Abstract
In this report, we challenge a common perception that tumor embolism is a size-limited event of mechanical arrest, occurring in the first capillary bed encountered by blood-borne metastatic cells. We tested the hypothesis that mechanical entrapment alone, in the absence of tumor cell adhesion to blood vessel walls, is not sufficient for metastatic cell arrest in target organ microvasculature. The in vivo metastatic deposit formation assay was used to assess the number and location of fluorescently labeled tumor cells lodged in selected organs and tissues following intravenous inoculation. We report that a significant fraction of breast and prostate cancer cells escapes arrest in a lung capillary bed and lodges successfully in other organs and tissues. Monoclonal antibodies and carbohydrate-based compounds (anti-Thomsen-Friedenreich antigen antibody, anti-galectin-3 antibody, modified citrus pectin, and lactulosyl-l-leucine), targeting specifically β-galactoside-mediated tumor-endothelial cell adhesive interactions, inhibited by >90% the in vivo formation of breast and prostate carcinoma metastatic deposits in mouse lung and bones. Our results indicate that metastatic cell arrest in target organ microvessels is not a consequence of mechanical trapping, but is supported predominantly by intercellular adhesive interactions mediated by cancer-associated Thomsen-Friedenreich glycoantigen and β-galactoside-binding lectin galectin-3. Efficient blocking of β-galactoside-mediated adhesion precludes malignant cell lodging in target organs.
Keywords: Cancer metastasis, endothelium, adhesion, Thomsen-Friedenreich antigen, galectins
Introduction
The current vision of the process of hematogenous cancer metastasis is based largely on two fundamental premises: the mechanical trapping theory and the seed and soil hypothesis, suggested by James Ewing and Stephen Paget, and further enhanced and developed by Isaiah J. Fidler, Lance A. Liotta, Garth Nicolson, and others (reviewed in Refs. [1,2]). Recent advances in intravital video microscopy techniques shed light onto numerous critical steps in tumor metastasis [3–9], suggesting that early metastasis-associated events, including initial micrometastases growth, may occur entirely intravascularly [3–5]. Nevertheless, despite extensive investigative efforts, several important questions related to how tumor cells lodge in a target organ vasculature remain unresolved.
For example, based on video microscopic observations, several groups suggested that metastatic cell arrest is a highly efficient, strictly mechanical process occurring in the first capillary bed encountered due to size limitation [5–7]. Thus, many view mechanical tumor embolism as an ultimate cause of metastatic deposit formation. However, recent results from Vantyghem et al. [8] documented the development of macroscopic extrapulmonary metastasis in the ovaries, peritoneal cavities, and abdominal mesenteries of mice injected intravenously with B16F10 melanoma cells. Given that survival of early metastatic colonies is a highly inefficient process [6], these results imply that a significant number of injected cells escaped mechanical arrest in the pulmonary microcirculation and landed successfully in other organs and tissues. Therefore, neoplastic cell arrest in the first capillary bed encountered could be a significantly less efficient process than previously thought. Our recent results, showing that metastatic cancer cells are capable of avoiding mechanical entrapment by adjusting their shape and passing through narrow microcirculatory compartments [9], further support this idea.
Taken together, these observations led us to hypothesize that mechanical factors alone are not sufficient for the ultimate tumor cell arrest in target organ circulation, and that specific adhesive interactions between metastatic cells and blood vessel endothelia are necessary for malignant cell arrest in microvessels. Indeed, many metastatic cells arrested in lung circulation, as shown recently by Al-Mehdi et al. [3] and Wong et al. [4], reside in precapillary arterioles of calibers far exceeding tumor cells in size. Similarly, Orr and Wang [10] documented colon carcinoma cell arrest in precapillary hepatic vessels larger than tumor cell diameters. In our experiments employing porcine dura mater model [11], we observed frequently stable breast and prostate cancer cell adhesion within 50- to 100-µm-wide precapillary arterioles [9,11,12]. These facts demonstrate unambiguously that blood-borne malignant cells could be arrested in a variety of organs and tissues through specific adhesive interactions with vessel walls in the absence of mechanical entrapment.
Further, in vitro metastatic breast and prostate carcinoma cell adhesion to microvascular endothelium derived from anatomic sites, including the lungs and bone marrow, is mediated largely by interactions between cancer-associated Thomsen-Friedenreich (TF) glycoantigen (Galβ1–3GalNAc) and β-galactoside-binding lectin galectin-3 [9,11–17]. We also demonstrated that in vitro TF antigen/galectin-3 interactions could be disrupted efficiently using function-blocking antibodies against galectin-3 [13,17] and TF antigen [12,15], or small-molecular-weight carbohydrate-based inhibitors specifically targeting β-galactoside-mediated adhesion such as modified citrus pectin (MCP) and lactulosyl-l-leucine (N-(1-deoxy-4-O-(β-d-galactopyranos-1-yl)-d-fructofuranos-1-yl)-(S)-2-amino-4-methylpentanoic acid) [15–17]. Thus, to test our hypothesis, we investigated in vivo patterns of organ-to-organ distribution of fluorescently labeled breast and prostate cancer cells, injected intravenously in mice. In the same model, we determined whether blocking of β-galactoside-mediated adhesion modified the formation of metastatic deposits in target organ microvasculature.
Materials and Methods
Antibodies, Chemicals, and Reagents
TIB-166, H18/7, DREG56, and WAPS 12.2 hybridoma cell lines, producing function-blocking monoclonal antibodies directed against galectin-3, E-selectin, L-selectin, and P-selectin, respectively, were obtained from ATCC (Manassas, VA). The JAA-F11 hybridoma producing anti-TF antigen mAb [18] was kindly provided by Dr. Kate Rittenhouse-Olson (State University of New York, Buffalo, NY). All hybridoma cell lines were grown using exactly the same media composition [RPMI 1640 medium supplemented with l-glutamine, 10% fetal bovine serum (FBS), sodium pyruvate, and nonessential amino acids]. Thus, when conditioned supernatants were used in in vivo metastatic deposit formation assay, they served as negative controls to each other. MCP and lactulosyl-l-leucine were obtained as described previously [19–22]. All other chemicals and reagents, unless otherwise specified, were from Sigma (St. Louis, MO).
Cancer Cell Lines and Cultures
The MDA-MB-435 human breast carcinoma cell line was kindly provided by Dr. Janet E. Price (M. D. Anderson Cancer Center, Houston, TX). The DU-145 human prostate carcinoma cells were purchased from ATCC. The RPMI 1640 medium supplemented with l-glutamine, 10% FBS, sodium pyruvate, and nonessential amino acids was used for tumor cell lines.
In Vivo Metastatic Deposit Formation Assay
Six-week-old male (for prostate cancer experiments) or female (for breast cancer experiments) HsdIcr:Ha(ICR)-scid mice (Harlan, Indianapolis, IN) were used in this study, in accordance with the University of Missouri-approved animal care protocol. Prior to intravenous injection, cancer cells were prelabeled for 5 minutes with 3 µg/ml solution of acridine orange in RPMI 1640 medium, rinsed three times with serum-free RPMI 1640 medium, and dissociated from plastic using a nonenzymatic cell dissociation reagent (Sigma). We demonstrated previously that labeling tumor cells with acridine orange does not affect their adhesive behavior in short time experiments [12]. Immediately following the dissociation, tumor cells were resuspended using one of the following: 1) complete RPMI 1640 medium (control); 2) complete RPMI 1640 medium supplemented with MCP (0.25% wt/vol final concentration); 3) complete RPMI 1640 medium supplemented with lactulosyl-l-leucine (2 mM final concentration); or 4) undiluted conditioned supernatant containing a corresponding function-blocking antibody directed against galectin-3, TF antigen, or E-selectin, L-selectin, or P-selectin, and pipetted to produce a single cell suspension. All subsequent manipulations with cancer cells were performed using the same media composition. To remove any remaining cell clumps, the tumor cell suspension was filtered through a 20-µm nylon mesh, and adjusted to contain 5 x 106 cells/ml. Next, 200 µl (1 x 106 cells) of a single-cell suspension of fluorescently labeled MDA-MB-435 or DU-145 cells was injected into a lateral tail vein of the experimental animal. Three hours postinjection, the animals were euthanized, and internal organs [lung, bones (vertebrae and sternum), liver, spleen, kidney, thyroid gland, and brain] were removed, examined by epifluorescence microscopy, and photographed using a QICAM high-performance digital CCD camera (Quantitative Imaging Corporation, Burnaby, Canada). At least two identical experiments were performed for each experimental set-up. In each animal, subpleural metastatic deposits were scored in four random observation fields. The results were calculated and presented as mean ± SD.
Results and Discussion
Metastatic Cell Arrest in the First Capillary Bed Encountered Is Less Efficient than Previously Thought
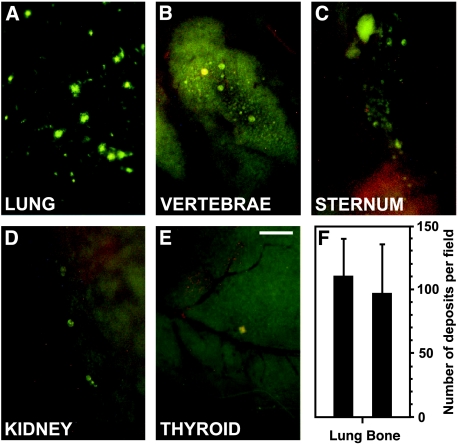
To test the hypothesis that a significant number of circulating neoplastic cells are capable of escaping mechanical entrapment in the first capillary bed encountered, we investigated in vivo patterns of organ-to-organ distribution of intravenously injected fluorescently labeled breast (MDA-MB-435) and prostate (DU-145) cancer cells. Three hours postinjection, numerous metastatic cells landed in the lungs (Figure 1A). However, as predicted, a significant number of tumor cells avoided arrest in lung circulation and lodged in bones (Figure 1, B and C). In addition, selected neoplastic cells were found in other organs such as the kidneys and thyroid gland (Figure 1, D and E). Of note, our experiments yielded comparable metastatic deposit counts in the lungs and bones (vertebrae), which did not differ significantly from each other (Figure 1F). These results demonstrate that neoplastic cell arrest in the first capillary bed encountered (lung) is less efficient than previously thought, suggesting that mechanical entrapment due to size limitation is not the ultimate cause of tumor cell arrest in lung.
Figure 1.
A significant fraction of intravenously injected cancer cells escapes mechanical entrapment in the first capillary bed encountered (lung) and reaches other organs and tissues. Metastatic deposits of fluorescently labeled cancer cells (DU-145 human prostate carcinoma shown) formed in vivo 3 hours postinjection of 1 x 106 cells in the lungs (A), vertebrae (B), sternum (C), kidney (D), and thyroid gland (E). Scale bar, 500 µm. (F) Metastatic deposit counts in lungs and bones (vertebrae). Bars, mean ± SD.
Thus, we have reasoned that specific adhesive interactions between metastatic cells and microvascular endothelia, rather than mechanical factors, could play the foremost role in mediating tumor cell arrest in distant organ microvessels. Recent results from our groups revealed that, in vitro, metastatic breast and prostate carcinoma cell adhesion to microvascular endothelial cells derived from various anatomic sites, including the lungs [17] and bone marrow [13,15], mediated largely by interactions between cancer-associated TF glycoantigen and β-galactoside-binding lectin galectin-3 [13–17]. Therefore, we investigated next whether blocking TF antigen and galectin-3 with monoclonal antibodies would modify the formation of breast and prostate cancer metastatic deposits in the lungs and bones in vivo.
Anti-TF Antigen and Anti-Galectin-3 Antibodies Inhibit Lung and Bone Metastatic Deposit Formation
In vitro, TF antigen/galectin-3 interactions mediate heterotypic adhesion between tumor cells and endothelia [9,15–17], as well as malignant cell homotypic aggregation with each other [9,15–17,23], both of which may contribute to metastatic cell arrest in microcirculation [9]. Further, galectin-3 expressed on both neoplastic and endothelial cells participates in these processes [9,17]. Thus, we investigated next whether blocking TF antigen and galectin-3 with monoclonal antibodies modified the formation of breast and prostate cancer metastatic deposits in the lungs and bones in vivo. In these experiments, conditioned supernatants of hybridoma cultures were used as function-blocking antibodies. All five hybridoma cell lines used in this study were grown using exactly the same media composition (RPMI 1640 medium supplemented with l-glutamine, 10% FBS, sodium pyruvate, and nonessential amino acids). The ability of TIB-166 and JAA-F11 antibodies, directed against galectin-3 and TF antigen, respectively, to inhibit tumor cell adhesion to the endothelium in vitro was demonstrated previously [12,15,17]. The supernatants of hybridomas producing antibodies directed against E-selectin, L-selectin, and P-selectin were tested for their ability to inhibit the rolling of peripheral mononuclear cells on TNF-α- or histamine-activated endothelial monolayers and P-selectin-coated plates, respectively. Consequently, all antibodies were applied at the concentrations exceeding their related in vitro IC50 at least 50- to 100-fold. Thus, when conditioned supernatants were used in in vivo metastatic deposit formation assay (Figures 2 and 3), they served as negative controls to each other.
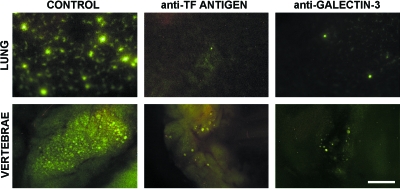
Figure 2.
The effect of anti-TF antigen and anti-galectin-3 antibodies on metastatic deposit formation in the lungs and bones in vivo. Both anti-TF antigen and anti-galectin-3 function-blocking monoclonal antibodies dramatically inhibit metastatic deposit formation in mouse lungs (top panel) and bones (bottom panel) in vivo. Scale bar, 500 µm.
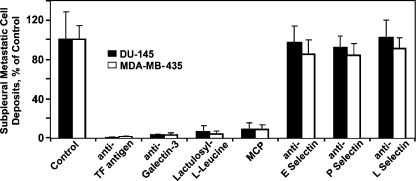
Figure 3.
The effect of various function-blocking monoclonal antibodies and carbohydrate-based compounds on subpleural metastatic deposit formation of DU-145 human prostate carcinoma and MDA-MB-435 human breast carcinoma cells. Anti-TF antigen, anti-galectin-3, lactulosyl-l-leucine, and MCP inhibit >90% subpleural metastatic deposit formation of prostate (closed bars) and breast (open bars) cancer cells in vivo, whereas all three of antiselectin antibodies tested fail to affect this process significantly. Bars, mean ± SD.
The results of these experiments demonstrated that blocking either TF antigen or galectin-3 with monoclonal antibodies dramatically inhibits metastatic deposit formation in both the lung and bones (Figure 2). It appears that efficient blockage of TF antigen/galectin-3-mediated adhesion precludes almost completely metastatic breast and prostate cancer cell arrest in distant organ microvessels. This outcome indicates that such arrest is consequent to adhesive interactions between tumor cells and microvascular endothelia, and not mechanical entrapment.
Intravenous injection of the fluorescently labeled tumor cells yields consistently reproducible counts of subpleural metastatic deposits in mouse lungs, and allows for assessing the inhibitory effect of various agents on this process. Thus, in the next series of experiments, we focused specifically on quantifying the effect of several potential antiadhesion compounds on subpleural metastatic deposit formation.
Breast and Prostate Cancer Cell Arrest in Murine Lung Supported Predominantly by β-Galactoside-Mediated Interactions
In these experiments, in addition to anti-TF antigen and anti-galectin-3 antibodies, two small-molecular-weight carbohydrate-based compounds, lactulosyl-l-leucine and MCP, were tested along with the function-blocking antibodies directed against E-selectin, L-selectin, and P-selectin for their ability to inhibit the formation of subpleural metastatic deposits. Of these, lactulosyl-l-leucine and MCP bind galectins and inhibit β-galactoside-mediated adhesion by mimicking essential structural features of naturally occurring carbohydrate structures [15,16]. For example, lactulosyl-l-leucine specifically blocks galectin-3 by mimicking cancer-associated TF antigen [15]. Importantly, these carbohydrate-based galectin-3 inhibitors were already shown to reduce efficiently both the incidence and number of spontaneous breast and prostate carcinoma metastases in vivo [16,20,22].
The results of these experiments demonstrated that all four compounds targeting β-galactoside-mediated interactions (anti-TF antigen, anti-galectin-3, lactulosyl-l-leucine, and MCP) inhibited almost completely the formation of breast and prostate carcinoma subpleural metastatic deposits in vivo (Figure 3). Specifically, anti-TF mAb, anti-galectin-3 mAb, lactulosyl-l-leucine, and MCP inhibited subpleural metastatic deposit formation by 99.3 ± 1.11%, 97.5 ± 2.26%, 96.3 ± 3.21%, and 92.7 ± 4.88% (mean ± SD), and 99.8 ± 0.32%, 97.6 ± 0.96%, 94.8 ± 6.65%, and 91.7 ± 7.30% (mean ± SD) of breast and prostate carcinoma cells, respectively. Thus, MDA-MB-435 and DU-145 metastatic cell arrest in lung microvessels is mediated predominantly by cancer-associated TF glycoantigen and galectin-3. Furthermore, although the inhibitory effect of these compounds on bone colonization was not quantified, our results (Figure 2, d–f) strongly suggest that β-galactoside-mediated intercellular adhesion is likely to play a pivotal role in supporting the homing of breast and prostate carcinoma cells to the bone microvasculature as well. However, in addition to inhibiting cell-to-cell adhesion, blocking galectin-3 may potentially increase tumor cell susceptibility to apoptosis including anoikis and, therefore, affect their viability and impact experimental outcomes. To ensure that the inhibitors of TF antigen/galectin-3 interactions do not affect cancer cell viability, we performed a series of control experiments, in which single-cell suspensions of tumor cells were incubated for 3 hours at 37°C in 5% CO2 atmosphere without (control) or with anti-TF antigen, anti-galectin-3, lactulosyl-l-leucine, and MCP at the same concentrations as in in vivo experiments. To prevent cancer cell adhesion to the plastic, these experiments were conducted in ultralow adhesion plates. The percentage of viable cells determined by a Trypan blue dye exclusion after 3 hours of incubation was as follows: control, 94.7 ± 4.8%; JAA-F11 (anti-TF antigen), 92.3 ± 5.2%; TIB-166 (anti-galectin-3), 90.9 ± 8.1%; lactulosyl-l-leucine, 92.3 ± 7.9%; and MCP, 88.7 ± 7.5% (mean ± SD). These results indicate that the galectin-3 and TF antigen inhibitors used in this study do not significantly affect tumor cell viability and, therefore, their effects on metastatic deposit formation in this experimental system could be attributed entirely to their antiadhesion effect. In contrast, it appears that selectins do not play a major role in breast and prostate carcinoma cell arrest in murine lung microvasculature. All three antiselectin antibodies tested failed to affect this process significantly (Figure 3). These results are consistent with observations by Satoh et al. [24] that multiple prostate carcinoma cell lines lack selectin-mediated adhesion despite expression of a sialyl-Lewis(x) antigen (the ligand for endothelial selectins). However, selectin-mediated adhesion plays an important role in colon cancer cell arrest in hepatic microvessels (Ref. [10] and reviewed in Ref. [25]). Similarly, in different types of cancer, other adhesion molecules could be crucial for metastatic cell lodging in their related target tissues.
The notion that mechanical entrapment is the primary means of metastatic cell arrest in distant organ microvessels is based largely on the fact that blood-borne metastatic cells lodge predominantly in precapillary vessels and capillaries (reviewed in Refs. [1,2]). In the absence of efficient inhibitors of tumor-endothelial cell adhesion, this phenomenon was interpreted by many as an evidence of tumor cell mechanical arrest in target organ microvessels. Here, we demonstrate that efficient blocking of tumor-endothelial cell adhesion precludes almost completely metastatic cell arrest and retention in lung and bones. It appears that mechanical tumor embolism does not occur when intercellular adhesive interactions are blocked, indicating that mechanical entrapment is not sufficient for the ultimate tumor cell arrest in microcirculation. Thus, we suggest that mechanical factors play a rather supportive role in mediating metastatic cell lodging in distant organs (i.e., mechanical factors reduce tumor cell traveling velocities and prolong neoplastic cell contact with microvascular endothelium, increasing greatly the chances for adhesive interactions to take place). However, as suggested previously by Fidler and Talmadge [26] in murine melanoma model, mechanical factors may cause an arrest of multicellular aggregates. We recently reported that both breast and prostate cancer cells form such aggregates intravascularly at the sites of their primary attachment to the endothelium [9] and, lately, the formation of multicellular metastatic deposits in vivo by human fibrosarcoma cells was elegantly shown by Yamamoto et al. [27] using color-coded fluorescently labeled cancer cells.
Here, we propose the model whereby mechanical and adhesive factors act cooperatively to support metastatic cell arrest in target organ microvessels. On the molecular level, we believe that TF antigen/galectin-3 interactions represent some of the earliest events in a multistep cell-to-cell adhesion process. Most likely, these carbohydrate-lectin interactions, which are rather weak and transient in nature, are important in initiating tumor-endothelial cell adhesive cascade and in mediating subsequent integrin-mediated stabilizing steps, which further determine the fate of metastatic deposits and organ specificity of hematogenous cancer metastasis. For example, the results from Wang et al. [28] demonstrated an important role for α3χ1 integrin in mediating the pulmonary arrest of several cancer cell lines, including the MDA-MB-435 breast carcinoma cells, which were used in this study. Further, most recently, Fukushi et al. [29] showed that galectin-3 physically associates with α3β1 integrin at the endothelial cell membrane. Taken together, these facts suggest a logical chain of subsequent molecular events, whereas TF antigen expressed on tumor cells mediates galectin-3 clustering on endothelial cell surfaces [9,12,15] and initiates transient adhesion, which is stabilized by α3χ1 integrin engagement and association with galectin-3. The galectin-3-mediated involvement of α3χ1 integrin provides for both the means of anchoring galectin-3 at the cell membrane and for initiating multiple downstream signaling cascades. In our opinion, this model offers answers to important questions regarding the microvascular pathophysiology of hematogenous cancer spread, and provides further justification for the unification of the seed and soil and mechanical trapping theories of cancer metastasis.
Acknowledgements
We thank J. E. Price for providing MDA-MB-435 cells, K. Rittenhouse-Olson for the gift of JAA-F11 hybridoma, and V. V. Mossine for synthesizing lactulosyl-l-leucine.
Abbreviations
- TF antigen
Thomsen-Friedenreich antigen
- FBS
fetal bovine serum
- MCP
modified citrus pectin
- Lactulosyl-l-leucine
N-(1-deoxy-4-O-(β-d-galactopyranos-1-yl)-d-fructofuranos-1-yl)-(S)-2-amino-4-methylpentanoic acid
Footnotes
This work was supported, in part, by the VA Merit Review Award (to V.V.G.), NASA NAG 5-12300 (to V.H.H.), and National Institutes of Health grants P-50 CA103130-01 (to V.V.G.), 1RO1CA89827-01 (to G.V.G), T32 HL07094 (to O.V.G.), R37 HL-42528-13 and PO1 HL52490-06 (to V.H.H.), P50 CA69568 (to K.J.P.), and RO1 CA 46120 (to A.R.).
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Fidler I. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 4.Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, Al-Mehdi AB. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749–753. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mook OR, Van Marle J, Vreeling-Sindelarova H, Jonges R, Frederiks WM, Van Noorden CJ. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology. 2003;38:295–304. doi: 10.1053/jhep.2003.50297. [DOI] [PubMed] [Google Scholar]
- 6.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, McDonald IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- 8.Vantyghem SA, Postenka CO, Chambers AF. Estrous cycle influences organ-specific metastasis of B16F10 melanoma cells. Cancer Res. 2003;63:4763–4765. [PubMed] [Google Scholar]
- 9.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 10.Orr FW, Wang HH. Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg Oncol Clin N Am. 2001;10:357–381. [PubMed] [Google Scholar]
- 11.Glinskii OV, Huxley VH, Turk JR, Deutscher SL, Quinn TP, Pienta KJ, Glinsky VV. Continuous real time ex vivo epifluorescent video microscopy for studying cancer cell interactions with dura mater microvasculature. Clin Exp Metastasis. 2003;20:451–458. doi: 10.1023/a:1025449031136. [DOI] [PubMed] [Google Scholar]
- 12.Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumor cells. J Physiol (London) 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst. 1998;90:118–123. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 14.Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP. Effects of Thomsen-Friedenreich antigen-specific peptide P-30 on β-galactoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res. 2000;60:2584–2588. [PubMed] [Google Scholar]
- 15.Glinsky VV, Glinsky GV, Rittenhouse-Olsen K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 16.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 17.Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, Glinsky GV, Sriramarao P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278:4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 18.Rittenhouse-Diakun K, Xia Z, Pickhardt D, Morey S, Baek M-G, Roy R. Development and characterization of monoclonal antibody to T-antigen: (Galβ1–3GalNAc-α-O) Hybridoma. 1998;17:165–173. doi: 10.1089/hyb.1998.17.165. [DOI] [PubMed] [Google Scholar]
- 19.Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconjug J. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- 20.Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, Donat TL, Tait L, Hogan V, Raz A. Inhibition of spontaneous metastasis in rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst. 1995;87:348–353. doi: 10.1093/jnci/87.5.348. [DOI] [PubMed] [Google Scholar]
- 21.Glinsky GV, Mossine VV, Price JE, Bielenberg D, Glinsky VV, Ananthaswamy HN, Feather MS. Inhibition of colony formation in agarose of metastatic breast carcinoma and melanoma cells by synthetic glycoamine analogs. Clin Exp Metastasis. 1996;14:253–267. doi: 10.1007/BF00053899. [DOI] [PubMed] [Google Scholar]
- 22.Glinsky GV, Price JE, Glinsky VV, Mossine VV, Kiriakova G, Metcalf JB. Inhibition of human breast cancer metastasis in nude mice by synthetic glycoamines. Cancer Res. 1996;56:5319–5324. [PubMed] [Google Scholar]
- 23.Inohara H, Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 1995;55:3267–3271. [PubMed] [Google Scholar]
- 24.Satoh M, Numahata K, Kawamura S, Saito S, Orikasa S. Lack of selectin-dependent adhesion in prostate cancer cells expressing sialyl Le(x) Int J Urol. 1998;5:86–91. doi: 10.1111/j.1442-2042.1998.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 25.McEver RP. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj J. 1997;14:585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- 26.Fidler IJ, Talmadge JE. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res. 1986;46:5167–5171. [PubMed] [Google Scholar]
- 27.Yamamoto N, Yang M, Jiang P, Xu M, Tsuchiya H, Tomita K, Moossa RM, Hoffman RM. Determination of clonality of metastasis by cell-specific color-coded fluorescent-protein imaging. Cancer Res. 2003;63:7785–7790. [PubMed] [Google Scholar]
- 28.Wang H, Fu W, Im JH, Zhou Z, Santoro SA, Iyer V, DiPersio CM, Yu QC, Quaranta V, Al-Mehdi A, et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164:935–941. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukushi JI, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and {alpha}3{beta}1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]