Abstract
The proteasome inhibitor Velcade (bortezomib/PS-341) has been shown to block the targeted proteolytic degradation of short-lived proteins that are involved in cell maintenance, growth, division, and death, advocating the use of proteasomal inhibitors as therapeutic agents. Although many studies focused on the use of one proteasomal inhibitor for therapy, we hypothesized that the combination of proteasome inhibitors Lactacystin (AG Scientific, Inc., San Diego, CA) and MG132 (Biomol International, Plymouth Meeting, PA) may be more effective in inducing apoptosis. Additionally, this regimen would enable the use of sublethal doses of individual drugs, thus reducing adverse effects. Results indicate a significant increase in apoptosis when LNCaP prostate cancer cells were treated with increasing levels of Lactacystin, MG132, or a combination of sublethal doses of these two inhibitors. Furthermore, induction in apoptosis coincided with a significant loss of IKKα, IKKβ, and IKKγ proteins and NFκB activity. In addition to describing effective therapeutic agents, we provide a model system to facilitate the investigation of the mechanism of action of these drugs and their effects on the IKK-NFκB axis.
Keywords: Prostate, apoptosis, proteasomal inhibitors, Lactacystin, MG132
Introduction
Removal of androgens induces apoptosis and drives androgen-sensitive prostate cancer into remission. Despite androgen depletion therapy and remission, prostate cancer survivors may develop a subclass of androgen-refractory cancer that is often more aggressive than the primary cancer [1]. This and other transformations have been associated with an increase in the prosurvival and proliferative activities of NFκB [2,3]. As a result, the regulation, processing, and disruption of NFκB are being explored as chemotherapeutic targets in cancer [4,5]. Of the members of the NFκB family, p65 is one of the most studied transcription factors, as it is involved in short-term and long-term cell survival and is active at both the nuclear and mitochondrial levels [4–8]. The transcriptional activity of p65 is regulated by inhibitor of κB (IκB) proteins that bind to the Rel Homology Domain of NFκB and prevent the translocation and transcriptional activity of NFκB [9]. To disrupt the binding of IκB to NFκB and to increase NFκB activity, IκB kinase proteins IKKα, IKKβ, and IKKγ are required. These proteins are located throughout the cytosol as either trimers of α, β, and γ subunits; dimers of β and γ; or monomers of IKKα [10,11]. Once phosphorylated, IKK proteins increase the transcriptional activity of NFκB directly and/or indirectly. For instance, phosphorylation of IκBα by IKKβ results in the immediate ubiquitination and degradation of IκBα by the 26S proteasome, thereby unmasking the nuclear localization signal of NFκB [4,12,13]. IKKα is a multifunctional protein responsible for the phosphorylation of IKKβ, phosphorylation of IκBα, direct phosphorylation of NFκB, and finally, phosphorylation of the nuclear histone H3 through CBP/p300 [14,15]; these cumulative effects facilitate the transcriptional activity of NFκB [7,16,17]. The activation of IKK proteins is necessary for the translocation of p65 into the nucleus [8] and the transcriptional activation of p65 in the nucleus. The process of NFκB activation and subsequent repression of apoptotic proteins may be deregulated when IKK proteins themselves or regulatory proteins upstream of IKK activation are blocked, missing, or mutated [3,18–21].
Of the drugs used for cancer therapy, proteasome inhibitors have shown promise in disrupting regulatory proteins, thereby reducing and/or eliminating various neoplastic cancers. The theory behind the use of proteasomal inhibitors is that, of all the cellular proteins involved in maintenance, differentiation, immunity, growth, division, and death, approximately 70% to 90% is eventually degraded by multicatalytic proteasomes [22–26]. By interfering with the normal function of proteasomes, cellular stress is induced, leading to cell cycle arrest and/or cell death [27–29]. Many compounds that either interfere or block proteasomal function have been identified. These include β-lactones such as clasto-Lactacystin, peptidyl aldehydes such as MG132 (carbobenzoxy-l-Leu-l-Leu-l-Leu), and peptidyl boronates such as Velcade (bortezomib/PS-341). Lactacystin (AG Scientific, Inc., San Diego, CA) binds covalently, whereas MG132 (Biomol International, Plymouth Meeting, PA) and Velcade bind reversibly to the N-terminal Thr residue of the β1 subunit within the 26S proteasome [30,31]. Aside from the antiproteasomal activity of Lactacystin and MG132, these compounds are known inhibitors of other proteases such as cathepsin A, cathepsin B, and calpain 1 [32,33], respectively. Regardless of the latter's protease activity, the primary effect of the inhibitors is directed on the proteasome. Proteasomal inhibitors increase the lifespan of short-lived proteins and block the modification of proteins involved in signaling pathways, resulting in changes that inhibit cell survival and proliferation. In early studies, Velcade was reported to induce DNA damage, arrest cellular cycling at the G1/S or G2/M phase, and reduce NFκB activity [34–37]. When used to treat cancer cells, Velcade induced a dose-dependent cleavage of polyadenosine-5′-diphosphate-ribose polymerase (PARP), significant apoptosis, expression of p21WAF1, and G2/M cell cycle arrest [38]. Based on these beneficial effects, Velcade is being tested in phase I to phase III clinical trials for a variety of solid tumors. Unfortunately, several side effects, including incomplete tumor regression, have been noted at the doses required for the induction of apoptosis [33,39–42], thus necessitating the identification of other drugs that are effective at lower doses.
Although proteasomal inhibitors are being tested in cancer therapy, available data on prostate cancer are sparse. Therefore, our goal was to examine the effect of proteasomal inhibitors on prostate cancer. We hypothesized that a combination of proteasome inhibitors (e.g., MG132 and Lactacystin) would induce more apoptosis at lower doses of the drugs compared to the use of either inhibitor alone. The following results highlight the advantages of using combination therapy, as the inhibitors synergistically induce apoptosis. We also demonstrate that the increase in apoptosis is associated with a decrease in proteins associated with the IKK-NFκB axis.
Materials and Methods
Cell Culture and Experimental Design
LNCaP and PC3 cells (American Type Culture Collection, Rockville, MD) were maintained in RPMI 1640 (Hyclone, Logan, UT) containing 10% FBS, 0.5% penicillin-streptomycin (0.05 U/ml) (InVitrogen Corporation, Carlsbad, CA), and 0.1% Fungizone (0.25 µg/ml) (InVitrogen Corporation). All experiments were conducted with the same stock media for 24 hours, but with an FBS concentration of 7.5%. Cells were treated with increasing concentrations of Lactacystin in Experiment 1 and with increasing concentrations of MG132 in Experiment 2. In Experiment 3, cells were treated with 5.0 µM Lactacystin, 250 µM MG132, or their combination. The activity of NFκB and p53 was determined in Experiments 4 and 5, respectively. After LNCaP cells were transfected with a plasmid-based NFκB-Luciferase or p53-Luciferase reporter construct, they were treated as in Experiment 3.
Induction of apoptosis was measured using an M30 Apoptosense kit (Peviva AB, Bromma, Sweden), which measured a neoepitope of cytokeratin 18 generated during apoptosis. Cell extracts were separated on Tris-glycine gels (10–15%) and were probed for IKKα, IKKβ, IKKκ, IκBα, IκBα(S32/36), phospho-p53(S15), p21WAF1, caspase-3, XIAP, NFκB1 (p50), and β-actin. Positive signals were developed using ECL-Plus (Amersham Biosciences Corp., Pittsburg, PA) and captured on a digital imager (Alpha Innotech, San Leandro, CA). Digitized signals were normalized against β-actin to control for differences in protein loading.
p53 and NFκB Activity Assays
Luciferase reporter activity was measured using a Luciferase reporter assay (Promega, Madison, WI). In each experiment, 3.5 x 106 cells were suspended in an electroporation buffer (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, 25 mM HEPES, 2 mM EGTA, 5 mM MgCl2, 50 mM glutathione, and 2 mM ATP, pH 7.6), transferred to a 4-mm electroporation cuvette, and mixed with 20 µg of a NFκB-Luciferase firefly luciferase reporter construct (BD Biosciences Clontech, Palo Alto, CA) or 20 µg of a p53-Luciferase firefly luciferase reporter construct (Panomics, Inc., Redwood, CA). The cells were electroporated using a BTX-830 electroporator (Gentronics, San Diego, CA) with 200-mV voltage, 10-millisecond pulse length, and two pulses at 500-millisecond intervals. After the cells had rested in the cuvette for 10 minutes, they were dispersed in RPMI media. The cells were seeded at 2.5 x 105 cells/well of a 12-well culture plate and allowed to adhere for 24 hours before treatment with the proteasome inhibitors.
Statistical Analysis
Apoptosense and actin-corrected densitometries of Western blot analysis data were analyzed by the GLM procedure of SAS (SAS Institute, Inc., Cary, NC) [43], and means were compared for NFκB and p53 activity data by Duncan's multiple range test (P < .01) only when a significant probability value of .05 was detected in the analysis of variance.
Results
Proteasomal Inhibitors MG132 and Lactacystin Induce Apoptosis
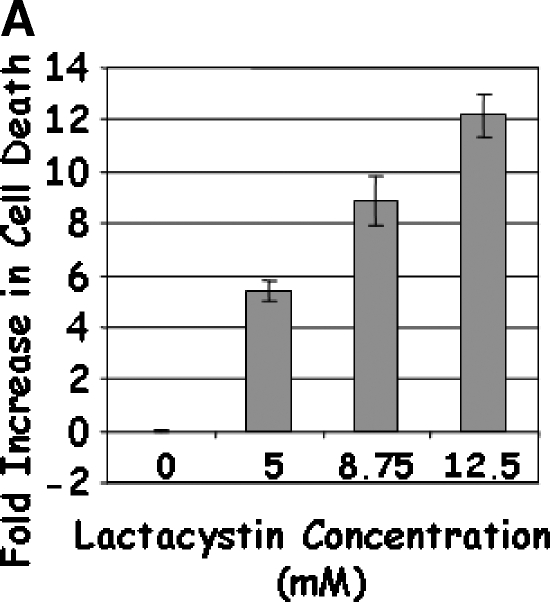
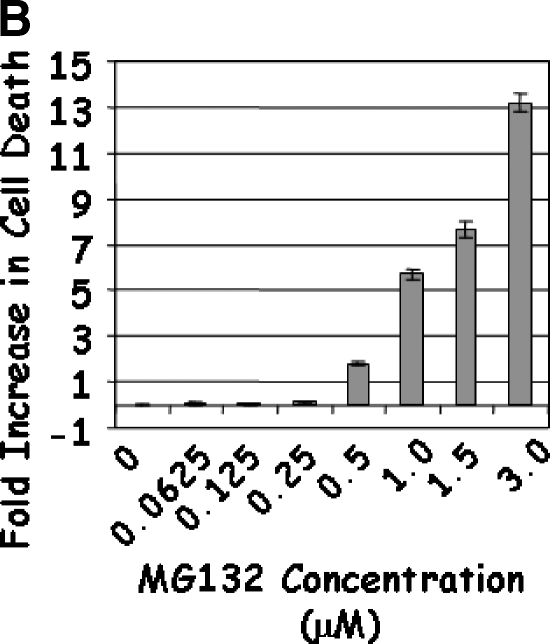
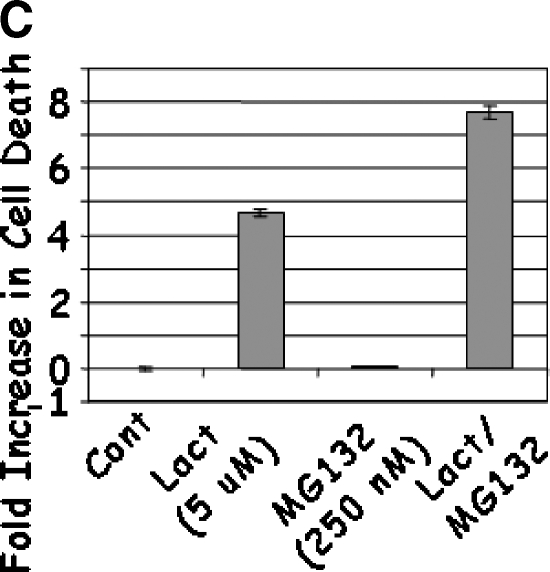
Treatment of LNCaP cells with Lactacystin induced apoptosis (greater than five-fold) at the lowest dose (5 µM) tested (Figure 1A). The apoptotic response continued linearly up to 12.5 µM Lactacystin (P < .0001; R2 = 0.956), indicating that Lactacystin is an effective apoptotic agent in LNCaP prostate cancer cells. No appreciable cell death was observed with lower doses of MG132, whereas 0.5 µM induced an approximately two-fold apoptosis (Figure 1B). Further increases in MG132 resulted in a curvilinear response (P < .0001; R2 = 0.985), leading to a 13-fold increase in apoptosis at the 3.00-µM dose. To test the hypothesis that the combination of the two proteasomal inhibitors would provide apoptotic advantage, LNCaP cells were treated with suboptimal doses of Lactacystin (5 µM) and MG132 (250 nM). Treatment with Lactacystin or MG132 alone confirmed the above results, whereas the combination yielded a highly significant increase in apoptosis (7.7-fold), indicating that this regimen may have therapeutic advantage (Figure 1C).
Figure 1.
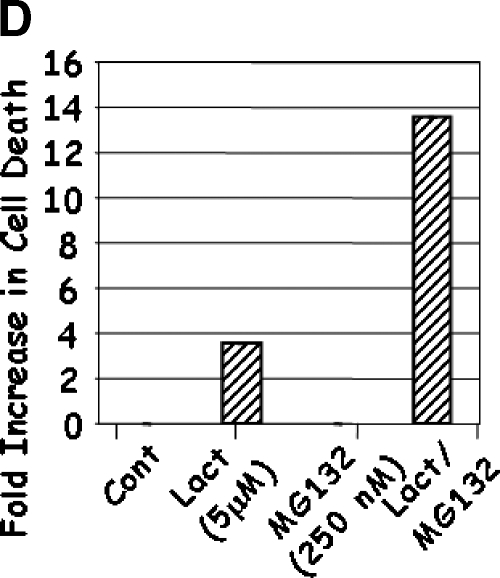
LNCaP cells were treated with 5.0 to 12.5 µM Lactacystin (panel A; Experiment 1), 0.06 to 3.0 µM MG132 (panel B; Experiment 2), or their combination (panel C; Experiment 3). PC3 cells were treated as in Experiment 3, and apoptosis was measured (panel D). Results are fold inductions of M30 activity. Values represent observations from two experiments with three to four replicates each.
The above results demonstrated that the proteasomal inhibitors Lactacystin and MG132 induced apoptosis in androgen-responsive LNCaP cells. As androgen-independent prostate cancer is a major concern in patients and is more difficult to treat, it is of interest to determine whether these proteasomal inhibitors are capable of inducing apoptosis in androgen-independent prostate cancer cells. Treatment of PC3 cells with the same suboptimal dose of Lactacystin induced apoptosis (Figure 1D). Furthermore, similar to LNCaP, treatment of PC3 with 250 nM MG132 induced very little or no apoptosis, whereas combination of Lactacystin and MG132 resulted in synergistic induction of apoptosis (10-fold increase compared to cells treated with Lactacystin alone). These results indicate that the proteasomal inhibitors Lactacystin and MG132 induced apoptosis in both androgen-responsive and androgen-independent prostate cancer cells, an important therapeutic advantage of these drugs.
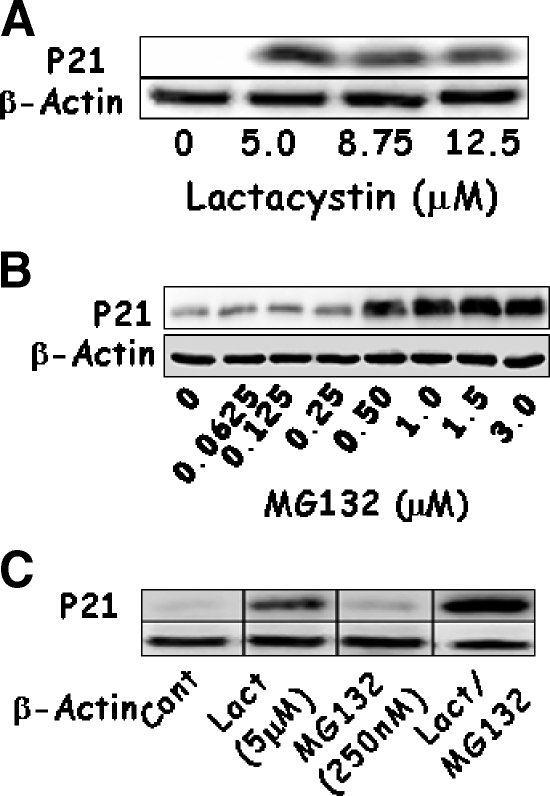
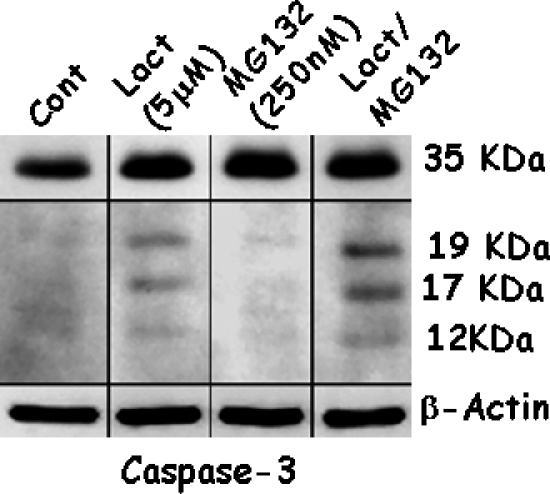
To confirm the results of apoptosis, the expression of p21WAF1 and caspase-3 was determined because an increase in active caspase-3 was associated with an increase in DNA cleavage and the expression of p21WAF1 in a p53-dependent manner. This not only facilitated apoptosis, but also induced cell cycle arrest. Expression of p21WAF1 increased with each dose of Lactacystin or MG132 (Figure 2, A and B). The lowest dose of Lactacystin tested was enough to significantly increase p21WAF1, whereas 500 nM MG132 was required to elicit a similar response. Combined treatment significantly increased the levels of p21WAF1 beyond that of either proteasome inhibitor alone (Figure 2C). A similar analysis showed that Lactacystin activated caspase-3, as noted by the presence of proteolytic fragments (Figure 3), whereas activation was not significant with MG132 alone. Combined treatment synergistically increased the processing of the 35-kDa procaspase to the 17- and 12-kDa active fragments.
Figure 2.
LNCaP cells were treated with increasing concentrations of Lactacystin (panel A; Experiment 1), MG132 (panel B; Experiment 2), or their combination (panel C; Experiment 3) for 24 hours. Expressions of p21 and β-actin were evaluated using Western blot analysis.
Figure 3.
LNCaP cells were treated with Lactacystin (Lact), MG132, or their combination for 24 hours. Expression levels of caspase-3, cleaved products of caspase-3, and β-actin were evaluated using Western blot analysis. Procaspase signals were obtained after 15 seconds of exposure, and signals from the cleaved caspase-3 fragments were obtained after a 3-minute exposure to a radiological film.
Proteasomal Inhibitors Decrease the Level of IKK Proteins
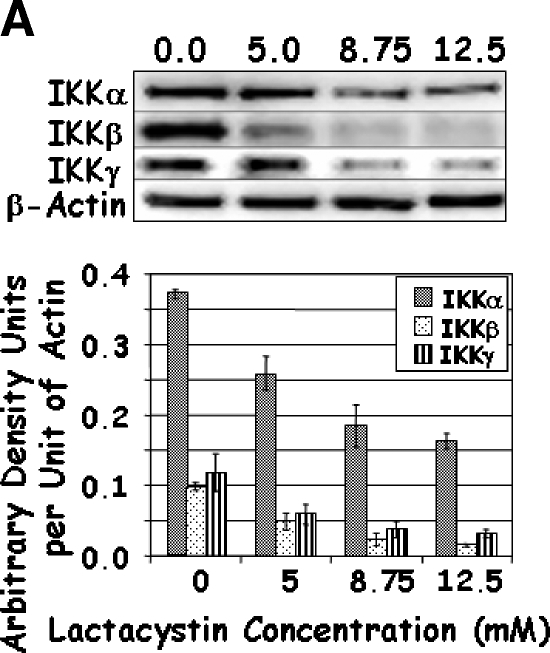
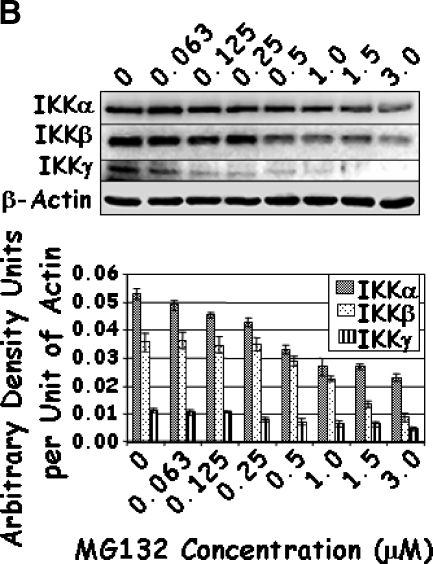
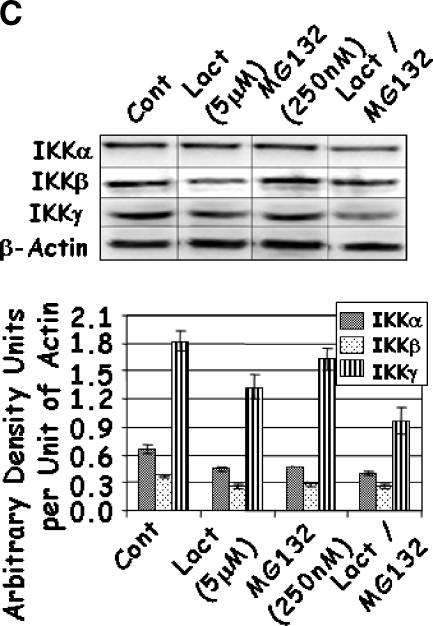
To further investigate the mechanism of action of these drugs, the IKK-NFκB axis was investigated starting with the expression of IKKα, IKKβ, and IKKγ. A dose-dependent decrease in the levels of each of the IKK proteins was observed with increasing concentrations of Lactacystin (Figure 4A); however, marginal reduction was observed for IKKα at the lower levels of Lactacystin. Where the addition of 5 µM Lactacystin reduced IKKα by 30%, the addition of 8.75 and 12.5 µM Lactacystin reduced IKKα by 50% and 56%, respectively. Although not to the same magnitude, this trend was similar for IKKβ and IKKγ. Results obtained with MG132 were similar to those of Lactacystin, except that IKK proteins decreased significantly only when treated with 0.25 to 3.0 µM MG132 (Figure 4B). In combination experiments, IKK proteins responded to the individual drugs as in the previous groups. However, the level of IKKγ was significantly reduced when compared to that of either proteasome inhibitor alone (Figure 4C). Together, these experiments demonstrated a significant decrease in all three IKK family members, which coincided with significant apoptosis with increasing concentrations or with a combination of Lactacystin and MG132.
Figure 4.
LNCaP cells were treated with increasing concentrations of Lactacystin (panel A; Experiment 1), MG132 (panel B; Experiment 2), or their combination (panel C; Experiment 3) for 24 hours. Immunoblots were analyzed for the expression of IKKα, IKKβ, IKKγ, and β-actin. The signal was captured in digital image analysis units, and arbitrary density units are expressed per actin value. Values are averages of three replicates from two experiments.
Lactacystin and MG132 Affect IκBα and NFκB Proteins
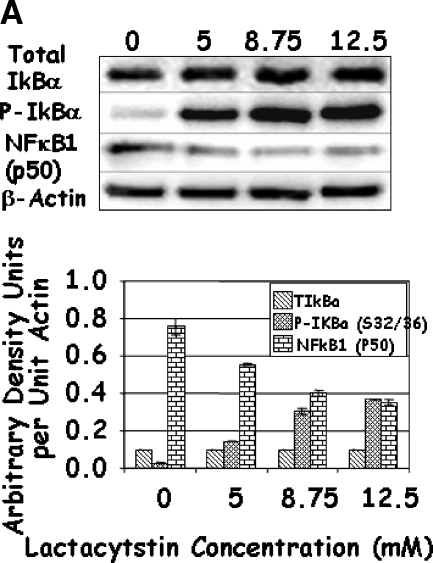
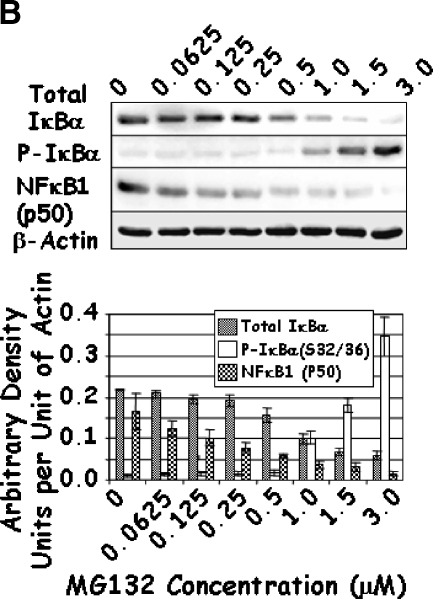
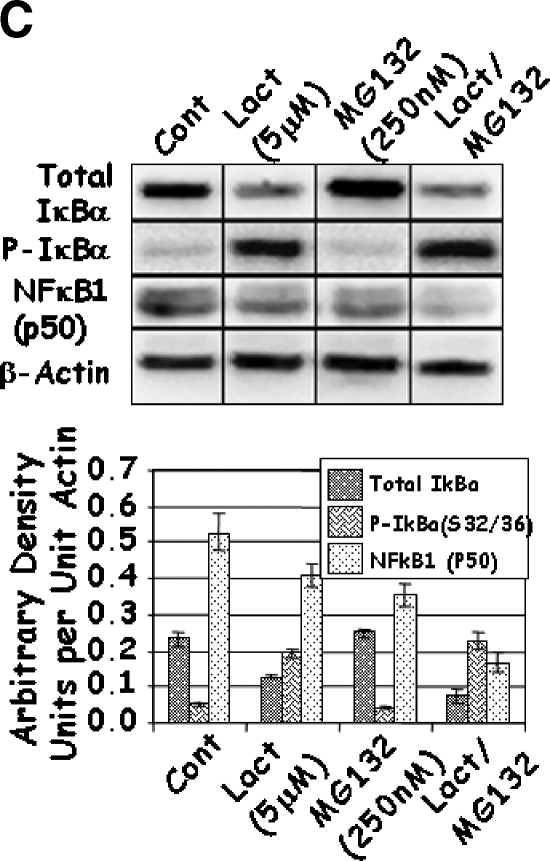
As changes in the function of IKK may affect the activity of NFκB through their effects on IκBα and the proteolytic modifications of p105 to p50, the levels of total and phosphorylated IκBα and NFκB1 (p50) were determined. Increasing concentrations of Lactacystin significantly increased the phosphorylation of IκBα, although it had no effect on the levels of total IκBα (Figure 5A). At first glance, these results seemed contradictive to this established molecular pathway, as the decrease in IKK should have resulted in a reduction in the phosphorylated form of IκBα. Under control conditions, phosphorylation of IκBα led to ubiquitination and degradation of IκBα by the 26S proteasome. However, inhibition of proteasomal activity by Lactacystin resulted in an increase in phosphorylated IκBα. Similarly, treatment with higher doses of MG132 increased IκBα phosphorylation (Figure 5B). Unlike Lactacystin, however, treatment with higher doses of MG132 resulted in an unexplainable decrease in total levels of IκBα (Figure 5B). Finally, combined treatment with Lactacystin and MG132 resulted in a significant reduction in total IκBα when compared to either individual treatment (Figure 5C); phosphorylation of IκBα increased significantly in cells treated with both inhibitors. These results indicate that treatment with proteasomal inhibitors Lactacystin and MG132 not only reduced the levels of IKK, but prolonged the half-life and levels of phosphorylated IκBα.
Figure 5.
LNCaP cells were treated with increasing concentrations of Lactacystin (panel A; Experiment 1), MG132 (panel B; Experiment 2), or their combination (panel C; Experiment 3) for 24 hours. The effect of treatment on total IκBα (IκBα), phosphorylated IκBαS32/36 (P-IκBα), and NFκB1 (p50) was analyzed by Western blot analysis. Values are averages of at least three replicates from two experiments.
A similar analysis of NFκB1 (p50) demonstrated a steady decrease in its levels with increasing concentrations of Lactacystin and/or MG132 (Figure 5), possibly attributed to the decrease in IκBα observed above. It is known that NFκB1 (p105) is processed to p50 by the proteasome in response to phosphorylation by IKKα. Thus, it is likely that the decrease in NFκB1 (p50) was due to proteasomal inhibition. Given the reduction in both IKK proteins and p50, it was of interest to determine whether these drugs affected the activity of NFκB.
Proteasomal Inhibitors Increase NFκB and p53 Activity
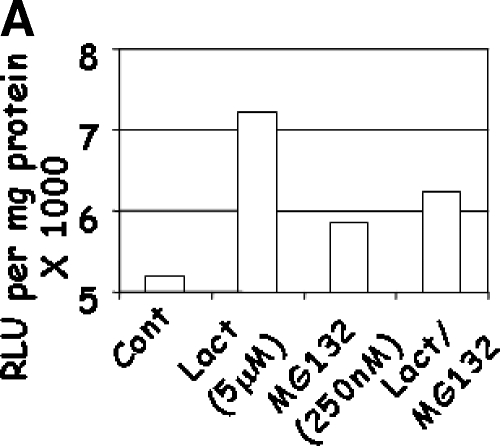
As NFκB is a key target of IKK proteins, the effects of Lactacystin and MG132 on the transcriptional activity of NFκB and p53 were determined. LNCaP cells were transfected with NFκB-Luciferase or p53-Luciferase constructs and treated with Lactacystin and MG132, individually or together. Treatment with Lactacystin induced significant NFκB-Luciferase activity (P < .0201; R2 = 0.78), whereas MG132 increased NFκB-Luciferase activity to a lesser extent (Figure 6A). These results were contrary to expectations, as IKK levels were reduced in these cells (Figure 4C). The significance of these results is discussed below.
Figure 6.
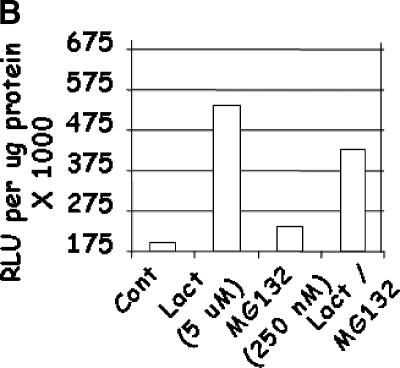
LNCaP cells were treated with a combination of Lactacystin and MG132 for 24 hours. NFκB (panel A; Experiment 4) and p53 (panel B; Experiment 5). Luciferase activity was expressed as relative light units (RLU) per microgram of cellular protein. Values are averages of two experiments with three replicates per treatment.
p53-Luciferase activity in Lactacystin- and MG132-treated cells (Figure 6B) agrees with p21WAF1 expression data (Figure 2C). Although the combination of Lactacystin and MG132 increased p53 activity (P < .0001; R2 = 0.96), it was lower than that of Lactacystin alone. This was at variance with the expression of p21WAF1 in these cells. This was probably due to the transcriptional regulation of the p53 construct by other members of the p53 protein family (such as p73).
Discussion
It is known that the proteasome is responsible for degrading 70% to 90% of all cellular proteins. The proteasome serves as a regulatory body that modifies proteins to render them functional (e.g., NFκB: p105 to p50), or that degrades proteins (e.g., p21WAF1 or active caspase-3) when they are no longer needed [44–46]. Although the proteasomal inhibitor Velcade is being tested in clinical trials, to date, there has been no report on the concurrent use of more than one class of proteasome inhibitors in the treatment of cancer. Therefore, the current study was designed to test the hypothesis that the combination of small doses of two different proteasome inhibitors would significantly induce apoptosis in prostate cancer when compared to the use of one proteasome inhibitor alone. Results from a series of experiments in this study indicate that the combination of Lactacystin and MG132 facilitates a high degree of cell death by inducing apoptosis, while simultaneously decreasing the expression of prosurvival proteins.
Cancer cells express a plethora of prosurvival proteins that override death-promoting signals in normal cells. Therefore, the goal of this study is to design therapy geared toward promoting the survival of death-inducing proteins. This is achieved by inhibiting the function of proteasomes. Our results showed a 39% increase in apoptosis when LNCaP cells were concurrently treated with Lactacystin and MG132. This effect may be due to changes in both the level and activity of proapoptotic and antiapoptotic proteins. Inhibitor-induced decrease in IKK proteins and processing of p105 to p50 may lead to a decrease in the function of prosurvival proteins, such as XIAP, BCL2, BCLXL, and MCL-1. Moreover, stabilization and expression of proapoptotic proteins in treated cells induced higher apoptosis and overcame the protection of survival proteins. These two scenarios are supported by the present results. Tang et al. [47] overexpressed caspase-3 in MCF-7 cells and observed a caspase-3-mediated cleavage of IKKβ when MCF-7 and HeLa cells were treated with TNFα. As observed, increased caspase-3 activity in treated cells may have led to an enhanced proteolytic cleavage of IKKβ. Despite the reduction in IKK proteins and contrary to expectations, phosphorylation of IκBα increased in Lactacystin- and MG132-treated cells due to the inhibition of proteasomal activity. The increase in Lactacystin-mediated IκBα phosphorylation was likely responsible for the observed increase in NFκB activity. Surprisingly, increased NFκB activity in Lactacystin-treated cells coincided with enhanced apoptosis, providing an interesting model that can be used to further explore the mechanisms involved in apoptotic response, including proapoptotic functions of NFκB.
Many short-lived proteins are known to induce apoptosis. Activated caspase-3 induces DNA damage through the cleavage of PARP and BRCA1, which signals ATM and ATR to directly phosphorylate p53, thereby increasing the stability and transcriptional activity of p53 [48,49]. Our results demonstrate increased p53 transcriptional activity in Lactacystin-treated cells correlating with apoptosis. Although MG132, by itself, did not increase transcriptional activity, a combination of Lactacystin and MG132 resulted in lower luciferase activity. These results are similar to other observations in which increased levels of Velcade were used to treat a variety of cancers. Williams and McConkey [50] reported an increase in not only the stability of nuclear MDM2-P53, but also in the ability of the complex to bind a p53 DNA consensus sequence. The increase in p53 activity observed in proteasomal inhibitor-treated cells is significant in light of the report that p53 repressed the expression of IKKα by competitively sequestering ETS-1 from the IKKα promoter [51]. This may explain the observed decrease in IKKα and the increase in p21WAF1, which may be responsible for the decreased activity of NFκB. The high degree of NFκB activity in proteasome inhibitor-treated LNCaP cells may be due to the crosstalk between NFκB and p53 [52,53]. Furthermore an NFκB-binding site has been demonstrated in the p53 gene, suggesting that an increase in NFκB activity could increase the level of p53 protein expression [54].
Conclusion
Arresting proteasome function with Velcade has certainly proven to be beneficial in arresting the progression of tumorigenesis. Preclinical and clinical trials demonstrate the need to improve the efficiency and efficacy of the drug [35,38,55–58]. The present study suggests that the treatment of prostate cancer with two chemically distinct proteasome inhibitors may be superior to the use of any one proteasome inhibitor alone. In this preliminary report, the effectiveness of proteasomal inhibitors is the sum total of its effects on both the apoptotic and the antiapoptotic pathways in cancer cells.
References
- 1.Syed S, Tolcher A. Innovative therapies for prostate cancer treatment. Rev Urol. 2003;5:S78–S84. [PMC free article] [PubMed] [Google Scholar]
- 2.Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 3.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 5.Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 6.Bottero V, Rossi F, Samson M, Mari M, Hofman P, Peyron JF. Ikappa b-alpha, the NF-kappa B inhibitory subunit, interacts with ANT, the mitochondrial ATP/ADP translocator. J Biol Chem. 2001;276:21317–21324. doi: 10.1074/jbc.M005850200. [DOI] [PubMed] [Google Scholar]
- 7.Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS., Jr NF-kappa B and I kappaB alpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappa B. J Biol Chem. 2003;278:2963–2968. doi: 10.1074/jbc.M209995200. [DOI] [PubMed] [Google Scholar]
- 8.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 9.Malek S, Huxford T, Ghosh G. Ikappa Balpha functions through direct contacts with the nuclear localization signals and the DNA binding sequences of NF-kappaB. J Biol Chem. 1998;273:25427–25435. doi: 10.1074/jbc.273.39.25427. [DOI] [PubMed] [Google Scholar]
- 10.Quirling M, Page S, Jilg N, Plenagl K, Peus D, Grubmuller C, Weingartner M, Fischer C, Neumeier D, Brand K. Detection of IKKbeta-IKKgamma subcomplexes in monocytic cells and characterization of associated signaling. J Biol Chem. 2004;279:37452–37460. doi: 10.1074/jbc.M312119200. [DOI] [PubMed] [Google Scholar]
- 11.Verma UN, Yamamoto Y, Prajapati S, Gaynor RB. Nuclear role of I kappa B kinase-gamma/NF-kappa B essential modulator (IKK gamma/NEMO) in NF-kappaB-dependent gene expression. J Biol Chem. 2004;279:3509–3515. doi: 10.1074/jbc.M309300200. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Elwood J, Greene WC. Both amino- and carboxylterminal sequences within I kappa B alpha regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 14.O'Mahony A, Lin X, Geleziunas R, Greene WC. Activation of the heterodimeric IkappaB kinase alpha (IKKalpha)-IKKbeta complex is directional: IKKalpha regulates IKKbeta under both basal and stimulated conditions. Mol Cell Biol. 2000;20:1170–1178. doi: 10.1128/mcb.20.4.1170-1178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Yin MJ, Gaynor RB. IkappaB kinase alpha (IKKalpha) regulation of IKKbeta kinase activity. Mol Cell Biol. 2000;20:3655–3666. doi: 10.1128/mcb.20.10.3655-3666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 18.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 20.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 21.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Jr, Whang YE. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277:11116–11125. doi: 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 22.Peters JM, Cejka Z, Harris JR, Kleinschmidt JA, Baumeister W. Structural features of the 26 S proteasome complex. J Mol Biol. 1993;234:932–937. doi: 10.1006/jmbi.1993.1646. [DOI] [PubMed] [Google Scholar]
- 23.Peters JM, Franke WW, Kleinschmidt JA. Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm. J Biol Chem. 1994;269:7709–7718. [PubMed] [Google Scholar]
- 24.Yoshimura T, Kameyama K, Takagi T, Ikai A, Tokunaga F, Koide T, Tanahashi N, Tamura T, Cejka Z, Baumeister W. Molecular characterization of the “26S” proteasome complex from rat liver. J Struct Biol. 1993;111:200–211. doi: 10.1006/jsbi.1993.1050. [DOI] [PubMed] [Google Scholar]
- 25.Koster AJ, Walz J, Lupas A, Baumeister W. Structural features of archaebacterial and eukaryotic proteasomes. Mol Biol Rep. 1995;21:11–20. doi: 10.1007/BF00990965. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Goldberg AL. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 27.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 28.Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;16:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hideshima T, Podar K, Chauhan D, Ishitsuka K, Mitsiades C, Tai YT, Hamasaki M, Raje N, Hideshima H, Schreiner G, et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene. 2004;23:8766–8776. doi: 10.1038/sj.onc.1208118. [DOI] [PubMed] [Google Scholar]
- 30.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific aminoterminal threonine modification by Lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 31.Myung J, Kim KB, Crews CM. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrowska H, Wojcik C, Omura S, Worowski K. Lactacystin, a specific inhibitor of the proteasome, inhibits human platelet lysosomal cathepsin A-like enzyme. Biochem Biophys Res Commun. 1997;234:729–732. doi: 10.1006/bbrc.1997.6434. [DOI] [PubMed] [Google Scholar]
- 33.Rivett AJ, Gardner RC. Proteasome inhibitors: from in vitro uses to clinical trials. J Pept Sci. 2000;6:478–488. doi: 10.1002/1099-1387(200009)6:9<478::AID-PSC285>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Fan XM, Wong BC, Wang WP, Zhou XM, Cho CH, Yuen ST, Leung SY, Lin MC, Kung HF, Lam SK. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001;93:481–488. doi: 10.1002/ijc.1373. [DOI] [PubMed] [Google Scholar]
- 35.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl-Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 36.Tenev T, Marani M, McNeish I, Lemoine NR. Pro-caspase-3 overexpression sensitises ovarian cancer cells to proteasome inhibitors. Cell Death Differ. 2001;8:256–264. doi: 10.1038/sj.cdd.4400808. [DOI] [PubMed] [Google Scholar]
- 37.An J, Sun Y, Fisher M, Rettig MB. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 2004;3:727–736. [PubMed] [Google Scholar]
- 38.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 39.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, et al. A Phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 40.Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 41.Delcros JG, Floc'h MB, Prigent C, Arlot-Bonnemains Y. Proteasome inhibitors as therapeutic agents: current and future strategies. Curr Med Chem. 2003;10:479–503. doi: 10.2174/0929867033368231. [DOI] [PubMed] [Google Scholar]
- 42.Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–369. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- 43.SAS Institute, author. SAS/STAT User's Guide. 1 and 2. Cary, NC: SAS Institute Inc.; 1999. Version 8. [Google Scholar]
- 44.Adams J. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol Med. 2002;8:S49–S54. doi: 10.1016/s1471-4914(02)02315-8. [DOI] [PubMed] [Google Scholar]
- 45.Ikezoe T, Yang Y, Saito T, Koeffler HP, Taguchi H. Proteasome inhibitor PS-341 down-regulates prostate-specific antigen (PSA) and induces growth arrest and apoptosis of androgen-dependent human prostate cancer LNCaP cells. Cancer Sci. 2004;95:271–275. doi: 10.1111/j.1349-7006.2004.tb02215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 47.Tang G, Yang J, Minemoto Y, Lin A. Blocking caspase-3-mediated proteolysis of IKKbeta suppresses TNF-alpha-induced apoptosis. Mol Cell. 2001;8:1005–1016. doi: 10.1016/s1097-2765(01)00380-x. [DOI] [PubMed] [Google Scholar]
- 48.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;26:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 49.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 50.Williams SA, McConkey DJ. The proteasome inhibitor bortezomib stabilizes a novel active form of p53 in human LNCaP-Pro5 prostate cancer cells. Cancer Res. 2003;63:7338–7344. [PubMed] [Google Scholar]
- 51.Gu L, Zhu N, Findley HW, Woods WG, Zhou M. Identification and characterization of the IKKalpha promoter: positive and negative regulation by ETS-1 and p53, respectively. J Biol Chem. 2004;279:52141–52149. doi: 10.1074/jbc.M407915200. [DOI] [PubMed] [Google Scholar]
- 52.Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 53.Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J Biol Chem. 1999;274:1879–1882. doi: 10.1074/jbc.274.4.1879. [DOI] [PubMed] [Google Scholar]
- 54.Benoit V, Hellin AC, Huygen S, Gielen J, Bours V, Merville MP. Additive effect between NF-kappaB subunits and p53 protein for transcriptional activation of human p53 promoter. Oncogene. 2000;19:4787–4794. doi: 10.1038/sj.onc.1203831. [DOI] [PubMed] [Google Scholar]
- 55.Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther. 2003;2:835–843. [PubMed] [Google Scholar]
- 56.Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Opin Chem Biol. 2002;6:493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 57.Adams J. Proteasome inhibitors as new anticancer drugs. Curr Opin Oncol. 2002;14:628–634. doi: 10.1097/00001622-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Nawrocki ST, Bruns CJ, Harbison MT, Bold RJ, Gotsch BS, Abbruzzese JL, Elliott P, Adams J, McConkey DJ. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2002;1:1243–1253. [PubMed] [Google Scholar]