells were observed to round up and detach from the edges of the assay accounting for these observations. Similar results were observed in the G112 cells. G112 cells expressing 20 MOI of β-galactosidase migrated at 55.9+8.3 µm/day, whereas those cells infected with 20 MOI FAK migrated at 11.6 ± 3.9 µm/day.
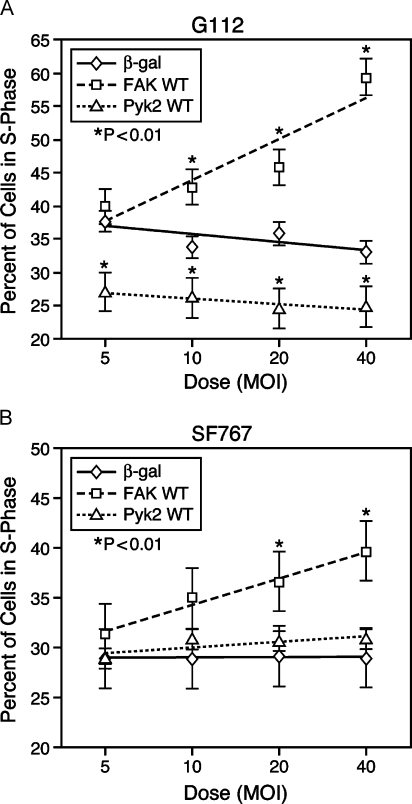
FAK Expression Induces Glioma Cell Proliferation
Overexpression of FAK in glioma cells resulted in statistically higher expression of the nuclear proliferation marker Ki67 [38] in both the core and rim populations in the radial migration assay format, whereas Pyk2 expression reduced Ki67 expression in these cell populations [24]. To substantiate these immunohistochemical data, we examined the effect of FAK or Pyk2 expression on glioma cell cycle progression. Subconfluent cultures of SF767 or G112 cells were infected with varying MOI of the control β-galactosidase virus, epitope-tagged wild-type Pyk2, or FAK, and the percentage of cells in S-phase was determined by flow cytometry (Figure 2). The G112 cell line was observed in all cases to proliferate to a greater degree than the SF767 cells. Increasing β-galactosidase MOI in G112 did not significantly affect the percentage of cells in S-phase (range 36.8-34.2, P = NS). However, increasing FAK expression increased the percentage of G112 cells in S-phase (range 40.1 ± 1.7% to 61.1 ± 2.3%) (Figure 2A). Infection of SF767 cells with β-galactosidase at increasing MOI also did not result in a significant change in the percentage of cells in S-phase (range 28.57–29.61; P = NS). Similar to the results obtained with G112 cells, expression of FAK in SF767 cells increased the percentage of cells in S-phase in a dose-dependent manner (range 32.5 ± 2.4% to 39.9 ± 3.1%) (Figure 2B).
Figure 2.
FAK expression stimulates glioma cell proliferation. Subconfluent cultures of G112 or SF767 cells were infected with varying MOI of the control β-galactosidase, wild-type FAK, or wild-type Pyk2. The percentage of cells in S-phase was determined by flow cytometry 24 hours after infection. (A) Expression of FAK significantly increased the percentage of G112 cells in S-phase in a dose-dependent manner (*P ≤ .01). In contrast, expression of Pyk2 decreased the percentage of cells in S-phase relative to control infected cells (*P < .05). (B) Expression of FAK increased the percentage of SF767 cells in S-phase in a dose-dependent manner (*P < .001). Pyk2 did not alter the percentage of SF767 cell in S-phase compared to the control β-galactosidase-infected cells.
Because WT FAK was observed to exert a positive effect on cell cycle progression, we investigated the effect of increasing expression of WT Pyk2 on the percentage of SF767 and G112 cells in S-phase. Increasing expression of Pyk2 in SF767 cells did not change the percentage of cells in S-phase relative to cells infected with a matching MOI of the control β-galactosidase virus. In contrast, expression of Pyk2 decreased the percentage of G112 cells in S-phase relative to control β-galactosidase-infected cells. Together, these results are consistent with the differential effects of FAK and Pyk2 on cell cycle progression [20,21,39].
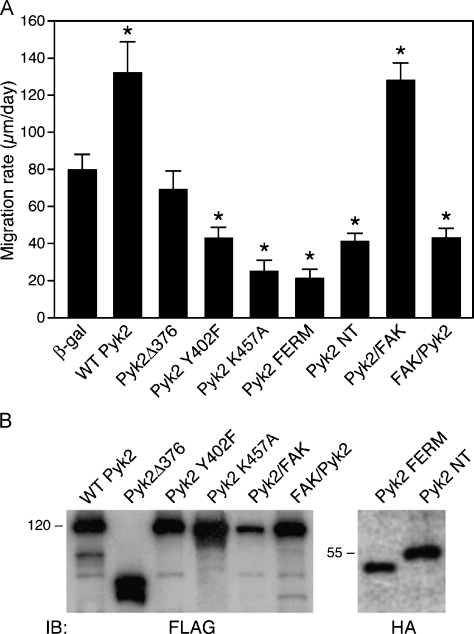
Stimulation of Glioma Cell Migration by Pyk2 Requires the N-terminal Domain
To identify the required structural or functional domains of Pyk2 required for its stimulatory effect on glioma cell migration, we generated several variant forms of Pyk2. These Pyk2 variants were expressed in SF767 cells (MOI = 20) and their effects on migration were analyzed with the radial migration assay (Figure 3). Pyk2 stimulation of cell migration required kinase activity because the expression of the catalytically defective mutant Pyk2 K457A inhibited SF767 cell migration. Similarly, the autophosphorylation site of Pyk2, Y402, was required for Pyk2 to stimulate migration because expression of the Pyk2 Y402F mutant inhibited cell migration relative to control SF767 cells infected with a matching MOI of β-galactosidase (43.0 ± 5.9 vs 78.8 ± 10.4 µm/day, respectively).
Figure 3.
Stimulation of glioma cell migration by Pyk2 requires the N-terminal domain. (A) SF767 cells were infected with adenoviruses encoding β-galactosidase, wild-type Pyk2, or the indicated Pyk2 variants (MOI = 20). Sixteen hours after infection, cells were seeded onto laminin (1 µg/ml) and migration rate was determined 24 hours postseeding. Values are mean ± SD of 10 replicate samples (*P < .01). (B) Expression of each of the epitope-tagged constructs was detected by blotting cell lysates with anti-Flag or anti-HA antibodies as indicated.
To further investigate the importance of the autophosphorylation site, we examined the effect of expressing a Pyk2 variant that is constitutively phosphorylated. Similar to the results observed following truncation of the N-terminal portion of FAK [40], deletion of the N-terminal 376 amino acids of Pyk2 results in a variant Pyk2 (Pyk2Δ376) that maintains a high level of constitutive phosphorylation at Y402 (data not shown). Interestingly, expression of Pyk2Δ376 did not stimulate migration relative to control cells (Figure 3). SF767 cells expressing Pyk2Δ376 migrated at 69.1 ± 10.2 µm/day compared to control β-galactosidase-expressing cells that migrated at 78.8 ± 10.4 µm/day (P = NS). Together, these results indicate that phosphorylation of Y402 was necessary but not sufficient to stimulate glioma cell migration. Moreover, these results suggested that the N-terminal domain of Pyk2 was required for the stimulation of glioma cell migration. To further investigate the requirement of the Pyk2 N-terminal domain, we examined the effect of expressing autonomous Pyk2 N-terminal domain fragments. SF767 cells were infected (MOI = 20) with a construct that encodes Pyk2 residues 1 to 367 (Pyk NT), or with a construct encoding Pyk2 residues 39 to 367 corresponding to the divergent FERM domain [41]. Expression of either the Pyk NT domain (47.9 ± 7.7 µm/day) or the Pyk2 FERM domain (21.0 ± 8.2 µm/day) significantly inhibited cell migration relative to cells expressing the control β-galactosidase protein (78.8 ± 10.4 µm/day) (Figure 3). Inhibition of cell migration by expression of the Pyk2 FERM domain was not due to a decrease in expression of endogenous Pyk2 (data not shown).
The requirement of the Pyk2 N-terminal domain for the stimulation of glioma cell migration was substantiated through the use of chimeric proteins. The chimera designated Pyk/FAK contains the N-terminal and kinase domains of Pyk2 fused to the C-terminal domain of FAK. The reciprocal chimera FAK/Pyk contains the N-terminal and kinase domains of FAK fused to the C-terminal domain of Pyk2. Expression of the Pyk/FAK chimera stimulated SF767 migration (Figure 3) to the same degree as wild-type Pyk2 (124.2 ± 10.5 µm/day for Pyk/FAK vs 132.1 ± 16.7 µm/day for WT Pyk2; P = NS). Conversely, expression of the FAK/Pyk construct significantly inhibited migration (40.5 ± 9.4 µm/day) compared to SF767 cells infected with an equivalent MOI of β-galactosidase (78 ± 10.4 µm/day) (Figure 3). These data indicate that the N-terminal domain of Pyk2 was required for stimulating cell migration, that the highly similar FAK N-terminal domain could not substitute for Pyk2, and that the C-terminal domain of Pyk2 was not required for stimulation of cell migration.
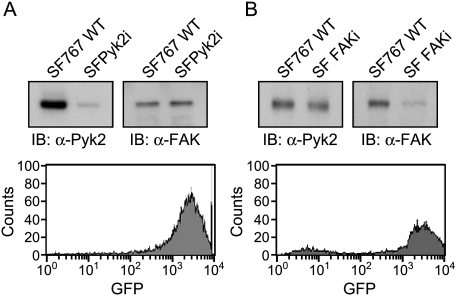
Effect of Silencing Pyk2 or FAK Expression on Glioma Cell Migration
The expression of both FAK and Pyk2 in the established glioma cell lines adds a level of complexity to the interpretation of experimental results. To overcome this limitation, we have utilized siRNA to knock down the expression of Pyk2 or FAK in SF767 cells. To generate glioma cell lines with a stable knockdown of Pyk2 or FAK expression, SF767 cells were transduced with a retrovirus expressing both a fluorescent tag and an siRNA targeting human Pyk2 or FAK. The fluorescent tag, humanized Renilla GFP, is expressed by the CMV immediate early promoter, whereas expression of the siRNA targeting Pyk2 is driven by the human U6 promoter. SF767 cells were infected with the retrovirus and GFP-positive knockdown cells (designated SFPyk2i or SFFAKi) were collected by mass sorting on a flow cytometer. Mass sorting of cells obviates concerns over results obtained with individual clones. Immunoblotting of SF767 or SFPyk2i cell lysates demonstrated that the Pyk2 siRNA effectively knocked down the expression of Pyk2 greater than 95% relative to parental SF767 cells (Figure 4A). The knockdown was specific for Pyk2 as immunoblots confirmed that the expression of FAK was equivalent in SF767 and SFPyk2i cells. Similarly, immunoblotting of SF767 and SFFAKi cell lysates demonstrated that the FAK siRNA effectively knocked down the expression of FAK, whereas expression of Pyk2 was unchanged between the SF767 and SFFAKi cell lines (Figure 4B).
Figure 4.
Silencing of Pyk2 and FAK expression in SF767 glioma cells. SF767 glioma cells were infected with a retrovirus expressing both a GFP tag and an siRNA targeting human Pyk2 or an siRNA targeting FAK. Forty-eight hours after infection, GFP-positive cells were collected by mass sorting on a FACS Vantage flow cytometer. (A) Top panel: Lysates of wild-type SF767 cells or SFPyk2i cells were immunoblotted with antibodies specific for Pyk2 or FAK. Bottom panel: FACS histogram of GFP expression in the mass-sorted cell population. (B) Top panel: Lysates of wild-type SF767 cells or SFFAKi cells were immunoblotted with antibodies specific for Pyk2 or FAK. Bottom panel: FACS histogram of GFP expression in the mass-sorted cell population.
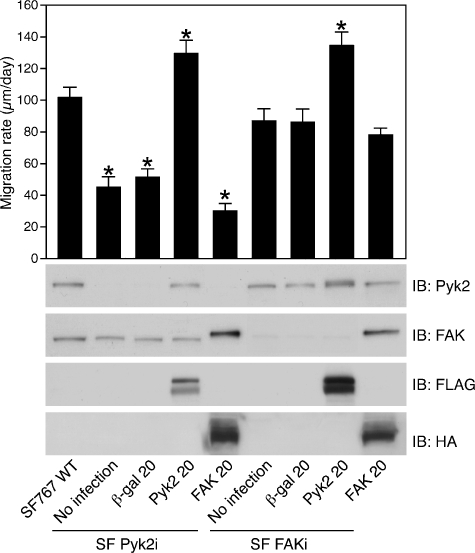
The effect of silencing expression of Pyk2 or FAK expression on SF767 migration was investigated with the in vitro migration assay (Figure 5). Knockdown of Pyk2 expression significantly reduced the migration rate of SFPyk2i cells compared to the wild-type SF767 cells in the radial migration assay. The wild-type SF767 cells migrated at 101.4 ± 6.6 µm/day compared to the SFPyk2i cell line, which migrated at 44.4 ± 7.1 µm/day. In contrast, knockdown of FAK expression only slightly reduced the migration rate of SFFAKi cells compared to the wild-type SF767 cells (86.5 ± 8.2 vs 101.4 ± 6.6 µm/day).
Figure 5.
Reconstitution of Pyk2 expression but not FAK restores glioma cell migration. Wild-type SF767, SFPyk2i, or SFFAKi cells were infected (MOI = 20) with recombinant adenoviruses expressing β-galactosidase, FLAG-tagged Pyk2, or HA-tagged FAK, plated on laminin (1 µg/ml), and migration rates were determined with the radial migration assay. Values represent mean ± SD of 10 replicate samples. Matching aliquots of cells were lysed and lysates were immunoblotted with the indicated antibodies (*P < .01).
Knockdown of Pyk2 expression in SF767 cells significantly inhibited cell migration despite the fact that endogenous FAK levels did not differ between the control SF767 cells and the SFPyk2i cells. This suggests the possibility that the residual migration observed in the SFPyk2i cells was due to the endogenous FAK. To further investigate the function of FAK and Pyk2 in glioma cell migration, we examined the effect of overexpression of FAK and reexpression of Pyk2 in the SFPyk2i cells. As shown in Figure 5, expression of wild-type FAK in SFPyk2i cells did not rescue their migration deficit relative to SFPyk2i cells (30 ± 4.6 vs 44.4 ± 7.1 µm/day). To reconstitute Pyk2 expression, SFPyk2i cells were infected with an epitope-tagged rat Pyk2 construct that differs from the corresponding human sequence by 1 bp over the targeted sequence. Immunoblotting of SKPyk2i cells infected with adenoviruses expressing rat Pyk2 demonstrated significant expression of Pyk2. In contrast to the effect of expression of FAK, reconstitution of Pyk2 expression restored migration to SFPyk2i cells. SFPyk2i cells re-expressing Pyk2 migrated at 129.5 ± 8.2 vs 44.4 ± 7.1 µm/day for the SFPyk2i cells and 50.9 ± 5.5 µm/day for SFPyk2i cells infected with a matching MOI of adenovirus expressing the control protein, β-galactosidase. Similarly, Pyk2 expression in SFFAKi cells resulted in a significant increase in the migration rate of the SFFAKi cells compared to control β-galactosidase-expressing cells (134.2 ± 8.2 vs 86.2 ± 8.0 µm/day). To reconstitute FAK expression in the SFFAKi cells, SFFAKi cells were infected with an epitope-tagged murine FAK construct that differs from the corresponding human sequence by 1 bp over the targeted sequence. FAK reconstitution in the SFFAKi cells did not significantly alter the migration rate of SFFAKi cells compared to β-galactosidase-expressing cells (77.8 ± 4.5 vs 86.2 ± 8.0 µm/day). This effect is different from that observed following expression of FAK in the SFPyk2i cells, which retain the expression of endogenous FAK. Expression of FAK in the SFPyk2i cells augments the endogenous FAK already present and further decreases the migration rate of SFPyk2i cells. In contrast, expression of FAK at 20 MOI in the SFFAKi cells brings the level of FAK in these cells to a level that is slightly greater than the amount of endogenous FAK in the parental SF767 cells. This is consistent with the observation that SFFAKi cells infected with 20 MOI of FAK migrate slower than wild-type SF767 cells (compare the first and last lanes of Figure 5). Together, these results confirm a central role for Pyk2 in glioma cell migration and suggest that the ultimate ratio between Pyk2 and FAK expression likely lies at the heart of the different functional responses.
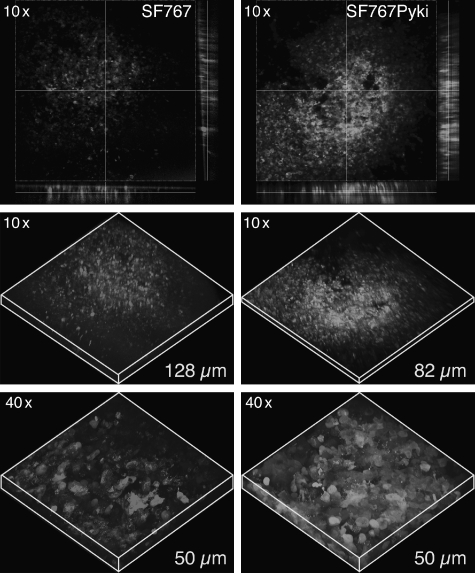
The effect of knockdown of Pyk2 expression on cell invasion was also examined in a more authentic brain matrix utilizing an ex vivo organotypic rat brain slice assay [37]. Wild-type SF767 cells stably transduced with a retrovirus expressing RFP or SFPyk2i cells (GFP) were seeded onto the hemicaudoputamen, a rat brain slice, and allowed to invade into the matrix for 5 days. Greater numbers of the SFPyk2i were present at the site of implantation on the surface of the slice after 5 days (Figure 6, top panel) compared to the wild-type SF767 cells, suggesting impaired invasion into the matrix. To investigate invasion into the slice, optical sectioning by confocal microscopy was performed. Greater numbers of SFPyk2i cells were also present 50 µm below the surface compared to the wild-type SF767 cells (Figure 6, bottom panel). Wild-type SF767 cells invaded to a depth of 128 µm compared to a depth of 82 µm for the SFPyk2i cells (Figure 6, middle panel). These data corroborate the in vitro migration assay results and further support a role for Pyk2 in migration and invasion both in vitro and in vivo.
Figure 6.
Silencing Pyk2 expression inhibits cell invasion in organotypic brain slice. SF767 cells stably transduced with RFP (left panels) or SFPyk2i cells expressing GFP (right panels) were seeded onto bilateral putamen of organotypic rat brain slice and cultured for 5 days. The extent of cell invasion was measured by optical sectioning by confocal microscopy. Images are at the surface of the slice (top), or at the indicated depths and magnification.
Discussion
The oncology of invasive gliomas is firmly rooted in the inverse relationship between migration and proliferation. Decreased proliferative activity and increased resistance to therapeutic cytotoxic agents accompany the increased commitment to invasion. The intracellular signaling networks and the critical signaling effectors that regulate temporal changes in glioblastoma proliferation and migration remain poorly defined. The current studies further define the functional role of Pyk2 and FAK in glioma cell migration and proliferation. The major findings of this report are: 1) expression of Pyk2 stimulated glioma cell migration in a dose-dependent manner; 2) expression of FAK inhibited glioma cell migration and stimulated cell cycle progression in a dose-dependent manner; 3) autophosphorylation of Pyk2 was required but not sufficient to stimulate cell migration; 4) the N-terminal domain of Pyk2 is required for stimulation of migration as an N-terminally deleted variant of Pyk2 failed to stimulate the migration and expression of an autonomous Pyk2 N-terminal fragment and inhibited cell migration; 5) silencing of Pyk2 expression by RNAi significantly inhibited cell migration in vitro and invasion in an authentic brain matrix; and 6) migration could be restored by reconstitution of Pyk2 expression but not by expression of FAK. These data confirm that the related focal adhesion kinases, Pyk2 and FAK, are important effectors in the temporal development of glioblastoma phenotype.
Results in the current report support our previous data [24] indicating that glioma cell migration correlates positively with Pyk2 activity. Expression of Pyk2 stimulated the migration of two different glioma cell lines in a dose-dependent manner, whereas inactive forms of Pyk2 inhibited cell migration. Silencing of Pyk2 expression by RNAi significantly inhibited, but did not completely abolish, migration. This effect in glioma cells is different from that commonly observed in a number of different cell types where FAK activity has been commonly linked to cell motility. This is particularly true for fibroblasts that notably do not express Pyk2. Interestingly, FAK-null fibroblasts express a significant amount of Pyk2, but its expression is not capable of compensating for their migration deficiency [42,43]. Because Pyk2 is not normally expressed in fibroblasts, the failure of Pyk2 to compensate for FAK in promoting the migration of these cells may potentially be due to the absence of cell type-specific elements required to interact with Pyk2 in order for it to mediate a promigratory effect. This might also be related to cellular localization as targeting Pyk2 to focal contacts rescued fibronectin-stimulated signaling and motility in FAK-null cells [44]. A role for Pyk2 in cell migration and cytoskeletal organization has been established in cells such as NK cells, monocytes, and osteoclasts that express Pyk2 but little or no FAK [29–32]. Definitive evidence for the Pyk2 function in migration of specific cell types has been demonstrated by studies performed with Pyk2-deficient mice. Although Pyk2-deficient mice are viable and fertile, macrophages from these mice fail to become polarized, to undergo membrane ruffling, or to migrate despite the normal expression of FAK in these cells [45]. Notably, integrin adhesion-mediated activation of Rho and PI-3 kinase was significantly compromised in Pyk2-null cells. Similarly, the migration of B-lymphocytes in the absence or presence of chemokines was impaired in Pyk2-null mice. Analogous to the results reported in this study, in studies with brain microvascular endothelial cells that, like glioma cells, express both Pyk2 and FAK, expression of Pyk2 stimulated migration whereas expression of an inactive Pyk2 variant substantially inhibited cell spreading and migration [46]. Pyk2 has also been reported to be a point of convergence between G protein-coupled receptors and receptor tyrosine kinases such as EGFR and FGFR [47–49], which play central roles in the activation of signaling networks critical to cell motility and proliferation. Furthermore, Pyk2 has also recently been identified as a comediator in triggering STAT3 activation [50] and has been implicated in promoting breast carcinoma invasion induced by heregulin stimulation [51]. Thus, the observed role of Pyk2 in the stimulation of glioma cell migration is consistent with a widening role for Pyk2 in cell type-specific migration.
Proliferation and invasion are integral to the malignant behavior of glioblastoma and its poor clinical prognosis. The “go or grow” hypothesis proposes that cell division and cell migration are temporally exclusive events in infiltrative gliomas. This implies that one behavior will have a direct influence on the other because cells cannot migrate and proliferate simultaneously. FAK has been previously demonstrated to stimulate cell cycle progression in vitro [20,21,39] and specifically in malignant astrocytoma cell proliferation in vivo [22]. In the current study, dose-response experiments demonstrated that FAK inhibited glioma cell migration, which may be correlated to some degree by its effects on proliferation. Thus, the FAK expression-stimulated cell cycle progression in both SF767 and G112 glioma cell lines was associated with decreased cell migration. Conversely, expression of Pyk2 stimulated the migration of both cell types and either inhibited or did not alter cell cycle progression, consistent with previous results indicating that Pyk2 and FAK differentially regulate cell cycle progression [39]. The observed differential effect of Pyk2 on cell cycle progression in the G112 cells versus the SF767 cells may potentially be linked to the relative balance between Pyk2 and FAK activity in these cell lines. Indeed, it has been previously proposed that Pyk2 and FAK may function in an antagonistic manner [39,52]. Therefore, the addition of exogenous Pyk2 to SF767 cells, which possess significant amounts of endogenous active Pyk2 [24], has relatively little further effect on cell cycle progression. Conversely, the high endogenous level of Pyk2 may antagonize the capacity of added exogenous FAK to increase cell cycle progression. In contrast, the addition of exogenous FAK to G112 cells, which possess low levels of endogenous active Pyk2, stimulates cell cycle progression to a greater extent than in the SF767 cells. Similarly, the addition of Pyk2 to G112 cells effectively antagonizes endogenous FAK and inhibits cell cycle progression. These results suggest that the balance of activity between Pyk2 and FAK in glioblastomas may be particularly relevant to the temporal regulation of tumor cell growth or migration.
Several Pyk2 mutants were constructed to characterize the important functional domains contributing to the pro-migratory effect of Pyk2 on in vitro migration. Pyk2-stimulated cell migration required catalytic activity, and an intact autophosphorylation site as expression of the kinase dead mutant K457A or the Y402F autophosphorylation mutant inhibited cell migration. However, an intact autophosphorylation site was not sufficient alone to stimulate migration. Expression of Pyk2Δ376, which lacks the NH2-terminal 376 amino acids and is constitutively phosphorylated on Y402, did not stimulate migration relative to expression of wild-type Pyk2. Notably, cells expressing this variant migrated at the same rate as cells expressing the control protein β-galactosidase, suggesting that residues in the Pyk2 NH2-terminal domain, in addition to the autophosphorylation site at Y402, are required for the stimulation of migration. The importance for Pyk2 N-terminal domain was further demonstrated through the use of chimeric proteins. The Pyk/FAK chimera, containing the Pyk2 N-terminal and kinase domains fused to the C-terminal domain of FAK, stimulated glioma cell migration to the same extent as wildtype Pyk2, indicating that the Pyk2 C-terminal domain was not required for this effect. Interestingly, expression of the reciprocal FAK/Pyk chimera inhibited glioma cell migration as effectively as expression wild-type FAK. This suggests that the FAK kinase domain is required for its inhibitory effect on migration and that the FAK N-terminal domain cannot effectively substitute for the Pyk2 N-terminal domain. The critical role of the Pyk2 N-terminus in the stimulation of glioma cell migration is supported by the observation that expression of an autonomous Pyk2 amino-terminal 367 amino acid fragment inhibited glioma cell migration. The aminoterminal domain of Pyk2 contains a divergent FERM-like domain [41], and expression of an autonomous Pyk2 FERM domain significantly inhibits cell migration. Although the mechanism for this inhibition remains to be determined, FERM domains have been shown to mediate protein-protein interactions, as well as to link cytoplasmic proteins to the plasma membrane [53]. Therefore, the Pyk2 aminoterminal domain, along with its divergent FERM motif, could serve an important functional role by directing Pyk2 membrane localization and by providing a scaffold for the interaction with important binding partners and signaling effectors. Alternatively, the N-terminal domain may function by regulating Pyk2 activity through intramolecular binding. An autoinhibitory function for the related N-terminal domain of FAK has been described [54]. However, we have not been able to observe an interaction of the Pyk2 N-terminal domain with endogenous Pyk2 in vitro, consistent with the results of a recent study [55] suggesting that it is unlikely that the Pyk2 N-terminal domain has a similar autoregulatory function as the FAK N-terminal domain. The mechanism for the effects of these chimeric proteins may be related to their subcellular localization and is an area of current investigation.
Proliferation and invasion are integral to the pathogenesis of glioblastoma, and both phenotypes ultimately need to be addressed for effective tumor management. Although actively proliferating cells may be targeted by surgery, localized radiation, and chemotherapeutic agents directed at DNA replication, initiation of the migratory phenotype substantially reduces the effectiveness of these approaches [1,2]. Indeed, coordinate modulation of apoptosis and migration is demonstrated by the observation that overexpression of bcl-2 promotes migration and invasion of glioma cells [56]. Conversely, inhibition of Rac activity inhibited glioma cell migration and induced apoptosis [57]. The present study provides further evidence that the related focal adhesion kinases, Pyk2 and FAK, function as important regulators of glioblastoma cell proliferation and migration. We conclude that Pyk2 plays a central role in glioma cell migration, whereas FAK activity plays an important role in glioma cell proliferation. Ongoing studies seek to determine if the roles for Pyk2 and FAK in determining the invasive or proliferative phenotypes in vitro are corroborated by studies with glioma cells within the context of the brain microenvironment. Understanding the intracellular signaling networks that trigger glioma cell migration and invasion could potentially lead to the development of novel anti-invasive therapies or approaches that effectively target migrating cells to increase their susceptibility to current treatment modalities.
Abbreviations
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- Pyk2
proline-rich tyrosine kinase 2
- siRNA
small interfering RNA
References
- 1.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 3.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, Berens T, Coons SW, Watts G, Trent JM, et al. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neuro-Oncol. 2001;53:161–176. doi: 10.1023/a:1012253317934. [DOI] [PubMed] [Google Scholar]
- 4.Puchner MJ, Giese A. Tamoxifen-resistant glioma-cell subpopulations are characterized by increased migration and proliferation. Int J Cancer. 2000;86:468–473. doi: 10.1002/(sici)1097-0215(20000515)86:4<468::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg. 2001;94:978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- 6.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 7.Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–560. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- 8.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin alpha3beta1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int J Cancer. 1998;76:63–72. doi: 10.1002/(sici)1097-0215(19980330)76:1<63::aid-ijc11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Paulus W, Baur I, Beutler AS, Reeves SA. Diffuse brain invasion of glioma cells requires beta 1 integrins. Lab Invest. 1996;75:819–826. [PubMed] [Google Scholar]
- 13.Paulus W, Tonn JC. Basement membrane invasion of glioma cells mediated by integrin receptors. J Neurosurg. 1994;80:515–519. doi: 10.3171/jns.1994.80.3.0515. [DOI] [PubMed] [Google Scholar]
- 14.Tysnes BB, Larsen LF, Ness GO, Mahesparan R, Edvardsen K, Garcia-Cabrera I, Bjerkvig R. Stimulation of glioma-cell migration by laminin and inhibition by anti-alpha3 integrin antibodies. Int J Cancer. 1996;67:777–784. doi: 10.1002/(SICI)1097-0215(19960917)67:6<777::AID-IJC5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase—a regulator of cell migration and invasion. IUBMB Life. 2002;53:115–119. doi: 10.1080/15216540211470. [DOI] [PubMed] [Google Scholar]
- 16.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 17.Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745–752. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 19.Hecker TP, Gladson CL. Focal adhesion kinase in cancer. Front Biosci. 2003;8:s705–s714. doi: 10.2741/1115. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Grammer JR, Cobbs CS, Stewart JE, Jr, Liu Z, Rhoden R, Hecker TP, Ding Q, Gladson CL. p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci. 2000;113(Pt 23):4221–4230. doi: 10.1242/jcs.113.23.4221. [DOI] [PubMed] [Google Scholar]
- 23.Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67:275–282. doi: 10.1002/(SICI)1097-0215(19960717)67:2<275::AID-IJC20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Lipinski CA, Tran NL, Bay C, Kloss J, McDonough WS, Beaudry C, Berens ME, Loftus JC. Differential role of proline-rich tyrosine kinase 2 and focal adhesion kinase in determining glioblastoma migration and proliferation. Mol Cancer Res. 2003;1:323–332. [PubMed] [Google Scholar]
- 25.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 28.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 29.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duong LT, Nakamura I, Lakkakorpi PT, Lipfert L, Bett AJ, Rodan GA. Inhibition of osteoclast function by adenovirus expressing antisense protein-tyrosine kinase 2. J Biol Chem. 2001;276:7484–7492. doi: 10.1074/jbc.M008368200. [DOI] [PubMed] [Google Scholar]
- 31.Gismondi A, Jacobelli J, Strippoli R, Mainiero F, Soriani A, Cifaldi L, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration. J Immunol. 2003;170:3065–3073. doi: 10.4049/jimmunol.170.6.3065. [DOI] [PubMed] [Google Scholar]
- 32.Watson JM, Harding TW, Golubovskaya V, Morris JS, Hunter D, Li X, Haskill JS, Earp HS. Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J Biol Chem. 2001;276:3536–3542. doi: 10.1074/jbc.M006916200. [DOI] [PubMed] [Google Scholar]
- 33.Gutenberg A, Bruck W, Buchfelder M, Ludwig HC. Expression of tyrosine kinases FAK and Pyk2 in 331 human astrocytomas. Acta Neuropathol (Berlin) 2004;108:224–230. doi: 10.1007/s00401-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 34.Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 35.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giese A, Rief MD, Loo MA, Berens ME. Determinants of human astrocytoma migration. Cancer Res. 1994;54:3897–3904. [PubMed] [Google Scholar]
- 37.Ohnishi T, Matsumura H, Izumoto S, Hiraga S, Hayakawa T. A novel model of glioma cell invasion using organotypic brain slice culture. Cancer Res. 1998;58:2935–2940. [PubMed] [Google Scholar]
- 38.Yu CC, Woods AL, Levison DA. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J. 1992;24:121–131. doi: 10.1007/BF01047461. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Zheng C, Guan J. Pyk2 and FAK differentially regulate progression of the cell cycle. J Cell Sci. 2000;113(Part 17):3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]
- 40.Schlaepfer DD, Hunter T. Signal transduction from the extracellular matrix—a role for the focal adhesion protein-tyrosine kinase FAK. Cell Struct Funct. 1996;21:445–450. doi: 10.1247/csf.21.445. [DOI] [PubMed] [Google Scholar]
- 41.Girault JA, Labesse G, Mornon JP, Callebaut I. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem Sci. 1999;24:54–57. doi: 10.1016/s0968-0004(98)01331-0. [DOI] [PubMed] [Google Scholar]
- 42.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of Fak in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance Fak(-) cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, Yano S, Naruse T, Nojima Y. Integrin-mediated signal transduction in cells lacking focal adhesion kinase p125FAK. FEBS Lett. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- 44.Klingbeil CK, Hauck CR, Hsia DA, Jones KC, Reider SR, Schlaepfer DD. Targeting Pyk2 to β1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J Cell Biol. 2001;152:97–110. doi: 10.1083/jcb.152.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM, Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- 47.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 48.Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade—role of focal adhesions and receptor tyrosine kinases. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- 49.Meyer AN, Gastwirt RF, Schlaepfer DD, Donoghue DJ. The cytoplasmic tyrosine kinase Pyk2 as a novel effector of fibroblast growth factor receptor 3 activation. J Biol Chem. 2004;279:28450–28457. doi: 10.1074/jbc.M403335200. [DOI] [PubMed] [Google Scholar]
- 50.Shi CS, Kehrl JH. Pyk2 amplifies epidermal growth factor and c-Src-induced stat3 activation. J Biol Chem. 2004;279:17224–17231. doi: 10.1074/jbc.M311875200. [DOI] [PubMed] [Google Scholar]
- 51.McShan GD, Zagozdzon R, Park SY, Zrihan-Licht S, Fu Y, Avraham S, Avraham H. Csk homologous kinase associates with RAFTK/Pyk2 in breast cancer cells and negatively regulates its activation and breast cancer cell migration. Int J Oncol. 2002;21:197–205. [PubMed] [Google Scholar]
- 52.Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization, PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci. 2001;114:2977–2987. doi: 10.1242/jcs.114.16.2977. [DOI] [PubMed] [Google Scholar]
- 53.Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- 54.Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23:8030–8041. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunty JM, Gabarra-Niecko V, King ML, Ceccarelli DF, Eck MJ, Schaller MD. FERM domain interaction promotes FAK signaling. Mol Cell Biol. 2004;24:5353–5368. doi: 10.1128/MCB.24.12.5353-5368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440:419–424. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- 57.Senger DL, Tudan C, Guiot MC, Mazzoni IE, Molenkamp G, LeBlanc R, Antel J, Olivier A, Snipes GJ, Kaplan DR. Suppression of Rac activity induces apoptosis of human glioma cells but not normal human astrocytes. Cancer Res. 2002;62:2131–2140. [PubMed] [Google Scholar]