Abstract
Amplification and/or overexpression of genes encoding tyrosine kinase receptors KIT and ERBB2 have been reported in testicular germ cell tumors (TGCTs). These receptors can bind the adaptor molecule GRB7 encoded by a gene adjacent to ERBB2 at 17q12, a region also frequently gained in TGCTs. GRB7 binding may be involved in the activation of RAS signaling and KRAS2 maps to 12p, which is constitutively gained in TGCT and lies within a minimum overlapping region of amplification at 12p11.2-12.1, a region we have previously defined. RAS proteins activate BRAF, and activating mutations of genes encoding these proteins have been described in various tumors. Here we determine the relationships between expression levels and activating mutations of these genes in a series of 65 primary TGCTs and 4 TCGT cell lines. High levels of expression and activating mutations in RAS were mutually exclusive events, and activating mutations in RAS were only identified in the seminoma subtype. Mutations in BRAF were not identified. Increased ERBB2 expression was associated with differentiated nonseminoma histology excised from lymph nodes postchemotherapy. Mutation, elevated expression, and correlations between expression levels of KRAS2, GRB7, and KIT are consistent with their involvement in the development of TGCTs.
Keywords: TGCT, RAS, GRB7, ERBB2, BRAF
Introduction
Testicular germ cell tumors (TGCTs) of adolescents and adults are the most common tumors in young men [1]. They are classified into two main histologic categories: seminoma (SE) and nonseminoma (NSE). SE resemble primordial germ cells, and NSE are composed of neoplastic tissues exhibiting either somatic, embryonal, or extraembryonal tissues [1]. Primordial germ cells are generally considered as the cells of origin of TGCT and are believed to give rise to carcinoma in situ (CIS), which is also known as intratubular germ cell neoplasia—a precursor lesion of both SE and NSE [2].
TGCTs consistently show gain of the short arm of chromosome 12 [3], and we have previously mapped a minimum overlapping region of gain at 12p11.2-p12.1 [4]. This region includes the KRAS2 gene, although one case with amplification of the region that does not include KRAS2 is reported [5]. RAS has a significant role in normal germ cell development, affecting proliferation and migration [6]. A high expression of KRAS2 has been reported in a small number of TGCT samples and cell lines, and activating mutations in RAS genes have been described in 10% to 40%; a review of the literature in 1995 determined the overall frequency at 10% [7–9]. Activating mutations or enhanced expressions of normal RAS genes are established enough to transform NIH3T3 cells and have been associated with many tumor types [10,11].
RAS activation can result in signaling through the RAS/RAF/MEK/ERK/MAP kinase pathway. Activation of ERK through phosphorylation has been recently reported in the majority of SE and NSE [9]. Activating mutations of BRAF have been identified in a wide range of tumors, including melanoma and colorectal neoplasms [12,13]. Activating mutations of BRAF also have the ability to transform NIH3T3 cells, and cell lines do not necessarily require RAS coactivation, suggesting functional redundancy in some tumor types [12]. However, the mutual exclusivity of activating mutations in RAS and BRAF is not the case in all tumors, and BRAF mutations have been found in pancreatic cancers with KRAS2 codon 12 mutations [14]. The MAP kinase pathway could therefore be affected by BRAF mutations in addition to other mechanisms and may potentially be involved in the pathogenesis of TGCT.
KIT and ERBB2 are receptor tyrosine kinases that are known to activate the RAS signaling cascade [15,16]. We have recently identified gain in the copy number of a small region at 4q12, and we have shown that KIT is the only gene amplified from this region in some cases [17] (McIntyre, submitted for publication). Specific amplification and previously described activating mutations for KIT suggest a role for this gene product in the development of TGCT [18,19]. ERBB2 maps to a region of recurrent gain at 17q12 in TGCTs (http://www.cgap.nci.nih.gov/Chromosomes/Mitelman; http://www.helsinki.fi/cmg/cgh_data.html); in one study, increased expression has been associated with adverse clinical outcomes in NSE [20].
GRB7 also maps to 17q12 and has been shown to be overexpressed in some TGCTs [21,22]. GRB7 is an adapter molecule that can bind directly to KIT and ERBB2 through its SH2 domain [23] and is also proposed to bind directly to RAS through its RAS association domain. In this way, GRB7 may have an important role in the regulation of the downstream signaling of these kinases. Furthermore, GRB7 is also known to play a role in cell migration [23].
The genomic alterations in TGCT described above and the potential functional links and roles for candidate genes from these regions prompted us to further investigate these genes in TGCTs. Here we have examined the copy number of KRAS2 in CIS adjacent to a tumor shown to have amplification of this gene and to have screened for activating BRAF mutations in TGCTs. Through analysis of a large number of samples, we have demonstrated the relationships between activating mutations, copy numbers, and expression levels of KRAS2, KIT, ERBB2, and GRB7, which are indicative of their involvement in the cellular behavior of TGCTs.
Materials and Methods
Tumor Samples
Snap-frozen primary tumor samples histologically verified as SE (32), NSE (27), and combined tumors (6) were all collected through the Royal Marsden Hospital NHS Trust. Six single samples (SE, five; NSE, one) were obtained from patients with sporadic bilateral disease, and two further cases of SE had a family history of TGCT. Patient consent and ethical approval were obtained for the use of this material in the study, in accordance with the tenets of the Helsinki Declaration. The cell lines Tera1, Tera2, GCT27, GCT44, HT-29, Colo205, and MAWI were cultured as described in Summersgill et al. [24] and ATCC (Manassas, VA; http://www.atcc.org). Genomic DNA was prepared using standard procedures, and RNA was extracted using Trizol, in accordance with the manufacturer's instructions (Life Technologies, Grand Island, NY). Commercially available normal pooled testis tissues from 45 samples were used as controls in expression experiments (BD Biosciences, Palo Alto, CA). CIS and corresponding tumor tissues from three samples were microdissected from Bouins-fixed paraffin-embedded tissues, and DNA was extracted as previously described [25].
Mutation Analysis of KRAS and NRAS
DNA from cell lines with known activating mutations of KRAS and NRAS at codons 12, 13, and 61 were used as controls. These and the test samples were screened by sequencing exons 2 and 3 of both genes [8]. The primers used to amplify the sequence of interest were as follows: KRAS2 exon 2: forward 5′-TTAACCTTATGTGTGACATGTTCTAA-3′, reverse 5′-CCTTTATCTGTATCAAAGAATGGTC-3′; KRAS2 exon 3: forward 5′-TCTTTGGAGCAGGAACAATG-3′, reverse 5′-TGCATGGCATTAGCAAAGAC-3′; NRAS exon 2: forward 5′-GGGTTTTCATTTCCATTGATT-3′, reverse 5′-ATTCTTTATACAGAATATGGGTAAA-3′; NRAS exon 3: forward 5′-GGCAGAAATGGGCTTGAATA-3′, reverse 5′AAGCTCTATCTTCCCTAGTGTGGT-3′. Polymerase chain reaction (PCR) products were bidirectionally screened by direct sequence analysis on an ABI3100 sequencer using the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Mutations were confirmed by repeat sequencing analysis from an independently amplified reaction. Negative controls (no DNA) and normal controls (pooled normal DNA) were included in every set of amplification and sequencing analysis.
Mutation Analysis of BRAF
DNA from cell lines HT-29, Colo205, and MAWI with known activating mutations of BRAF in exon 11 at G1403C (G468A) and in exon 15 at T1796A (S599E) and C1786G (L596V) were used as controls [12]. These and the TGCT samples were screened by conformational sensitive gel electrophoresis (CSGE). Exon 11 was amplified using the forward primer 5′-TTGGCTTGACTTGACTTT-3′ and the reverse primer 5′-ATCCTATTATGACTTGTC-3′. Exon 15 was amplified using the forward primer 5′-TTCATAATGCTTGCTCTGATAGG-3′ and the reverse primer 5′-TGTGAATACTGGGAACTATGAAAA-3′. Primers were 5′-end-labeled with 32P. DNA were amplified in 20-µl PCR reactions of 30 seconds at 94°C; 30 seconds at 68°C to 50°C touchdown and 55°C, respectively; followed by 1 minute at 72°C for 30 cycles. In exon 15, the positive control cell lines HT-29, Colo205, and MAWI were used and were consistently positive. The products were resolved on a 6% acrylamide gel under semidenaturing conditions. No positive controls were available for exon 11; therefore, 4 of 65 random samples were also sequenced. PCR products were bidirectionally screened by direct sequence analysis on an ABI3100 sequencer using the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Negative controls (no DNA) and normal controls (pooled normal DNA) were included in every set of amplification and CSGE and sequencing analyses.
Quantitative PCR Analyses
Quantitative PCR and reverse transcription (RT)-PCR were performed using the ABI PRISM 7700 Sequence Detection System, in accordance with the manufacturer's instructions (Applied Biosystems). B2-microglobulin (B2M) was used as endogenous control in expression studies, whereas POLR2D at 2q21 was used as endogenous control in genomic studies as this region rarely shows loss or gain in samples according to reported karyotype and comparative genomic hybridization (CGH) analyses (http://www cgap.nci.nih.gov/Chromosomes/Mitelman; http://www.helsinki.fi/cmg/cgh_data.html). Expression data were normalized to pooled normal testis RNA, and genomic data were normalized to normal male DNA. Primer and probe sets for the genes GRB7, ERBB2, and the control B2M were purchased from Applied Biosystems (assays Hs00180450, Hs00170433, and Hs99999907). Primers and probes designed for genomic analysis of POLR2D (chromosome 2 control), KRAS2, and expression analysis of KRAS2 were as follows: RBP4: forward 5′-CCCAGGTGACATGGAATCTTG-3′, probe 5′-AGCCTTGTGCAGTGGCAGCCAGT-3′, reverse 5′-GCAGAGGCACGTTCAGGAA-3′; KRAS2 genomic: forward 5′-TCTCGACACAGCAGGTCAAGA-3′, probe 5′-AGTACAGTGCAATGAGGGACCAGTACATGAGG-3′, reverse 5′-CTTCAAATGATTTAGTATTATTTATGGCAAAT-3′; KRAS2 expression: forward, probe, reverse. Both the B2M and POLR2D control used were Vic-labeled. All test sets were labeled with Fam. Quantification of each sample was determined by averaging the results from three separate reactions.
Results
Mutation Analysis of KRAS2, NRAS, and BRAF
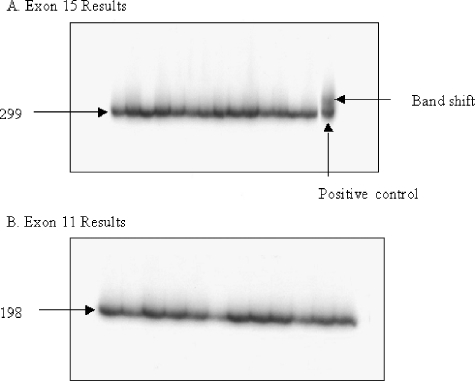
Activating mutations in codons 12 and 13 of KRAS2 and NRAS were identified through direct sequencing in four and one primary tumor samples, respectively (8% of all TGCTs). The four KRAS2 mutations were as follows: two G12V, one G12C, and one G13N. The NRAS mutation was G12V. All five mutations were determined in primary tumors of the SE subtype (16%). No activating RAS mutations were determined in the NSE primary tumors or cell lines. No mutations of BRAF were detected in any of the TGCT primary samples or cell lines. An example of these results can be seen in Figure 1.
Figure 1.
BRAF CSGE mutation screen. The results shown are a representation of the 65 primary tumor samples and the 4 cell lines screened. The results show no double-band products, which would indicate the presence of a mutation, in any sample except in the exon 15-positive control. (A) Exon 15 results. (B) Exon 11 results.
Quantitative PCR Analyses
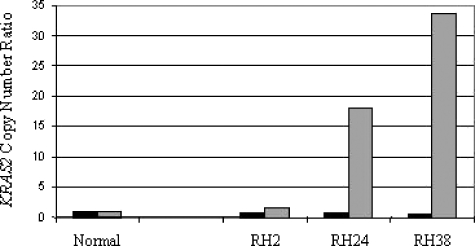
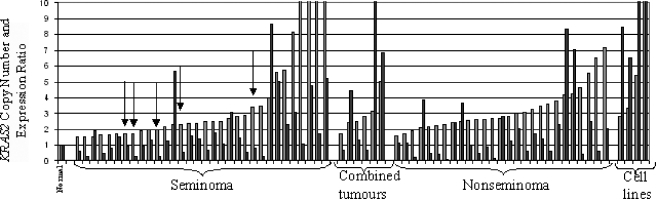
Using quantitative techniques, we determined the copy number and expression levels of KRAS2 and the expression of ERBB2 and GRB7 in the 65 primary TGCT and 4 cell lines. KRAS2 copy number was increased in all cases, in keeping with the gain of 12p found in all TGCTs. However, KRAS2 copy number was not increased in CIS (Figure 2), which was microdissected as previously described [25]. Twenty-eight percent of SE, 50% of combined tumors, 39% of NS, and all the cell lines showed an increased expression of KRAS2, which was two-fold greater than that of normal testis control (Figure 3). KRAS2 expression in normal testis was greater than in any normal tissue examined, with an expression level that was 5.5-fold greater than the average of a range of 17 other normal tissues examined. Increased expressions of KRAS2 and activating RAS mutations were mutually exclusive events in the samples analyzed here. A significant positive correlation was determined between the genomic copy number and the expression of KRAS2 in TGCTs (ρ = 0.456, P < .01, n = 65).
Figure 2.
KRAS2 copy number data for CIS adjacent to tumor. Dark bars represent the copy number ratio in CIS adjacent to invasive tumor; lighter bars denote copy number ratios of the tumor.
Figure 3.
Copy number and expression ratios for KRAS2 in TGCTs. Dark bars represent the copy number ratio of tumor samples and cell lines; lighter bars denote corresponding expression ratios. Samples with activating mutations in KRAS2 or NRAS are indicated with arrows.
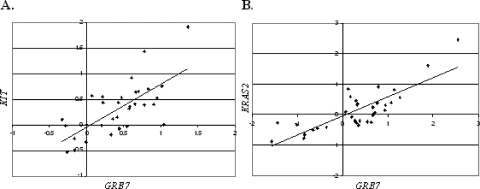
Increased levels of GRB7 expression levels relative to testis were noted in 63% of primary tumor samples and in cell lines showing an expression ratio greater than two-fold that of normal testis. Seven primary tumor samples (NSE, five; SE, two) and all four cell lines showed an increased expression of ERBB2 greater than two-fold. All five NSE cases with high expression of ERBB2 were cases wherein the material was from extragonadal sites retrieved postchemotherapy with differentiated teratoma histology. The two SE cases with increased ERBB2 expression were both primary site tumors associated with no known metastasis or relapse. The expression levels quantified for KRAS2, ERBB2, and GRB7 and our previously reported results for KIT [26] were compared to examine correlations in their expression patterns. In SE, a significant positive correlation was determined between the expression of three genes: KIT and KRAS2 (ρ = 0.569, P < .01, n = 32), KIT and GRB7 (ρ = 0.632, P < .01, n = 37), and KRAS2 and GRB7 (ρ = 0.726, P < .01, n = 32). In NS, a significant positive correlation was determined between the expression of KRAS2 and GRB7 (ρ = 0.712, P < .01, n = 33), but not between KIT and GRB7 (ρ = 0.311, P = .078, n = 32) (examples of correlations can be seen in Figure 4). ERBB2 expression did not correlate with the expression of the other genes studied here.
Figure 4.
Examples of correlations between gene expression levels in TGCT. (A) Correlation between log 2 ratio expression levels of KIT and GRB7 in SE (ρ = 0.632, P < .01, n = 31). (B) Correlation between log 2 ratio expression levels of KRAS2 and GRB7 in NS (ρ = 0.712, P < .01, n = 33).
Discussion
Mutation, increased copy number, and overexpression of genes are associated with malignant phenotypes. Previous definition of minimum overlapping regions of gain at 12p11.2-12.1 and 4q12 and reports of gain at 17q12 in TGCTs encompass the genes KRAS2, KIT, ERBB2, and GRB7 as candidates for involvement in the development of TGCTs. [4,17,21,22]. The relationships between mutation status and/or expression levels of these genes and BRAF have been determined here in a series of 65 primary TGCT samples and 4 NSE cell lines in the light of their genomic positions and known functional interactions.
The copy number analysis of KRAS2 that maps to 12p did not identify copy number gain in the precursor lesion CIS, whereas KRAS2 copy number increase was found in all TGCT tumor samples. This is in keeping with previous CGH analyses that did not show 12p gains in CIS adjacent to the tumor [25]. These data are consistent with an increased copy number of KRAS2/12p being involved in tumor progression [25,27,28]. A positive correlation was determined between KRAS2 copy number and level of expression; therefore, a gain in the region is associated with an increased expression of KRAS2. Increased expression of wild-type RAS will transform NIH3T3 cells [10,11]. It is therefore possible that increased expression of KRAS2 activates RAS signaling in 35% of TGCTs, and activating mutations of RAS are found in a further 8% of the TGCTs. Activating RAS mutations were confined to the SE subtype here, although they have previously been reported in NSE [7–9]. No increased expression of KRAS2 was identified in the five samples with activating RAS mutations. Therefore, activation of RAS through either overexpression or mutation may be important in the progression from CIS to TGCT.
Activating mutations of BRAF can also transform NIH3T3 cells, and cell lines with an activating mutation of BRAF do not require RAS activation, suggesting functional redundancy in some tumor types [12]. Here we sought to determine whether activating mutations of BRAF were present in TGCTs, particularly in samples without overexpression or mutation of RAS. No BRAF mutations were identified, consistent with the results of a previous study that included six TGCT cell lines [12]. Recently, activating BRAF mutations have been found within the embryonal carcinoma component of 9% of NSE samples, although we did not look specifically at individual NSE components in this study [9].
Increased expression of ERBB2 is associated with other types of malignancy, including breast carcinoma, where it is known to increase the activation of RAS signaling through its association with GRB2 and SOS [16]. GRB7 is also known to bind directly to both ERBB2 and KIT and is proposed also to bind to RAS proteins through its RAS association domain. Here we found increased expression of ERBB2 only in NSEs of differentiated teratoma histology, excised from the lymph nodes postchemotherapy. This is consistent with another study demonstrating an increased expression of ERBB2 by immunohistochemistry in differentiated NSEs and highlighting an association with adverse clinical outcome [20]. In addition, we found increased expression levels in two SE samples with localized disease. All the cell lines derived from NSE had increased expression of ERBB2. There is no difference in expression between the cell line GCT27 and its clonally selected derivative GCT27R, which is resistant to cisplatin. Further molecular studies are required to elucidate the association between adverse clinical outcome and increased ERBB2 expression.
GRB7 is known to have a role in cell migration through its association with EPHB1 and is proposed to modulate the pattern of RAS signaling [23]. Greater-than-two-fold expression levels of GRB7 relative to normal testis were noted in 63% of primary tumor samples and in cell lines. This is consistent with previous work showing increased GRB7 RNA and protein in TGCTs [21,22]. No distinct pattern of phosphorylation of MAPK, STAT3, or AKT has been found in cases with or without activating KIT mutations, and activated ERK has been recently been reported in almost all SEs and NSEs [9,18].
A significant correlation between expressions of genes encoding interacting proteins has been shown for lower organisms and has been recently demonstrated for Homo sapiens [29]. The correlations between expression levels in the tumors examined in this study indicate interactions between KIT, KRAS2, and GRB7 in SE and only between KRAS2 and GRB7 in NSE, where KIT is rarely overexpressed. Gain of KRAS2 and specific amplification of KIT, in combination with coordinated patterns of mutation and expression of KRAS2, KIT, and GRB7 genes, imply their involvement in TGCT development, particularly the involvement of KIT in SE. This may maintain or reestablish in TGCT the migratory and proliferative behavior of primordial germ cells, which has been shown to involve KIT and RAS activation in mice [30].
The implication of KIT, ERBB2, RAS, or GRB7 in TGCTs and the further unraveling of the signaling involved should provide specific points at which therapies could be targeted [31–33].
Acknowledgements
The authors thank Jeremy Clark for his helpful comments and Martin Pera for providing the cell lines GCT27 and GCT44.
Footnotes
The authors are grateful to the Lance Armstrong Foundation and Cancer Research UK for support.
References
- 1.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 2.Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 3.Atkin NB, Baker MC. Specific chromosome change, i(12p), in testicular tumours? Lancet. 1982;2:1349. doi: 10.1016/s0140-6736(82)91557-4. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez S, Jafer O, Goker H, Summersgill BM, Zafarana G, Gillis AJ, van Gurp RJ, Oosterhuis JW, Lu YJ, Huddart R, et al. Expression profile of genes from 12p in testicular germ cell tumors of adolescents and adults associated with i(12p) and amplification at 12p11.2-p12.1. Oncogene. 2003;22:1880–1891. doi: 10.1038/sj.onc.1206302. [DOI] [PubMed] [Google Scholar]
- 5.Zafarana G, Gillis AJ, van Gurp RJ, Olsson PG, Elstrodt F, Stoop H, Millan JL, Oosterhuis JW, Looijenga LH. Coamplification of DAD-R, SOX5, and EKI1 in human testicular seminomas, with specific overexpression of DAD-R, correlates with reduced levels of apoptosis and earlier clinical manifestation. Cancer Res. 2002;62:1822–1831. [PubMed] [Google Scholar]
- 6.Li J, Xia F, Li WX. Coactivation of STAT and Ras is required for germ cell proliferation and invasive migration in Drosophila. Dev Cell. 2003;5:787–798. doi: 10.1016/s1534-5807(03)00328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moul JW, Theune SM, Chang EH. Detection of RAS mutations in archival testicular germ cell tumors by polymerase chain reaction and oligonucleotide hybridization. Genes Chromosomes Cancer. 1992;5:109–118. doi: 10.1002/gcc.2870050204. [DOI] [PubMed] [Google Scholar]
- 8.Olie RA, Looijenga LH, Boerrigter L, Top B, Rodenhuis S, Langeveld A, Mulder MP, Oosterhuis JW. N- and KRAS mutations in primary testicular germ cell tumors: incidence and possible biological implications. Genes Chromosomes Cancer. 1995;12:110–116. doi: 10.1002/gcc.2870120205. [DOI] [PubMed] [Google Scholar]
- 9.Sommerer F, Hengge UR, Markwarth A, Vomschloss S, Stolzenburg JU, Wittekind C, Tannapfel A. Mutations of BRAF and RAS are rare events in germ cell tumours. Int J Cancer. 2004;113(2):329–335. doi: 10.1002/ijc.20567. [DOI] [PubMed] [Google Scholar]
- 10.Chang EH, Furth ME, Scolnick EM, Lowy DR. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982;297:479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- 11.McCoy MS, Toole JJ, Cunningham JM, Chang EH, Lowy DR, Weinberg RA. Characterization of a human colon/lung carcinoma oncogene. Nature. 1983;302:79–81. doi: 10.1038/302079a0. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Ikehara N, Semba S, Sakashita M, Aoyama N, Kasuga M, Yokozaki H. BRAF mutation associated with dysregulation of apoptosis in human colorectal neoplasms. Int J Cancer. 2005;115(6):943–950. doi: 10.1002/ijc.20957. [DOI] [PubMed] [Google Scholar]
- 14.Ishimura N, Yamasawa K, Karim Rumi MA, Kadowaki Y, Ishihara S, Amano Y, Nio Y, Higami T, Kinoshita Y. BRAF and K-ras gene mutations in human pancreatic cancers. Cancer Lett. 2003;199:169–173. doi: 10.1016/s0304-3835(03)00384-7. [DOI] [PubMed] [Google Scholar]
- 15.Hong L, Munugalavadla V, Kapur R. c-Kit-mediated overlapping and unique functional and biochemical outcomes via diverse signaling pathways. Mol Cell Biol. 2004;24:1401–1410. doi: 10.1128/MCB.24.3.1401-1410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre A, Summersgill B, Jafer O, Rodriguez S, Zafarana G, Oosterhuis JW, Gillis AJ, Looijenga L, Cooper C, Huddart R, et al. Defining minimum genomic regions of imbalance involved in testicular germ cell tumors of adolescents and adults through genome wide microarray analysis of cDNA clones. Oncogene. 2004;23(56):9142–9147. doi: 10.1038/sj.onc.1208115. [DOI] [PubMed] [Google Scholar]
- 18.Kemmer K, Corless CL, Fletcher JA, McGreevey L, Haley A, Griffith D, Cummings OW, Wait C, Town A, Heinrich MC. KIT mutations are common in testicular seminomas. Am J Pathol. 2004;164:305–313. doi: 10.1016/S0002-9440(10)63120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looijenga LH, de Leeuw H, van Oorschot M, van Gurp RJ, Stoop H, Gillis AJ, de Gouveia Brazao CA, Weber RF, Kirkels WJ, van Dijk T, et al. Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ-cell tumors. Cancer Res. 2003;63:7674–7678. [PubMed] [Google Scholar]
- 20.Mandoky L, Geczi L, Bodrogi I, Toth J, Csuka O, Kasler M, Bak M. Clinical relevance of HER-2/neu expression in germ-cell testicular tumors. Anticancer Res. 2004;24:2219–2224. [PubMed] [Google Scholar]
- 21.Skotheim RI, Monni O, Mousses S, Fossa SD, Kallioniemi OP, Lothe RA, Kallioniemi A. New insights into testicular germ cell tumorigenesis from gene expression profiling. Cancer Res. 2002;62:2359–2364. [PubMed] [Google Scholar]
- 22.Skotheim RI, Abeler VM, Nesland JM, Fossa SD, Holm R, Wagner U, Florenes VA, Aass N, Kallioniemi OP, Lothe RA. Candidate genes for testicular cancer evaluated by in situ protein expression analyses on tissue microarrays. Neoplasia. 2003;5:397–404. doi: 10.1016/s1476-5586(03)80042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han DC, Shen TL, Guan JL. The Grb7 family proteins: structure, interactions with other signaling molecules and potential cellular functions. Oncogene. 2001;20:6315–6321. doi: 10.1038/sj.onc.1204775. [DOI] [PubMed] [Google Scholar]
- 24.Summersgill BM, Jafer O, Wang R, Goker H, Niculescu-Duvaz I, Huddart R, Shipley J. Definition of chromosome aberrations in testicular germ cell tumor cell lines by 24-color karyotyping and complementary molecular cytogenetic analyses. Cancer Genet Cytogenet. 2001;128:120–129. doi: 10.1016/s0165-4608(01)00414-9. [DOI] [PubMed] [Google Scholar]
- 25.Summersgill B, Osin P, Lu YJ, Huddart R, Shipley J. Chromosomal imbalances associated with carcinoma in situ and associated testicular germ cell tumours of adolescents and adults. Br J Cancer. 2001;85:213–220. doi: 10.1054/bjoc.2001.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre A, Summersgill B, Grygalewicz B, Gillis AJM, Stoop J, van Gurp RJHLM, Dennis N, Fisher C, Huddart R, Cooper C, et al. Amplification and overexpression of the KIT gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. 2005;65(18):1–5. doi: 10.1158/0008-5472.CAN-05-0471. (in press) [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg C, Van Gurp RJ, Geelen E, Oosterhuis JW, Looijenga LH. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene. 2000;19:5858–5862. doi: 10.1038/sj.onc.1203950. [DOI] [PubMed] [Google Scholar]
- 28.Ottesen AM, Skakkebaek NE, Lundsteen C, Leffers H, Larsen J, Rajpert-De Meyts E. High-resolution comparative genomic hybridization detects extra chromosome arm 12p material in most cases of carcinoma in situ adjacent to overt germ cell tumors, but not before the invasive tumor development. Genes Chromosomes Cancer. 2003;38:117–125. doi: 10.1002/gcc.10244. [DOI] [PubMed] [Google Scholar]
- 29.Hahn A, Rahnenfuhrer J, Talwar P, Lengauer T. Confirmation of human protein interaction data by human expression data. BMC Bioinformatics. 2005;6:112. doi: 10.1186/1471-2105-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Miguel MP, Cheng L, Holland EC, Federspiel MJ, Donovan PJ. Dissection of the c-Kit signaling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proc Natl Acad Sci USA. 2002;99:10458–10463. doi: 10.1073/pnas.122249399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pero SC, Oligino L, Daly RJ, Soden AL, Liu C, Roller PP, Li P, Krag DN. Identification of novel non-phosphorylated ligands, which bind selectively to the SH2 domain of Grb7. J Biol Chem. 2002;277:11918–11926. doi: 10.1074/jbc.M111816200. [DOI] [PubMed] [Google Scholar]
- 32.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 33.Ross JS, Schenkein DP, Pietrusko R, Rolfe M, Linette GP, Stec J, Stagliano NE, Ginsburg GS, Symmans WF, Pusztai L, et al. Targeted therapies for cancer 2004. Am J Clin Pathol. 2004;122:598–609. doi: 10.1309/5CWP-U41A-FR1V-YM3F. [DOI] [PubMed] [Google Scholar]