Abstract
Gluconate is one of the preferred carbon sources of Escherichia coli, and two sets of gnt genes (encoding the GntI and GntII systems) are involved in its transport and metabolism. GntR represses the GntI genes gntKU and gntT, whereas GntH was previously suggested to be an activator for the GntII genes gntV and idnDO-gntWH. The helix-turn-helix residues of the two regulators GntR and GntH exhibit extensive homologies. The similarity between the two regulators prompted analysis of the cross-regulation of the GntI genes by GntH. Repression of gntKU and gntT by GntH, as well as GntR, was indeed observed using transcriptional fusions and RNA analysis. High GntH expression, from cloned gntH or induced through 5-ketogluconate, was required to observe repression of GntI genes. Two GntR-binding elements were identified in the promoter-operator region of gntKU and were also shown to be the target sites of GntH by mutational analysis. However, the GntI genes were not induced by gluconate in the presence of enhanced amounts of GntH, whereas repression by GntR was relieved by gluconate. The repression of GntI genes by GntH is thus unusual in that it is not relieved by the availability of substrate. These results led us to propose that GntH activates GntII and represses the GntI genes in the presence of metabolites derived from gluconate, allowing the organism to switch from the GntI to the GntII system. This cross-regulation may explain the progressive changes in gnt gene expression along with phases of cell growth in the presence of gluconate.
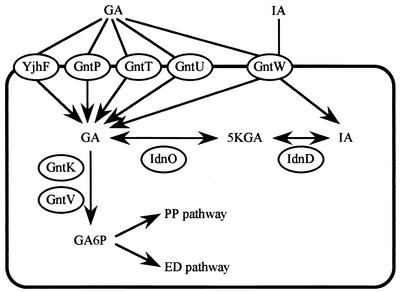
Gluconate uptake and initial catabolism in Escherichia coli involve two different systems, GntI and GntII (10). GntI, functioning as the main system, consists of high- and low-affinity gluconate permeases and a thermoresistant gluconate kinase encoded by the gntT, gntU, and gntK genes, respectively (2, 39; A. Hung, A. Orozco, and N. Zwaig, Bacteriol. Proc., p. 148, 1970) (Fig. 1). The GntII system includes another high-affinity gluconate permease and a thermosensitive gluconate kinase encoded by the gntW and gntV genes, respectively, and has been thought to be a subsidiary system (2, 39; Hung et al., Bacteriol. Proc., 1970). Recently, Bausch et al. (3) demonstrated that the gntW and gntV genes are involved in l-idonate uptake and catabolism, although GntW is capable of importing both d-gluconate and l-idonate to a similar extent. The genes for the GntI and GntII systems are located around 77 and 97 min, respectively, on the E. coli genome (8, 19, 21, 39). Another high-affinity gluconate permease, encoded by gntP, was discovered in E. coli, and its gene expression was shown to be repressed by the presence of gluconate or glucose (15). GntP was thus hypothesized to allow the entry of d-gluconate into cells for induction of other gnt genes. Additionally, YjhF, a homologue of GntT, was found in E. coli by database searching (24, 38).
FIG. 1.
Gluconate uptake and metabolism in E. coli. Gluconate (GA) is imported by high-affinity permeases, GntP, GntT, and GntW, and by a low-affinity permease GntU. GntT and GntU are members of the GntI system, whereas stationary-phase-specific GntW is a component of the GntII system. The gntP gene encoding GntP is located separately from the GntI and GntII genes and was shown to be repressed by the presence of gluconate (15). The imported gluconate is phosphorylated by either GntK or GntV, the thermoresistant gluconate kinase in GntI or thermosensitive gluconate kinase in GntII, respectively. The resulting gluconate-6-phosphate is metabolized by the pentose phosphate (PP) or Entner-Doudoroff (ED) pathway. Gluconate is also converted to 5-ketogluconate (5KGA) and then to idonate (IA) by 5-ketogluconate reductase (IdnO) and idonate dehydrogenase (IdnD), respectively, in GntII. GntW imports idonate as well as gluconate.
The gntT gene and gntRKU operon for the GntI system were cloned and characterized (12, 13, 25, 26, 34). The gntRKU operon has a promoter for gntRKU and another for gntKU. Both gntT and gntKU are regulated positively by the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex and negatively by GntR; one GntR-binding element for gntK has been proposed (12, 34), and two GntR-binding elements for gntT were experimentally defined (26). GntR also acts as a repressor for edd (encoding 6-phosphogluconate dehydratase) and eda (encoding 2-keto-3-deoxy-6-phosphogluconate aldolase) (10, 39), which are constituents of the Entner-Doudoroff pathway. Moreover, transcriptional attenuation occurs after the gntK gene, by which the gntU expression may be reduced to modulate the production of the low-affinity gluconate permease depending on the concentration of gluconate available. Permeases of GntT and GntU were characterized on the basis of transport activity and substrate specificity (13, 26, 34). The function of GntK was also determined after overexpression and purification (11). On the other hand, the genes for GntII system are transcribed at least by three promoters, one for the gntV gene, one for the idnDO-gntWH operon, and one for gntH, of which the first two are 5-ketogluconate or idonate inducible and the third is constitutive (reference 3 and unpublished data). The idnD and idnO genes are involved in the metabolism of idonate and encode idonate dehydrogenase (oxidizing idonate to 5-ketogluconate) and 5-ketogluconate reductase (reducing 5-ketogluconate to gluconate), respectively (3). GntH, with 42% similarity to GntR (12), has been suggested (3) or shown (unpublished data) to be an activator for the GntII genes, and 5-ketogluconate or idonate has been proposed as a coactivator (3).
In this study we have shown the negative regulation mechanism of the GntI system by GntR and also by GntH, an activator for the GntII system. The studies were performed with single-copy and multicopy lacZ operon fusions, disruptants of the regulator genes, mutants with and mutations in GntR-binding sequences. This allows us to propose a novel cross-regulation such that one system is negatively controlled by an activator for the different but related system in addition to its own regulator. We also discuss a possible mutual regulation between the GntI and GntII systems and its physiological significance.
The impingement of one regulator on another system has been demonstrated for Trp repressor protein, which is capable of intruding into other amino acid biosynthetic systems (4, 14), but its molecular mechanism has not been clarified. A well-characterized example of cross-regulation is found in the alternative control between lysogenic and lytic pathways by cI and Cro regulators in λ phage (27). This ingenious λ control, however, is achieved by the presence of closely located divergent promoters. Thus, cross-regulation between GntI and GntII genes located far from each other provides a new example of metabolic control.
MATERIALS AND METHODS
Materials.
Restriction enzymes and T4 DNA ligase were purchased from Takara Shuzo and New England Biolabs. The DNA sequencing kit was from Amersham Pharmacia Biotech Ltd. Primers were synthesized by Sawady Technology (Table 1). Other chemicals were of analytical grade.
TABLE 1.
Primers used in this study
| Name | Sequence |
|---|---|
| P1 | 5′-ACCGAATTCATGAAAAAGAAAAGACCC-3′ |
| P2 | 5′-ACAGTCGACTTAAATAGATCCGCCCGG-3′ |
| P3 | 5′-CAGGGATCCAGGTGGTGAAAGGCAAT-3′ |
| P4 | 5′-CTCAAGCTTCAGACCCTACTGCTGTT-3′ |
| P5 | 5′-CGCTCTAGATGTTTATTATCGCTGGC-3′ |
| P6 | 5′-TTAGGATCCTTGGCATCGGTGCGCAT-3′ |
| P7 | 5′-GTGAAGCTTGTGTCTACCTGACCAGTG-3′ |
| P8 | 5′-CCGGGATCCGATCGTTCATTGTATTAT-3′ |
| P9 | 5′-AATGGATCCGCCTGGGTAAATATGGCG-3′ |
| P10 | 5′-AATGTCGACCAGACCGGTGATCACTAG-3′ |
| P11 | 5′-AACCCCGGGCCCGACGCACTTTGCGCC-3′ |
| P12 | 5′-TCACCCGGGCACTTATTCAGGCGTAGC-3′ |
| P13 | 5′-AACGGATCCCCCGACGCACTTTGCGCC-3′ |
| P14 | 5′-TCAGGATCCCACTTATTCAGGCGTAGC-3′ |
| P15 | 5′-ATGGAATTCGGCGAATCTGTGACACC-3′ |
| P16 | 5′-ATGGGATCCTTCGGATTACCTTCACG-3′ |
| P17 | 5′-ATGGAATTCCTTTTGTAGATTGCCCG-3′ |
| P18 | 5′-ATGGGATCCATTCCTTGCATTAATCC-3′ |
| P19 | 5′-GAGCACGACTAACCATGA-3′ |
| P20 | 5′-TAACCTCAATGGTGCTTG-3′ |
| P21 | 5′-ACCGAATTCGTTATGCGCAATCACAGA-3′ |
| P22 | 5′-TCCCTGTACATCCATCAA-3′ |
| P23 | 5′-GATGTCGACCTAAAGCGTGTTGCCGTG-3′ |
| P24 | 5′-CGGCGAACACTCCTACTACT-3′ |
| P25 | 5′-GCAACAATGACTAATGGCAT-3′ |
| P26 | 5′-AACGCCTGCTGGCGCGT-3′ |
| P27 | 5′-CGACCGCAGATTTGCCGCTG-3′ |
Bacterial strains, plasmids, and media.
The bacterial strains used in this study were derivatives of E. coli K-12. Their relevant genotypes and plasmids are shown in Table 2. The cells were grown in modified Luria-Bertani (LB) medium (1% Bacto Tryptone, 0.5% yeast extract, 0.5% NaCl) (18) or minimum medium A (MMA) (19). MMA (pH 7.0) consisted of 27.2 mM (NH4)2SO4, 1.8 mM MgSO4, 4.5 × 10−2 mM CaCl2, 6.6 × 10−3 mM FeSO4, 0.3 × 10−2 mM thiamine hydrochloride, 32 mM K2HPO4, 32 mM NaH2PO4, 50 mM morpholinepropanesulfonic acid (MOPS), and 0.4% sugar.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| TG1 | supE hsdΔ5 thi Δ(lac-proAB) F′ traΔ36 proAB lacIqlacZΔM15 | 29 |
| CT690 | recB21 recC22 thi-1 thr-1 leu-6 lacY1 mxl-1 xyl-1 ara14 galK2 his-4 proA2 argF3 rpsL31 tsx-33 sup-37 sbcB15 | M, Tsuda |
| P90C | ara Δ(lac-pro) thi | 19 |
| NK7049 | ΔlacX74 galOP308 rpsL | 33 |
| YU506 | NK7049 λ Φ(gntK-lacZ) | This work |
| YU507 | NK7049 λ Φ(gntT-lacZ) | This work |
| YU563 | NK7049 gntR::cml | This work |
| YU564 | NK7049 gntH::cml | This work |
| YU565 | YU563 λ Φ(gnK-lacZ) | This work |
| YU566 | YU564 λ Φ(gnK-lacZ) | This work |
| YU567 | YU563 λ Φ(gntT-lacZ) | This work |
| YU568 | YU564 λ Φ(gntT-lacZ) | This work |
| YU577 | NK7049 gntR::Tn10 gntH::cml | This work |
| YU578 | YU577 λ Φ(gntK-lacZ) | This work |
| YU579 | YU577 λ Φ(gntT-lacZ) | This work |
| YU632 | NK7049 idnO::cml | This work |
| Plasmids | ||
| pACYC177 | Ampr Kanr | 5 |
| pCB192 | AmprlacZ galK | 31 |
| pUC119 | AmprlacZα | 35 |
| pMBL18 | AmprlacZα | 22 |
| pRS551 | Ampr Kanr promoterless lacZ | 33 |
| pKF18k | Kanr (dual amber mutant) lacZα | 9 |
| pGEX4T-1 | Ampr GST gene | Amersham Pharmacia Biotech |
| pGNT2 | pACYC177 with gntV, idnD, idnO, gntW, and gntH | This work |
| pGNT5 | pBR322 with gntK | 12 |
| pGNTT20 | pBR322 with gntT | 13 |
| pGNTK-CAT1 | pKK232-8 with the BamHI-PstI fragment from pGNT5 | 12 |
| pYY2 | Frameshift mutation of the bla gene on pACYC177 | 37 |
| pGNTR1 | pACYC177 with gntR | 12 |
| pGNTH | pACYC177 with gntH | This work |
| pGNTR18 | pMBL18 with gntR | This work |
| pGNTH119 | pUC119 with the 1.7-kb DraI fragment from pGNT2 | This work |
| pGNTH18 | pMBL18 with gntH | This work |
| pGNTR177 | pACYC177 with gntR | This work |
| pGNTR177-CM | pGNTR177 with cml | This work |
| pGNTH-DIS2 | pUC119 with gntH | This work |
| pGNTH-DIS2-CM | pGNTH-DIS2 with cml | This work |
| pBRIDNO | pBR322 with idnO | This work |
| pBRIDNO-CM | pBRIDNO with cml | This work |
| pGST-GNTR | pGEX4T-1 with gntR | This work |
| pGST-GNTH | pGEX4T-1 with gntH | This work |
| pGNTT-LAC4 | pCB192 with the 460-bp Sau3AI fragment from pGNTT20 | 12 |
| pGNTK-LAC | pCB192 with the 1.2-kb SmaI-HindIII fragment from pGNTK-CAT1 | This work |
| pKFGNTK | pKF18k with the 1.2-kb SmaI-HindIII fragment from pGNTK-CAT1 | This work |
| pGNTK-LACMR1 | pGNTK-LAC derivative with mutations at the R1 sequence of the gntK operator (MR1) | This work |
| pGNTK-LACMR2 | pGNTK-LAC derivative with mutations at the R2 sequence of the gntK operator (MR2) | This work |
| pGNTK-LACMR12 | pGNTK-LAC derivative with mutations at the R1 and R2 sequences of the gntK operator (MR12) | This work |
| pRSGNTK | pRS551 with the 500-bp fragment including the promoter-operator of gntK | This work |
| pRSGNTT | pRS551 with the 460-bp fragment including the promoter-operator of gntT | This work |
DNA manipulation and sequencing.
Conventional recombinant DNA techniques were applied (29). Nucleotide sequencing was carried out by the dideoxy-chain termination method (30). The nucleotide sequences of the cloned genes and the deduced amino acid sequences were compared with those listed in the DNA and protein databases by GENETYX software (Software Development, Tokyo, Japan).
Cloning of the GntII genes and gntR.
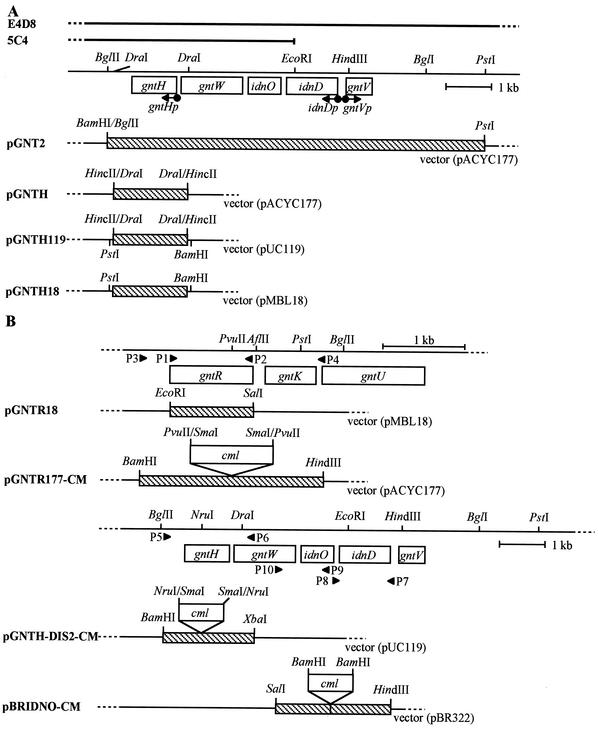
Plasmid pGNT2 bearing gntV, idnD, idnO, gntW, and gntH in the GntII-encoding locus was constructed by inserting the 8.4-kb BglII-PstI fragment from a Kohara clone, E4D8 (16), into the BamHI-PstI site of pACYC177 (Fig. 2A). The gntH gene was subcloned by inserting the 1.7-kb DraI fragment from pGNT2 into the HincII site of pACYC177 and into the HincII site of pUC119, generating pGNTH and pGNTH119, respectively. The 1.7-kb PstI-BamHI fragment from pGNTH119 was inserted into the PstI-BamHI site of pMBL18, generating pGNTH18. The gntR gene was cloned by inserting the PCR fragment, which was amplified with a primer set consisting of P1, containing the EcoRI site (Table 1), and P2, containing the SalI site, and pGNTR1 DNA (13) as a template and digested with EcoRI and SalI, into the EcoRI-SalI site of pMBL18, generating pGNTR18 (Fig. 2B). For the construction of the gntR- and gntH-disrupted mutants (as described below), pGNTR177 bearing gntR (pGNTR177-CM in Fig. 2B) and pGNTH-DIS2 bearing gntH (pGNTH-DIS2-CM in Fig. 2B) were made by inserting PCR fragments, amplified with primers P3, containing the BamHI site, and P4, containing the HindIII site, and pGNTR1 DNA as a template and with primers P5, containing the BamHI site, and P6, containing the XbaI site, and pGNT2 DNA as a template, into the BamHI-HindIII site of pACYC177 and the BamHI-XbaI site of pUC119, respectively. To construct an idnO-disrupted mutant (as described below), two PCR fragments were amplified with primers P7, containing the HindIII, site and P8, containing the BamHI site, and with primers P9, containing the BamHI site, and P10, containing the SalI site, and pGNT2 DNA as a template, and digested with HindIII and BamHI and with BamHI and SalI, respectively. The two fragments were then inserted together into the HindIII-SalI site of pBR322. The resultant pBRIDNO bearing idnO (pBRIDNO-CM in Fig. 2B) is lacking 72 bp immediately downstream of the initiation codon and has a new BamHI site at the same point.
FIG. 2.
Organization of the GntI and GntII genes, and clones for disruptants and lacZ operon fusions. (A) The GntII genes, gntV, idnD, idnO, gntW, and gntH, located on the E. coli W3110 genome are represented by open boxes. Parts of the Kohara clones, E4D8 and 5C4, are shown at the top. The direction of their transcription from promoters, gntVp, idnDp, and gntHp, are shown by arrows. Plasmid pGNT2 with the 8.4-kb BglII-PstI fragment from E4D8 bears all five genes. pGNTH and pGNTH119 with the 1.7-kb DraI fragment bear the gntH gene. pGNTH18 containing the 1.7-kb PstI-BamHI fragment from pGNTH119 bears the gntH gene. (B) The open boxes indicate the GntI and GntII genes. pGNTR18 containing the 1.0-kb PCR fragment bears gntR. pGNTR177-CM, pGNTH-DIS2-CM, and pBRIDNO-CM bear the gntR, gntH, and idnO genes, respectively, which have insertion of the cml gene (open boxes) and are used for gene disruption. (C and D) The gntRKU and gntT genes (open boxes) are shown at the top. The regions inserted in the gnt-lacZ operon fusions and the promoterless lacZ genes are shown by hatched and dotted boxes, respectively. The promoter-operator regions of gntKU (C) and gntT (D), based on the previous data (12, 13, 25), are schematically represented at the middle and bottom, respectively. Arrows indicate the initiation sites and direction of the gntKU and gntT mRNAs. The corresponding promoter sequences with the −10 and −35 sequences are shown by brackets. The GntR- and cAMP-CRP-binding sites are represented by boxes, and the ribosome-binding sites (SD) and the start codons of gntK and gntT are also shown. Three mutants (MR1, MR2, and MR12) and their mutation sites (boxed) in the GntR-binding elements of the gntK promoter-operator region are shown at the bottom of panel C. The procedures for cloning and mutant construction are described in Materials and Methods. Hatched boxes represent the DNA fragments inserted into vectors, which were derived from the genomic DNA, and arrowheads P1 to P18 represent the position and direction of primers corresponding to those in Table 1.
Construction of gntR-, gntH-, and idnO-disrupted mutants.
gntR-, gntH-, and idnO-disrupted mutant derivatives of NK7049 were constructed. A DNA fragment of the chloramphenicol resistance gene (cml), obtained by PCR with pACYC184 DNA as the template and primer sets P11, bearing the SmaI site, and P12, bearing the SmaI site, or primer sets P13, bearing the BamHI site, and P14, bearing the BamHI site, was inserted into the PvuII site in pGNTR177, the NruI site in pGNTH-DIS2, or the BamHI site in pBRIDNO to disrupt the open reading frames and produce pGNTR177-CM, pGNTH-DIS2-CM, and pBRIDNO-CM, respectively (Fig. 2B). The DNA fragments bearing the disrupted gntR, gntH, and idnO genes were amplified by PCR using primers P3 and P4, P5 and P6, and P7 and P10, respectively, and pGNTR177-CM, pGNTH-DIS-CM, and pBRIDNO-CM DNAs, respectively, as templates. Each amplified fragment was introduced into CT690 (recBC sbcB) to allow homologous recombination between the cml-inserted gene on the fragment and the corresponding gene on the chromosome, and the recombinant was screened on LB plates containing chloramphenicol (15 μg/ml). The recombination in the recBC sbcB background is dependent on the RecF pathway (7). The gene disruption of each construct was confirmed by PCR using the same primers as shown above and its genomic DNA, followed by digestion with EcoRI, whose recognition site is located in the cml gene. The recombinant gntR::cml, gntH::cml, and idnO::cml were then transferred to NK7049 by P1 transduction (19), generating YU563, YU564, and YU632, respectively. A gntR and gntH double-disrupted mutant of NK7049 was constructed, based on the fact that YU506 bearing a single-copy gntK-lacZ operon fusion on the chromosome catabolizes lactose only very poorly because the gntK expression was repressed by GntR in the absence of gluconate. The gntR-disrupted mutant was thus screened on minimum medium plates containing 0.4% lactose and tetracycline (8 μg/ml) after transposon mutagenesis with λNK1323 (23). The mutants obtained as large colonies were subjected to PCR with the same primers (P3 and P4) as those used for construction of the cml-inserted mutant to confirm the transposon insertion. The gntR::Tn10 in YU506 was transferred to YU564 by P1 transduction, generating YU577.
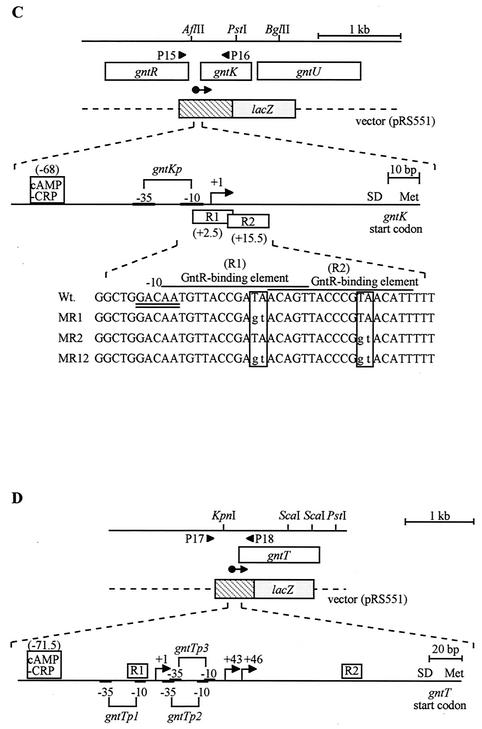
Construction of single-copy gntK-lacZ and gntT-lacZ operon fusions on the E. coli chromosome.
Single-copy gntK-lacZ (YU506, YU565, YU566, and YU578) and gntT-lacZ (YU507, YU567, YU568, and YU579) operon fusions on the chromosome were constructed by the procedure of Simons et al. (33). The PCR fragments encompassing the 5′-flanking regions to parts of the coding regions of the gntK and gntT genes were subcloned into the EcoRI-BamHI site of pRS551 to generate pRSGNTK and pRSGNTT, respectively. To prepare the PCR fragments, the following primer sets (Fig. 2C and D) and the templates were used: P15 bearing the EcoRI site, P16 bearing the BamHI site, and pGNTK-LAC DNA for gntK; P17 bearing the EcoRI site, P18 bearing the BamHI site, and pGNTT20 DNA (13), for gntT. E. coli strain P90C transformed with pRSGNTK or pRSGNTT was used as a host strain for growth of phage λRS45 (33) to prepare phage lysate by the standard method (32). E. coli strains NK7049, YU563, YU564, and YU577 were infected with the lysate, and phage lysogens were screened on LB plates containing kanamycin (35 μg/ml), streptomycin (50 μg/ml), and 0.005% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). As a result, YU506, YU565, YU566, YU578, YU507, YU567, YU569, and YU578 were constructed.
Construction of a multicopy gntK-lacZ operon fusion and site-directed mutagenesis.
The operon fusion of the gntK gene with the lacZ gene was constructed to examine the function of GntR-binding elements. Plasmid pGNTK-LAC is a pCB192 (31) derivative with the 1.2-kb SmaI-HindIII fragment bearing the promoter-operator region of gntKU from pGNTK-CAT1 (12). The SmaI-HindIII fragment from pGNTK-LAC was subcloned into pKF18k (9) as the vector for the oligonucleotide-directed dual amber method (9), resulting in pKFGNTK. Mutations were introduced by the method using the Mutan-Super Express Km kit (Takara Shuzo) into the GntR-binding elements in pKFGNTK. Plasmid pKF18k contains dual amber codons in the kanamycin resistance gene, and thus after PCR using a mutagenic primer and a “selection primer” that restores the amber mutations and introduction into a sup0 strain, the mutant clones can be screened on LB plates containing kanamycin. Mutagenic primers used for R1 and R2 elements are CGGCTGGACAATGTTACCGAGTACAGTTACCC and CAGTTACCCGGTACATTTTT, respectively, and the constructed mutants MR1, MR2, and MR12 have the mutations on R1, R2, and both R1 and R2, respectively; their mutated sequences are shown in Fig. 2C. Each mutated DNA fragment was subcloned into the SmaI-HindIII site of pCB192, generating mutant gnt-lacZ fusion plasmids, pGNTK-LACMR1, pGNTK-LACMR2, or pGNTK-LACMR12, and was cointroduced into YU577 with pYY2 (37), as a control plasmid, pGNTR1 bearing gntR, or pGNTH bearing gntH. Double-transformants were grown on LB plates containing ampicillin (100 μg/ml) and kanamycin (50 μg/ml) and subjected to a β-galactosidase assay (19).
RT-PCR analysis.
To examine the gntK and gntH gene expression, reverse transcription-PCR (RT-PCR) analysis was performed with the mRNA selective RT-PCR kit (Takara Shuzo). Primer sets P19 plus P20 and P21 plus P22 for gntK and gntH, respectively, were used. Total RNAs were isolated by the hot-phenol method (1) immediately after cultivation in LB or minimum medium. Using LB medium, the cells were precultured at 37°C for 16 h. The preculture was diluted 30-fold with the same medium, and incubation was carried out for 2 h. Incubation was further continued for 2 h after the addition of gluconate or 5-ketogluconate (at a final concentration of 0.5%). With minimum medium containing 0.4% glycerol as the sole carbon source, the cells were precultured at 37°C for 24 h, diluted 30-fold with minimum medium containing 0.4% gluconate, and incubated at 37°C for 4, 9, or 14 h. The RT reaction was carried out at 50°C for 15 min with 0.1 μg of each total RNA and the P20 or P22 primer, and PCR (15 to 35 cycles) consisting of denaturation at 85°C for 1 min, annealing at 45°C for 1 min, and extension at 72°C for 1 min was then performed using the two primers for each gene. The PCR products were analyzed by agarose gel electrophoresis (0.9% agarose) and stained with ethidium bromide. As a control, 10-μg samples of total RNAs were subjected to agarose gel electrophoresis (1.2% agarose) and stained with ethidium bromide. The relative amounts of RT-PCR products on the gel were compared by measuring band density after the color of the image obtained was reversed by using a model GS-700 imaging densitometer (Bio-Rad). Linearity of the amplification was observed at least up to the 25th cycle. This experiment was repeated at least twice, and the experimental error was estimated to be less than twofold. Under our conditions, the RNA-selective RT-PCR was able to specifically detect mRNA because no band was observed when reverse transcriptase was omitted.
GntR and GntH purification.
GntR and GntH were expressed and purified as specified by the manufacturer of the glutathione S-transferase (GST) gene fusion system (Amersham Pharmacia Biotech). To construct pGST-GNTR and pGST-GNTH, encoding GST-GntR and GST-GntH protein fusions, respectively, the PCR fragments bearing the whole gntR and gntH genes were amplified using primers set P1 plus P2 and P21 plus P23, respectively, with pGNTR1 and pGNTH119 DNAs, respectively, as templates. The amplified fragments were digested with EcoRI and SalI and inserted into the EcoRI-SalI site of pGEX4T-1. The fusion protein was expressed in TG1 cells after induction with 0.1 mM isopropyl-β-d-thiogalactopyranosida (IPTG) in LB medium for 6 h and purified on glutathione-Sepharose column as recommended by the manufacturer (Amersham Pharmacia Biotech). Purified GntR and GntH were used for gel shift analysis after removal of the GST portion by thrombin protease cleavage.
Gel shift analysis.
The specific DNA binding of GntR or GntH was tested by gel shift analysis by the method of Miwa et al. (20) with some modifications. PCR products prepared as follows were used as DNA fragments for the gel shift analysis. The 402-bp fragment bearing the promoter-operator region of gntT was amplified with the primer set P24 and P25, with pGNTT-LAC4 DNA as the template. The 295-bp fragment bearing the promoter-operator region of gntKU was amplified with the primer set P26 and P27, with pGNTK-LAC, pGNTK-LACMR1, pGNTK-LACMR2, or pGNTK-LACMR12 DNA as the template. DNA fragments (4.1 to 5.0 pmol) were mixed with purified GntR (11.6 to 27.1 pmol) or GntH (15.7 to 51.0 pmol) at 30°C for 30 min in a 30-μl mixture of 30 mM Tris-HCl (pH 7.5), 0.6 mM EDTA, 0.6 mM dithiothreitol, 30 mM KCl, and sugar (if required). After incubation, samples were applied to a 5% polyacrylamide gel and run in 45 mM Tris-borate (pH 7.8) containing 6.2 mM EDTA at room temperature. The DNA bands on the gel were then stained with ethidium bromide. This analysis was performed at least twice independently.
Enzyme assay.
Cells harboring a lacZ operon fusion plasmid or cells with the lacZ fusion on the chromosome were grown at 37°C for 16 h in 3 ml of LB medium containing both ampicillin (100 μg/ml) and kanamycin (50 μg/ml) or both streptomycin (50 μg/ml) and kanamycin (35 μg/ml), respectively. The preculture was diluted 30-fold with the same medium containing antibiotics and further incubated for the appropriate times. Portions of the cell cultures were then taken and subjected to a β-galactosidase assay (19, 23). When necessary, gluconate or 5-ketogluconate was added to the medium at a final concentration of 0.5%.
RESULTS
Cloning of gntH and characteristics of the GntH structure.
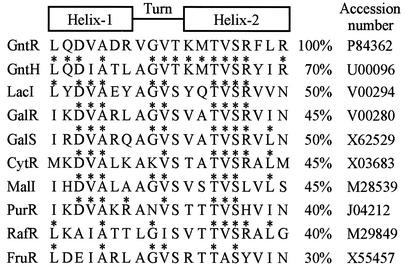
The gntH gene and other gnt genes in the GntII system were subcloned from a Kohara phage clone, E4D8 (Fig. 2A). The gntH open reading frame consisting of 999 bases starts with an ATG initiation codon at position 66 downstream of the gntW gene. A possible promoter, TTGCACX16TGTGAT, corresponding to −35 and −10 sequences, exists at position 48 upstream of the initiation codon. GntH is predicted to consist of 332 amino acid residues with a molecular mass of 37,567 Da. Like GntR, GntH appears to belong to the GalR-LacI family of bacterial regulators (36). Comparison of the GntH primary structure with those of other members in this family reveals that GntH has a helix-turn-helix (HTH) motif, LQDIATLAGVTKMTVSRYIR, at its N terminus, which may be responsible for DNA binding. As shown in Fig. 3, the HTH of GntH has quite high similarity to that of GntR compared with those of other members. The second helix in the HTH is involved mainly in binding to DNA, and most amino acid residues for DNA binding in LacI and MalI seem to be within the first six residues in the second helix (17, 28). Notably, the corresponding six residues are completely conserved between GntH and GntR. These observations led us to conclude that GntH might bind to GntR elements and might repress the GntI genes.
FIG. 3.
Sequence comparison of the HTH of GntR with that of GntH and with those of the GalR-LacI family. A 20-amino-acid sequence of the HTH of GntR was compared with that of GntH and those of the members of GalR-LacI family. The identities to GntR are expressed as percentages, and identical residues to GntR are indicated by asterisks.
Effect of GntH on GntI gene expression.
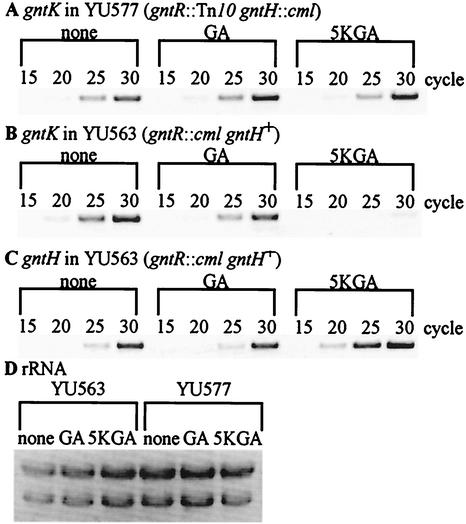
To test the notion mentioned above, we investigated gntK expression by RT-PCR analysis with total RNAs from YU563 (gntR::cml gntH+) and YU577 (gntR::Tn10 gntH::cml), as shown in Fig. 4A and B. Expression was compared on the basis of the intensity of bands at each PCR cycle shown in Fig. 4A to C relative to a reference consisting of rRNA in each extract (Fig. 4D). Comparisons in the absence of inducers indicated that the presence of GntH did not reduce or increase gntK expression. However, gntK expression was extremely low in YU563 compared to that in YU577 in the presence of 5-ketogluconate. Interestingly, gluconate did not have the same effect. These results suggested that GntH could repress gntK, but only in a 5-ketogluconate-dependent manner. The 5-ketogluconate-dependent repression of gntK by GntH might be due to the increased affinity of GntH for the elements in GntI genes by binding of 5-ketogluconate or its derivative, as occurs with GntH-inducer complex in the activation of the GntII genes (3; unpublished data). Alternatively, repression in the presence of 5-ketogluconate could have been due to the elevation in the number of GntH molecules. Indeed, by RT-PCR it was observed that gntH expression from the idnDO-gntWH operon was increased by the addition of 5-ketogluconate (Fig. 4C). This result suggested that elevated levels of GntH were required for binding to GntI promoters, and this idea was tested using overexpressed GntH, as shown below. However, it could not be ruled out that the elevated level of GntH might still need 5-ketogluconate as a corepressor.
FIG. 4.
RT-PCR analysis of the gntK and gntH expression in a gntR gntH double disruptant and a gntR single disruptant. (A to C) YU577 (gntR::Tn10 gntH::cml) (A) and YU563 (gntR::cml gntH+) (B and C) were grown in LB medium at 37°C for 2 h and further incubated for 2 h in the presence of 0.5% gluconate (GA) or 5-ketogluconate (5KGA). Total RNAs were then prepared and subjected to RT-PCR analysis with primers specific for gntK (A and B) or gntH (C) as described in Materials and Methods. The numerals represent cycles of PCR. (D) rRNA was used as a control. Total RNAs (10 μg) in panels A through C were used.
Effect of a multi copy gntH on the GntI gene expression in single-copy gnt-lacZ operon fusions.
To further examine the effect of GntH on the GntI genes, we constructed single-copy gntK-lacZ and gntT-lacZ operon fusions in several different E. coli genetic backgrounds and measured their expression in LB medium containing gluconate or 5-ketogluconate (Table 3). Also, we cloned gntH and gntR into a moderate-copy-number plasmid (pMBL18), generating pGNTH18 and pGNTR18, respectively (Fig. 2A and B), which were then introduced into YU578 [λ Φ(gntK-lacZ) gntR::Tn10 gntH::cml] and YU579 [λ Φ(gntT-lacZ) gntR::Tn10 gntH::cml], as shown in Table 3.
TABLE 3.
Expression of single-copy gntK-lacZ and gntT-lacZ operon fusions on the genome in LB medium
| Strain | β-Galactosidase activity (Miller units)a at:
|
|||||
|---|---|---|---|---|---|---|
| 4 h
|
24 h
|
|||||
| No addition | GAb addition | 5KGAb addition | No addition | GAb addition | 5KGAb addition | |
| gntK-lacZ | ||||||
| YU578 (gntR::Tn10 gntH::cml) | ||||||
| + pACYC177c | 6,200 ± 150 | 3,800 ± 160 | 4,700 ± 580 | 9,800 ± 800 | 5,500 ± 100 | 6,100 ± 100 |
| + pGNTR18c | 86 ± 15 | 3,500 ± 140 | 1,600 ± 130 | 43 ± 3.0 | 1,800 ± 100 | 530 ± 10 |
| + pGNTH18c | 99 ± 0 | 59 ± 3.5 | 63 ± 1.6 | 130 ± 10 | 34 ± 3.0 | 45 ± 4.0 |
| YU506 (gntR+ gntH+) | 42 ± 2.0 | 2,800 ± 700 | 420 ± 70 | 23 ± 2.0 | 1,600 ± 100 | 91 ± 29 |
| YU565 (gntR::cml gntH+) | 4,600 ± 1,000 | 3,200 ± 26 | 2,300 ± 300 | 12,000 ± 1,000 | 7,200 ± 300 | 3,300 ± 100 |
| YU566 (gntR+ gntH::cml) | 34 ± 1.0 | 2,300 ± 100 | 1,000 ± 100 | 44 ± 2.0 | 1,300 ± 100 | 430 ± 50 |
| YU578 (gntR::Tn10 gntH::cml) | 4,900 ± 800 | 3,500 ± 300 | 4,900 ± 1,100 | 6,500 ± 300 | 5,400 ± 600 | 7,700 ± 1,700 |
| gntT-lacZ | ||||||
| YU579 (gntR::Tn10 gntH::cml) | ||||||
| + pACYC177 | 1,400 ± 43 | 730 ± 25 | 1,100 ± 19 | 1,800 ± 100 | 1,100 ± 200 | 1,100 ± 100 |
| + pGNTR18 | 76 ± 11 | 830 ± 6.3 | 660 ± 61 | 45 ± 1.0 | 470 ± 0 | 280 ± 10 |
| + pGNTH18 | 170 ± 0 | 140 ± 5.0 | 130 ± 0 | 290 ± 30 | 100 ± 0 | 110 ± 10 |
| YU507 (gntR+ gntH+) | 38 ± 9.0 | 730 ± 100 | 230 ± 30 | 27 ± 1.0 | 220 ± 60 | 94 ± 2.0 |
| YU567 (gntR::cml gntH+) | 1,300 ± 100 | 600 ± 90 | 480 ± 50 | 2,400 ± 400 | 1,400 ± 100 | 810 ± 70 |
| YU568 (gntR+ gntH::cml) | 33 ± 2.0 | 560 ± 30 | 360 ± 30 | 26 ± 2.0 | 270 ± 30 | 230 ± 20 |
| YU579 (gntR::Tn10 gntH::cml) | 1,000 ± 70 | 510 ± 50 | 550 ± 50 | 1,800 ± 200 | 1,100 ± 200 | 1,100 ± 100 |
Reported values are means and standard deviations of more than two independent experiments performed in triplicate.
0.5% gluconate (GA) or 5-ketogluconate (5KGA) was added after a 2-h incubation.
pACYC177, pGNTR18, and pGNTH18 were used as the control plasmid, the plasmid bearing gntR, and the plasmid bearing gntH, respectively.
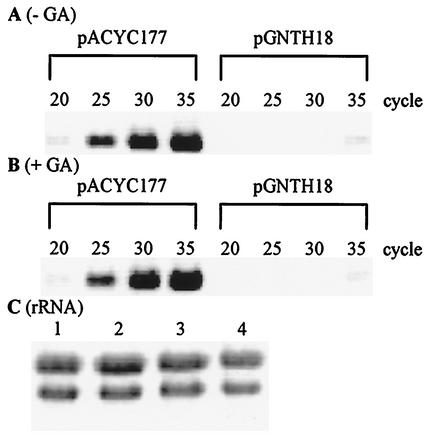
As expected, negative control of gntK and gntT gene expression by cloned GntR was observed. There was greatly decreased β-galactosidase activity in the cells harboring pGNTR18 compared to that in cells harboring a control plasmid, pACYC177. More interestingly, β-galactosidase activities from bacteria harboring pGNTH18 were also much lower than those from the cells harboring pACYC177. Hence, cloned GntH was indeed a repressor of both GntI genes. Repression of the gntK-lacZ and gntT-lacZ operon fusions in the presence of pGNTH18 was still observed irrespective of the addition of gluconate or 5-ketogluconate, suggesting that a high level of GntH bypasses any requirement for corepressor. This is in contrast to GntR encoded by pGNTR18, with which a significant induction of the fusions was detected with gluconate or 5-ketogluconate. Consistent with the fusion results, RT-PCR analysis of gntK expression in total RNA indicated a strong repression by GntH only when cloned GntH was present. As shown in Fig. 5, YU563 harboring pGNTH18 contained far less gntK RNA than did strains harboring pACYC177. Only faint bands were observed in the case of pGNTH18 with or without gluconate, whereas strong bands appeared in the case of the control plasmid. Taken together, GntH seems to repress the expression of the gntKU and gntT genes, and the repression occurs even in the presence of gluconate, which could be physiologically important, as discussed below.
FIG. 5.
Repression of the gntK expression by GntH derived from a multicopy plasmid, pGNTH18. (A and B) YU563 (gntR::cml gntH+) harboring pACYC177 as a control plasmid or pGNTH18 bearing gntH was grown in LB medium at 37°C for 2 h and further incubated for 2 h in the presence of 0.5% gluconate (GA). Total RNAs were then prepared and subjected to RT-PCR analysis with primers specific for gntK as described in Materials and Methods. The numbers above the lanes represent cycles of PCR. (C) rRNA was used as a control. Lanes 1 and 2 contain total RNAs (10 μg) from cells harboring pACYC177 and cells harboring pGNTH18 grown in the medium without GA, respectively, and lanes 3 and 4 contain total RNAs from cells harboring pACYC177 and harboring pGNTH18 grown with GA, respectively.
Control in expression of single-copy lacZ operon fusions of the GntI genes.
The single-copy fusions were also used to test the expression of gntK and gntT in the absence of cloned regulators (Table 3). Minimum medium was not used in these experiments because the gntR-disrupted mutants grew extremely slowly. Although there is no clear explanation for this slow growth, cells might be sensitive to gluconate overaccumulation or to membrane perturbation by excess production of gluconate permease molecules. Consistent with previous reports (12, 13, 25), β-galactosidase activities of the lacZ operon fusion strains, YU506 [λ Φ(gntK-lacZ) gntR+ gntH+] and YU507 [λ Φ(gntT-lacZ) gntR+ gntH+], were increased by the addition of gluconate. These activities moderately increased in the presence of 5-ketogluconate. This partial increase may be due to the intracellular conversion of 5-ketogluconate to gluconate, which induces expression of the GntI genes by interacting with GntR and releasing it from the operator (see below). Greatly increased expression of β-galactosidase due to the gntR disruption was clearly observed in the gntK-lacZ operon fusions of YU565 (gntR::cml) and YU578 (gntR::Tn10 gntH::cml) and in the gntT-lacZ operon fusions of YU567 (gntR::cml) and YU579 (gntR::Tn10 gntH::cml). These results confirm that GntR is a negative regulator of the GntI genes, as demonstrated previously (12, 13, 25).
In Table 3, with the single-copy fusions in the absence of plasmid, there are three additional pieces of data regarding the involvement of GntH in the GntI gene expressional control. First, in the gntR::cml and gntH+ background, β-galactosidase activities were lower in the presence of 5-ketogluconate than in the presence of gluconate. Second, the gntR gntH doubly disrupted strains, YU578 and YU579, showed higher activities than the gntR singly disrupted strains, YU565 and YU567, respectively in the presence of 5-ketogluconate. No such difference, however, was observed in the presence of gluconate. The reduction ratio by 5-ketogluconate was relatively small compared to the RT-PCR results in Fig. 4, which may be due to the continued stability of highly expressed β-galactosidase in the gntR background before the addition of 5-ketogluconate. Finally, although the gntH-disrupted strains of YU566 [λ Φ(gntK-lacZ) gntR+ gntH::cml] and YU568 [λ Φ(gntT-lacZ) gntR+ gntH::cml] showed similar patterns in expression of the fusion genes to that of the wild-type strains, their β-galactosidase activities in the presence of 5-ketogluconate were 1.6- to 4.7-fold higher than those from the wild-type strains, respectively. Taking all the above data together, it is concluded that the gntH gene is involved in the negative control of the GntI genes when 5-ketogluconate is present.
Involvement of GntH in the growth phase regulation of GntI gene expression.
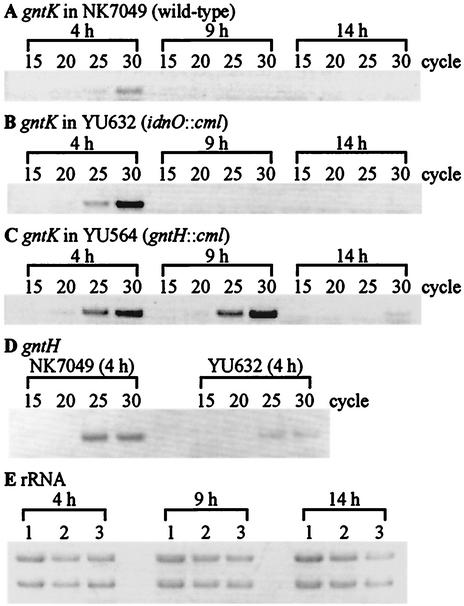
It was reported that induction of a gntT::lacZ fusion in LB medium containing gluconate peaked at early logarithmic phase and then subsided gradually throughout the remaining logarithmic phase (26). We therefore tested the idea that GntH is involved in the decrease of GntI gene expression, in which 5-ketogluconate or idonate is required as a corepressor and/or functions as a coactivator for expressing the gntH gene by induction of the idnDp that transcribes the idnDO-gntWH operon. Enzymes encoded by the GntII genes have been demonstrated to catalyze the reversible conversion from gluconate to 5-ketogluconate and then to idonate (3). We thus constructed an idnO-disrupted strain, YU632, that is defective in converting gluconate to 5-ketogluconate. The effect of the idnO mutation was evaluated by comparison of its gntK expression by RT-PCR analysis with that of the wild type during different growth phases (Fig. 6A to C). Bacteria were grown for 4 h (early logarithmic phase), 9 h (late logarithmic phase), and 14 h (early stationary phase) in gluconate minimum medium, from which total RNAs were prepared. The intensity of each band at each cycle revealed that the gntK expression in the wild-type strain decreased at the late logarithmic phase. In contrast, the gntH-disrupted strain, YU564, did not show a decrease until early stationary phase, and the subsequent decrease in expression was assumed to be caused by GntR owing to the low level of intracellular gluconate during that period. Furthermore, the gntK gene expression in the idnO-disrupted strain appeared to become low, as in the wild-type strain after 9 h, but the expression at early logarithmic phase was about eightfold higher than that in the wild type. These results suggest that in the presence of gluconate, the expression of the GntI genes was induced at early logarithmic phase and was then repressed by a GntH-dependent mechanism. As expected, the gntH expression was reduced in the idnO::cml background (Fig. 6D), where the gntH gene may be transcribed almost only by the constitutive gntHp. Therefore, 5-ketogluconate or idonate derived from gluconate may play a crucial role via GntH in the decline of GntI gene expression at the beginning of the logarithmic phase.
FIG. 6.
Change of gntK expression along with the cell cycle in the medium containing gluconate. (A to D) NK7049 (A), YU632 (idnO::cml) (B), and YU564 (gntH::cml) (C) were grown in minimum medium containing gluconate as the sole carbon source at 37°C for 4 h (early logarithmic phase), 9 h (late logarithmic phase), and 14 h (early stationary phase). Total RNAs were then prepared and subjected to RT-PCR analysis with primers specific for gntK (A to C) or gntH (D) as described in Materials and Methods. The numbers above the lanes represent cycles of PCR. (E) rRNA was used as a control. Lanes 1 to 3 contain total RNAs (10 μg) from NK7049, YU632, and YU564, respectively.
Mutational analysis of GntR-binding elements in the gntKU promoter-operator region.
GntR-binding elements were predicted to occur around the promoters of the GntI genes (12, 13, 26), and two GntR-binding elements in the gntT gene were analyzed in detail (25). We found two possible GntR-binding elements, AATGTTACCGATAACAGT (R1) and ACAGTTACCCGTAACATT (R2), overlapping with or close to the −10 sequence of the gntKU promoter (Fig. 2C). To examine the function of the elements, mutations were introduced into them by site-directed mutagenesis, which changed nucleotide sequences conserved among the predicted GntR-binding elements in the GntI genes, and the mutant derivatives of gntK-lacZ operon fusions, pGNTK-LACMR1, pGNTK-LACMR2, and pGNTK-LACMR12, containing mutations at R1, R2, and both R1 and R2 sequences, respectively, were constructed (Fig. 2C).
The β-galactosidase activity of YU577 (gntR::Tn10 gntH::cml) cells harboring each of two plasmids, pGNTR1 or pGNTH, or the control plasmid, pYY2, together with the mutant gntK-lacZ fusions, was then measured (Table 4). The activity from pGNTK-LAC was strongly reduced by GntR and moderately reduced by GntH derived from the plasmid pGNTR1 and pGNTH, respectively, in the absence of gluconate. Notably, the down-regulation by GntR was weakened by the addition of gluconate, but that by GntH was not. β-Galactosidase activities from pGNTK-LACMR1 and pGNTK-LACMR12 were moderately and were slightly reduced by cloned GntR and GntH, respectively, under the tested conditions without gluconate. On the other hand, the activity from pGNTK-LACMR2 was reduced by cloned GntR and moderately reduced by cloned GntH under the conditions without gluconate as in pGNTK-LAC, but its derepression ratio by gluconate was higher than that in pGNTK-LAC. These results suggest that both GntR-binding sequences, R1 and R2, are responsible for the repression of gntKU expression, although the affinity of GntR or GntH for R1 seems to be different from that for R2. This, together with the data from the gel shift analysis as shown below, suggest the presence of two GntR-binding elements in the gntKU promoter-operator region, contrary to the original prediction of only one element (12, 26). It is possible that GntR and/or GntH binds as a tetramer to the two partially overlapping elements. These results not only confirm the negative control by GntH of the gntKU expression but also suggest its binding to the same GntR-binding elements. This is because the repression pattern by GntH of the wild-type and mutant fusions was similar to that by GntR when gluconate was absent.
TABLE 4.
Effect of mutations in possible GntR-binding sequences on the expression of a multicopy gntK-lacZ fusion in LB medium
| Gnt-lacZ fusion plasmid in YU577 | β-Galactosidase activity (Miller units)a
|
|||||
|---|---|---|---|---|---|---|
| No addition
|
GAb addition
|
|||||
| pYY2c | pGNTR1c | pGNTHc | pYY2 | pGNTR1 | pGNTH | |
| pGNTK-LAC | 480 ± 84 | 42 ± 4.8 | 260 ± 29 | 450 ± 166 | 120 ± 23 | 220 ± 1.4 |
| pGNTK-LACMR1 | 530 ± 72 | 280 ± 47 | 510 ± 9.9 | 470 ± 48 | 480 ± 55 | 530 ± 45 |
| pGNTK-LACMR2 | 390 ± 14 | 41 ± 2.7 | 240 ± 36 | 470 ± 1.5 | 260 ± 35 | 220 ± 20 |
| pGNTK-LACMR12 | 530 ± 28 | 300 ± 2.3 | 390 ± 15 | 410 ± 12 | 490 ± 53 | 430 ± 41 |
Cells were incubated at 37°C for 4 h, and β-galactosidase activity was measured as described in Materials and Methods. Reported values are means and standard deviations of more than two independent experiments performed in triplicate.
0.5% gluconate (GA) was added after a 2-h incubation.
pYY2 as the control plasmid, pGNTR1 bearing gntR, or pGNTH bearing gntH was cointroduced into YU577.
Porco et al. (26) also found two operators at the regulatory region of gntT, which were proposed to allow formation of a DNA loop structure bridged by a tetrameric GntR, as demonstrated for LacI and GalR (6). In contrast, the two operators for gntKU overlap each other without an intervening sequence, so that no such DNA loop structure can be formed.
Gel shift analysis of GntR-binding elements in the gntKU promoter-operator region.
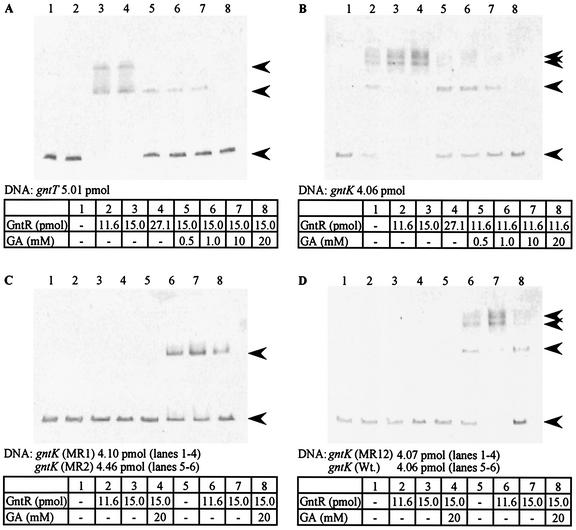
To further confirm the existence of two GntR-binding elements for gntKU, we compared the binding patterns of purified GntR to DNA fragments including the GntR-binding elements of the gntT and gntKU genes. Two and three shifted bands were observed in the presence of GntR in the gntT and gntK DNA fragments, respectively (Fig. 7A, lanes 3 and 4, and Fig. 7B, lanes 2 to 4). These bands are consistent with the presence of at least two GntR-binding sites in each DNA fragment. When gluconate was added, the upper shifted bands disappeared at 0.5 mM but the lower shifted bands were still retained up to 10 mM. Thus, the two shifted bands at the top for the gntK DNA fragment might be due to the conformational difference of the GntR-DNA complex. The upper shifted band(s) and the lower shifted band may be the complex composed of at least two GntR molecules and one DNA fragment and of one GntR and one DNA fragment, respectively, as demonstrated previously for gntT (25).
FIG. 7.
Gel shift analysis of the binding of GntR to the gntT and gntKU operators and effects of mutations in the GntR-binding elements of gntKU on its binding. Gel shift analysis was performed as described in Materials and Methods. DNA fragments from pGNTT-LAC4 (A), pGNTK-LAC (B and D), pGNTK-LACMR1 (C), pGNTK-LACMR2 (C), and pGNTK-LACMR12 (D) were used. pGNTK-LACMR1, pGNTK-LACMR2, and PGNTK-LACMR12 have mutations in the R1, R2, and both R1 and R2 sequences, respectively, of the gntKU promoter-operator region. The shifted bands at the top and in the middle as well as free DNA at the bottom are indicated by arrowheads.
We also attempted gel shift analysis with purified GntH (data not shown). Perhaps surprisingly, no band shifts were observed, even in the presence of 5-ketogluconate. It may require higher concentrations of GntH than used in vitro to see the binding suggested by the repression observed with cloned GntH. GntH was tested up to 1.7 μM, about twice as high as the highest concentration of GntR in Fig. 7. Alternatively, it may be speculated that idonate derived from 5-ketogluconate is the real corepressor of GntH. This speculation could not be proven because of the commercial unavailability of idonate.
Another interesting feature of the gel-shift results was that when 0.4 μM GntR (11.6 pmol) was present, shifted band was detected for gntK but not for gntT. The negative influence of GntR on the transcription of gntKU may thus be stronger than on the transcription of gntT owing to the different affinity of GntR for each operator. The difference would be crucial for the differential expression of gntKU and gntT in the GntI system.
Gel shift analysis was also performed with DNA fragments from pGNTK-LACMR1, pGNTK-LACMR2, and pGNTK-LACMR12 containing mutations in the GntR-binding elements of the gntKU promoter-operator region (Fig. 7C and D). One shifted band, corresponding to the lower shifted band of the wild-type fragment, was observed only in the fragments from pGNTK-LACMR2, whereas no shifted band was observed in the fragments from pGNTK-LACMR1 and pGNTK-LACMR12. These results appear to be consistent with those from the experiments with lacZ operon fusions, as shown in Table 4, and it is likely that the binding of GntR to a single element (the R1 sequence) is capable of decreasing the transcription of the gntKU genes. The complex of GntR with the DNA fragment of the MR2 mutant, however, may be less stable than that with the DNA fragment of the wild type because the derepression ratio by the gluconate was higher in the R2 mutant than in the wild type (Table 4). Both the R1 and R2 regions contribute to repression, judging from the fact that the mutations at the R1 and R2 sequences had distinct influences on the binding of GntR in the gel shift analysis and on the expression of the lacZ operon fusions. Also, the mutation at the R1 sequence disturbed the binding of GntR to the R2, suggesting that GntR has a different affinity for the R1 and R2 sequences and that the binding of GntR to the R1 sequence may lead to the interaction of GntR with the R2. Taken together with the results of the β-galactosidase assay in Table 4, we conclude that the R1 and R2 sequences in the gntKU promoter-operator region are GntR-binding elements and are targeted by GntR and presumably by GntH.
DISCUSSION
A number of genes are involved in gluconate uptake and catabolism and in the conversion between gluconate and idonate in E. coli, as shown in Fig. 1. It is still unknown why E. coli possesses many permeases for gluconate and why two systems of GntI and GntII are present in the organism.
Significant primary sequence homology between GntR and GntH, especially in their HTH motifs for DNA binding, led us to hypothesize that GntH, an activator for the GntII genes, represses the GntI genes via GntR-binding elements. The hypothesis was tested by several experiments including RT-PCR quantification of transcripts in gntR and gntH mutants, β-galactosidase measurements in strains overexpressing the regulators and containing the mutations in the regulators, and gel shift experiments of the GntR regulator to various wild-type and mutant operator sequences. These results strongly suggest that GntH negatively controls the GntI genes, presumably by binding to GntR-binding elements. In contrast to the case for GntR, the GntI genes in the presence of cloned GntH were negligibly induced by gluconate (Table 3; Fig. 5). The expression of gntK, a representative of the GntI genes, was repressed by the presence of 5-ketogluconate, but not gluconate, in the gntR-disrupted strain (Fig. 4), suggesting that 5-ketogluconate or its derivative, but not gluconate, is a corepressor for GntH. Notably, GntH was able to repress the GntI genes even in the presence of gluconate. It is noteworthy that GntH molecules free from the corepressor might bind to the GntR-binding elements with low affinity because the expression activity of gntK-lacZ or gntT-lacZ was very low in strains overexpressing GntH in the medium lacking 5-ketogluconate (Table 3).
These observations and the suggestion described above led us to further examine the expressional control of the GntI genes by GntH along with cell growth in gluconate minimum medium. The results of RT-PCR experiments presented in Fig. 6 suggest that the expression of the gntK gene peaks during the logarithmic phase and is followed by a decrease, consistent with previous reports (34), and that the peak was delayed in the gntH-defective strain. Therefore, it is likely that GntH plays a crucial role in the decrease in expression after the peak where gluconate still remains in the medium. At the same time, GntH may induce the expression of the GntII genes; this is supported by evidence showing that the GntII genes have an expression peak at the late logarithmic or stationary phase (unpublished data). We postulate that GntH is responsible for repression of the GntI genes when GntR was released from the operator by interaction with gluconate, and for induction of the GntII genes. The dual function of GntH may direct the switching of the two systems during cell growth, as proposed in the model shown in Fig. 8.
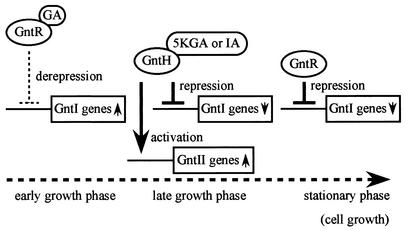
FIG. 8.
Model of expression regulation of the gnt genes. In early growth phase, the GntI genes are induced by release of GntR from their operators via binding of gluconate. As cell growth is proceeding (late logarithmic phase), gluconate (GA) may gradually accumulate and is then converted to 5-ketogluconate (5KGA) or idonate (IA) by IdnO and IdnD. The products 5-ketogluconate and idonate bind to GntH, resulting in activation of the GntII genes and repression of the GntI genes. Finally, gluconate depletion inside the cell leads to the repression of the GntI genes by GntR again. The dotted arrow represents the time course of cell growth.
In the model, gluconate is first imported by gluconate permease, GntP, as demonstrated (15), and interacts with the GntR molecule to induce the GntI system genes. As the intracellular gluconate concentration is increased, gntP expression diminishes (15). Instead, expressed GntT and GntU in the GntI system function mainly in the uptake of gluconate. Imported gluconate is metabolized via the pentose phosphate and Entner-Doudoroff pathways after phosphorylation by GntK. Such an early growth phase event may result in intracellular gluconate pools (late logarithmic phase), which may promote the production of 5-ketogluconate or idonate. This should lead to induction of the GntII genes, including gntH. The production of GntH may repress the GntI genes by forming a complex with 5-ketogluconate or idonate and further induce GntII genes, whereas GntR molecules are being released from the operators in the state of interacting with gluconate. Consequently, a metabolic transition from the GntI to the GntII system is smoothly achieved. Such a transition would be important for cells to avoid overaccumulation of gluconate inside cells. The GntII system would thus be involved in conversion of gluconate to 5-ketogluconate or idonate. Although high-affinity gluconate permease, GntW, in GntII is also induced, it is assumed to be physiologically not functional in this situation for the following reasons. (i) GntW is classified as a high-affinity permease, but the affinity for gluconate is 10-fold lower than that of GntT (24). (ii) Its expression may be low because of only readthrough transcription from idnDp. Another possibility is that idonate inhibits transporters such as GntW from accumulating gluconate, but no evidence is available for this notion. Finally, gluconate levels inside cells drop and GntR molecules free of gluconate repress the GntI genes again. The model implies that the physiological function of the GntI system may be the utilization of gluconate at the early growth phase, and uptake of gluconate in excess of the metabolizable amount would be prevented by GntH, with extra gluconate inside cells being converted to 5-ketogluconate or idonate by the GntII system.
Even though the the GntII system may function principally in the uptake and catabolism of idonate, as demonstrated by Bausch et al. (3), the negative regulation of the GntI genes by GntH followed by the expression of GntII genes appears to be important in the rational metabolism of gluconate. Additionally, in situations when the GntI system is defective, GntII performs the task of gluconate utilization, as demonstrated by genetic studies (2, 39; Hung et al., Bacteriol. Proc., 1970). The expressional control of the GntII genes by GntR has also recently been found (unpublished data). We thus assume the existence of a mutual regulation between the GntI and GntII systems, where GntR and GntH are mainly involved. Further studies are necessary for the elucidation of the physiological significance of the cross-regulation through the control of GntH as repressor.
Acknowledgments
We thank O. Adachi and H. Toyama for helpful discussions.
This work was partially supported by grants from the Ministry of Education, Science and Culture of Japan. H.I. was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Bächi, B., and H. L. Kornberg. 1975. Genes involved in the uptake and catabolism of gluconate by Escherichia coli. J. Gen. Microbiol. 90:321-335. [DOI] [PubMed] [Google Scholar]
- 3.Bausch, C., N. Peekhaus, C. Utz, T. Blais, E. Murray, T. Lowary, and T. Conway. 1998. Sequence analysis of the GntII (subsidiary) system for gluconate metabolism reveals a novel pathway for L-idonic acid catabolism in Escherichia coli. J. Bacteriol. 180:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogosian, G., and R. Somerville. 1983. Trp repressor protein is capable of intruding into other amino acid biosynthetic systems. Mol. Gen. Genet. 191:51-58. [DOI] [PubMed] [Google Scholar]
- 5.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived form the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choy, H., and S. Adhya. 1996. Negative control, p. 1287-1299. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 7.Degryse, E. 1995. Evaluation of Escherichia coli recBC sbcBC mutants for cloning by recombination in vivo. J. Biotechnol. 15:181-187 [DOI] [PubMed] [Google Scholar]
- 8.Faik, P., and H. L. Kornberg. 1973. Isolation and properties of E. coli mutants affected in gluconate uptake. FEBS Lett. 32:260-264. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto-Gotoh, T., K. Yasojima, and A. Tsujimura. 1995. Plasmids with kanamycin-resistance gene for site-directed mutagenesis using the oligodeoxyribonucleotide-directed dual amber method. Gene 167:333-334. [DOI] [PubMed] [Google Scholar]
- 10.Istúriz, T., E. Palmero, and J. Vitelli-Flores. 1986. Mutations affecting gluconate catabolism in Escherichia coli. Genetic mapping of the locus for the thermosensitive gluconokinase. J. Gen. Microbiol. 132:3209-3219. [DOI] [PubMed] [Google Scholar]
- 11.Izu, H., O. Adachi, and M. Yamada. 1996. Purification and characterization of the Escherichia coli thermoresistant gluconokinase encoded by the gntK gene. FEBS Lett. 394:14-16. [DOI] [PubMed] [Google Scholar]
- 12.Izu, H., O. Adachi, and M. Yamada. 1997. Gene organization and transcriptional regulation of the gntRKU operon involved in gluconate uptake and catabolism of Escherichia coli. J. Mol. Biol. 267:778-793. [DOI] [PubMed] [Google Scholar]
- 13.Izu, H., T. Kawai, Y. Yamada, H. Aoshima, O. Adachi, and M. Yamada. 1997. Characterization of the gntT gene encoding a high-affinity gluconate permease in Escherichia coli. Gene 199:203-210. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, D. I., and R. L. Somerville. 1983. Evidence that repression mechanisms can exert control over the thr, leu, and ilv operons of Escherichia coli K-12. J. Bacteriol. 155:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemm, P., S. Tong, H. Nielsen, and T. Conway. 1996. The gntP gene of Escherichia coli involved in gluconate uptake. J. Bacteriol. 178:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, M., G. Chang, N. C. Horton, M. A. Kercher, H. C. Pace, M. A. Schumacher, R. G. Brennan, and P. Lu. 1996. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science 271:1247-1254. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Miwa, Y., and Y. Fujita. 1988. Purification and characterization of a repressor for the Bacillus subtilis gnt operon. J. Biol. Chem. 263:13252-13257. [PubMed] [Google Scholar]
- 21.Nagel de Zwaig, R., N. Zwaig, T. Istúriz, and R. S. Sánchez. 1973. Mutations affecting gluconate metabolism in Escherichia coli. J. Bacteriol. 114:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1995. Construction of a series of pACYC-derived plasmid vectors. Gene 162:157-158. [DOI] [PubMed] [Google Scholar]
- 23.Nitta, T., H. Nagamitsu, M. Murata, H. Izu, and M. Yamada. 2000. Function of the σE regulon in dead-cell lysis in stationary-phase Escherichia coli. J. Bacteriol. 182:5231-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peekhaus, N., S. Tong, E. Murray, J. R. Reizer, M. Saier, and T. Conway. 1997. Characterization of a novel transporter family that includes multiple Escherichia coli gluconate transporters and their homologues. FEMS Microbiol. Lett. 147:233-238. [DOI] [PubMed] [Google Scholar]
- 25.Peekhaus, N., and T. Conway. 1998. Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J. Bacteriol. 180:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porco, A., N. Peekhaus, C. Bausch, S. Tong, T. Istúriz, and T. Conway. 1997. Molecular genetic characterization of the Escherichia coli gntT gene of GntI, the main system for gluconate metabolism. J. Bacteriol. 179:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptashne, M., A. D. Johnson, and C. O. Pabo. 1982. A genetic switch in bacterial virus. Sci. Am. 247:128-130. [DOI] [PubMed] [Google Scholar]
- 28.Reidl, J., K. Römisch, M. Ehrmann, and W. Boos. 1989. MalI, a novel protein involved in regulation of the maltose system of Escherichia coli, is highly homologous to the repressor proteins GalR, CytR, and LacI. J. Bacteriol. 171:4888-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider, K., and C. F. Beck. 1986. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene 42:37-48. [DOI] [PubMed] [Google Scholar]
- 32.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 34.Tong, S., A. Porco, T. Istúriz, and T. Conway. 1996. Cloning and molecular characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J. Bacteriol. 178:3260-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 36.Weickert, M. J., and S. Adhya. 1992. A family of bacterial regulators homologous to Gal and Lac repressors. J. Biol. Chem. 267:15869-15874. [PubMed] [Google Scholar]
- 37.Yamada, Y., M. Yamada, and A. Nakazawa. 1995. A ColE-encoded gene directs entry exclusion of plasmid. J. Bacteriol. 177:6064-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada, M., K. Kawai, and H. Izu. 1996. Analysis of the Escherichia coli gntT and gntU genes and comparison of the products with their homologues. Biosci. Biotechnol. Biochem. 60:1548-1550. [DOI] [PubMed] [Google Scholar]
- 39.Zwaig, N., R. Nagel de Zwaig, T. Istúriz, and M. Wecksler. 1973. Regulatory mutations affecting the gluconate system in Escherichia coli. J. Bacteriol. 114:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]