Abstract
Dendritic cell (DC) defects are an important component of immunosuppression in cancer. Here, we assessed whether cancer could affect circulating DC populations and its correlation with tumor progression. The blood DC compartment was evaluated in 136 patients with breast cancer, prostate cancer, and malignant glioma. Phenotypic, quantitative, and functional analyses were performed at various stages of disease. Patients had significantly fewer circulating myeloid (CD11c+) and plasmacytoid (CD123+) DC, and a concurrent accumulation of CD11c-CD123- immature cells that expressed high levels of HLA-DR+ immature cells (DR+IC). Although DR+IC exhibited a limited expression of markers ascribed to mature hematopoietic lineages, expression of HLA-DR, CD40, and CD86 suggested a role as antigen-presenting cells. Nevertheless, DR+IC had reduced capacity to capture antigens and elicited poor proliferation and interferon-γ secretion by T-lymphocytes. Importantly, increased numbers of DR+IC correlated with disease status. Patients with metastatic breast cancer showed a larger number of DR+IC in the circulation than patients with local/nodal disease. Similarly, in patients with fully resected glioma, the proportion of DR+IC in the blood increased when evaluation indicated tumor recurrence. Reduction of blood DC correlating with accumulation of a population of immature cells with poor immunologic function may be associated with increased immunodeficiency observed in cancer.
Keywords: Solid tumors, dendritic cell subsets, immune dysfunction, immature antigen-presenting cell, breast cancer
Introduction
Generation of anticancer immunity requires antigen-presenting cells (APC) that recognize tumors, and process and present antigens to T-lymphocytes, which subsequently target malignant cells [1]. Dendritic cells (DC) are the key APC population for initiating and coordinating antitumor responses [1,2]. Despite the potential for tumor control, the immune system often fails. Numerous mechanisms, including deletion of tumor-specific cytotoxic T-lymphocytes [3] and recruitment of regulatory T-lymphocytes [4] and inhibitory cell types [5], have been implicated in this failure. It has also been suggested that a tumor's incapacity to recruit DC significantly contributes to immune evasion [6–8].
More recently, the suppressive effects of tumors on DC maturation and differentiation have been reported to play a crucial role in the systemic failure of the host to mount an effective antitumor response [9–11]. Several tumor-derived factors [vascular endothelial growth factor (VEGF), interleukin (IL) 6, macrophage colony-stimulating factor (M-CSF), gangliosides, prostanoids, and polyamines] affect DC differentiation from progenitors, both in vitro and in vivo [12–18]. This is consistent with the fact that reduced DC counts are frequently found in the peripheral blood of cancer patients [13,16,19,20]. A review of the effects of tumor-derived factors on DC was recently compiled [21].
In spite of this evidence, only a few studies have directly assessed the functional status of DC populations circulating in vivo in patients with cancer [8,13,16,22]. Circulating blood DC can be identified as mononuclear cells expressing major histocompatibility complex (MHC) II molecules (HLA-DR) but lacking common lineage markers (Lin) such as CD3, CD14, CD19, CD20, CD56, and CD34 [23]. This blood DC compartment (Lin-HLA-DR+ cells) includes two different subsets that are discernible into myeloid or plasmacytoid DC based on their reciprocal expression of CD11c (α-integrin) and CD123 (IL-3 receptor α) [24].
The aim of this study was to assess the blood DC compartment in patients with cancer to ascertain whether alterations in DC subset distribution could correlate with tumor progression. For this purpose, we analyzed a large cohort of patients with different types of cancer (including breast cancer, prostate cancer, and malignant glioma) at various stages of disease. A 12-week chronological monitoring was also performed in patients with malignant glioma to relate any changes in the composition of the blood DC compartment with tumor recurrence in individual patients. Our results indicate that, in contrast to healthy donors, patients with cancer demonstrate a marked alteration in the distribution of myeloid (CD11c+DC) and plasmacytoid (CD123+DC) subtypes with a significant accumulation of CD11c-CD123- immature cells. Notably, accumulation of these immature cells with poor APC function correlates with disease status and tumor growth. These findings may prove to be relevant in understanding DC pathophysiology in cancer progression.
Materials and Methods
Patients and Donors
A total of 120 female patients (43–80 years of age) with histologically confirmed breast adenocarcinoma were enrolled in the study. Of these, 96 patients presented with early disease, either local (stage I [T1N0M0]: tumor < 2 cm [T1], no lymph node involvement [N0], and no distant metastases [M0]; n = 37) or nodal (stage II [T2N1M0]: tumor between 2 and 5 cm [T2], ipsilateral lymph node involvement [N0–N1], and no distant metastases [M0]; n = 59), and 24 patients presented with advanced metastatic disease (stage IV [TNM1]: any tumor size/node involvement with distant metastases to other organs [M1]). All patients were newly diagnosed, except for those with advanced disease who presented with recurrence after a disease-free interval and had no prior therapy for at least 6 months. Staging was performed in accordance with International Union Against Cancer: TNM Classification of Malignant Tumors [25]. In addition, 10 male patients (68–80 years of age) with histologically confirmed prostate cancer were enrolled in the study. All patients had hormone-refractory tumors with elevated and rising prostate-specific antigen (PSA) levels on at least two consecutive occasions, ranging from 11 to 890 ng/ml, in the presence of castrate serum testosterone levels. Although the International Union Against Cancer system does not have a clear category for the first seven patients who had consistently rising serum PSA values (all > 4 ng/ml) in the absence of bone or soft tissue metastases, the conditions of the remaining three patients were classified as stage IV ([TNM1]: any tumor size/node involvement with distant metastases to other organs [M1]). Of these, one patient had multiple soft tissue secondary deposits and two had bone metastases. Three patients had some of their soft tissue tumor materials harvested as a source of antigens in a vaccine study. In addition, a total of six patients (three females and three males, 26–68 years of age) with newly diagnosed supratentorial high-grade malignant glioma (grade IV) were enrolled in the study. Grading was performed in accordance with the World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Nervous System [26]. For the follow-up study, glioma patients who were initially treated and underwent complete macroscopic resection were monitored. Blood samples were collected starting at 4 weeks and then at 6, 8, and 12 (24) weeks after tumor resection. To assess tumor recurrence, each patient underwent computed tomography (CT) and/or magnetic resonance imaging (MRI) of the brain and comprehensive clinical examination at 4, 6, 8, and 12 (24) weeks postsurgery. According to uniform clinical protocols, two patients received symptomatic management with phenytoin and diazepam during the follow-up period. One patient received temozolamide and two underwent additional surgical excision of tumor recurrence after the follow-up period. Finally, 20 healthy donors (12 females and 8 males, 22–73 years of age) volunteered for the study and served as controls. For phenotypic and cell sorting experiments, 50 or 350 ml of venous blood was collected into heparinized tubes and processed immediately. The research ethics committees of both clinical (Wesley Medical Centre, Royal Brisbane and Women Hospital and Mater Misericordiae Hospital) and scientific (Queensland Institute of Medical Research) institutions approved the study protocols.
Monoclonal Antibodies, Reagents, and Cytokines
The following monoclonal antibodies were used in this study: CD3, CD14, CD19, CD20, CD56, CD34, CD2, CD4, CD33, CD7, CD11c, HLA-DR, CD15, CD127, CD123, CD80, CD86, DC-SIGN, and IgG1, IgG2a, and IgG2b isotype controls from BD Pharmingen (BD Biosciences, San Jose, CA); CD62L, CD4, HLA-DR, CD40, CD83, CD19, and IgG1 isotype control from Beckman Coulter (Fullerton, CA); and BDCA-2, BDCA-3, BDCA-4, and CD1c from Miltenyi Biotech (Bergisch Gladbach, Germany). CD10, CD79a, CD11b, CD13, myeloperoxidase (MPO), CD41, CD61, CD235a, and CD71 (all from BD Biosciences) and SIgλ, SIgκ, and Cµ (all from DakoCytomation, Fort Collins, CO) were kindly provided by Dr. Greg Bryson (Royal Brisbane Hospital, Brisbane, Australia). Fluorescein isothiocyanate (FITC)-, PE-, biotin-, APC-, or PE-Cy5-conjugated antibodies were used. For indirect staining with biotinylated antibodies, streptavidin APC (BD Biosciences) was used. For exclusion of dead cells, samples were stained with 7-amino-actinomycin D (7-AAD; BD Biosciences). Tetanus toxoid (TT) obtained from CSL (Melbourne, Victoria, Australia) was conjugated with FITC (FITC-TT) in 0.5 M bicarbonate buffer (pH 9.5) and dialyzed in phosphate-buffered saline (PBS) for 48 hours before use. Dialysis membranes (membra cell; Polylabo, Strasbourg, France) with a Mw cutoff of under 10,000 to 14,000 were used. Sheep red blood cells were obtained from Equicell (Melbourne, Victoria, Australia). Complete media included RPMI 1640 supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 µg/ml), l-glutamine (2 mM), HEPES (25 mM), and nonessential amino acids (all purchased from Gibco Life Technologies, Gaithersburg, MD). The combination of proinflammatory cytokines [27] consisted of IL-1β (10 ng/ml), IL-6 (10 ng/ml), and TNF-α (10 ng/ml) (all obtained from R&D Systems, Minneapolis, MN) plus prostaglandin E2 (PGE2; 1 µg/ml; Sigma, St. Louis, MO). The CpG oligodeoxynucleotide 2216 (CpG ODN; 3 µg/ml) [28] was acquired from Geneworks (Melbourne, Victoria, Australia). Lipopolysaccharide (LPS; 50 ng/ml) and double-stranded RNA (poly I:C; 50 µg/ml) [29] were purchased from Sigma.
Cell Purification and Microscopy
DC and DR+IC were purified from peripheral blood mononuclear cells (PBMC). Briefly, PBMC from patients with breast cancer were stained with lineage mixture (CD3, CD14, CD19, CD20 and CD56) and CD34 (all FITC), HLA-DR (PE), and CD11c (APC), and then indirectly stained with biotinylated CD123 followed by streptavidin (APC). CD34 was added to the lineage mixture (Lin) to exclude circulating hematopoietic stem cells. 7-AAD was used as a viability indicator. Viable DC (Lin-HLA-DR+CD11c+CD123+) and DR+IC (Lin-HLA-DR+CD11c-CD123-) were sorted in parallel (99% purity) using MoFlo Sorter (DakoCytomation) and resuspended in complete medium. For light microscopy (LM), cytospins were made by preparing 2 x 104 to 3 x 104 sorted cells onto a glass slide. Cytospins were air-dried and stained using May-Grunwald-Giemsa. For electron microscopy (EM), 2 x 104 to 3 x 104 sorted cells were fixed in 3% glutaraldehyde and 4% paraformaldehyde plus 0.8% calcium chloride before being embedded in epoxy resin. Ultrathin sections were cut and stained for EM.
Phenotype, Antigen Uptake, and Cell Counts
Four-color flow cytometry was used to analyze the phenotype and antigen uptake of DC and DR+IC in PBMC. For phenotypic analyses, cells were stained with the lineage mixture (FITC), HLA-DR (PE-Cy5), CD11c, CD123 (APC), and antigen of interest (PE). For phenotypic maturation, PBMC were cultured (107 cells/ml) in six-well plates for 18 to 36 hours in complete medium in the presence of a combination of inflammatory cytokines (IL-1β, IL-6, and TNF-α plus PGE2; Cytokine Cocktail [CC]), LPS, poly I:C, or CpG ODN. Cells were stained with the lineage mixture (FITC), HLA-DR (PE-Cy5), CD11c, and CD123 (APC) andCD40, CD80, CD83, and CD86 (PE). Doses and incubation times were optimized in preliminary experiments. For antigen uptake, PBMC were analyzed fresh or after activation with poly I:C or CC. Following activation, cells were resuspended in complete medium for incubation (60 minutes) with FITC-TT (0.5 mg/ml) at either 4°C or 37°C. Cells were washed four times in cold PBS and then stained. Antigen capture was calculated as the difference in mean fluorescence intensity (ΔMFI) between the test (37°C) and the control (4°C). In all experiments, 5 x 105 to 10 x 105 events were collected within the mononuclear cell gate. Where indicated, absolute counts (106 l-1) were calculated from the number of PBMC estimated by the automated cell counter (Advia 120, Hematology System or Technicon H.3 RTX; Bayer, Tarrytown, NY) multiplied by the percentage of DR+IC, CD11c+DC, and CD123+DC, as determined by fluorescence-activated cell sorter (FACS) analysis. Data were acquired on a FACS Calibur flow cytometer and analyzed using CellQuest 3.1 (BD Biosciences), FloJo (TreeStar, San Carlos, CA), or Summit (Cytomation) software.
Mixed Lymphocyte Reaction (MLR) and Interferon (IFN) γ Secretion
The capacity of DC and DR+IC to stimulate allogeneic T-cell proliferation was tested in MLR. Allogeneic T-cells were obtained by rosetting PBMC with neuraminidase-treated sheep red blood cells (≥ 90% CD3+ cells). DC and DR+IC were purified from patients with breast cancer using MoFlo Sorter, as described above. Varying numbers of DC and DR+IC were cultured (37°C, 5% CO2) in triplicate with 105 allogeneic T-cells for 5 days in complete medium. Sixteen hours prior to harvesting, 1 µCi/well [3H]thymidine was added to each well. [3H]thymidine incorporation was measured in a β-scintillation counter (MicroBeta Trilux Scintillation Counter; Wallac, Turku, Finland). For measurements of IFN-γ secretion, after 5 days in culture, supernatants were collected, pooled, and assayed using IFN-γ ELISA kit (Mabtech, Stockholm, Sweden), according to the manufacturer's instructions.
Statistical Analysis
Comparisons of samples for the establishment of statistical significance were determined by two-tailed Student's t test. Results were considered to be statistically significant when P < .05.
Results
Blood DC Subset Composition in Cancer
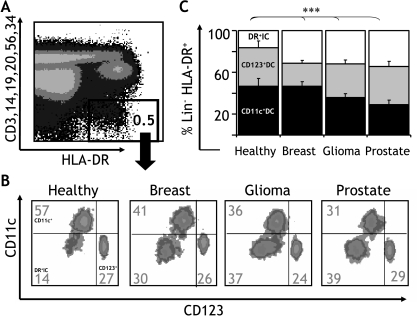
The blood DC compartment (Lin-HLA-DR+ population) (Figure 1A) includes two different DC subsets that can be distinguished into myeloid and plasmacytoid lineages based on their reciprocal expression of CD11c and CD123 antigens [24]. Given that dissimilar alterations in the frequency of these subsets have been reported in patients with cancer [16,20], we set out to carefully analyze the CD11c+DC and CD123+DC subset compositions of the blood DC compartment in a cohort of 34 patients with different types of solid tumors. We assessed patients with breast cancer (stage II; n = 18), malignant glioma (grade IV; n = 6), and hormone-refractory prostate cancer (n = 10). In addition, 11 age-matched healthy donors served as controls (Figure 1B). In healthy donors, the proportion of CD11c+DC (46.6 ± 7.4%) and CD123+DC (36.9 ± 6.6%) accounted for 83.5 ± 1.7% of the total blood DC compartment. In contrast, in patients with breast cancer, both DC subtypes accounted for 67.9 ± 3.1% (CD11c+DC: 46.0 ± 4.2% and CD123+DC: 21.9 ± 2.8%); in malignant glioma, both DC subtypes accounted for 67.9 ± 3.6% (CD11c+DC: 35.8 ± 3.8% and CD123+DC: 32.1 ± 3.9%); and in prostate cancer, both DC subtypes accounted for 65.7 ± 3.9% (CD11c+DC: 29.0 ± 4.5% and CD123+DC: 36.7 ± 5.0%) of the total blood DC compartment. As shown in Figure 1B, this finding points to the existence of a significant population of CD11c-CD123- cells expressing high levels of HLA-DR and lacking markers for mature hematopoietic lineages (HLA-DR+ immature cells, DR+IC). Interestingly, although this population represented only 16.4 ± 1.7% of the blood DC compartment in healthy donors, it represented a significantly larger proportion in patients with breast cancer (31.2 ± 3.1%), glioma (32.0 ± 3.6%), and prostate cancer (34.2 ± 3.9%; Figure 1C). These results were further confirmed when a second cohort of 35 patients with breast cancer was assessed (CD11c+DC: 44.0 ± 3.6%, CD123+DC: 24.5 ± 2.9%, and DR+IC: 31.4 ± 4.6%). Interestingly, when these patients were grouped according to stage of disease (stage I, n = 17; stage II, n = 10; and stage IV, n = 8), analysis revealed that the DR+IC population represented 18.1 ± 4.3% of the blood DC compartment in patients with local disease (stage I), 28.3 ± 8.3% in patients with nodal disease (stage II), and 63.5 ± 7.0% in patients with metastatic disease (stage IV), suggesting an association with disease extension (Figure 2A).
Figure 1.
DC subset composition in cancer. (A) The blood DC compartment (Lin-HLA-DR+ cells) in PBMC can be further separated (B) based on the expression of CD11c (y axis) and CD123 (x axis) into myeloid (CD11c+DC) and plasmacytoid (CD123+DC) subtypes and a minor population of CD11c-CD123- cells (DR+IC). In patients with cancer, this population can be significantly increased. (C) The composition of the blood DC compartment was analyzed to determine the proportion of CD11c+DC (black), CD123+DC (grey), and DR+IC (clear) in a cohort of healthy donors (n = 11) and patients with cancer, including breast cancer (stage II, n = 18), glioma (grade IV, n = 6), and prostate cancer (n = 10). Error bars indicate SEM. Statistically significant differences are indicated (***P < .001).
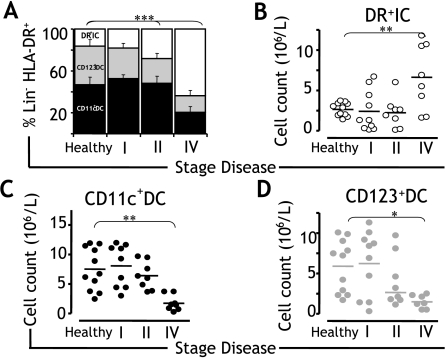
Figure 2.
Correlation with disease status. (A) In a cohort of 35 patients with breast cancer (stage I, n = 17; stage II, n = 10; and stage IV, n = 8) and 11 healthy donors, the composition of the blood DC compartment was analyzed to determine the proportion of CD11c+DC (black), CD123+DC (grey), and DR+IC (clear) according to the stage of disease. (B–D) In a cohort of 28 patients with breast cancer (stage I, n = 10; stage II, n = 10; and stage IV, n = 8) and 11 healthy donors, absolute numbers of (B) DR+IC, (C) CD11c+DC, and (D) CD123+DC were quantified from the number of PBMC (106 l-1) in the blood, determined by a hematology cell counter, multiplied by the percentage of cells determined by FACS, and plotted according to the stage of disease. Means are shown as horizontal lines. Statistically significant differences are indicated (*P < .05; **P < .01; ***P < .001).
Correlation with Tumor Burden
Given that our data demonstrated a larger increment in the proportion of DR+IC in patients with advanced breast cancer, we set out to assess whether numeric changes could correlate with status of disease. To test this, we estimated the absolute numbers of circulating DR+IC in the peripheral blood of 28 patients with breast cancer, and we subsequently analyzed their distribution according to the stage of disease (stage I, n = 10; stage II, n = 10; and stage IV, n = 8). We found that although the absolute numbers of DR+IC were comparable in healthy donors and early-disease patients, DR+IC counts were significantly increased in patients with advanced disease (Figure 2B). This is in keeping with the alteration in the percentage of DR+IC estimated as a proportion of the blood DC compartment (healthy: 16.4 ± 1.7%; stage I: 16.4 ± 6.1%; stage II: 22.0 ± 9.2%; and stage IV: 65.7 ± 6.4%). In contrast to DR+IC, the absolute numbers of CD11c+DC and CD123+DC in patients with advanced disease were significantly reduced when compared to those of healthy donors and early-disease patients (Figure 2, C and D), confirming that accumulation of DR+IC coincides with a reduction in the number of competent CD11c+DC and CD123+DC.
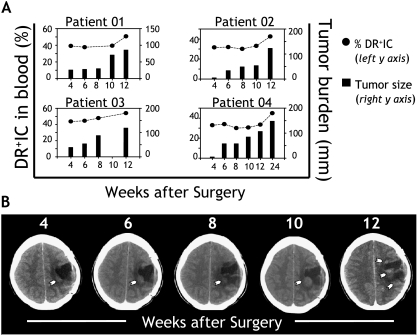
We also assessed whether accumulation of DR+IC could correlate with tumor burden in individual patients. For this purpose, tumor progression was assessed in a cohort of six patients with completely resected high-grade malignant glioma. Given that systemic metastases of primary cerebral malignant gliomas are very rare, accounting for less than 0.5% of cases [30], accurate estimation of tumor burden in individual patients is possible by carefully monitoring disease status within the central nervous system. Patients were monitored for clinical and radiological signs of tumor recurrence (CT and MRI) at 4, 6, 8, and 12 (24) weeks after surgical excision (Figure 3, A and B). Tumor burden was estimated as the sum of the maximum perpendicular diameters (x, y, z) registered for each lesion. Each lesion was monitored at the same anatomic level throughout the follow-up period. In parallel, the frequency of DR+IC as a percentage of the blood DC compartment was estimated. Repeated measurements performed on eight samples from six patients demonstrated a small variation (< 16.2%, with an average of 9.2%) on these estimates. Cumulatively, our data demonstrated that the increase in DR+IC in the circulation correlated with recurrence and enlargement of tumor lesions on follow-up. Indeed, when clinical and imaging evaluation demonstrated tumor progression, the proportion of DR+IC increased in the peripheral blood (Figure 3, A and B).
Figure 3.
Correlation with tumor burden. (A) The percentage of DR+IC within the blood DC compartment (y axis, left) was analyzed in a group of patients with fully resected malignant glioma starting at 4 weeks and then at 6, 8, and 12 (24) weeks after surgery (x axis). Each patient underwent CT and/or MRI of the brain and comprehensive clinical examination to determine tumor progression. Tumor burden (y axis, right) was estimated as the sum of the maximum perpendicular diameters (x, y, z) registered for any given lesion detected at the time of evaluation. When multiple lesions were present, sizes were added. Results shown correspond to four patients, representative of six patients who were assessed. (B) Longitudinal MRI follow-up corresponds to “patient 02” (shown above), indicating tumor progression. Arrows point to recurring lesion(s), and numbers indicate time points of assessment (weeks postsurgery). Means are shown as horizontal lines. Statistically significant differences are indicated (*P < .05).
Morphology and Costimulatory Phenotype
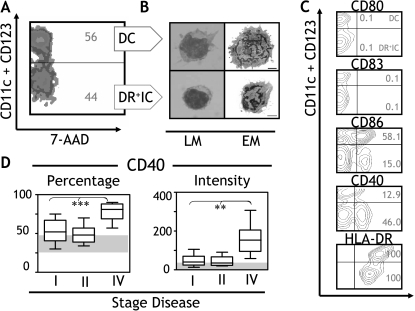
DC are heterogeneous and encompass populations with different morphologies, phenotypes, and properties. Given that DR+IC represented a significant proportion of the blood DC compartment in cancer patients, we analyzed their morphology and costimulatory phenotype by carrying out comparisons with their DC counterparts. PBMC were isolated from patients with breast cancer (stage II, n = 3), and Lin-HLA-DR+ cells (Figure 1A) were separated into DC (CD11c+CD123+) and DR+IC (CD11c-CD123-) based on the expression of CD11c and CD123 (Figure 4A). Each population was examined by LM (May-Grunwald-Giemsa staining) and EM. Freshly isolated DC were relatively large cells (7–8 µm diameter) featuring an irregular surface with abundant cytoplasm and veiled projections (Figure 4B; DC and LM). At an ultrastructural level, several cytoplasmic organelles (i.e., mitochondria and smooth endoplasmic reticulum) and ribosomes were evident. The nucleus was lobulated or indented and mainly euchromatic (Figure 4B; DC and EM). In contrast, DR+IC were smaller cells (4–5 µm diameter) showing a relatively irregular surface with some projections and a large, rounded heterochromatic nucleus (Figure 4B; DR+IC and LM). The volume of the cytoplasm compared with that of the nucleus was small. The cytoplasm had fewer inclusions and organelles than DC. The relative absence of organelles, the scanty cytoplasm, and the largely condensed chromatin in the nucleus suggested a more immature cell compared to DC (Figure 4B; DR+IC and EM). Given that the expression of HLA-DR and other costimulatory molecules such as CD40, CD80, CD83, or CD86 is known to reflect the maturation status of APC, we assessed their expression in DR+IC. As shown in Figure 4C, these cells revealed a low expression of CD80 (< 1%) and CD83 (< 1%), a moderate expression of CD86 (15%), and a high expression of CD40 (50%) and HLA-DR (100%). Notably, these data are consistent with an evaluation of CD40 expression in the blood DC compartment (encompassing DC and DR+IC) of 41 patients with breast cancer (stage I, n = 20; stage II, n = 12; and stage IV, n = 9). Patients with advanced disease had significantly increased CD40 expression (intensity and percentage; Figure 4D) and concomitantly the largest proportion of circulating DR+IC (Figure 2A).
Figure 4.
Morphology and phenotype. (A) The blood DC compartment was separated into DC (CD11c+CD123+) or DR+IC (CD11c-CD123-) based on the expression of CD11c and CD123 (y axis). Viable cells (7-AAD-; x axis) were sort-purified. (B) DC and DR+IC were analyzed by LM (left panel) and EM (right panel). Size bars represent 1 µm. Micrographs shown are representative of three breast cancer patients (stage II) who were assessed. (C) PBMC purified from patients with cancer were separated into DC or DR+IC (y axis), and the percentage of cells positive (x axis) for CD80, CD86, CD83 CD40, and HLA-DR was determined. Results shown are representative of 17 breast cancer patients who were assessed (stage II, n = 10; stage IV, n = 7). (D) In a cohort of 41 patients with breast cancer (stage I, n = 20; stage II, n = 12; and stage IV, n = 9), the percentage and intensity of expression of CD40 within the blood DC compartment (encompassing DC and DR+IC) were analyzed and plotted. The normal reference range from healthy donors (n = 12) is indicated as shaded areas. Means are shown as horizontal lines, and error bars indicate SEM. Statistically significant differences are indicated (**P < .01; ***P < .001).
Ability to Capture and Present Antigens to T-cells
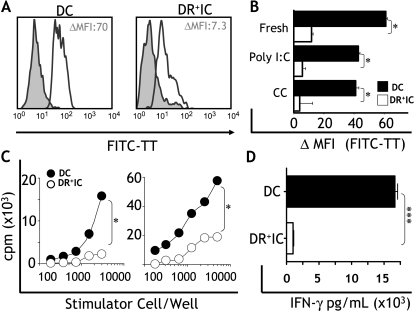
The expression of HLA-DR and costimulatory molecules indicated a potential role of DR+IC as APC. To test this possibility, we set out to assess their capacity to capture and present antigen, and to induce T-cell activation. First, we studied the ability of DR+IC isolated from patients with breast cancer (stage II, n = 5) to capture a soluble antigen (FITC-TT). In fresh samples, DC had a higher capacity to take up antigens (ΔMFI: 61.2 ± 8.7) compared to DR+IC (ΔMFI: 9.4 ± 2.2) (P < .05; Figure 5A). Given that DC are known to downregulate their capacity to capture antigens on activation [31], we also assessed whether DR+IC could modulate their FITC-TT uptake in response to exogenous stimuli. Two classes of inflammatory mediators known to activate DC [29] were tested: 1) a combination of inflammatory cytokines (TNF-α, IL-1β, IL-6, and PGE2; CC) [27] and 2) synthetic double-stranded RNA (poly I:C). We found that stimulated DC exhibited a small (not significant) reduction in antigen capture compared to unstimulated cells (Figure 5B). As with DC, DR+IC downregulated this capacity (Figure 5B), suggesting a certain level of functional modulation. We then assessed the ability of DR+IC to present antigens and to induce the proliferation of alloreactive T-cells. We found that DR+IC purified from patients with breast cancer (stage II, n = 5) induced a significantly reduced (P < .05) proliferation of allogeneic T-cells in MLR compared to DC (Figure 5C). Finally, we examined the capacity of DR+IC to induce IFN-γ secretion in allogeneic T-cells. We found that DR+IC purified from cancer patients (stage II, n = 5) were poor stimulators of IFN-γ secretion. Conversely, DC induced significantly (P < .001) higher levels of IFN-γ secretion by T-cells (Figure 5D).
Figure 5.
Antigen uptake and allostimulatory capacity. (A) Antigen uptake (FITC-TT, x axis) by DC and DR+IC from patients with cancer. Filled histograms indicate uptake at 4°C (control), and empty histograms represent uptake at 37°C (test). Numbers indicate ΔMFI between test and control. (B) FITC-TT uptake by DC and DR+IC was analyzed ex vivo (fresh) or following maturation with viral double-stranded RNA (poly I:C) or a combination of inflammatory cytokines (CC; TNF-α, IL-1β, IL-6, and PGE2). Uptake is expressed as ΔMFI (x axis) between test and control for each condition. Data shown are representative of five breast cancer patients who were assessed (stage II). (C) Increasing numbers of DC and DR+IC purified from cancer patients were tested for their capacity to stimulate the proliferation of allogeneic T-cells. Results shown correspond to two breast cancer patients (stage II) and are representative of five patients who were examined. (D) DC and DR+IC purified from five cancer patients (stage II) were cultured with alloreactive T-cells and, after 5 days in culture, supernatants were analyzed for IFN-γ content by ELISA. Error bars correspond to SEM. Statistically significant differences between DC and DR+IC are indicated (*P < .05; ***P < .001).
Response to Inflammatory Factors
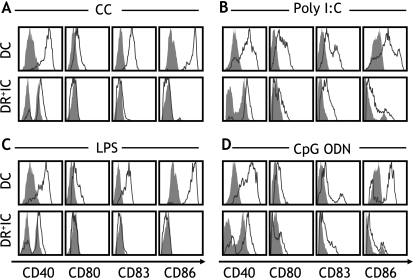
Another feature of APC is their capacity to mature and respond to activation signals. As with antigen uptake, this ability is regulated by different types of inflammatory mediators [32]. Therefore, we assessed the phenotypic maturation of DR+IC and DC in response to a range of 1) proinflammatory mediators (CC) and 2) pathogen-derived factors, including ligands for toll-like receptors 4 (LPS), 3 (poly I:C), and 9 (bacterial oligodeoxynucleotide; CpG ODN)—all known to induce maturation of APC [27,29,32]. We analyzed 16 patients with breast cancer (stage II, n = 9; stage IV, n = 7) and found that freshly isolated DC were phenotypically immature, expressing low levels of CD40, CD80, CD83, and CD86 (Figure 6). Similarly, DR+IC expressed low levels of CD80, CD83, and CD86, although, as noted previously, expression of CD40 was elevated in 40% to 50% of cells (Figure 6). Interestingly, DR+IC responded weakly to inflammatory mediators and pathogen-derived products. Expression of HLA-DR (Table 1) and CD40 (Figure 6, A–D) was increased, and a modest upregulation of CD83 and CD86 expression was noted in response to CpG ODN (Figure 6D) and occasionally to poly I:C (Figure 6B). In contrast, DC responded vigorously to all proinflammatory and pathogen-derived factors (Figure 6 and Table 1), upregulating the expression of all costimulatory and activation markers (CD40, CD80, CD83, CD86, and HLA-DR).
Figure 6.
Response to proinflammatory and pathogen-derived factors. Phenotypic maturation was evaluated by assessing the expression of CD40, CD80, CD83, and CD86 (x axis) on DC and DR+IC (y axis) following incubation with (A) a combination of proinflammatory cytokines TNF-α, IL-1β, IL-6, and PGE2 (CC); (B) viral double-stranded RNA (poly I:C); (C) LPS; or (D) bacterial oligodeoxynucleotide (CpG-ODN). Filled histograms indicate expressions on unstimulated cells and empty histograms following stimulation. Data shown are from two breast cancer patients (stage II) and are representative of 16 patients who were assessed (stage II, n = 9; stage IV, n = 7).
Table 1.
Response to Proinflammatory and Pathogen-Derived Factors.
| CC | LPS | Poly I:C | CpG ODN | |||||
| DC | DR+IC | DC | DR+IC | DC | DR+IC | DC | DR+IC | |
| CD40 | 351.9 ± 52.3** | 82.4 ± 8.7** | 344.4 ± 45.6** | 122.0 ± 12.9** | 175.6 ± 76.5** | 57.9 ± 28.4** | 218.3 ± 21.5 | 93.9 ± 33.3 |
| CD80 | 17.3 ± 4.6** | 0.6 ± 1.4** | 34.7 ± 8.3** | 4.4 ± 1.5** | 22.6 ± 6.6** | 3.5 ± 0.8** | 10.0 ± 1.7 | 3.7 ± 3.3 |
| CD83 | 65.2 ± 12.9** | 9.14 ± 2.8** | 40.9 ± 5.0*** | 10.0 ± 2.3*** | 20.3 ± 9.2** | 6.4 ± 1.9** | 77.9 ± 13.2* | 24.3 ± 16.1* |
| CD86 | 729.51 ± 100.*** | 314.5 ± 6.3*** | 703.6 ± 85.6*** | 22.9 ± 10.6*** | 348.7 ± 159.5* | 21.8 ± 5.3* | 477.2 ± 32.9*** | 3.6 ± 1.6*** |
| HLA-DR | 1046.5 ± 20.6* | 273.9 ± 47.3* | 1044.1 ± 56.7* | 168.9 ± 60.7* | 458.0 ± 70.8** | 135.8 ± 29.0** | 663.6 ± 209.5 | 297.5 ± 120.7 |
Phenotypic maturation of DC and DR+IC was analyzed in PBMC following incubation with a combination of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and PGE2; CC) or pathogen-derived factors, including ligands for TLR4 (LPS), TLR3 (poly I:C), and TLR9 (CpG ODN). Data were collected from 16 patients with breast cancer (stage II, n = 9; stage IV, n = 7). The magnitude of the response is expressed as ΔMFI (MFI of stimulated cells - MFI of unstimulated cells) ± SEM. Statistically significant differences between DC and DR+IC are indicated.
P < .05.
P < .01.
P < .001.
Lineage Composition
Finally, given that DR+IC lacked an expression of markers associated with mature hematopoietic (CD3, CD14, CD19, CD20, and CD56) and circulating stem (CD34) cells, lineage composition of these cells was further evaluated (Table 2). To obtain representative data, phenotypic analyses were conducted in a cohort of 21 patients with breast cancer (stage II, n = 17; stage IV, n = 4). It was found that variable proportions of DR+IC expressed molecules associated with DC [33], including HLA-DR (100%), CD2 (7%), CD4 (3%), and CD1c (22%). Similarly, a consistent proportion of DR+IC expressed some early B-cell markers such as CD79a, SIgκ, SIgλ, and cytoplasmic Cµ (14–20%); and early progenitor markers such as CD7, CD10, CD13, CD33, and CD71 (3–15%). Less than 5% of DR+IC expressed markers for the polymorphonuclear (PMN) or erythroid lineages such as MPO, CD15, or CD235a; and 5% to 30% expressed integrins such as CD11b, CD62L, CD41, and CD61. Altogether, these data suggested that multiple small subpopulations of immature cells ascribed to different lineages coexist within the DR+IC population.
Table 2.
Phenotypic Characterization of DR+IC*
| Names | Definition/Function | % Positive |
| Lineage markers | ||
| CD3 | T-cell, TCR signaling | ≤1 |
| CD14 | Monocyte, LPS recognition | ≤1 |
| CD19 | B-cell, signal transduction | ≤1 |
| CD20 | B-cell, activation | ≤1 |
| CD56 | NK cell, adhesion | ≤1 |
| CD34 | Progenitor, adhesion | ≤1 |
| DC markers | ||
| HLA-DR | MHC-II | 100 |
| HLA-ABC | MHC-I | 100 |
| CD11c | Integrin, binds fibrinogen | ≤1 |
| CD2 | Costimulation | 7.2±0.9 |
| CD4 | MHC-II coreceptor | 2.9±0.7 |
| CD1c | MHC-I-like molecule | 21.5±3.4 |
| CD123 | IL-3 receptor α chain | ≤1 |
| BDCA2 | Type II C-type lectin | ≤1 |
| BDCA3 | Type II C-type lectin | ≤1 |
| BDCA4 | Type II C-type lectin | 1.2±0.7 |
| DC-SIGN | Type II C-type lectin | 2.3±1.9 |
| Costimulatory molecules | ||
| CD40 | Activation, binds CD40L | 47.1±6.2 |
| CD80 | Binds CD28 | ≤1 |
| CD83 | Activation marker | ≤1 |
| CD86 | Binds CD28 | 12.5±3.0 |
| Adhesion molecules | ||
| CD11b | Integrin, binds ECM | 23.6±2.6 |
| CD62L | L-selectin, leukocyte tethering | 27.0±1.2 |
| CD41 | Integrin, binds fibrinogen | 5.5±0.7 |
| CD61 | Integrin, binds ECM | 13.0±4.3 |
| PMN markers | ||
| Cytoplasmic MPO | Enzymatic degradation | 4.3±0.6 |
| CD15 | Adhesion | ≤1 |
| Erythroid marker | ||
| CD235a, glycophorin A | Anion transport | 5.0±2.8 |
| Early B-cell markers | ||
| Cytoplasmic CD79a | Signal transduction | 20.0±3.7 |
| Igk | Immunoglobulin light chain | 14.4±4.1 |
| Igk | Immunoglobulin light chain | 20.0±2.0 |
| Cytoplasmic Cm | Immunoglobulin M heavy chain | 14.0±9.2 |
| Precursor markers | ||
| CD7 | Lymphoid, costimulation | 4.6±0.7 |
| CD10 | Lymphoid, enzymatic activity | 3.0±1.0 |
| CD13 | Myeloid, metalloproteinase | 4.5±1.2 |
| CD33 | Myeloid, metalloproteinase | 5.7±1.7 |
| CD71 | Transferrin receptor | 15.0±3.8 |
Phenotype of DR+IC in PBMC ex vivo. Data were collected from a cohort of 21 patients with breast cancer (stage II, n = 17; stage IV, n = 4). Values indicate the proportion of DR+IC that are positive for each marker.
Discussion
Despite the potential for tumor control, the immune system often fails to prevent cancer progression. Substantial evidence now indicates that defects in DC function have a crucial role in this process. Hence, research into the biology of DC tumor interactions has been the focus of much effort. Most knowledge in this field has emerged from in vitro studies whereby DC are generated from hematopoietic progenitors following culture with cytokines [14,15,34,35]. However, cytokine-driven activity of cultured DC is unlikely to reflect the functional status of DC populations that are circulating in vivo. Therefore, despite inherent methodological constraints, we evaluated the blood DC compartment in a large cohort of patients with various types of cancer. We report that, in these patients, a population of HLA-DR+CD11c-CD123- cells (DR+IC) distinct from the recognized myeloid (CD11c+DC) and plasmacytoid (CD123+DC) subtypes emerged as a significant proportion of the DC compartment. Indeed, although DR+IC in healthy donors represented 5% to 15% of Lin-HLA-DR+ cells, it accounted for a significantly larger proportion in patients with cancer (30–65%). Moreover, the relative proportion of DR+IC in the circulation increased with advancing disease. Although patients with locally limited breast cancer (stages I and II) had twice the normal number, patients with advanced breast cancer (stage IV) had a four-fold increase in DR+IC compared to healthy donors.
One intriguing aspect of these findings is how cancer progression could contribute to the selective accumulation of immature cells in the circulation. It may be related to 1) tumor-derived factors (granulocyte-macrophage colony-stimulating factor) that promote the mobilization of precursors from the bone marrow [36], or 2) tumor products (VEGF, IL-6, M-CSF, gangliosides, prostanoids, and spermine) that alter the differentiation of APC from their progenitors [12–18]. Interestingly and supporting a role for tumor products in this process, we demonstrate a close correlation between accumulation of immature cells in the blood and tumor burden. Indeed, patients with metastatic disease (stage IV) showed a larger number of DR+IC in the blood than patients with nodal (stage II) or local disease (stage I). In addition, in patients with fully resected malignant glioma, the proportion of DR+IC in the circulation increased when clinical and imaging evaluation demonstrated tumor progression. These results confirmed that the presence of DR+IC within the blood DC compartment was associated with tumor status and correlated with clinical behavior of the disease.
It is also tempting to speculate that, although the systemic accumulation of immature cells could facilitate generalized immune dysfunction as a late event, immature APC present at the tumor site or lymphoid organs could play a role at an earlier phase in tumor progression. Indeed, in patients with head and neck squamous cell carcinoma, tumor infiltration with immature cells has been correlated with increased rate of recurrence and metastases [37]. In our hands, however, the direct identification of DR+IC in tumor stroma has remained difficult due to their lack of specific markers and heterogeneity (unpublished data). Nevertheless, purification and characterization of DR+IC were possible from the peripheral blood. Phenotypic characterization revealed minimal expression of CD80 and CD83, moderate levels of CD86, and high expression of CD40 and HLA-DR. Furthermore, 100% of DR+IC were MHC-I-positive, and 20% expressed CD1c. All these molecules are involved in APC-T-cell interactions, costimulation, and antigen presentation. Because these data suggested that DR+IC could potentially perform as APC, we assessed their capacity to 1) capture antigens, 2) stimulate T-cell proliferation/IFN-γ secretion, and 3) mature in vitro on stimulation. Different types of stimuli or “danger signals,” including inflammatory cytokines, as well as ligands for toll-like receptors 4 (LPS), 3 (poly I:C), and 9 (CpG ODN), were tested. We found that, compared to DC, DR+IC had lower antigen capture, presentation, and phenotypic maturation in response to these inflammatory mediators. It may be suggested that this nonresponsiveness could be due to limited expression of cytokine or toll-like receptors. However, upregulation in HLA-DR and CD40 expression was evident with all stimuli (Table 1). Similarly, elevated expression of CD83 and CD86 was noticeable in response to poly I:C and CpG ODN, suggesting that DR+IC could respond, at least to some extent, to inflammatory mediators. It may be that DR+IC are functionally impaired due to an effect of the tumor. In this regard, tumor-derived factors have been shown to induce abnormal intracellular signaling (STAT-3 and NFκB) in APC progenitors, thus hampering their differentiation and function [38,39]. Alternatively, it could be related to the fact that most DR+IC are represented by immature cells at early stages of differentiation. The latter is more likely the case because stimulation with CD40 ligation induces DR+IC differentiation/maturation, as shown in an accompanying paper.1
We also confirmed the immaturity of DR+IC by morphology and phenotypic analyses. In contrast to DC, DR+IC were small cells with short projections, poorly developed organella, and largely condensed chromatin in the nucleus. Similarly, lineage characterization suggested that multiple subpopulations of immature cells were present. In accordance with these results, it has been reported that disease progression in cancer patients is associated with reduction of DC numbers and the appearance of a large number of immature macrophages, granulocytes, DC, and precursors of the myelo/monocytic lineage in the peripheral blood [11,36,40]. Other studies indicate that accumulation of immature cells is not only an indicator for tumor progression but may also promote immune suppression. For instance, in patients with head and neck, lung, and breast cancers, immature cells have been shown to suppress responses to recall antigens and to inhibit antigen-specific immunity [40,41]. In contrast to those studies, the DR+IC described here express HLA-DR and other molecules associated with antigen presentation (CD86, CD1c, and CD40), and capture and present antigens, suggesting a role as inefficient APC rather than cells with suppressive function. This assumption is further supported by the finding that DR+IC do not inhibit T-cell proliferation in either allogeneic or antigen-specific manner when competent DC are present, as described in our accompanying paper.
Interestingly, in contrast to the expression of HLA-DR, CD86, and CD1c, the expression of CD40 was significantly higher in DR+IC (51.0 ± 8.9%) when compared to DC (13.0 ± 5.5%). It has been demonstrated that CD40 ligation on DC increases their resistance to tumor-induced apoptosis [42]. In an accompanying paper, we show that DR+IC's expression of CD40 renders them exquisitely sensitive to signaling through this pathway. Moreover, DR+IC demonstrate resistance to tumor-induced apoptosis. Thus, accumulation of CD40+ immature cells probably represents accumulation of cells resistant to the apoptotic effect of the tumor, and not merely mobilization of progenitors from the bone marrow. In this context, tumors may produce numerous suppressive factors that induce a significant decline in the numbers of CD11c+DC and CD123+DC (by altered differentiation or apoptosis) with the concurrent accumulation of immature cells (by recruitment or resistance to apoptosis), thus displacing competent APC and favoring tumor evasion [21].
The findings reported here are relevant due to the large effort devoted to harnessing blood DC for the immunotherapy of cancer. In fact, blood DC have already been used for vaccination in patients with multiple myeloma, albeit demonstrating limited induction of tumor-specific immunity [43]. Similarly, preclinical studies have demonstrated that, although DC generated in vitro from progenitors purified from cancer patients are capable of stimulating T-cell responses, blood DC isolated from the same patients are deficient in their APC capacity [8,13]. Our study indicates that the defective function of circulating DC could, at least in part, be the result of decreased frequency of competent DC and accumulation of immature cells with poor APC function rather than suppressive function. As demonstrated in our accompanying paper (HLA-DR+ Immature Cells Exhibit Reduced Antigen-Presenting Cell Function But Respond to CD40 Stimulation), when T-cells primed with DR+IC were compared with T-cells primed with DC, a different activation “pattern,” as assessed by the expression of activation markers and cytokines, was noted. Indeed, a smaller proportion of T-cells expressed activation markers that were upregulated following adequate T-cell activation, and Th2 bias in cytokine secretion was detected. We propose that the significant accumulation of immature cells (DR+IC) with poor APC function could contribute to tumor immune evasion by displacing competent DC, presenting antigens inadequately and inducing Th2 bias, thus failing to generate effective antitumor responses.
In summary, we document the significant accumulation of a novel population of cells within the blood DC compartment of patients with cancer. This population exhibits heterogeneous and immature phenotype, limited response to “danger signals,” and poor APC function. Increased numbers of these cells closely correlate with disease stage and tumor progression. It is possible that the accumulation of these cells could be associated with decreased immune function and compromised clinical status in patients with cancer. Our data should also be taken into account when assessing immune competence (i.e., DC enumeration/characterization in patients with cancer), and they necessitate prudence when using the peripheral blood DC compartment as a source of cells for DC-based cancer immunotherapy protocols.
Acknowledgements
The authors are grateful to Grace Chojnowski, Paula Hall, and Clay Winterford for technical assistance with FACS, MoFlo sorting, and ultrastructural analysis, respectively. We also thank Greg Bryson for antibody supply and Geoff Hill for useful discussions. We are grateful to Maureen Gleeson, Sonia Tepes, and Georgina Crosbie (Mater Medical Research Institute, Australian Red Cross Blood Service, and Sullivan Nicolaides Pathology Laboratories, respectively) for blood samples and logistic assistance, and mostly to our patients and donors without whom this study would have not been possible.
Abbreviations
- DC
dendritic cells
- DR+IC
HLA-DR+ immature cells
- APC
antigen-presenting cells
- Lin
lineage markers
Footnotes
This work was funded by the National Breast Cancer Foundation, Australia. A.P.C. was supported by the University of Queensland International Postgraduate Research and the Paul Mackay Bolton Cancer Research Scholarships.
HLA-DR+ immature cells exhibit reduced APC function but respond to CD40 stimulation.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Dworacki G, Gooding W, Lotze MT, Whiteside TL. Spontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanoma. Clin Cancer Res. 2000;6:1351–1364. [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–316. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Troy AJ, Davidson PJ, Atkinson CH, Hart DN. CD1a dendritic cells predominate in transitional cell carcinoma of bladder and kidney but are minimally activated. J Urol. 1999;161:1962–1967. [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 9.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–593. [PubMed] [Google Scholar]
- 11.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 13.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 14.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 15.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333–4343. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 16.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 18.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 19.Lissoni P, Vigore L, Ferranti R, Bukovec R, Meregalli S, Mandala M, Barni S, Tancini G, Fumagalli L, Giani L. Circulating dendritic cells in early and advanced cancer patients: diminished percent in the metastatic disease. J Biol Regul Homeost Agents. 1999;13:216–219. [PubMed] [Google Scholar]
- 20.Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8:1787–1793. [PubMed] [Google Scholar]
- 21.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–467. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 22.Satthaporn S, Robins A, Vassanasiri W, El-Sheemy M, Jibril JA, Clark D, Valerio D, Eremin O. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53:510–518. doi: 10.1007/s00262-003-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savary CA, Grazziutti ML, Melichar B, Przepiorka D, Freedman RS, Cowart RE, Cohen DM, Anaissie EJ, Woodside DG, McIntyre BW, et al. Multidimensional flow-cytometric analysis of dendritic cells in peripheral blood of normal donors and cancer patients. Cancer Immunol Immunother. 1998;45:234–240. doi: 10.1007/s002620050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Sobin LH, Wittekind C. International Union Against Cancer: TNM Classification of Malignant Tumors. New York: Wiley-Liss; 2002. [Google Scholar]
- 26.Kleihues P, Cavenee WK. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Nervous System. Lyon, France: IARC Press; 2000. [Google Scholar]
- 27.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20:A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 28.Krug A, Rothenfusser S, Selinger S, Bock C, Kerkmann M, Battiany J, Sarris A, Giese T, Speiser D, Endres S, et al. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol. 2003;170:3468–3477. doi: 10.4049/jimmunol.170.7.3468. [DOI] [PubMed] [Google Scholar]
- 29.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster H, Jellinger K, Gund A, Regele H. Extracranial metastases of anaplastic cerebral gliomas. Acta Neurochir (Wien) 1976;35:247–259. doi: 10.1007/BF01406121. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jefford M, Schnurr M, Toy T, Masterman KA, Shin A, Beecroft T, Tai TY, Davis ID, Shackleton M, Davis ID, et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 34.Kanto T, Kalinski P, Hunter OC, Lotze MT, Amoscato A. Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol. 2001;167:3773–3784. doi: 10.4049/jimmunol.167.7.3773. [DOI] [PubMed] [Google Scholar]
- 35.Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 36.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73:663–669. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 37.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL, Petruzzelli GJ. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 38.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 39.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 40.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 41.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 42.Esche C, Gambotto A, Satoh Y, Gerein V, Robbins PD, Watkins SC, Lotze MT, Shurin MR. CD154 inhibits tumor-induced apoptosis in dendritic cells and tumor growth. Eur J Immunol. 1999;29:2148–2155. doi: 10.1002/(SICI)1521-4141(199907)29:07<2148::AID-IMMU2148>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Reichardt VL, Okada CY, Liso A, Benike CJ, Stockerl-Goldstein KE, Engleman EG, Blume KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]