Abstract
Erythropoietin (Epo) is used for managing anemia in cancer patients. However, recent studies have raised concerns for this practice. We investigated the expression and function of Epo and the erythropoietin receptor (EpoR) in tumor biopsies and cell lines from human head and neck cancer. Epo responsiveness of the cell lines was assessed by Epoetin-α-induced tyrosine phosphorylation of the Janus kinase 2 (JAK2) protein kinase. Transmigration assays across Matrigel-coated filters were used to examine the effects of Epoetin-α on cell invasiveness. In 32 biopsies, we observed a significant association between disease progression and expression of Epo and its receptor, EpoR. Expression was highest in malignant cells, particularly within hypoxic and infiltrating tumor regions. Although both Epo and EpoR were expressed in human head and neck carcinoma cell lines, only EpoR was upregulated by hypoxia. Epoetin-α treatment induced prominent JAK2 phosphorylation and enhanced cell invasion. Inhibition of JAK2 phosphorylation reduced both basal and Epo-induced invasiveness. Our findings support a role for autocrine or paracrine Epo signaling in the malignant progression and local invasiveness of head and neck cancer. This mechanism may also be activated by recombinant Epo therapy and could potentially produce detrimental effects in rhEpo-treated cancer patients.
Keywords: Erythropoietin, HIF, cancer, invasion, hypoxia
Introduction
Erythropoietin (Epo) treatment increases hematocrit and improves fatigue in anemic cancer patients [1]. However, recent studies have raised the possibility that rhEpo treatment may also exert direct biologic actions on human cancer cells [2–5]. Two recent clinical trials in fact suggested possible clinical worsening associated with rhEpo use [6,7]. One of these trials evaluated 351 head and neck cancer patients and found poorer locoregional progression-free survival in rhEpo-treated patients versus the placebo group [7]. The other trial, which enrolled 939 patients with metastatic breast cancer, was terminated prematurely because of an increased incidence of disease progression and a higher early mortality in patients receiving rhEpo. [6]. Although the underlying mechanism for these findings remains unknown, these studies raise the question of whether Epo can act on erythropoietin receptors (EpoRs) expressed by tumor cells to enhance their malignant properties.
Expression of the Epo and EpoR genes in neoplastic lesions has recently been documented and correlated with poor prognosis in several human cancers including breast [8], cervical [3], and endometrial carcinomas [9]. It is possible that some of the newly appreciated, nonhematopoietic biologic activities of Epo signaling, such as promotion of angiogenesis [10] and inhibition of apoptosis [11], may contribute to disease progression in human cancers. We performed this study to determine whether Epo signaling mechanisms were expressed by, and had biologic effects in, head and neck cancer. We investigated the expression of Epo and EpoR in human head and neck cancer specimens and explored the biologic effects of Epo on head and neck carcinoma cell lines. Our results are consistent with a role for autocrine or paracrine Epo signaling in head and neck squamous cell carcinoma (HNSCC) progression and invasion. This mechanism may contribute to adverse outcomes associated with tumor hypoxia and with exogenous rhEpo treatment of cancer patients.
Methods
Clinical Samples and Clinical Data
Study protocols involving human material were approved by the University of Pennsylvania Institutional Review Board (Philadelphia, PA). Thirty-two cases of HNSCC biopsies or tumor resections (larynx—nine; aryepiglottic fold—five; epiglottis—four; tongue—four; retromolar trigone—four; cervical lymph node—six) were selected from the Surgical Pathology files of the University of Pennsylvania Medical Center. Hematoxylin and eosin (H&E)-stained slides of all cases were reviewed and the diagnoses were confirmed. Invasive carcinomas as well cases of carcinoma in situ were also evaluated. All specimens were primary resection or pretreatment biopsies from patients with no prior treatment with Epo.
Immunohistochemistry and Immunocytochemistry
Immunohistochemical assays were performed on formalin-fixed paraffin-embedded sections as described previously [3,8]. Five-micrometer-thick sections were cut and deparaffinized in xylene and rehydrated in graded alcohols. All slides were steamed in 0.01 M sodium citrate buffer (pH 6.0) for 20 minutes. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol for 20 minutes. Slides were incubated with the antibodies against Epo (rabbit polyclonal, H-162, 1:200 dilution; Santa Cruz Biotechnologies, Santa Cruz, CA) and EpoR (rabbit polyclonal, C-20, 1:400 dilution; Santa Cruz Biotechnologies) overnight at 4°C. Slides were then washed five times with Tris-buffered saline containing Tween 20 (TBST, pH 7.6; DAKO, Carpinteria, CA) and incubated for 30 minutes at room temperature with horseradish peroxidase-labeled dextran polymer coupled to antirabbit antibody (DAKO En-Vision + System HRP; DAKO), developed with diaminobenzidine for 10 minutes and counterstained with hematoxylin. For Epo and EpoR immunohistochemistry, slides of fetal liver and placenta were used as positive controls. The specificity of the Epo and EpoR antibodies was confirmed previously [2]. In addition, the specificity of the EpoR and Epo immunoreactivity was also evaluated by the antibody absorption test: the primary antibody was preincubated with blocking peptide for EpoR (Santa Cruz Biotechnologies) or human recombinant Epo (rHuEpo; R&D Systems, Minneapolis, MN) (10:1 peptide/antibody ratio), which resulted in complete abolishment of immunohistochemical staining. The specificity of the immunostaining reaction is further supported by other experiments using a mouse monoclonal anti-Epo (clone 9C21D11; R&D Systems) and a rabbit polyclonal anti-EpoR antibody (Upstate Biotechnology, Inc., Lake Placid, NY) [3,8,9], which resulted in an immunostaining pattern similar to that obtained with antibodies used in the current study. For cell line staining, cells were fixed with 10% formalin and stained for Epo as described above.
Interpretation of Immunohistochemical Stains
Immunohistochemical stains for Epo and EpoR were interpreted semiquantitatively by assessing the intensity and extent of staining on the entire tissue sections present on the slides according to a four-tiered (0–3) scale [3]. For Epo, cytoplasmic—for EpoR, cytoplasmic and/or membrane—immunoreactivity was considered positive. In the case of dysplasias or in situ carcinomas, first, the percentage of total epithelial thickness showing positive staining was determined (e.g., 50% if the basal half or 75% if the basal three-fourths of the squamous epithelium showed positive immunostaining, etc.). In the case of invasive tumors, first, the total percentage of positively staining tumor cells was determined. Then the percentage of: 1) weakly, 2) moderately, and 3) strongly staining cells was determined, so that the sum of these categories equated with the overall percentage of positivity. A staining score was then calculated as follows: Score (out of maximum of 300) = Σ of 1 x percentage of weak, 2 x percentage of moderate, and 3 x percentage of strong staining.
Statistical Analysis
The Wilcoxon signed rank test was used for the comparison of median EpoR and Epo immunohistochemical expression levels in invasive squamous cell carcinoma, squamous cell dysplasia, and adjacent benign squamous epithelium. Median EpoR and Epo immunohistochemical expression levels in benign epithelia, dysplasia, and invasive carcinoma were compared using the Kruskal-Wallis one-way analysis of variance by ranks followed by Dunn's multiple comparison test, when appropriate. Statistical significance was established if the two-sided P value of a test was less than .05.
Cell Culture and Hypoxia Treatments
Human JHU-O22SCC (from here on referred to as 022) and UM-SCC-22B (from here on referred to as 22B) cancer cells were cultured in RPMI 1640 (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) containing a high amount of glucose (25 mM) with 10% FBS, respectively. Hep3B and DU145 cells obtained from the ATCC (Manassas, VA) were cultured with high-glucose DMEM and RPMI 1640, respectively. All media were supplemented with 1% (vol/vol) penicillin/streptomycin. Cell lines were maintained in 21% O2, 5% CO2, and 74% N2 in a humidified cell incubator at 37°C. For hypoxia treatments, culture dishes were sealed in a humidified chamber and flushed with a gas mixture of 1% O2, 5% CO2, and 94% N2, and incubated at 37°C for the time indicated.
Western blot analysis and Immunoprecipitations
For cell extract preparation, cell pellets from 100% confluent 10-cm culture dishes were lysed in RIPA buffer [0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 5 mM EDTA, 0.5% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-HCl, with 2 mM DTT and protease inhibitors cocktail] for 60 minutes on ice. Lysates were centrifuged (4°C) at 16,000g for 10 minutes and the supernatant was collected for Western blot analysis. For hypoxia-inducible factor 1 (HIF-1) α and EpoR Western blot analysis, whole cell lysates were resolved using 4% to 12% polyacrylamide SDS gel (100 µg for HIF-1α and 50 µg for EpoR). Proteins were transferred to nitrocellulose membrane, blocked with 5% nonfat dry milk in TBS-T (50 mM Tris, pH 7.6, 150 mM NaCl, and 0.1% Tween-20), and probed with HIF-1α monoclonal antibody 1:350 (Transduction Laboratories, San Diego, CA) and EpoR rabbit polyclonal antibodies (C20) 1:1500 (Santa Cruz Biotechnologies) overnight at 4°C as previously described by us [2,3,8]. Horseradish peroxidase-conjugated secondary antibodies were used to probe membranes: sheep antimouse (Amersham Pharmacia Biotech, Piscataway, NJ) for HIF-1α 1:2000 and goat antirabbit for EpoR (1:5000). Immunoreactive bands were visualized using chemiluminescence (SuperSignal WestPico Chemiluminescence kit; Pierce, Rockford, IL). Phospho-Janus kinase 2 (p-JAK2) immunoprecipitations were performed as previously described [12]. Briefly, cells were lysed and immunoprecipitations were performed with 5 g of JAK2 antibody (Upstate Biotechnology, Inc.) and protein A-Sepharose beads (Boehringer Mannheim, Indianapolis, IN). Immunoprecipitates were separated on 8.75% polyacrylamide SDS gel, transferred to nitrocellulose membrane, and probed with a monoclonal antiphosphotyrosine 4G10 antibody 1:1000 (Upstate Biotechnology, Inc.). HIF-1 and EpoR Western blot protein levels were quantified using densitometry. Immunoreactive bands were captured on high-performance chemiluminescence film (Amersham Biosciences, Pascataway, NJ) and scanned. Densitometry of bands was quantified with ImageJ (http://rsb.info.nih.gov/ij/). Equal protein loading was determined by BioRad (Hercules, CA) protein assay and independently verified by β-actin immunoreactivity. Three independent experiments were performed for each protein, results were graphed, and a two-tailed Student's t test was performed to determine significance. P values less than .05 were considered significant.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR Analysis for Epo Gene Expression
Total RNA from cells was isolated using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA). cDNA was generated from 5 µg of total RNA using iScript cDNA Synthesis Kit (BioRad). PCR was conducted using MAXIscript SP6 (Ambion, Austin, TX) with 1 µl of cDNA template and 0.3 µM of forward and reverse primers. The primers for Epo were: forward (5′-TCACTGTCCCAGACACCAAA-3′) and reverse (5′-GGGAAGAGTTGACCAACAGG-3′), which correspond to base pairs 378 to 518. PCR cycling conditions were 40 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 60 seconds. PCR products were run on a 4% agarose gel along with a 50-bp ladder (Invitrogen, Corp., Carlsbad, CA) Primers for control gene HPRT were: forward (5′-TGACACTGGCAAAACAATGCA-3′) and reverse (5′-GGTCCTTTTCACCAGCAAGCT-3′). For quantitative real-time PCR analysis, the SYBR Green PCR Master Mix (Perkin Elmer, Boston, MA) and the BioRad Detection System were used. Single-band amplification was verified through multicomponent analysis. Primers for EPOR were: forward (5′-GGCAGTGTGGACATAGTGGC-3′) and reverse (5′-AGCAGGATGGATTGGGCAGA-5′-AGCAGGATGGATTGGGCAGA-3′); primers for GLUT3 were: forward (5′-TGACGATACCGGAGCCAATG-3′) and reverse (5′-TCAAAGGACTTGCCCAGTTT-3′). Primers for control gene GUS were: forward (5′-GAAAATATGTGGTTGGAGAGCTCATT-3′) and reverse (5′-CCGAGTGAAGATCCCCTTTTTA-3′).
Cell Invasion Assay
Cell invasion experiments were performed using 24-well Biocoat Matrigel Invasion Chambers with an 8-µm pore polycarbonate filter according to the manufacturer's instructions (cat no. 35-4480; Becton Dickinson Labware, Bedford, MA). Growth factor-reduced Biocoat Matrigel Invasion Inserts were used for Hep3B cells and DU145 cells (Epo invasion-enhancing dose 200 U/ml). Prior to experimentation, all invasion chamber inserts were hydrated according to the manufacturer's protocol. Briefly, cells in the growing phase were trypsinized and resuspended at a concentration of 2 x 105 cells/ml in media with 0.5% FBS. The lower compartment of the plates received 750 µl of serum-free media. All drug treatments were added to the lower compartment of the plate prior to cell plating. An amount of 1 x 105 cells was plated in each insert and allowed to invade for 48 hours at 37°C in a humidified incubator with 21% O2. Cells that remained inside the insert after 48 hours were thoroughly wiped with a cotton swab and invading cells were fixed and stained using Diff-Quick Stain Solution (Dade Behring, Newark, DE). Images of invading cells were captured and quantified by counting the number of stained cells in five predetermined fields at x20 magnification (the average number of cells per field for O22 cells and 22B cells under serum-free conditions was 5 and 61, respectively). All treatments groups were performed with an n of six inserts. The difference in invasion between treatment groups was statistically analyzed using a two-tailed Student's t test. Recombinant Epo was purchased from AMGEN (Thousand Oaks, CA). AG490 was from Sigma.
Results
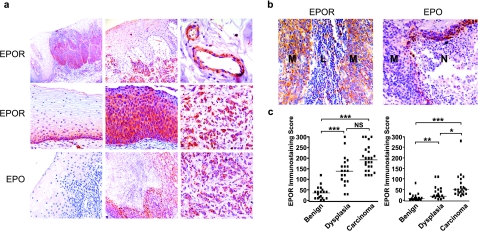
To investigate whether Epo signaling might play a direct role in HNSCC progression, we examined the immunohistochemical expression of Epo and EpoR proteins in biopsy samples obtained from oral cavity, oropharyngeal, hypopharyngeal, and laryngeal lesions of patients not previously treated with rhEpo. Our analysis revealed high levels of both Epo and EpoR expression in the carcinomas examined (25/32 and 32/32, respectively). In normal tissues, EpoR staining was low and confined to the basal epithelial layer (Figure 1a). Strong EpoR staining was seen throughout the dysplastic epithelium, in invasive carcinoma cells, and in lymph node metastases. Tumoral vascular elements also showed prominent EpoR immunoreactivity. Epo staining of normal elements was undetectable in most samples (Figure 1a). Within tumors, however, Epo immunoreactivity was typically seen in the perinecrotic rims, which are known to be severely hypoxic [13]. Discretely intense Epo staining was also seen in invasive carcinoma cells, but was not as uniformly expressed as EpoR staining. A statistically significant correlation between tumor progression and immunohistochemical staining for EpoR (Figure 1b) and Epo (Figure 1c) was demonstrated.
Figure 1.
EpoR and Epo immunohistochemistry in HNSCC. (a) Top row: A prominent increase in EpoR staining (brown color) is seen in biopsies with dysplastic (left panel) and invasive carcinoma cells (middle panel) as well as in tumoral vasculature (right panel). Middle row: EpoR immunoreactivity in normal epithelium (left panel), dysplasic epithelium (middle panel), and invasive carcinoma (right panel). Bottom row: Epo immunoreactivity in normal epithelium (left panel), perinecrotic tumor region (middle panel), and invasive carcinoma (right panel). (b) EpoR and Epo expression in lymph node metastasis. EpoR staining is seen in metastatic cancer cells (M) but not in normal lymphocytes (L). Epo staining is most prominent in the malignant cells bordering necrotic regions (N). (c) Correlation of EpoR and Epo immunoreactivity with malignant progression. P values of EpoR staining were calculated for benign and dysplasia (**P < .01), benign and carcinoma (***P < .001), and dysplasia and carcinoma (P > .05). P values of Epo staining were calculated for benign and dysplasia (***P < .001), benign and carcinoma (***P < .001), and dysplasia and carcinoma (*P < .05). Bars indicate median immunostaining score values. NS = not significant.
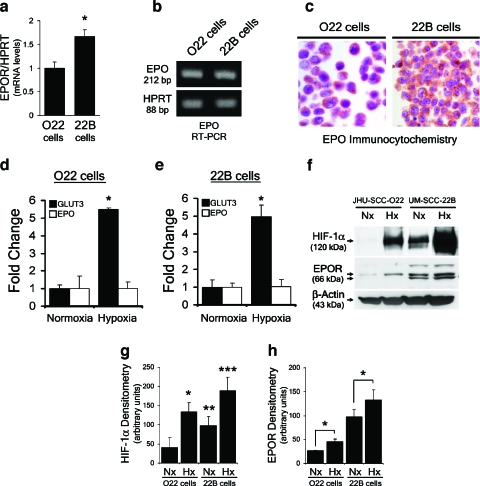
We next explored the biologic regulation and actions of Epo signaling in cancer cells using human HNSCC cell lines. The O22 and the 22B HNSCC cell lines were specifically chosen because they are known to differ significantly in their basal expression levels of the oxygen-responsive HIF-1α subunit of HIF-1 as well as in their relative invasiveness through Matrigel [14]. Expression of EpoR mRNA was detected by RT-PCR in both cell lines (Figure 2a). However, despite the basal expression of Epo mRNA and immunoreactivity (Figure 2, b and c), neither cell lines displayed an upregulation of Epo expression on treatment with hypoxia (1% O2) for 24 hours (Figure 2, d and e). Epogene expression is regulated by the HIF-1 [15]. However, the absence of hypoxic Epo regulation was not due to a lack of hypoxic responsiveness as demonstrated by hypoxic induction of the HIF-1-regulated glucose transporter gene Glut-3 in both cell lines (Figure 2, d and e). Moreover, hypoxia clearly induced nuclear accumulation of the oxygen-regulated HIF-1α protein in both cell lines and also upregulated EpoR protein expression (Figure 2, f–h) as reported previously for other human cancers [2,3].
Figure 2.
Differential invasiveness of HNSCC cell lines correlates with higher HIF and EpoR expression. (a) Quantitative real-time PCR analysis of EpoR from O22 and 22B cell cDNA with HPRT as control gene. (b) PCR amplification of Epo from O22 and 22B cell cDNA with HPRT as control gene. (c) Epo immunocytochemistry demonstrated protein expression in normoxic 022 cells and 22B cells, Epo antibody concentration 1:200, and no primary control exhibited no staining. (d) Quantitative real-time PCR analysis of Epo and GLUT3 mRNA levels in O22 cells after 24 hours of treatment with hypoxia. The amount of each mRNA in samples was normalized to the average of HPRT1 mRNA and GUS mRNA in the same sample. (e) Quantitative real-time PCR analysis of Epo and GLUT3 mRNA levels in 22B cells cultured for 24 hours under hypoxia. The amount of each mRNA in samples was normalized to the average of HPRT1 mRNA and GUS mRNA in the same sample. (f) Differential expression of HIF-1 and EpoR expression in O22 and 22B HNSCC cells. For hypoxia treatment, cells were exposed to 1% O2 for 24 hours. (g) HIF-1 protein levels from (f) were quantified using densitometry. Densitometry values from three independent experiments were graphed and a two-tailed Student's t test was performed to compare relative HIF-1 levels of indicated treatment groups. (*, **, ***P < .05; all treatment groups were compared to HIF-1 levels of normoxic O22 cells). (h) EpoR protein levels from (f) were quantified using densitometry. Densitometry values from three independent experiments were graphed and a two-tailed Student's t test was performed to compare EpoR levels of normoxia- versus hypoxia-treated cells (*P < .05).
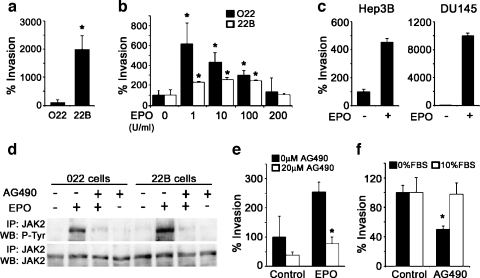
The 22B cells also display greater invasiveness through Matrigel-coated Boyden chambers than the 022 cells [14] (Figure 3a). To find out whether Epo signaling could influence the invasive behavior of HNSCC cells, we treated both cell lines with increasing doses of rhEpo (in the form of Epoetin-α). Remarkably, Epoetin-α promoted invasiveness in both cell lines (Figure 3b). The O22 cells displayed a greater response to Epo when expressed as percent change, although this may have been due to the much higher basal invasiveness seen in the 22B cells. A bell-shaped dose-response relationship was observed for Epo-induced invasiveness and similar responses have been previously reported for other cellular actions of Epo [16]. Epoetin-α (200 U/ml) also prominently induced invasiveness in the human hepatoma cell line, Hep3B, and the human prostate cancer cell line, DU145 (Figure 3c). Both of these cancer cell types have been shown to express EpoR and have specifically been proposed as useful experimental models for studying the potential for growth regulation by Epo-EpoR in an autocrine or paracrine manner [17,18]. We next examined the involvement of EpoR signaling in mediating Epoetin-α-induced invasiveness in HNSCC cells. On binding Epo, dimerization of EpoR recruits and activates the JAK2 tyrosine kinase, which then phosphorylates itself along with other signaling components [19]. As shown in Figure 3d, Epoetin-α promoted tyrosine phosphorylation of JAK2 in both 022 and 22B cells. Moreover, Epoetin-α stimulation of JAK2 phosphorylation was blocked by the specific JAK2 inhibitor, AG490 [20]. Epoetin-α-induced HNSCC cell invasion was also blocked by AG490, thus implicating the involvement of the EpoR-JAK2 signaling pathway in this effect (Figure 3e). Moreover, the high basal invasiveness of 22B cells was also blunted by AG490 (Figure 3f), suggesting that an autocrine Epo signaling mechanism may play a role in the invasion of some HNSCC.
Figure 3.
Epo signaling mediates invasion in HNSCC cell lines. (a) 22B cells display higher invasive potential as assayed with Matrigel coated Boyden chambers for a 48-hour period under serum-free conditions (*P < .05). (b) Exogenous rhEpo promotes cell invasion of O22 and 22B cells through Matrigel-coated Boyden chambers under serum-free conditions (48 hours; *P < .05). (c) Exogenous rhEpo promotes cell invasion of hepatoma (Hep3B) and prostate (DU145) cancer cell lines through Matrigel-coated Boyden chambers under serum-free conditions (48 hours; *P < .05). (d) Exogenous rhEpo (10 U/ml) treatment enhances phosphorylation of JAK2 and this activation is blocked with AG490 (20 µM; *P < .05). (e) Epo (10 U/ml)-induced invasion in O22 cells is blocked with AG490 (20 µM) treatment (*P < .05). (f) Basal invasion of 22B cells is reduced with AG490 (20 µM) treatment only under serum-free conditions (*P < .05).
Discussion
We have shown that Epo signaling elements are prominently expressed in head and neck cancers. Other studies have recently identified biologically active Epo signaling in human breast and uterine cancers and have correlated the expression of Epo and EpoR with poor prognosis [3,8,9]. The correlation we report here between Epo and EpoR expression and malignant progression in head and neck cancer is consistent with these previous observations. Although it is possible that some of the Epo immunostainings associated with cancer cells may be accumulated from peripheral sources, the detection of Epo mRNA and protein in cultured HNSCC cell lines demonstrates that this hormone can be ectopically produced in this type of cancer. The mechanism underlying Epo and EpoR gene expression in cancer cells is not entirely clear. EpoR, but not Epo, expression was observed in the basal cell layer of normal oral mucosa (Figure 1a). Such expression patterns, which were also seen previously in cervical squamous epithelium [3], suggest that the recently recognized role for Epo signaling in gut epithelium development [27] may also extend to other epithelia. Expression of both Epo and EpoR can be stimulated by hypoxia [21,22], and the HNSCC cell lines we examined displayed hypoxia-inducible upregulation of EpoR expression rather than Epo. The higher normoxic expression of EpoR in the 22B cell line is correlated with their higher basal HIF-1α expression and invasiveness [14]. HIF-1α is the key regulatory subunit of HIF-1, a transcription factor that controls the gene expression of Epo and other hypoxia-responsive genes. High basal and hypoxia-inducible HIF-1 expression is observed in solid tumors [23] and has been linked to increased angiogenesis [24], enhanced invasiveness [14,24,25], and poor clinical outcome [26]. It is possible that some of the adverse effects correlated with HIF-1 expression in cancer are mediated by Epo signaling.
Although hypoxia did not induce Epo mRNA expression in either cell line, RT-PCR experiments and immunocytochemical detection of Epo protein support an active constitutive production of the cytokine. Epo is known to exhibit tissue-restricted expression with hypoxia and several known growth factors can modulate its expression levels [27]. The mechanisms regulating the normoxic expression of Epo in these cells are unknown. Methylation of the CpG sites in the Epo promoter interferes with HIF-1 binding, which ultimately restricts Epo gene expression under hypoxia [28]. Fetal liver, uterine epithelium, and embryonal carcinoma cells can express Epo in a hypoxia-independent fashion through the action of GATA 4 [29], steroid receptor [30], and retinoic acid receptor [31] transcription factors, respectively. It is possible that one or more of these mechanisms contribute to constitutive Epo expression in HNSCC cell lines. The association of Epo staining with perinecrotic hypoxic regions in head and neck tumor biopsies also suggests that HIF-1 may regulate Epo expression in many such cancers. Although the ability of Epo to promote angiogenesis and improve cell survival has been suggested to play a role in human cancer, our report is the first to demonstrate an effect of Epo on cancer cell invasiveness. Exogenous Epo activated JAK2 phosphorylation and stimulated cell invasion of both HNSCC cell lines, whereas a JAK2 inhibitor blocked this effect. JAK2 can activate several intracellular signaling cascades including the phosphorylation of the STAT family of transcription factors [19]. STATs have been implicated in tumorigenesis [32] previously but have yet to be examined for a role in invasiveness. Activation of the JAK-STAT signaling pathway by Epo is well appreciated in erythroid precursors and endothelial cells. Epo is known to induce an invasive, pro-angiogenic phenotype in endothelial cells as well as neovascularization in vivo [33]. These processes correlate with Epo-induced JAK2 phosphorylation and matrix metalloprotease-2 production in endothelial cells [33,34]. Moreover, rhEpo can promote migration of enterocytes [35], in addition to stimulating the migration of burst-forming unit erythroids (BFU-E) from the bone marrow to the spleen [36]. The ability of Epo signaling to increase the migratory or invasive behavior of cells may thus be a widespread but underappreciated activity important for normal development and physiology [37,38]. As suggested by our demonstration of Epoetin-α-induced invasion of hepatoma and prostate cancer cells, constitutive or hypoxia-inducible expression of this activity may contribute to the invasiveness of several different human cancers.
Biologic actions of Epo signaling in cancer cells are just beginning to be appreciated. The adverse clinical outcome in rhEpo-treated patients recently reported in two clinical trials has heightened the importance of understanding Epo effects on cancer cells. Although our data do not completely explain the findings of these trials, our demonstration of functional EpoR expression and Epoetin-α-induced biologic effects on HNSCC cells does show that rhEpo can directly impact head and neck cancer. We propose that autocrine or paracrine Epo signaling can enhance cancer invasion and that the indiscriminate treatment of cancer patients with rhEpo should be re-examined.
Acknowledgement
JHU-SCC-O22 cells were kindly provided by Joseph Califano.
Abbreviations
- Epo
erythropoietin
- rhEpo
recombinant human Epo
- EpoR
erythropoietin receptor
- JAK2
Janus kinase 2
- HIF-1
hypoxia-inducible factor 1
- HNSCC
head and neck squamous cell carcinoma
Footnotes
The opinions or assertions contained herein are those of the authors, and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
This study was funded by National Institutes of Health grant no. NS37814 and a grant to A.V. from the Mary Kay Ash Charitable Foundation.
References
- 1.Quirt I, Robeson C, Lau CY, Kovacs M, Burdette-Radoux S, Dolan S, Tang SC, McKenzie M, Couture F Canadian Eprex Oncology Study Group, author. Epoetin alfa therapy increases hemoglobin levels and improves quality of life in patients with cancer-related anemia who are not receiving chemotherapy and patients with anemia who are receiving chemotherapy. J Clin Oncol. 2001;19:4126–4134. doi: 10.1200/JCO.2001.19.21.4126. [DOI] [PubMed] [Google Scholar]
- 2.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 3.Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83:1477–1487. doi: 10.1097/01.lab.0000090156.94795.48. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al. Erythropoietin regulates tumor growth, of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 6.Leyland-Jones B BEST Investigators and Study Group, author. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 7.Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al. Erythropoietin to treat head and beck cancer patients with anemia undergoing radiotherapy: randomized, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 8.Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969–981. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- 9.Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100:2376–2386. doi: 10.1002/cncr.20244. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen SL, Lai SY, Longmore GD, Liu KD, Goldsmith MA. Genetic evidence for an additional factor required for erythropoietin-induced signal transduction. Blood. 1999;94:74–86. [PubMed] [Google Scholar]
- 11.Ribatti D, Marzullo A, Nico B, Crivellato E, Ria R, Vacca A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology. 2003;42:246–250. doi: 10.1046/j.1365-2559.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 12.Carlini RG, Alonzo EJ, Dominguez J, Blanca I, Weisinger JR, Rothstein M, Bellorin-Font E. Effect of recombinant human erythropoietin on endothelial cell apoptosis. Kidney Int. 1999;55:546–553. doi: 10.1046/j.1523-1755.1999.00266.x. [DOI] [PubMed] [Google Scholar]
- 13.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 14.Cohen NA, Lai SY, Ziober AF, Ziober BL. Dysregulation of hypoxia inducible factor-1alpha in head and neck squamous cell carcinoma cell lines correlates with invasive potential. Laryngoscope. 2004;11:418–423. doi: 10.1097/00005537-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weishaupt JH, Rohde G, Polking E, Siren AL, Ehrenreich H, Bahr M. Effect of erythropoietin on axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 17.Arcasoy MO, Amin K, Vollmer RT, Jiang X, Demark-Wahnefried W, Haroon ZA. Erythropoietin and erythropoietin receptor expression in human prostate cancer. Mod Pathol. 2004 doi: 10.1038/modpathol.3800288. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Ohigashi T, Yoshioka K, Fisher JW. Autocrine regulation of erythropoietin gene expression in human hepatocellular carcinoma cells. Life Sci. 1996;58:421–427. doi: 10.1016/0024-3205(95)02307-0. [DOI] [PubMed] [Google Scholar]
- 19.Watowich SS. Activation of erythropoietin signaling by receptor dimerization. Int J Biochem Cell Biol. 1999;31:1075–1088. doi: 10.1016/s1357-2725(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 20.Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 21.Chin K, Yu X, Beleslin-Cokic B, Liu C, Shen K, Mohrenweiser HW, Noguchi CT. Production and processing of erythropoietin receptor transcripts in brain. Brain Res Mol Brain Res. 2000;81:29–42. doi: 10.1016/s0169-328x(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 22.Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, Noguchi CT. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–2080. doi: 10.1182/blood-2004-02-0744. [DOI] [PubMed] [Google Scholar]
- 23.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 24.Semenza GL. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19:59–65. doi: 10.1023/a:1026544214667. [DOI] [PubMed] [Google Scholar]
- 25.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- 26.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 27.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 28.Wenger RH, Kvietikova I, Rolfs A, Camenisch G, Gassmann M. Oxygen-regulated erythropoietin gene expression is dependent on a CpG methylation-free hypoxia-inducible factor-1 DNA-binding site. Eur J Biochem. 1998;253:771–777. doi: 10.1046/j.1432-1327.1998.2530771.x. [DOI] [PubMed] [Google Scholar]
- 29.Dame C, Sola MC, Lim KC, Leach KM, Fandrey J, Ma Y, Knopfle G, Engel JD, Bungert J. Hepatic erythropoietin gene regulation by GATA-4. J Biol Chem. 2004;279:2955–2961. doi: 10.1074/jbc.M310404200. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- 31.Kambe T, Tada J, Chikuma M, Masuda S, Nagao M, Tsuchiya T, Ratcliffe PJ, Sasaki R. Embryonal carcinoma P19 cells produce erythropoietin constitutively but express lactate dehydrogenase in an oxygen-dependent manner. Blood. 1998;91:1185–1195. [PubMed] [Google Scholar]
- 32.Song JI. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 33.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell'Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 34.Fuste B, Serradell M, Escolar G, Cases A, Mazzara R, Castillo R, Ordinas A, Diaz-Ricart M. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb Haemost. 2002;88:78–85. [PubMed] [Google Scholar]
- 35.Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythropoietin present in human milk? Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res. 1999;46:263–268. doi: 10.1203/00006450-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Kato Y, Sugiyama Y. Mechanism of the upregulation of erythropoietin-induced uptake-clearance by the spleen. Am J Physiol. 1999;276:E887–E895. doi: 10.1152/ajpendo.1999.276.5.E887. [DOI] [PubMed] [Google Scholar]
- 37.Knabe W, Knerlich F, Washausen S, Kietzmann T, Siren AL, Brunnett G, Kuhn HJ, Ehrenreich H. Expression patterns of erythropoietin and its receptor in the developing midbrain. Anat Embryol. 2004;207:503–512. doi: 10.1007/s00429-003-0365-y. [DOI] [PubMed] [Google Scholar]
- 38.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]