Abstract
Malignant astrocytomas are the most common and highly vascularized of all primary adult brain tumors. The histopathological hallmarks of malignant astrocytomas are microvascular proliferation and formation of vascular entities, which are referred to as “glomeruloid bodies.” The significance of glomeruloid bodies and the molecular mechanisms driving the abnormal vascular architecture in malignant astrocytomas are not understood. We have observed that overexpression of angiopoietin-1 (Ang1) in both subcutaneous and intracranial xenograft models of malignant astrocytomas reproduces many of the vascular features of these tumors, including glomeruloid bodies. To confirm that the formation of glomeruloid bodies was directly dependent on Ang1, we performed experiments where levels of Ang1 expression were regulated under tetracycline control, and we found a direct correlation between levels of Ang1 expression and the occurrence of glomeruloid bodies in xenografts. Additionally, we inhibited the action of Ang1 by blocking its cognate receptor Tie2, and we found that the formation of glomeruloid bodies was inhibited. Collectively, these results support our hypothesis that Ang1 is a key molecular regulator of pathological vascularization characteristic of malignant astrocytomas.
Keywords: Glomeruloid bodies, astrocytoma, angiopoietin, tumor angiogenesis, Tie2 receptor
Introduction
Astrocytomas are the most common primary brain tumor. According to World Health Organization classification, the most malignant grades are anaplastic astrocytoma (AA; grade III) and glioblastoma multiforme (GBM; grade IV) [1]. Malignant astrocytomas are histopathologically characterized by microvascular proliferation—a feature that represents a switch to a malignant growth phase of an astrocytoma [1,2]. Microvascular proliferation is characterized by hyperproliferation and piling of endothelial cells (ECs) around a vessel lumen—forming entities referred to as glomeruloid bodies—because they phenotypically resemble renal glomeruli [1,2]. To date, there has been very little investigation on the significance of glomeruloid bodies and the molecular regulators responsible for this florid pathological vascular development in GBMs. Although vascular endothelial growth factor-A (VEGF-A) and its receptors (vascular endothelial growth factor receptors) have been clearly demonstrated to play a uniform potent proangiogenic role in the malignant progression of astrocytomas, it has not been directly associated with the formation of glomeruloid bodies [1–3]. Expanding on our previous observations that angiopoietins are also important regulators of astrocytoma angiogenesis [4–6], in this study, we examined the role of angiopoietin-1 (Ang1) as a potential key molecular regulator of glomeruloid body formation in malignant astrocytomas.
Angiopoietins act with VEGF-A in a closely coordinated fashion to regulate normal vessel development and angiogenesis [7–10]. Unlike VEGF-A, angiopoietins are not mitogenic for ECs but are involved in the maturation of newly formed vessels by regulating interactions between ECs and mesenchymal supportive cells such as pericytes (PCs) and smooth muscle cells (SMCs) of the extracellular matrix (ECM) [7–10]. Ang1 is a highly soluble activating ligand [10–12] and is secreted by parenchymal cells, activating the Tie2 receptor expressed by ECs in a paracrine fashion and thus leading to stabilization and maturation of the newly formed vasculature. Additional mechanisms by which angiopoietins regulate vessel biology beyond the above paradigm have been identified, with angiopoietins shown to regulate EC survival, adhesion, motility, and development of the lymphatic system [13–21].
To date, the role of angiopoietins in tumor angiogenesis has been seemingly contradictory, as they have shown different angiogenic effects in varying tumor models [11,21–28]. Their role in astrocytoma angiogenesis has been investigated by a few groups, including our own; however, their specific contribution to pathological vessel formation has not been explored. We previously established that angiopoietin expression and Tie2 activation increase with increasing malignancy grade of astrocytomas [29]. Furthermore, we demonstrated that inhibiting Tie2 activation can significantly decrease the growth of GBM xenograft models, through disruption of tumor angiogenesis [4]. We found that, in GBMs, Ang1 acts in a proangiogenic capacity in the presence of VEGF-A, synergizing the vascular response triggered by VEGF and promoting the overall growth of GBMs [30]. In this study, we have focused on the role of Ang1 in regulating the formation of glomeruloid bodies, which is characteristic of human malignant astrocytomas.
Materials and Methods
Cell Lines
Established human GBM cells (U87-MG) were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and maintained in Dulbecco's minimal essential medium (DMEM; Cellgro, Herndon, VA) supplemented with 10% FBS and penicillin-streptomycin. Human umbilical vein endothelial cells were obtained from ATCC and maintained in Ham's medium. 3T3-Tie2 cells were a gift from Chris Kontos (Duke University, Durham, NC) and were maintained in DMEM plus 500 mg/ml G418. All of the above cells were grown in 37°C at 5% CO2. Sf9 insect cells were grown and maintained as described previously [4].
Stable Clones
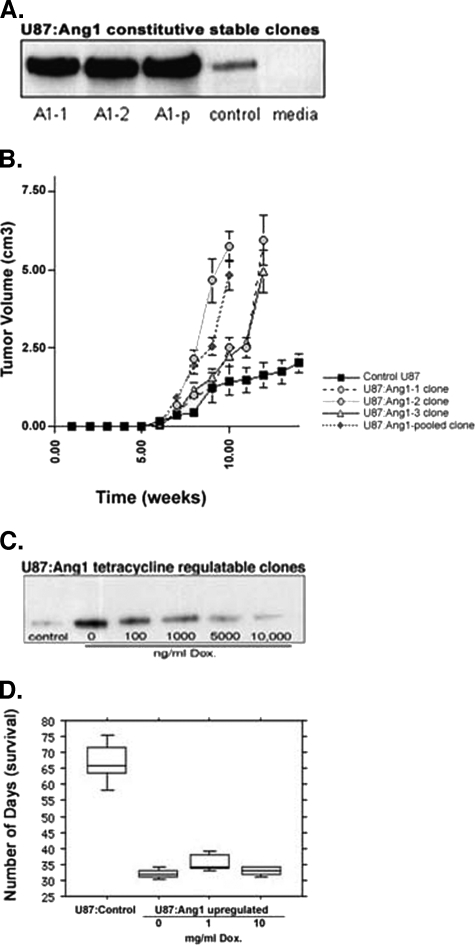
Constitutively overexpressing angiopoietin clones Full-length human Ang1 cDNA was a gift from K. Alitalo (Helsinki, Finland) and was subcloned into the mammalian expression vector pSECTagB/Myc-HIS (Invitrogen, Mississauga, Ontario, Canada). The Ang-Myc/HIS sequence was subcloned into the pCAGG vector, containing a Cytomegalovirus promoter with a chicken β-actin enhancer. Stable cell lines were generated by transfection of this vector into the U87-MG cells using Lipofectamine-2000 (Gibco/BRL, Burlington, Ontario, Canada), as per the manufacturer's instructions. Stable clones were selected with 1 mg/ml Zeocin (Invitrogen), and cells were expanded. Twenty stable clones were examined for Ang1 expression levels using immunoprecipitation and Western blot analysis, as described below. Two of the highest-producing Ang1 clones (A1-1 and A1-2), plus one pooled clone (A1-p), were selected for in vivo studies (Figure 2A). Potential clonal variability was accounted for by using multiple clones, pooled clones, and empty vector transfectants. These stable clones were expanded and maintained in DMEM plus 300 µg/ml Zeocin. For control stable lines, a similar transfection was undertaken with empty vector constructs.
Figure 2.
In vivo growth effect of Ang1 on subcutaneous and intracranial xenograft models of GBMs. (A) The level of Ang1 expressed above baseline control by the corresponding stable U87-MG:Ang1 clones (A1-1, A1-2, and one pooled clone A1-p) is demonstrated at the bottom of the growth curve. (B) U87-MG:Ang1 astrocytoma stable clones grown as subcutaneous xenograft models in NOD-SCID mice demonstrate a faster growth rate and a final tumor size comparable to that of control empty vector-transfected clones. (C) Varying levels of Ang1 expression by addition of Dox (bottom) did not result in a statistically significant difference in survival, which were all decreased compared to control. (D) Intracranial xenografts of Tet-Off-regulated U87-MG:Ang1 tumors demonstrate decreased survival concordant with a faster growth rate, compared to U87-MG:Control (vector transfectants).
Tetracycline-inducible clones overexpressing angiopoietins As described previously, stable Tet-Off U87-MG cells have been established [31,32]. Briefly, U87-MG cell lines were transfected with the pTet-Off (Clontech, Palo Alto, CA) vector, and stable clones were selected and maintained in 1 mg/ml and 500 µg/ml G418, respectively. Thirty of the Tet-Off clones were assayed by transfecting with pTRE-LUC and, using a luciferase assay, the highest tetracycline-inducible clone was selected for generating double-stable Tet-Off cell lines (data not shown). Double-stable Tet-Off U87-MG cell lines overexpressing Ang1 were generated by cotransfecting U87-MG:Tet-Off stable cells with pTRE-Ang1 using the pTK-puromycin vector. Stable clones were selected in 3 µg/ml puromycin, and 20 clones were tested for induction of Ang1 expression using immunoprecipitation followed by Western blot analysis, as described below. For control U87-MG:Tet-Off double-stable cell lines, pTRE-Red vector (Clontech) expressing the dsRed fluorescent protein was used. In vitro testing of tetracycline induction was determined using varying doses of doxycycline (Dox), with the most tightly regulated clones expressing Ang1 selected for in vivo experiments (Figure 2B).
Characterization of Angiopoietins Secreted by Stable Clones
Stable clones of Ang1 were plated (P100) to the same number of cells as that of control empty vector transfectants. After 96 hours, the conditioned medium (CM) was collected and centrifuged to clear debris. For Tet-Off clones, the cells were plated to an equal number in P100 plates and exposed to varying doses (0 ng/ml, 10 ng/ml, 100 ng/ml, 500 ng/ml, 1 mg/ml, and 5 mg/ml) of Dox (Sigma, St. Louis, MO), according to standard protocol, for 36 hours. The CM was subsequently collected, and protein levels were quantified using the bicinchoninic acid assay to ensure equal loading for immunoblot analysis of Ang1, as outlined below. Immunoprecipitation of Ang1 was performed with 5 µg of Ang1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) recovered by protein G Sepharose beads (Invitrogen) at 4°C. Beads were recovered, washed with HNTG buffer three times, eluted with twice-concentrated SDS sample buffer, boiled at 100°C for 10 minutes, separated using SDS-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. Standard Western blot analysis using anti-Ang1 antibody at 1:5000 dilution in 5% skim milk-Tris-buffered saline Tween 20 was performed. A horseradish peroxidase-conjugated anti-protein G antibody, coupled with an enhanced chemiluminescence system reagent (NEN Life Science Products, Boston, MA), was used to visualize specific bands for Ang1 at approximately 70 kDa.
In vivo Tumor Models
Subcutaneous models Subcutaneous xenografts were generated by growing U87-MG stable clones overexpressing Ang1 in the flanks of NOD-SCID mice. For each stable clone, seven mice were injected with 10E7 cells suspended in 300 µl of PBS, with five mice injected with control empty vector transfectants. Tumor growth was measured biweekly, using calipers, by two observers in a blinded fashion. Tumor volume was calculated using the formula: (diameter2 x length)/2. As per animal protocol, mice were sacrificed by cervical dislocation after 100 mg/kg bromodeoxyuridine (BrDu) (Sigma-Aldrich, Toronto, Canada) injection. Tumors were cut in cross sections, with two cross sections kept in formaldehyde for paraffin blocks and immunohistochemical analysis and with the remaining tumor stored in liquid nitrogen. All in vivo tumor models were repeated in duplicate.
Intracranial models For orthotopic xenograft models, Tet-Off-regulated human U87-MG:Ang1 cells (10E6) were stereotactically injected 3 mm deep into the frontal cortex of NOD-SCID mice. Mice were treated with Dox in drinking water, with doses of 0, 1, and 10 mg/ml. These doses were selected based on prior published studies demonstrating that Dox crosses the blood-brain barrier efficiently to regulate gene expression in the brain [32]. When animals exhibited symptoms consistent with failure to thrive or when their intracranial pressure increased, the mice were sacrificed by perfusion fixation after BrDu injection and tail vein injection of 2% Evans blue solution (2 ml/kg) to determine intraluminal blood flow and vessel permeability. The time interval between the injection of Evans blue and the perfusion and killing of the mice was approximately 30 minutes [32]. All in vivo experiments were repeated in duplicate.
Inhibition of Ang1 in Orthotopic Intracranial Tumor Models
ExTek is a soluble protein that contains only the extracellular portion of the Tie2 receptor, which we have shown to inhibit Tie2 activation both in vitro and in vivo [4]. ExTek is purified using a baculovirus expression system, as detailed previously [4]. To inhibit the effect of Ang1, ExTek treatment was started 2 weeks after intracranial injection of U87-MG: Ang1 cells, with repeat delivery into the tumor center using a guide-screw technique, as described previously [4]. A total of 30 to 50 µg of purified ExTek, suspended in 10 to 20 µl of PBS, was delivered into the tumor center every other day, as described previously, with a 32% decrease in tumor growth rate in intracranial U87-MG xenografts [4]. A total of 15 mice received ExTek, 5 received PBS, and 5 received no treatment. When animals exhibited symptoms consistent with failure to thrive, as per animal care protocols, the mice were sacrificed by perfusion fixation after BrDu and Evans blue injections. The brain and the tumors were analyzed in a fashion similar to the subcutaneous xenografts above.
Tumor Vascularity
Four different tumor portions were each cut at 5-µm consecutive paraffin sections and stained with the EC-specific marker anti-factor VIII (1:2000; Dako, Carpinteria, CA), followed by detection with an avidin-biotin complex method, 3,3′-diaminobenzidine (VectaStain Elite; Vector Laboratories, Burlingame, CA) system. Microvessel density (MVD) was calculated by counting the number of hollow lumen vessels in 10 high-power fields (HPF; x500) and in 5 HPF at vascular “hot spots.” Areas that included abnormal vascular structures, such as glomeruloid bodies, were not included in the MVD count as the functional status of these vascular units in both human and xenograft tumors is not known. All analyses were carried out using the Micro-Computer Image Device (MCID-Imaging Research, Inc., St. Catharines, Ontario, Canada) linked to a color charge-coupled device camera (DXC 970 MD; Sony, Crofton, MD) mounted on a transmitted-light microscope (Zeiss Axioskop, Carl Zeiss AG, Vertrieb, Germany). The extent of EC and SMC colocalization in a vessel was determined by double staining using fluorescein isothiocyanate-conjugated factor VIII antibody and chromogenic smooth muscle antigen (SMA) staining. Paraffin sections were analyzed using laser microscopy to detect the red fluorescence of Evans blue that was injected through the tail vein prior to animal sacrifice. This technique allowed for the detection of blood flow in vessel lumens and the extent of plasma leakage from vessels.
Immunohistochemistry
Standard hematoxylin and eosin (H&E) staining and immunohistochemical analysis were performed on 5-µm tissue sections from paraffin-embedded tissue blocks. Primary antibodies used include the following: Ki-67 (polyclonal rabbit antibody no. A0047, used at 1:400; Dako), factor VIII (rabbit polyclonal antibody no. A0082, used at 1:2500, Dako), CD34 (human monoclonal antibody), polyclonal goat anti-Ang1 and anti-Ang2 antibody (1:200 and 1:400; Santa Cruz Biotechnology), and rabbit polyclonal anti-Tie2 (1:400; Santa Cruz Biotechnology). The secondary antibody was a goat antimouse antibody (Zymed, Markham, Ontario, Canada) used at 1:200, and antigens were detected using the avidin-biotin complex method (Vector Laboratories) and diaminobenzidine substrate. All slides were reviewed independently by two observers and our neuropathology colleague (P.S.).
Statistical Analysis
All analyses were completed using StatView 4.1 for Macintosh (Abacus Concepts, Berkeley, CA). All errors were calculated as the standard error of the mean (SEM). One-tailed Student's t test was used to compare means (two samples, unequal variance), with P < .05 considered statistically significant.
Results
Characterization of Human GBM-Associated Glomeruloid Bodies
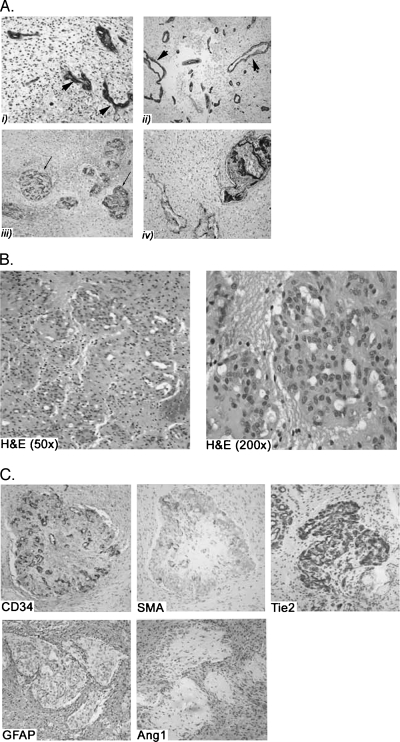
Human GBM vasculature demonstrates a wide range of architectural aberrations, with a repertoire of pathological alterations as illustrated in Figure 1A. The vessels are dilated, take on ectatic serpentine forms (Figure 1A, i and ii), and are often thrombosed when present in association with regions of tumor necrosis [1,33]. Additionally, the vessel lumens are lined with multiple layers of ECs, instead of a single layer of ECs, as seen in normal vessels. Piling of vascular ECs leads to formation of structures that resemble glomeruloid bodies and are otherwise also referred to as vascular tufts [Figure 1, A (iii and iv) and B]. The structure of glomeruloid bodies has not been characterized extensively in the past, and their pathophysiology and significance remain to be deciphered. Although, on H&E (Figure 1B), cells composing a glomeruloid body have the phenotypic appearance of ECs, they do not stain uniformly for EC-specific markers, as seen with the patchy CD34 staining pattern (Figure 1C), suggesting diversity of cells composing glomerular bodies in GBMs. Another population of cells within the heterogeneous population of cells in glomeruloid bodies are the SMCs, positively staining for SMA (Figure 1C). Of interest, all of the cells within the glomeruloid bodies uniformly express Tie2, the receptor for Ang1, regardless of whether the cells are ECs or SMCs (Figure 1C). As predicted, human glomeruloid bodies do not stain positively for the astrocyte marker glial fibrillary acidic protein (GFAP) (Figure 1C). More importantly, we found that astrocytes surrounding glomeruloid bodies express Ang1 uniformly, as seen with consecutive GFAP and Ang1 staining (Figure 1C).
Figure 1.
Characterization of human GBM glomeruloid bodies. (A) The repertoire of pathological vascular structures seen in GBMs is illustrated in this figure. EC-specific immunohistochemical staining of human GBM sections allows identification of vascular structures. There are microvascular proliferation and piling of ECs around the vessel lumen (arrowheads) with dilated, ectatic, and serpentine structures (i and ii). The vessels form structures referred to as glomeruloid bodies that are generated from the piling of ECs around vessel lumens and by mimicking of glomeruli seen in human kidneys (arrow) (iii and iv). (B) H&E sections of human GBM illustrating the vascular units called glomeruloid bodies that very closely resemble renal glomeruli. The cells composing a glomeruloid body, as seen on H&E, correspond to ECs. (C) Immunohistochemical characterization of the expression profile of cells composing glomeruloid bodies shows a mixed population of ECs (as illustrated by EC-specific stain: CD34) and smooth muscle stains (as illustrated with smooth muscle actin stain: SMA). A majority of cells of a glomeruloid body express Tie2 uniformly, regardless of whether they are EC- or SMA-positive. However, Ang1 expression is restricted to astrocytoma cells surrounding the glomeruloid bodies, as illustrated by the parallel staining pattern for GFAP and Ang1.
Characterization of U87-MG Cells Overexpressing Ang1
We have previously published studies on the expression profile of VEGF and angiopoietin in U87-MG cells [29]. U87-MG:Control cells express moderate amounts of endogenous VEGF and Ang1, without any detectable Tie2 expression. Overexpression of Ang1 did not alter the in vitro proliferation rate, extent of apoptosis, morphology of U87-MG cells, or baseline VEGF-A expression, compared to parental controls (data not shown). The two highest clones of the constitutively overexpressing U87-MG:Ang1 stable transfectants (A1-1 and A1-2), plus one pooled clone (A1-p), were selected for in vivo studies (Figure 2A). Tet-Off-regulated U87-MG:Ang1 stable clones were also established, with the most tightly regulated clones selected for in vivo studies (Figure 2C). In U87-MG:Ang1 Tet-Off clone, 10,000 ng/ml Dox decreased Ang1 levels to close to parental levels of Ang1 expression (Figure 2, A and C). The levels of Dox required to regulate the decrease in Ang1 expression in U87-MG cells were found to be higher than those reported for other cell types, likely due to some degree of leakiness of the Tet-Off system and the presence of moderate amounts of endogenous Ang1 expressed by parental U87-MG cell lines.
We have previously established and published the characteristics of the stable cell lines used in this study and have demonstrated that the exogenous Ang1 secreted in the CM of the stable clones is biologically active using two in vitro assays [4,30]. Briefly, established Tie2 phosphorylation and microtubule bioassays were used to ensure that the exogenous Ang1 can activate the Tie2 receptor and induce ECs to grow as tubule structures on a fibrin matrix.
Effect of Ang1 on Tumor Growth and Proliferation
We have previously shown that Ang1 overexpression confers a growth advantage on U87-MG xenografts, with an overall increase in tumor cell proliferation and a decrease in apoptosis rate [30]. This was also observed in this study with both subcutaneous xenografts (∼3.5- and 5-fold increase in tumor size and proliferative index, respectively; Figure 2B, Table 1) and intracranial xenografts (∼2.6-fold increase in proliferative index and decreased survival; Table 2). However, the growth advantage in response to Ang1 was not dose-dependent, as seen by our Tet-Off-regulated U87-MG:Ang1 clones (Figure 2D, Table 2). Varying levels of Ang1 expression did not alter the overall survival, proliferative index, or MVD. At all three levels of Ang1 expression above, baseline tumor growth and proliferation were significantly increased compared to control tumors. The lack of tight in vivo regulation of Tet-Off stable clones and the leakiness of the system can be potential explanations for this apparent lack of dose-dependent response to Ang1. However, these results may also indicate that even small amounts of Ang1 above the baseline parental levels are sufficient to stimulate an increased growth of the U87-MG xenografts.
Table 1.
Effect of Ang1 on Subcutaneous Xenografts Models of GBM.
| U87-MG:Control (n = 10) | U87-MG:Ang1 (n = 15) | |
| Tumor size | 2.01 (0.3) | 6.96 (0.5) [P = 6 x 10-5] |
| Proliferation index | 0.23 | 0.90 [P = .001] |
| MVD | 2.12 (0.1) | 3.9 (0.2) [P = .001] |
SEM values are presented inside parentheses.
Table 2.
Effect of Ang1 on Intracranial U87-MG Xenografts.
| U87-MG:Control (n = 10) | U87-MG:Ang1 | |||
| No Dox (n = 10) | 1 mg/ml Dox (n = 10) | 10 mg/ml Dox (n = 10) | ||
| Overall survival (days) | 63.7 (2.3) | 35.2 (1.6)* [P = 4.5 x 10-9] | 35.7 (0.9)* [P = 5.1 x 10-6] | 32.7 (0.4)* [P = 6.1 x 10-9] |
| Proliferation index | 0.040 (0.01) | 0.107 (0.01)* [P = .0007] | 0.104 (0.01)* [P = .0018] | 0.094 (0.01)* [P = .0007] |
| MVD (vessels/HPF; mean of 10 counts) | 5.8 (0.55) | 9.5 (0.48)* [P = .0017] | 9.4 (1.14)* [P = .0093] | 9.8 (1.59)* [P = .0016] |
SEM values are presented inside parentheses.
Statistically significant compared to control.
Effect of Ang1 on Tumor Vascularity
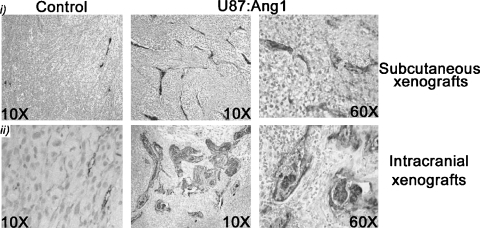
Tumor vascularity is increased with overexpression of Ang1. In both subcutaneous and intracranial models of U87-MG:Ang1, there was an increase in tumor angiogenesis, as measured by MVD, which was elevated by ∼1.7-fold in both subcutaneous and intracranial tumors (Tables 1 and 2). The striking and intriguing finding was the alteration of vascular architecture with overexpression of Ang1. In the U87-MG: Ang1 xenografts, many of the vessels take on a highly serpentine structure and abnormal multilayering of ECs (Figure 3), mimicking the characteristic glomeruloid bodies seen in human GBMs (Figure 1, A and B). In comparison, the U87-MG control xenografts had normal vascular architecture, with the lumens being well formed and lined with a single layer of ECs (Figure 3). Although present in the subcutaneous U87-MG: Ang1 xenografts, the number and the size of the glomeruloid bodies and overall abnormal vascular structures were more prominent in the intracranial xenografts (Figure 3). We postulate this difference to be due to the impact of microenvironmental factors and the physical confines of the intracranial space. The occurrence of these pathological vascular alterations was not restricted to a particular site within the xenografts, as they were present equally at the center and at the periphery of the tumor, and no direct correlation with necrotic zones was observed.
Figure 3.
Effect of Ang1 on tumor vascularity. Vascular structures in U87-MG:Ang1 xenografts stained with factor VIII demonstrate pathological piling of ECs, serpentine and ectatic vessels, and vascular structures that closely resemble glomeruloid bodies or tufts. This was not observed in the U87-MG:Controls and was more prominent in intracranial (i) versus subcutaneous (ii) xenografts.
Characterization of Glomeruloid Bodies Seen in U87-MG:Ang1 Xenografts
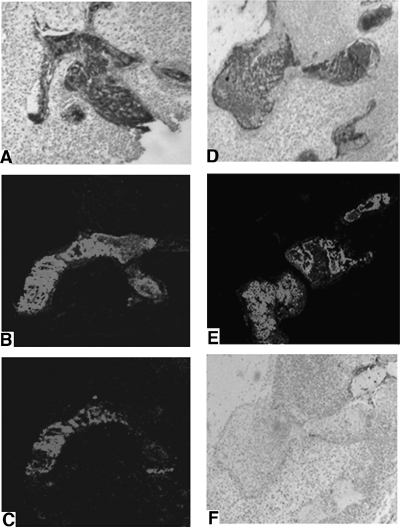
The cells composing glomeruloid bodies seen in U87-MG:Ang1 xenografts are principally ECs (Figure 4A) and a few SMCs (Figure 4F). These ECs uniformly express Tie2 (Figure 4D), similar to glomeruloid bodies in human GBM specimens (Figure 1C). Similar to human GBMs, the glomeruloid bodies in U87-MG:Ang1 xenografts do not stain for GFAP, whereas the surrounding GFAP-positive tumor cells express Ang1, suggestive of a paracrine stimulation of ECs and SMCs to form glomeruloid bodies (Figures 1 and 4). We also examined the correlation of SMCs and ECs in our xenografts by double labeling with fluorescent factor VIII (Figure 4E) and chromogenic SMA stain (Figure 4F). There was no significant pattern or correlation observed between ECs and SMCs within a glomeruloid body, similar to our observation in human glomeruloid bodies (Figure 1C). Using intravascular injection of Evans blue, detected as a red fluorescence signal, the extent of blood flow through the vascular lumens of glomeruloid bodies was assessed (Figure 4C). The glomeruloid bodies had areas with EC piling with no effective blood flow, as well as regions where the ECs appeared to form multiple small channels and lumens with effective blood flow.
Figure 4.
Characterization of glomeruloid bodies in U87:Ang1 xenografts. (A) The extent of ECs composing the glomeruloid bodies was confirmed by EC-specific stains. Consecutive sections of tumors were stained for factor VIII, fluorescent factor VIII (B), and intraluminal injection of Evans blue (C). The extent of blood flow through the glomeruloid bodies was determined by Evans blue injection, showing areas where ECs form multiple lumens with blood flow and no increase in vessel permeability. The cells of a glomeruloid body stain strongly for Tie2 (D), and these correspond directly with the factor VIII-positive cells. Double staining with fluorescent factor VIII (E) and chromogenic SMA (F) demonstrated minimal SMCs present in the glomeruloid bodies.
Glomeruloid Bodies Are Ang1-Dependent
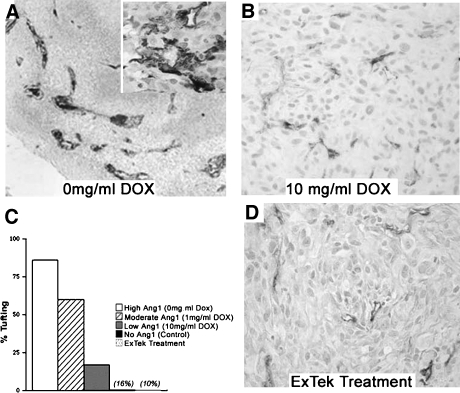
To determine whether the abnormal vascular structures seen in U87-MG:Ang1 xenografts are a direct consequence of Ang1 upregulation, we used several strategies. First, varying the levels of Ang1 expression using the Dox-regulated Tet-Off system, we found the extent of glomeruloid body or tufting in the U87-MG:Ang1 intracranial xenografts to be dependent on levels of Ang1 expression (Figure 5). At 0 mg/ml Dox (high Ang1 expression; Figure 2B), 88% of the intracranial xenografts had glomeruloid bodies present, compared to only 16% of tumors with 10 mg/ml Dox (low Ang1 expression; Figure 2B). This figure is comparable to that seen in U87-MG:Control xenografts (10%) that have basal endogenous Ang1 expression (Figure 5C). The formation of glomeruloid body was dependent on levels of Ang1 expression, as illustrated with the tetracycline-regulated xenografts, despite Ang1 levels not altering the overall tumor growth or angiogenesis, as discussed above. This would suggest that glomeruloid bodies may not directly contribute to the vascular growth of GBMs.
Figure 5.
Glomeruloid body formation is directly dependent on Ang1. There was a direct correlation between the extent of glomeruloid body formation in U87-MG:Ang1 xenografts and the level of Ang1 expression induced. With high levels of Ang1, or (A) 0 mg/ml Dox in the drinking water, typical EC piling (tortuous, thrombosed, and serpentine vessels with an increase in MVD) was present in 88% of the tumors. With low levels of Ang1 expression ((B) 10 mg/ml Dox), only (C) 16% of tumors illustrated glomeruloid body formation, comparable to the extent of glomeruloid bodies seen in control tumors (10%). (D) Inhibition of Ang1-activating Tie2 using ExTek, a kinase-deficient dominant-negative soluble Tie2 peptide, resulted in loss of glomeruloid body formation and other pathological vascular structures induced by Ang1.
Our second strategy to confirm that glomeruloid body formation was directly Ang1-dependent was to inhibit the activation of Ang1's cognate receptor Tie2, using a soluble kinase-dead dominant-negative Tie2 construct ExTek [4]. Intracranial U87-MG:Ang1 xenografts treated with ExTek showed loss of abnormal EC piling and glomeruloid body formation (Figure 5, C and D). Third, and to further support our postulate that Ang1 is the primary molecular regulator of glomeruloid bodies in GBMs, are our observations in xenografts, which we had generated previously to express low levels of VEGF-A while still maintaining overexpression of Ang1 [29]. These xenografts had decreased growth and angiogenesis, however, without any alterations in the incidence of glomeruloid bodies [30].
Collectively, these experimental results, in addition to the expression profile of angiopoietins and Tie2 in human GBM-associated glomeruloid bodies, are highly supportive of our hypothesis that glomeruloid bodies result from increased expression of Ang1 in GBM tumor cells, which, in a paracrine manner, activate the Tie2 receptors present in the tumor ECs.
Discussion
Astrocytomas are the most common brain tumor in adults, with necrosis and vascular proliferation differentiating malignant astrocytomas from their lower-grade counterparts [2,34]. The pathognomonic features of malignant astrocytomas are increase in microvascular proliferation and piling of ECs around a vessel lumen, forming glomeruloid bodies or vascular tufts (Figure 1A, i, iii, and iv) [2,34]. Glomeruloid bodies are thought to be vascular channels lined by hyperplastic ECs, surrounded by basal lamina and an incomplete layer of PCs. The molecular pathogenesis of glomeruloid bodies is not well understood, and their functional significance is yet undetermined [2,3]. Results from our study suggest that Ang1 expressed by astrocytoma cells, through a paracrine stimulation of its receptor Tie2, regulates the occurrence of glomeruloid bodies seen in human malignant astrocytomas.
It is established that there is overexpression of Ang1 and Tie2, as well as increased activation of Tie2, with increasing malignancy grade of astrocytomas [11,16,26,28,29,35,36]. We have previously demonstrated that overexpression of Ang1 in human GBM xenografts promotes tumor angiogenesis and growth in a VEGF-A dependent manner [30]. During these experiments, we also observed an association between Ang1 overexpression and glomeruloid body formation, and that the formation of these vascular structures was not dependent on concomitant VEGF-A expression [30]. These observations led to our hypothesis and current study that Ang1-mediated activation of Tie2 in a paracrine manner is an important molecular regulator of glomeruloid body formation in human malignant astrocytomas.
The glomeruloid bodies seen in human GBMs and our U87-MG:Ang1 xenografts demonstrate a very similar vascular architecture and expression profile for Ang1 and Tie2, supporting our U87-MG:Ang1 model as a useful model for studying the pathogenesis of the pathological vascular structures seen in human GBMs. All cells of these glomeruloid bodies uniformly express Tie2, whereas Ang1 expression is restricted to the surrounding astrocytoma cells. We postulate that activation of Tie2 by Ang1, in a paracrine fashion, leads to increased EC survival and accumulation, thereby generating these vascular tufts or glomeruloid bodies [37,38]. In support of our postulate, using the Tet-Off system, we have demonstrated that there is a direct correlation between the level of Ang1 expression and the extent of glomeruloid body formation. Furthermore, inhibition of Ang1-mediated activation of Tie2, using the soluble kinase-deficient dominant-negative mutant ExTek protein, results in a loss of glomeruloid body formation. To date, there has been only one study where the possible mechanism of glomeruloid body formation was investigated [3]. In this study, adenoviral-mediated delivery of VEGF-A to the skin of mice resulted in vascular structures resembling glomeruloid bodies. However, a similar experimental paradigm by another group with intracranial VEGFA injection failed to induce glomeruloid body formation [39]. Of relevance, we have found that VEGF-A upregulation in GBM cells leads to cross-modulation and spontaneous overexpression of Ang1, although the contrary (upregulation of VEGF by Ang1) is not seen (data not shown). Therefore, we speculate that the glomeruloid bodies induced by adenoviral VEGF-A injection into the skin [3] may also, in fact, be due to a secondary elevation of Ang1 rather than a direct consequence of VEGF-A. In addition, our prior published results, with VEGF-A demonstrating decreased tumor angiogenesis and overall growth but with preservation of glomeruloid body formation in U87-MG:Ang1 intracranial xenografts, also suggest that glomeruloid body formation is primarily linked to Ang1, rather than to VEGF-A [30].
In summary, our cumulative data strongly suggest that Ang1 is a key molecular regulator of glomeruloid bodies, as seen in malignant human astrocytomas. First, we have seen that there are many structural and cellular similarities between the glomeruloid bodies induced by Ang1 overexpression in human GBM xenografts and those found in human astrocytomas. Second, Ang1 induced glomeruloid bodies in a dose-dependent process. Third, inhibiting Ang1 activation of Tie2 led to a loss of glomeruloid body formation. These and our previous observations suggest that Ang1, in addition to playing an important proangiogenic and growth-promoting role in malignant astrocytomas, is also a key molecular regulator of glomeruloid body formation. The functional status of glomeruloid bodies as vascular units still remains unknown, with the U87-MG:Ang1 xenografts providing a good model to further understand the biologic relevance of this process in human astrocytomas. In this study, we were able to establish that components of the glomeruloid bodies have effective blood flow through the luminal channels, as evidenced by Evans blue intraluminal tracing. A noteworthy observation is that, despite blood flow within the vascular channels, there was no correlation between the extent of glomeruloid body formation and tumor growth. However, we do know that the presence of glomeruloid bodies in pathological examinations of human adult astrocytomas is closely linked with malignant progression and ultimate prognosis. Ongoing additional studies utilizing the U87-MG:Ang1 model are necessary to help better understand the functional relevance of glomeruloid bodies.
Footnotes
G.Z. was supported by fellowship funding from the American Brain Tumor Association, National Cancer Institute of Canada. Operating funds granted to A.G. were from the Canadian Institute of Health Research, the National Cancer Institute of Canada, and the Heart and Stroke Foundation of Canada.
References
- 1.Burger PC, Scheithauer BW. Tumors of the Central Nervous System (Atlas of the Tumor Pathology) 10. Vol. 3. Washington, DC: Armed Forces Institute of Pathology; 1994. [Google Scholar]
- 2.Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol. 2001;158(3):789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundberg CNJ, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158(3):1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zadeh G, Qian B, Sabha N, Okhowat A, Kontos C, Guha A. Targeting the Tie2/Tek receptor in astrocytomas. Am J Pathol. 2004;164(2):467–476. doi: 10.1016/S0002-9440(10)63137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zadeh G, Guha A. Angiogenesis in nervous system disorders. Neurosurgery. 2003;53(6):1362–1374. doi: 10.1227/01.neu.0000093425.98136.31. (discussion 1374–1376) [DOI] [PubMed] [Google Scholar]
- 6.Zadeh G, Guha A. Molecular regulators of angiogenesis in the developing nervous system and adult brain tumors (review) Int J Oncol. 2003;23(3):557–565. [PubMed] [Google Scholar]
- 7.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, et al. Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system. Ann NY Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 10.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 11.Zadeh G, Guha A. Neoangiogenesis in human astrocytomas: expression and functional role of angiopoietins and their cognate receptors. Front Biosci. 2003;8:e128–e137. doi: 10.2741/964. [DOI] [PubMed] [Google Scholar]
- 12.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 13.Jones N, Dumont DJ. Tek/Tie2 signaling: new and old partners. Cancer Metastasis Rev. 2000;19(1–2):13–17. doi: 10.1023/a:1026555121511. [DOI] [PubMed] [Google Scholar]
- 14.Carlson TR, et al. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276(28):26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, et al. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19(39):4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 16.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 17.Kim I, et al. Characterization and expression of a novel alternatively spliced human angiopoietin-2. J Biol Chem. 2000;275(24):18550–18556. doi: 10.1074/jbc.M910084199. [DOI] [PubMed] [Google Scholar]
- 18.Thurston G, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 19.Suri C, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282(5388):468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 20.Huang YQ, Li JJ, Karpatkin S. Identification of a family of alternatively spliced mRNA species of angiopoietin-1. Blood. 2000;95(6):1993–1999. [PubMed] [Google Scholar]
- 21.Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13(1):19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad SA, et al. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92(5):1138–1143. doi: 10.1002/1097-0142(20010901)92:5<1138::aid-cncr1431>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Hayes AJ, et al. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83(9):1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etoh T, et al. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61(5):2145–2153. [PubMed] [Google Scholar]
- 25.Stratmann A, et al. Differential inhibition of tumor angiogenesis by tie2 and vascular endothelial growth factor receptor-2 dominant-negative receptor mutants. Int J Cancer. 2001;91(3):273–282. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1054>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Koga K, et al. Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res. 2001;61(16):6248–6254. [PubMed] [Google Scholar]
- 27.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158(2):563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Audero E, et al. Expression of angiopoietin-1 in human glioblastomas regulates tumor-induced angiogenesis: in vivo and in vitro studies. Arterioscler Thromb Vasc Biol. 2001;21(4):536–541. doi: 10.1161/01.atv.21.4.536. [DOI] [PubMed] [Google Scholar]
- 29.Ding H, et al. Expression and hypoxic regulation of angiopoietins in human astrocytomas. Neuro-Oncology. 2001;3(1):1–10. doi: 10.1093/neuonc/3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zadeh G, Koushman K, Shannon P, Guha A. Interaction of angiopoietins and VEGF in astrocytomas. J Neuropathol Exp Neurol. 2004;63(9):978–989. doi: 10.1093/jnen/63.9.978. [DOI] [PubMed] [Google Scholar]
- 31.Tsugu A, et al. Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence. Am J Pathol. 2000;157(3):919–932. doi: 10.1016/S0002-9440(10)64605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, et al. Conditional gene expression in human intracranial xenograft tumors. Biotechniques. 2001;31(1):196–202. doi: 10.2144/01311dd04. [DOI] [PubMed] [Google Scholar]
- 33.Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neuro-Oncol. 1997;32(3):253–265. doi: 10.1023/a:1005746320099. [DOI] [PubMed] [Google Scholar]
- 34.Burger PC, Scheithauer BW, Vogel FS. Brain Tumors. New York, NY: Churchill Livingstone Inc; 1991. Surgical pathology of the nervous system and its coverings; pp. 193–437. [Google Scholar]
- 35.Zagzag D, et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159(2):391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- 36.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153(5):1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak HJ, et al. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448(2–3):249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3V-kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 39.Vogel J, et al. Heterologous expression of human VEGF165 in rat brain: dose-dependent, heterogeneous effects on CBF in relation to vascular density and cross-sectional area. J Cereb Blood Flow Metab. 2003;23(4):423–431. doi: 10.1097/01.WCB.0000054757.97390.BE. [DOI] [PubMed] [Google Scholar]