Abstract
We evaluated deguelin and silibinin in A/J mice treated with the tobacco-specific carcinogen benzo(a)pyrene (BP) for their ability to inhibit pulmonary adenoma formation and growth. Animals were treated with either deguelin (5.0 or 10.0 mg/kg body weight, by gavage) or silibinin at doses of 0.05% and 0.1% in the diet, approximately 10 days before a single intraperitoneal dose of BP. We found that oral administration of deguelin reduced tumor multiplicity by 56% and tumor load by 78%, whereas silibinin treatment at doses of 0.05% and 0.1% in the diet did not show any significant efficacy on either tumor multiplicity or tumor load. The result indicates that deguelin significantly inhibits pulmonary adenoma formation and growth in A/J mice. Finding new and effective agents that can prevent lung cancer is urgently needed because cancer of the lungs remains the principal cause of cancer deaths in the United States and because effective chemoprevention of this cancer type remains elusive. Thus, deguelin appears to be a promising new preventive agent for lung cancer and may be considered for further studies in other animal models and in clinical trials.
Keywords: Chemoprevention, deguelin, silibinin, lung cancer, A/J mice
Introduction
Lung cancer is the leading cause of cancer deaths in men and women in the United States and western Europe [1]. Epidemiological and laboratory animal model studies have demonstrated that smoking and environmental exposure to carcinogens are closely linked to increased lung cancer risk [2–5]. Tobacco exposure has been implicated in 90% of lung carcinomas, and smokers have a 20-fold greater risk of developing lung cancer compared with persons who have never smoked [6–9]. Chemoprevention is a potentially important approach to reduce the large number of tobacco-caused cancer deaths in both current and former smokers. Chemoprevention is the use of pharmacological or natural agents to inhibit the development of cancer. Numerous studies have found that chemoprevention can prevent a wide variety of cancers in multiple animal models [10]. This approach is especially useful in targeting persons who are at high risk for developing cancer, such as those who are at high risk for developing a second primary tumor after surgical removal of a tumor, those who have genotypes that put them at high risk for specific forms of cancer, and those who have preexisting preinvasive lesions [10]. Targets for pharmacological intervention are various stages of tumor development, including hyperplasia and dysplasia. There are two major classes of cancer chemopreventive agents: blocking agents and suppressing agents [11–13]. Blocking agents prevent metabolic activation of carcinogens to reduce the likelihood of DNA damage or to enhance the repair of damaged DNA. Suppressing agents block expansion of carcinogen-initiated cells by suppressing cell replication, stimulating cell differentiation, or causing apoptosis of precancerous or cancerous cells.
Because of similarities in histopathology and tumor progression stages between mouse and human lung adenocarcinomas, the mouse lung tumor model has been used extensively to evaluate the efficacy of putative lung cancer chemopreventive agents [3,14]. Among more than 50 different agents tested, several groups of chemicals, including glucocorticoids and isothiocyanates, have shown significant efficacy against mouse lung tumor development. Glucocorticoids have proven to be successful during the progression stage of tumor development, whereas isothiocyanates have proven particularly effective in blocking carcinogenesis [3,15]. The present investigation is a continuing effort to develop an effective chemoprevention of carcinogenesis of the lungs. There is a great need for agents that can inhibit pulmonary neoplasia with minimal or no toxicity and are effective during different periods of the carcinogenic process. We evaluated two novel agents (deguelin and silibinin) in A/J mice treated with the tobacco-specific carcinogen benzo(a)pyrene (BP) for their ability to inhibit pulmonary adenoma formation and growth.
Deguelin is a rotenoid derived from plant roots [16]. Rotenone and rotenoid-containing botanicals are important insecticides and fish poisons that act by inhibiting NADH: ubiquinone oxidoreductase, an enzyme complex present in mitochondrial oxidative phosphorylation [16]. In addition and potentially more relevant to cancer prevention, deguelin can inhibit the PI3K/AKT pathway. Deguelin has shown chemopreventive efficacy in several in vivo and in vitro models [17–20]. In an animal experimental in vivo system, deguelin reduced tumor incidence in CD-1 mice skin carcinogenesis [16] and tumor multiplicity of mammary tumorigenesis in Sprague-Dawley rats [16]. In addition, treatment with deguelin suppressed the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice [17]. In several in vitro experiments, deguelin has been reported to inhibit the growth of colon cancer cells [20]. It could specifically inhibit the growth of transformed human bronchial epithelial cells and non-small cell lung cancer (NSCLC) cells [18,19].
Silibinin is a naturally occurring polyphenolic flavonoid. Silymarin is composed of mainly silibinin (90%), with small amounts of other silibinin stereoisomers. Several studies in rodent and cell cultures have shown that silibinin is a strong antioxidant that scavenges both free radicals and reactive oxygen species, thereby providing significant protection against different cancers of epithelial origin [21,22]. It has been reported that silibinin inhibits the growth of advanced human prostate carcinoma in nude mice [23] and of skin tumors in SENCAR mice [24]. To date, there are no data on the chemopreventive efficacy of silibinin on lung tumorigenesis.
The goal of this investigation was to discover and develop effective chemopreventive agents against lung tumorigenesis. We determined the capacity of the two agents to prevent pulmonary adenoma formation and growth, and we found that deguelin is a potent inhibitor of lung tumorigenesis in A/J mice.
Materials and Methods
Reagents and Animals
BP, tricaprylin, and silibinin were purchased from Sigma Chemical Co. (St. Louis, MO), whereas deguelin was purchased from Gaia Chemical Corporation (Gaylordsville, CT). Female A/J mice were obtained from Jackson Laboratories (Bar Harbor,ME) at 6 weeks of age. Animals were quarantined for 1 week and housed with wood chip bedding in environmentally controlled, clean-air rooms with a 12-hour light-dark cycle and a 50% relative humidity. Drinking water and diet were supplied ad libitum. The study was approved by the Washington University's Institutional Animal Care and Use Committee.
Animal Experiments
Mice were randomly divided into one control group and two treatment groups. For deguelin testing, the groups consisted of a control group and two groups of animals treated with deguelin at 5.0 or 10.0 mg/kg body weight. Deguelin was dissolved in corn oil just before administration and was administered by gavage (intragastrically) 5 days/week for the duration of the study. Control animals were treated with corn oil (administered intragastrically) throughout the study. For silibinin testing, the groups consisted of a control group treated with AIN-76A purified pellet diet and two groups treated with silibinin at 0.05% and 0.1% (wt/wt) doses mixed with AIN-76A purified powder diet. Foods were prepared once a week with a powerful KitchenAid mixer (St. Joseph, MI) mixing for at least 1 hour. Treatments were initiated 10 days before carcinogen introduction. After 10 days of treatment, all groups received a single intraperitoneal dose of BP (100 mg/kg body weight) in 0.2 ml of tricaprylin. Body weights of mice were measured every 2 weeks.
During the duration of the study, the health condition of the mice was monitored everyday, and body weights were measured every 2 weeks. Mice were sacrificed by CO2 asphyxiation 20 to 21 weeks after carcinogen treatment. For the phenotyping of lung tumors in all bioassays described above, lung tissues were fixed in Tellyesniczky's solution overnight, and then in 75% EtOH. Lung tumor development was estimated by two investigators, using a Leica MZ75 dissecting microscope, to measure the number (N), volume (V), and total tumor load (NV), as reported previously [25]. Volume calculation was based on the formula: V (mm3) = 4/3πr3. Histopathological examinations were performed to determine the diagnosis of lung tumors.
Statistical Analysis
We hypothesized that BP-induced lung tumors are more likely to occur in the carcinogen control group than in the treatment groups. To test this hypothesis, Student's t test was used. The data were obtained from the BP control groups and the different treatment groups in each experiment. We applied square root transformation on tumor numbers because the original data did not follow normal distribution. The transformed data were of normal distribution. Accordingly, Student's t test was used to test the differences between the control groups and the treatment groups.
Results
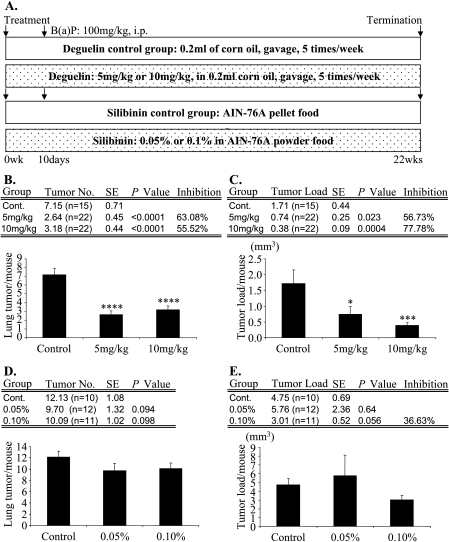
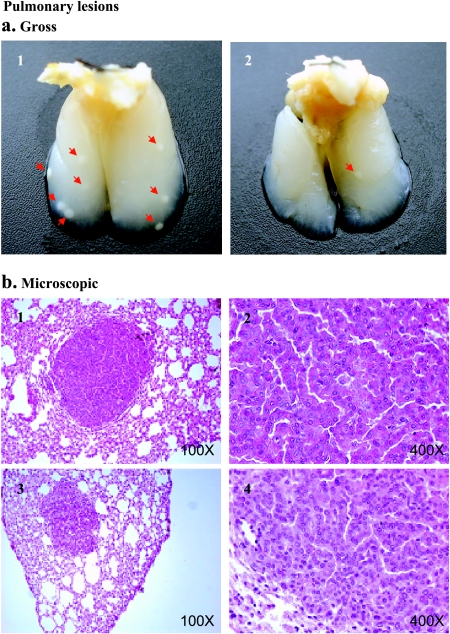
Preventive agents can block tumor initiation either by blocking carcinogen activation, by scavenging reactive carcinogens, or potentially by enhancing DNA damage repair. In addition, agents can suppress the progression of initiated cells. In this study, we used the complete chemoprevention protocol, which involves intervention at 1 to 2 weeks before carcinogen administration. Preventive agents are used throughout the study. This protocol is designed to determine both blocking and suppressing effects on all stages of lung tumorigenesis (initiation, promotion, and progression). Because the two compounds are novel agents, deguelin and silibinin were tested using this protocol based on the rationale that any agent that was ineffective or minimally effective did not warrant further consideration at the doses employed. The mice treated with either deguelin or silibinin showed no signs of gross toxicity or loss of body weight during the experiment (data not shown). The administration of deguelin by gavage decreased tumor multiplicity. The 5-mg/kg dose of deguelin decreased tumor multiplicity from 7.15 ± 0.72 tumors per mouse (n = 15) in the control group to 2.64 ± 0.45 tumors per mouse (n = 22, P < .0001). The higher dose of 10 mg/kg reduced tumor multiplicity to 3.18 ± 0.44 tumors per mouse (n = 22, P < .0001) (Figure 1). Therefore, tumor multiplicity was reduced by 63% and 56% in the 5- and 10-mg/kg groups, respectively (Figure 1). Deguelin treatment showed substantial effects on tumor load: low-dose treatment (5 mg/kg) reduced the tumor load by 57%, whereas high-dose treatment (10 mg/kg) inhibited the tumor load by 78% (Figure 1). Finally, dietary feeding of silibinin at 0.05% and 0.1% (wt/wt) doses to A/J mice with BP-induced lung tumorigenesis did not show significant preventive efficacy (Figure 1). All of the lung nodules found in this experiment were diagnosed as lung adenomas (Figure 2).
Figure 1.
Effects of deguelin and silibinin on BP-induced lung tumorigenesis in A/J mice. (A) Experimental design. We used the complete protocol. The upper panel shows the protocol for deguelin, and the lower panel shows the protocol for silibinin. After 10 days of administration of deguelin and silibinin, all mice were subjected a single dose of BP (100 mg/kg body weight). The total treatment duration was 22 weeks. (B) Effect of deguelin on lung tumor multiplicity. Deguelin significantly decreased tumor multiplicity by 63% and 56% in the 5- and 10-mg/kg groups, respectively. (C) Effect of deguelin on lung tumor load. Deguelin decreased tumor load by 57% and 78% in a dose-dependent manner. (D) Effect of silibinin on lung tumor multiplicity. The treatment of silibinin did not show significant inhibitory effects on tumor multiplicity in this entire study. (E) Effect of silibinin on lung tumor load. High-dose silibinin (0.1% in the diet) showed a mild (but not statistically significant) inhibitory effect. Error bars indicate standard error. *P < .05, ***P < .001, and ****P < .0001, compared with the control group.
Figure 2.
BP-induced pulmonary lesions in A/J mice. Representative lung nodules seen in control groups (a1) and in treated groups (a2) after perfusion with Tellyesniczky's solution through the trachea. Red arrows indicate tumors. (b) Light photomicrographs of representative adenomas from the control groups (b1 and b2) and the treatment groups (b3 and b4) at x100 and x400 magnifications, respectively.
Discussion
Effective chemoprevention of lung cancer—the principal cause of cancer deaths in the United States—has not been achieved. Characterization and use of effective chemopreventive agents have become important issues in the prevention and control of this deadly disease, particularly in the case of former smokers who are known to remain at high risk. Aiming to identify novel chemopreventive agents for lung cancer, we have determined the efficacy of two agents (deguelin and silibinin) in preventing lung tumorigenesis in mice. We found that deguelin was an effective chemopreventive agent in our mouse model of lung cancer at doses that caused no significant toxicity. Both doses of 5 and 10 mg/kg deguelin decreased tumor multiplicity by 63% and 56%, respectively (both P < .0001; Figure 1). When examining tumor load, both doses reduced tumor load in a dose-dependent manner by 57% in the 5-mg/kg group (P < .05) and by 78% in the 10-mg/kg group (P < .001) (Figure 1), respectively.
A systematic pharmacokinetic study of deguelin was performed in rats [26]. Deguelin has a long mean residence time (6.98 hours) and half-life (9.26 hours) in rat plasma, with reasonable levels distributed in the lungs. For example, the relative levels of tissue distribution were as follows: perirenal fat > heart > mammary gland > colon > kidney > liver > lung > brain > skin, following intragastric administration [26]. Deguelin has shown chemopreventive efficacy in several in vivo models using dose levels similar to those of the present study [17,26]. In a recent study, deguelin suppressed the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice when it was given intragastrically at doses of 2.5, 5.0, and 10.0 mg/kg body weight [17]. The bioavailability of silibinin in mouse models is well-documented in the literature [23,27,28]. Oral feeding of silibinin at 200 mg/kg resulted in physiologically available silibinin levels in both lungs (20 µg/g lung tissue) and plasma (60 µM = 30 µg/ml) [27]. Using 0.05% and 0.1% silibinin (wt/wt) in diets for 60 days, tumor volume was significantly decreased (35–58%; P < .05–.001) in silibinin-fed mice [28]. We believe that silibinin should be bioavailable in lung tissues because we used identical doses of silibinin (0.05% and 0.1%, mixed in AIN-76A purified powder diet) for more than 20 weeks, as reported previously [28].
Recently, advances have been reported in the development of small molecule inhibitors for Akt, which plays an important role in cell survival and proliferation through a number of downstream effectors. The majority of small molecule inhibitors of Akt are adenosine triphosphate-competitive inhibitors, phosphatidylinositol (PI) analogs, and allosteric inhibitors with pleckstrin homology domain [29]. Deguelin, a natural inhibitor of PI3K and AKT, can inhibit cell proliferation by blocking cells in the G2/M phase of the cell cycle and by increasing apoptosis in premalignant and malignant cells [16–18]. One of the major mechanisms of action for the observed chemopreventive efficacy of deguelin is through its inhibitory effect on PI3K/Akt signaling [18]. Furthermore, the PI3K signaling pathway has been reported to be involved in the early stages of lung carcinogenesis [30,31]; increased expression of activated Akt was observed in the early stages of tobacco-induced lung carcinogenesis [18,30]. Deguelin was previously shown to inhibit the formation of preneoplastic lesions when mouse mammary glands were exposed to 7,12-dimethylbenz(a)anthracene (DMBA), skin carcinogens in CD-1 mice, and MNU-induced mammary carcinogens in Sprague-Dawley rats [16]. Deguelin was also effective in suppressing the formation of carcinogen-induced aberrant crypt foci by more than 70% in the colon of CF-1 mice, presumably through the induction of apoptosis and cell cycle arrest [17,20]. The results from these studies and from the present study clearly demonstrate that deguelin has a significant chemopreventive efficacy against tumorigenesis in multiple organ sites.
Silibinin is a major bioactive flavanone in milk thistle and has shown efficacy against tumor growth in prostate, skin, and colon cancer models [27,32,33]. Recently, oral silibinin was found to suppress human NSCLC A549 xenograft growth and to enhance therapeutic response to doxorubicin in athymic mice, suggesting that silibinin may be a potential chemopreventive agent for lung tumorigenesis [26]. However, administration of silibinin in the diet did not reduce lung tumor multiplicity or tumor load, indicating that silibinin has no significant efficacy at the doses employed in BP-induced mouse lung tumorigenesis. At present, it is not clear why silibinin failed to inhibit lung tumorigenesis as sufficient bioavailability could be achieved with oral feeding of silibinin in the in vivo setting [28].
In summary, the results of this study show that deguelin is a novel lung chemopreventive agent in A/J mice. Identification of new and effective agents against lung cancer is of the highest importance because cancer of the lungs remains the principal cause of cancer deaths and because effective chemoprevention has not been found. For example, carotene, retinol, and vitamins E and C have been shown to have little, or negative, effect on human lung cancer development in smokers [15]. Thus, deguelin is among the more promising preventive agents for lung cancer and should be further tested in clinical trials.
Footnotes
This work was supported by Public Health Service grants CA058554 and CA9696401.
References
- 1.Beckett WS. Epidemiology and etiology of lung cancer. Clin Chest Med. 1993;14:1–15. [PubMed] [Google Scholar]
- 2.Fielding JE. Smoking: health effects and control. N Engl J Med. 1985;313:491–498. doi: 10.1056/NEJM198508223130807. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CR, Lubet RA, You M. Genetic alterations in mouse lung tumors: implications for cancer chemoprevention. J Cell Biochem Suppl. 1997;29:49–63. [PubMed] [Google Scholar]
- 4.Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- 5.Witschi H, Espiritu I, Peake JL, et al. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18:575–586. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- 6.Shopland DR, Eyre HJ, Pechacek TF. Smoking-attributable cancer mortality in 1991: is lung cancer now the leading cause of death among smokers in the United States? J Natl Cancer Inst. 1991;83:1142–1148. doi: 10.1093/jnci/83.16.1142. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. Br Med J. 1952;2:1271–1286. doi: 10.1136/bmj.2.4797.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minna JD. The molecular biology of lung cancer pathogenesis. Chest. 1993;103:449S–456S. doi: 10.1378/chest.103.4_supplement.449s. [DOI] [PubMed] [Google Scholar]
- 9.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA. 2005;294:1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- 10.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ, Boone CW, Steele VE, Crowell JA, Lubet R, Sigman CC. Progress in cancer chemoprevention: perspectives on agent selection and short-term clinical intervention trials. Cancer Res. 1994;54:2015s–2024s. [PubMed] [Google Scholar]
- 12.Jordan VC. Tamoxifen: the herald of a new era of preventive therapeutics. J Natl Cancer Inst. 1997;89:747–749. doi: 10.1093/jnci/89.11.747. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, MacMahon B. Diet and cancer—an overview. N Engl J Med. 1984;310:633–638. doi: 10.1056/NEJM198403083101006. [DOI] [PubMed] [Google Scholar]
- 14.Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52:2670s–2676s. [PubMed] [Google Scholar]
- 15.You M, Bergman G. Preclinical and clinical models of lung cancer chemoprevention. Hematol/Oncol Clin North Am. 1998;12:1037–1053. doi: 10.1016/s0889-8588(05)70040-x. [DOI] [PubMed] [Google Scholar]
- 16.Udeani GO, Gerhauser C, Thomas CF, Moon RC, Kosmeder JW, Kinghorn AD, Moriarty RM, Pezzuto JM. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer Res. 1997;57:3424–3428. [PubMed] [Google Scholar]
- 17.Murillo G, Kosmeder JW, II, Pezzuto JM, Mehta RG. Deguelin suppresses the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice. Int J Cancer. 2003;104:7–11. doi: 10.1002/ijc.10901. [DOI] [PubMed] [Google Scholar]
- 18.Lee HY. Molecular mechanisms of deguelin-induced apoptosis in transformed human bronchial epithelial cells. Biochem Pharmacol. 2004;68:1119–1124. doi: 10.1016/j.bcp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Clin Cancer Res. 2004;10:1074–1079. doi: 10.1158/1078-0432.ccr-0833-3. [DOI] [PubMed] [Google Scholar]
- 20.Murillo G, Salti GI, Kosmeder JW, II, Pezzuto JM, Mehta RG. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer. 2002;38:2446–2454. doi: 10.1016/s0959-8049(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 21.Racz K, Feher J, Csomos G, Varga I, Kiss R, Glaz E. An antioxidant drug, silibinin, modulates steroid secretion in human pathological adrenocortical cells. J Endocrinol. 1990;124:341–345. doi: 10.1677/joe.0.1240341. [DOI] [PubMed] [Google Scholar]
- 22.Muzes G, Deak G, Lang I, Nekam K, Gergely P, Feher J. Effect of the bioflavonoid silymarin on the in vitro activity and expression of superoxide dismutase (SOD) enzyme. Acta Physiol Hung. 1991;78:3–9. [PubMed] [Google Scholar]
- 23.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulinlike growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 24.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA, You M. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]
- 26.Udeani GO, Zhao GM, Shin YG, Kosmeder JW, II, Beecher CW, Kinghorn AD, Moriarty RM, Moon RC, Pezzuto JM. Pharmacokinetics of deguelin, a cancer chemopreventive agent in rats. Cancer Chemother Pharmacol. 2001;47:263–268. doi: 10.1007/s002800000187. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomark Prev. 2003;12:933–939. [PubMed] [Google Scholar]
- 29.Barnett SF, Bilodeau MT, Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr Top Med Chem. 2005;5:109–125. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- 30.Chun KH, Kosmeder JW, II, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 31.Crowell JA, Steele VE. AKT and the phosphatidylinositol 3-kinase/AKT pathway: important molecular targets for lung cancer prevention and treatment. J Natl Cancer Inst. 2003;95:252–253. doi: 10.1093/jnci/95.4.252. [DOI] [PubMed] [Google Scholar]
- 32.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 33.Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002;101:461–468. doi: 10.1002/ijc.10625. [DOI] [PubMed] [Google Scholar]