Abstract
In this work, we investigated the effects of Casiopeina II-gly (Cas IIgly)—a new copper compound exhibiting antineoplastic activity—on glioma C6 cells under both in vitro and in vivo conditions, as an approach to identify potential therapeutic agents against malignant glioma. The exposure of C6 cells to Cas IIgly significantly inhibited cell proliferation, increased reactive oxygen species (ROS) formation, and induced apoptosis in a dose-dependent manner. In cultured C6 cells, Cas IIgly caused mitochondrio-nuclear translocation of apoptosis induction factor (AIF) and endonuclease G at all concentrations tested; in contrast, fragmentation of nucleosomal DNA, cytochrome c release, and caspase-3 activation were observed at high concentrations. Administration of N-acetyl-l-cystein, an antioxidant, resulted in significant inhibition of AIF translocation, nucleosomal DNA fragmentation, and caspase-3 activation induced by Cas IIgly. These results suggest that caspase-dependent and caspase-independent pathways both participate in apoptotic events elicited by Cas IIgly. ROS formation induced by Cas IIgly might also be involved in the mitochondrio-nuclear translocation of AIF and apoptosis. In addition, treatment of glioma C6-positive rats with Cas IIgly reduced tumor volume and mitotic and cell proliferation indexes, and increased apoptotic index. Our findings support the use of Cas IIgly for the treatment of malignant gliomas.
Keywords: Casiopeina II-gly, apoptosis, reactive oxygen species, apoptosisinducing factor, glioma C6 cells
Introduction
Apoptosis occurs spontaneously in malignant tumors, and this event is often potentiated by chemotherapeutic agents [1,2]. Morphologically, the process of apoptosis is characterized by cellular condensation, nuclear fragmentation, and engulfment of cell debris by phagocytes [3]. The induction of apoptosis may involve either extracellular triggering signals (such as a tumor necrosis factor) or endogenous signals [such as cytochrome c (cyt c)] [4], which are followed by the activation of caspases [5] and endonucleases [6,7], thus causing disassembly of nuclear chromatin and degradation of oligonucleosomal DNA. Moreover, the translocation of apoptosis induction factor (AIF) leads to apoptosis through a caspase-independent pathway, producing the condensation of nuclear chromatin, as well as large-scale (∼50 kb) DNA fragmentation [8–10]. In addition, it has been suggested that Fe3+ and Cu2+, two redox-active metal ions, are involved in reactive oxygen species (ROS) generation, and hence in apoptosis [11,12].
Oxidative stress is a putative mediator of apoptosis through many different mechanisms [13]. Among them, we can mention the following: 1) the intracellular increase of ROS [14] or the depletion of endogenous antioxidants [15]; 2) the action of some antioxidants—such as N-acetyl-l-cystein (NAC)—which act as intracellular ROS scavengers, thus inhibiting the activation of caspases [16]; 3) the overexpression of Mn superoxide dismutase (SOD), which restores the mitochondrial transmembrane potential, thus inhibiting apoptosis [16,17]; 4) the overexpression of Cu/Zn-SOD, which delays apoptosis by scavenging O2.- [18,19]; and 5) the overexpression of the mitochondrial phospholipid hydroperoxide glutathione peroxidase (GPx) that inhibits the generation of ROS formation [20].
However, malignant gliomas are known to be resistant to different forms of chemotherapy, and this is consistent with evidence demonstrating that glioma cells are prone to developing drug resistance during treatment. Therefore, the search for novel and more effective chemotherapeutic agents against glioma cells becomes more relevant than ever. Moreover, the concept, that drugs based on endogenous (essential) metallic components may be more effective and less toxic than other currently used agents, prompted several groups to develop copper-based drugs with potential use as therapeutic agents. In our case, we have recently completed the synthesis of mixed copper-chelating compounds with the generic name of casiopeinas, which present the following basic formula: Cu(N-N)(A-A)]NO3 (A-AN-O, O-O) [21,22]. Furthermore, it has been demonstrated that some copper-based generic compounds may exhibit a higher antineoplastic potency than cisplatin for human ovarian carcinoma (CH1), murine leukemia (L1210), and various cervico-uterine carcinomas [23,24]. However, casiopeinas still need to be characterized in their properties against tumors from the nervous system. Therefore, the present study was undertaken to investigate the effects of Casiopeina II-gly (Cas IIgly) [Cu(4,7-dimethyl-1, 10-phenanthroline)(glycine)(H2O)]NO3 in rats with glioma C6 and in cultured glioma C6 cells. Our data show that Cas IIgly inhibited cell growth and induced programmed cell death (PCD) in vitro and in vivo, thus involving an active role of ROS formation in the positive actions of casiopeina through both caspase-dependent and caspase-independent mechanisms.
Materials and Methods
Reagents
Cas IIgly was synthesized as previously described [25] and dissolved in sterile water. All other reagents were obtained from known commercial sources.
In Vitro Experiments
Glioma cell culture Cultured glioma C6 cells from rats (American Tissue Culture Collection, Rockville, MD) were maintained at 37°C in 5% CO2 under sterile conditions in Dulbecco's modified Eagle's medium (Sigma Chemical Co., St. Louis, MO), supplemented with 5% fetal bovine serum (Sigma Chemical Co.), and treated during 24 hours with increasing concentrations of Cas IIgly (1, 2.5, 5, and 10 µg/ml; equivalent to 4.26 x 10-4, 1.0 x 10-3, 2.13 x 10-3, and 4.26 x 10-3 µM, respectively), either in the presence or absence of 20 mM NAC (Sigma Chemical Co.).
Cell viability assay The effect of Cas IIgly on the survival of glioma C6 cells was determined by the MTT (3[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assay (Roche Diagnostics, Mannheim, Germany), which measures mitochondrial activity. MTT is a yellow-colored salt that is taken up and cleaved only by metabolically active cells, reducing it to a colored, water-insoluble formazan salt. The solubilized formazan products were quantified in a 96-well-format spectrophotometer at 570 nm (Labsystem Uniskan, Manchester, UK). Briefly, 5 x 104 cells were seeded in cell culture plates and preincubated overnight. After exposure to increasing concentrations of Cas IIgly for 24 hours at 37°C, 10 µl of MTT (5 mg/ml) was added, and the cells were then incubated at 37°C for 4 hours. The tetrazolium crystals were solubilized by the addition of 10% SDS in 0.01 N HCl. Absorbance was measured after overnight incubation at 37°C. To observe cell morphology, 5 x 104 cells were seeded on chamber slides and exposed to Cas IIgly for 24 hours. Air-dried cells were fixed with 4% paraformaldehyde and stained with hematoxylin-eosin (DakoCytomation, Carpinteria, CA), and cell morphology was observed with a light microscope at x20.
Determination of apoptosis To assess apoptosis, fragmented DNA was isolated and analyzed by electrophoresis on 2% (wt/vol) agarose gels and stained with ethidium bromide as described in the Quantum PrepR AquaPure Genomic DNA Kit (Bio-Rad, Hercules, CA). Induction of cell death was also monitored as the appearance of the sub-G0 peak in cell cycle analysis [26]. Briefly, control and treated cells (1 x 106) were centrifuged and fixed overnight in 70% ethanol at 4°C; then cells were washed three times with PBS, incubated for 1 hour in the presence of 1 mg/ml RNase A and 20 µg/ml propidium iodide at room temperature, and analyzed for different cell cycle phases with a Becton Dickinson (San Jose, CA) FACScan flow cytometer.
Apoptosis was assayed by a second method using the in situ Cell Death Detection Kit, AP (Roche Diagnostics): cells were seeded on 0.7-cm2 glass cover slides in eight-well plates (Daigger, Vernon Hills, IL). After treatment with Cas IIgly, cells were washed twice with PBS and fixed with 4% paraformaldehyde, washed with PBS, permeabilized with 0.1% Triton X-100 in a 0.1% sodium citrate solution, and incubated with the terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) reaction mixture (consisting of calf thymus deoxynucleotidyl transferase and nucleotide mixture in a reaction buffer) for 60 minutes at 37°C. The slides were rinsed three times with PBS and Converter-AP was added (antifluorescein antibody Fab fragment from sheep, conjugated with alkaline phosphatase) during 30 minutes at 37°C. The slides were rinsed again three times with PBS, and the substrate solution was then added [NBT (4-nitro blue tetrazolium chloride) and BCIP (5-bromide-4-chloride-3-indolyl-phosphate 4-toluidine salt)]. Apoptotic cells were visualized with a light microscope.
Apoptosis was also analyzed by a third method using a caspase-3 Colorimetric Assay (RyD System, Minneapolis, MN): briefly, following the treatment with Cas IIgly for 24 hours, cells were washed in PBS, resuspended in lysis buffer, and incubated on ice for 10 minutes. After centrifugation at 10,000g during 1 minute, caspase-3 activity was measured in the supernatant at 37°C in 96 microplates; the reaction contained the caspase-3 colorimetric substrate (DEVD-pNA). The absorbance was measured at 405 nm using a Labsystem Uniskan plate reader.
An apoptotic-related protein, AIF, was also analyzed using Western blot analysis and immunocytochemistry techniques. For immunocytochemistry, treated and control cells were cultured on 0.7-cm2 glass cover slides in eight-well plates (Daigger), fixed with 4% paraformaldehyde in PBS for 10 minutes, washed three times with PBS, and permeabilized with DAKO target retrieval solution (Dako-Cytomation) for 30 minutes at 95°C, and then blocked with 3% hydrogen peroxide for 10 minutes at room temperature. Cells were incubated with an AIF antibody (diluted 1:100) in PBS for 30 minutes at room temperature, washed three times, and then incubated with horseradish peroxidase-conjugated antimouse IgG (DakoCytomation) at a 1:1000 dilution during 15 minutes. After three more washes, the slides were processed by avidin-biotin-peroxidase and visualized with a light microscope at x20.
Mitochondrial transmembrane potential assay Mitochondrial energization was evaluated through the analysis of mitochondrial retention of the cationic fluorescent dye, rhodamine 123 (R123) [27]. Briefly, 1 x 105 cells were treated with Cas IIgly for 24 hours, washed with PBS, and incubated with 20 µg/ml R123 at 37°C for 20 minutes. After washing cells twice again with PBS, R123-related fluorescence was analyzed by cytometry using a FACSCalibur cytometer (Becton Dickinson) and analyzed with Cellquest 3.1f analysis software.
Measurement of ROS formation 2′,7′-Dichlorofluorescein diacetate (DCFH-DA) is a stable, nonfluorescent molecule that is hydrolized by esterases to the nonfluorescent DCFH. DCFH is then oxidized in the presence of ROS (superoxide anion, hydrogen peroxide, and hydroxyl radical) to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) [28]. For ROS analysis, the DCFH-DA probe was used as previously described [29]. Briefly, lysed cells were diluted 1:10 with 40 mM Tris (pH 7.4) and loaded with 5 µM DCFH-DA (Molecular Probes, Eugene, OR) in methanol for 15 minutes at 37°C. Afterward, the fluorescence was recorded before and after 60 minutes of incubation. The formation of the fluorescent oxidized derivative of DCFH, named DCF, was monitored at an excitation wavelength of 525 nm (slit, 5 nm). The bucket holder was thermostatically maintained at 37°C. Autofluorescence of the cellular lysate was always less than 6%. The fluorescent signals of both methanol (as vehicle) and substrates were recorded at the baseline, before the calculation of DCF formation. DCF formation was quantified from a standard curve (Sigma Aldrich, St. Louis, MO) in methanol. Analysis was performed with a Perkin-Elmer (Boston, MA) LS50-B luminescence spectrometer.
Assay of lipid peroxidation The amounts of aldehydic products generated by lipoperoxidation were determined in lysated glioma C6 cells from control and Cas IIgly-treated groups 24 hours after incubation, using the thiobarbituric acid reaction (Sigma Aldrich) [30], and modified as previously reported [31]. Results were listed as nanomoles of thiobarbituric acid reactive substances (TBARS) per milligram of protein.
Subcellular fractionation Control and treated glioma C6 cells were harvested, washed once with ice-cold PBS, resuspended in 200 µl of ice-cold Cell Lysis-M Buffer (Sigma Chemical Co.) plus protease inhibitors [10 mg/ml leupeptin, 1.0 µg/ml aprotinin, and 0.1 mM phenymethylsulfonyl fluoride (PMSF)] (Sigma Chemical Co.), and then sonicated. Insoluble debris was removed by centrifugation at 12,000g for 15 minutes, and the supernatants were stored at -80°C. The nuclear and cytoplasmic extractions were performed with the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL), as previously described [32]. Protein concentrations of each sample were determined using the Bio-Rad protein assay system.
Western blot analysis The levels of proliferating cell nuclear antigen (PCNA) protein expression were evaluated by Western blot analysis. Samples containing equal amounts of protein (30 µµg) were mixed with an equal volume of 2x sample buffer (125 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS3, 0.02% bromophenol blue, and 10% 2-mercaptoethanol) and boiled during 5 minutes. The samples were cooled on ice for 5 minutes, centrifuged for a short time, and exposed to 10% to 15% SDS polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to a nitrocellulose membrane for 2 hours at 70 V with 25 mM Tris-HCl, pH 8.0, 195 mM glycine, and 10% methanol. The membrane was blocked with 5% light milk in 1x PBS for 1 hour, and the PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, Ca) was added to the membrane for 24 hours at 4°C. After three consecutive washes in PBS, the membrane was incubated with a sheep antimouse IgG-horseradish peroxidase complex (Santa Cruz Biotechnology) during 1 hour at room temperature, followed by three washes with PBS. Then, chemiluminescence was visualized using the enhanced chemiluminescence (ECL) kit (Santa Cruz Biotechnology). The blot was exposed to Kodak XAR-5-ray film (Sigma Chemical Co.) for 10 minutes and then developed. A similar procedure was used for AIF, cyt c, β-actin, lamin B (Santa Cruz Biotechnology), and endonuclease G (Endo G) primary antibodies (Chemicon International, Temecula, CA). The band intensities were quantified in a Molecular Dynamics (Durham, NC) computing densitometer using ImageQuant software version 3.2.2.
In Vivo Experiments
Tumor model and treatment Cultured C6 cells (1 x 107) were intraperitoneally inoculated into a 12-week-old Wistar: Hsd rat. Fifteen days later, a multilobulated peritoneal tumor developed. The tumor was mechanically dissociated at 4°C, and a suspension of 1 x 107 cells in 500 µl of saline solution was subcutaneously inoculated into the back of 12-week-old male Wistar:Hsd rats as previously described [33]. Subcutaneous tumors developed in 80% of the animals, reaching a diameter of 1.5 cm, 14 to 17 days after cell implantation [33]. These rats were randomly allocated to one of three groups: group A (n = 30) was used as control and injected subcutaneously with 0.5 ml of water every 24 hours for 21 days, whereas groups B (n = 30) and C (n = 30) were injected intraperitonerally every 24 hours for 21 days with 0.4 and 0.8 mg/kg Cas IIgly, respectively.
Antineoplastic evaluation and collateral effects of Cas IIgly Twenty-one days after treatment, animals from all groups were bled by intracardiac puncture to study chemical blood parameters and then killed; the corresponding tumor was removed from each animal and its volume was measured by fluid displacement [34]. The tumors were dissected and cut by the middle into eight parts; sections were stained by the hematoxylin-eosin method for the morphologic study. The mitotic index was determined by mean percentages of mitoses in 10 different fields. For studies of cell proliferation, sections were stained by immunohistochemistry with monoclonal antibodies against the PCNA (DAKO). The cell proliferation index was obtained by the mean number of positive cells in 10 different microscopic fields. All histologic evaluations (x40) were made blindly to avoid bias.
The determination of apoptosis in 3 µm of glioma C6 sections was also analyzed using the in situ Cell Death Detection Kit, AP (Roche Diagnostics; technique previously described). The fraction of apoptotic cells was expressed as an apoptotic rate, which represented the number of apoptotic cells among 1000 glioma C6 nucleated cells.
Statistical analysis All in vitro studies were made in triplicate. Data from in vitro and in vivo experiments were analyzed with the Student's t test.
Results
Cas IIgly Inhibits Cell Proliferation and Induces Morphologic Changes
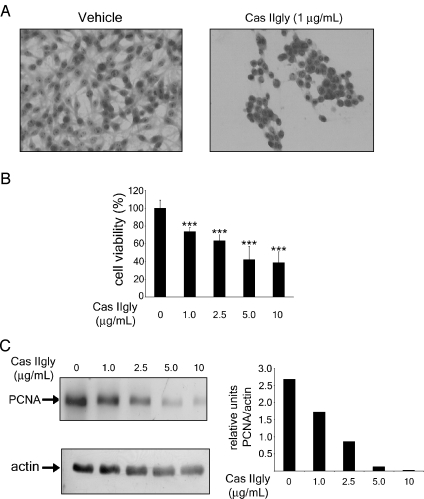
Treatment with Cas IIgly induced growth inhibition and morphologic changes in a dose-dependent manner. Figure 1A shows morphologic alterations in glioma C6 cells treated with 1 µg/ml Cas IIgly for 24 hours; such cells contracted, became rounded, and detached from the culture dishes. In contrast, nontreated glioma C6 cells remained morphologically unchanged. Figure 1B depicts the dose-dependent effect of Cas IIgly on glioma C6 cell viability; most cells died after treatment with 5 and 10 µg/ml Cas IIgly for 24 hours. In addition, the Western blot analysis revealed that the levels of PCNA were significantly decreased in cells treated with higher concentrations of Cas IIgly (Figure 1C). These results show that Cas IIgly has an antineoplastic effect on glioma C6 cells through the inhibition of cell proliferation, in a mechanism apparently involving the induction of apoptosis.
Figure 1.
Cas IIgly inhibits cell proliferation and induces morphologic changes in tumor cells. (A) Morphology of untreated glioma C6 cells (left panel) and glioma C6 cells treated with 1 µg/ml Cas IIgly for 24 hours (right panel). Cell morphology was visualized with a light microscope at x20 using H&E staining. (B) Dose-dependent effect of Cas IIgly on cell viability in C6 glioma cells. Cell viability was measured by the MTT assay; data represent the mean ± SD (*P ≤ .05, **P ≤ .001, and ***P ≤ .0001) from six independent experiments. (C) Dose-dependent effect in the expression of PCNA in cell lysates of control and Cas IIgly-treated glioma C6 cells (left panel). The bar graphs indicate the relative units of PCNA normalized with actin (right panel). Each bar represents the mean ± SD (*P ≤ .05, **P ≤ .001, and *** P ≤ .0001) of three independent experiments.
Treatment with Cas IIgly Induces Apoptosis
To investigate whether the Cas IIgly-induced decrease in cell viability was the result of an apoptosis induction, glioma C6 cells were treated with 1, 2.5, 5, and 10 µg/ml Cas IIgly for 24 hours and analyzed by flow cytometry with propidium iodide. Figure 2A shows dose-dependent changes in the percentage of cell death. Apoptotic cells were identified by TUNEL staining. Treatment with 1, 2.5, 5, and 10 µg/ml Cas IIgly caused 25%, 58%, 83%, and 95% of apoptotic cells, respectively, whereas only 4% of apoptotic cells was found in controls (Figure 2B). The induction of apoptosis by DNA analysis showed the generation of large-scale DNA fragments at doses of 1 and 2.5 µg/ml Cas IIgly, and oligonucleosomal cleavage of DNA in cells treated with 5 and 10 µg/ml casiopeina (Figure 2C).
Figure 2.
Treatment with Cas IIgly induces apoptosis in tumor cells. (A) Cell death of control and 24-hour Cas IIgly-treated glioma C6 cells was measured by flow cytometry after propidium iodine staining. The data represent the mean ± SD (*P ≤ .05, **P ≤ .001, and ***P ≤ .0001) of three independent determinations. (B) Dose-dependent effect of Cas IIgly on apoptosis. Apoptosis of control and 24-hour Cas IIgly-treated glioma C6 cells was determined by TUNEL assay. The results are the mean ± SD (*P ≤ .05 and ***P ≤ .0001) of three independent determinations. Quantitative estimation of TUNEL-positive nuclei at different doses was determined by counting five fields at x10 for each determination. (C) Effect of Cas IIgly on DNA. The marker is a 100-bp DNA ladder (left lane). DNA was isolated from control and 24-hour Cas IIgly-treated glioma C6 cells.
Cas IIgly Induced the Loss of Mitochondrial Membrane Potential and the Release of Apoptogenic Factors
Mitochondria play a critical role in apoptosis caused by drugs such as chemotherapeutic and DNA-damaging agents [35]. Because mitochondria manifest signs of outer and/or inner membrane permeabilization when exposed to a variety of proapoptotic drugs, we determined the mitochondrial membrane potential in glioma C6 cells using the fluorescent dye R123 and analyzed it by flow cytometry. Cas IIgly produced a reduction in the mitochondrial membrane potential in a dose-dependent manner (Figure 3A). Mitochondrial damage may result in the release of cyt c to cytosol and further activation of caspase-3. Western blot analysis (Figure 3B) revealed a significant release of cyt c at doses of 5 and 10 µg/ml Cas IIgly when compared with control. As a key executioner of apoptosis, the activation of caspase-3 was measured in glioma C6 cells treated with Cas IIgly (Figure 3C); whereas there was no significant activation of caspase-3 at doses of 1 and 2.5 µg/ml Cas IIgly when compared with untreated controls, doses of 5 and 10 µg/ml increased the protease activation by 1.5- and 3-fold, respectively. Another mechanism for apoptosis induction, independent of caspase activity, is through AIF—a mitochondrial protein that is translocated into the nucleus on induction of apoptosis. Within the nucleus, AIF, in cooperation with a second mitochondrial protein Endo G, induces chromatin condensation and large-scale DNA fragmentation [36]. Western blot analysis (Figure 3D) revealed the presence of AIF and Endo G in all nuclear extracts obtained from glioma C6 cells treated with Cas IIgly. A significant dose-dependent increase in AIF and Endo G levels was observed. These results clearly show that Cas IIgly induces both the loss of mitochondrial membrane potential and apoptosis through mechanisms dependent and independent of caspases, suggesting that, at lower doses, apoptosis is mediated by a caspase-independent mechanism-involving AIF and Endo G, whereas at higher doses, apoptosis is due both to caspase-dependent and caspase-independent pathways. Moreover, several reports have proposed that AIF is indeed a caspase-dependent death effector [37], implicating that the translocation of AIF into the nucleus is a caspase-dependent event. However, we have found that a broad-spectrum caspase inhibitor, ZVAD-FMK, failed to attenuate AIF translocation induced at all doses of Cas IIgly, suggesting that the casiopeina induces nuclear redistribution of AIF in a caspase-independent manner during the apoptotic process (data not shown).
Figure 3.
Cas IIgly induced the loss of mitochondrial membrane potential and the release of apoptogenic factors. (A) Mitochondrial membrane potential from control and 24-hour Cas IIgly-treated glioma C6 cells was measured by accumulated R123 fluorescence determined by flow cytometry. The data represent the mean ± SD (*P ≤ .05, **P ≤ .001, and ***P ≤ .0001) of three independent determinations. (B) Western blot analysis for cyt c, the cytosolic fraction obtained from control and 24-hour Cas IIgly-treated C6 cells (left panel). The bar graphs indicate the relative amounts of cyt c normalized to the respective actin level (right panel). Each bar represents the mean of three independent experiments. (C) Caspase-3 activity is presented as fold increases over control. Each bar represents a mean of three independent experiments. (D) Western blot analysis for AIF and Endo G in the nuclear fraction obtained from control and 24 h Cas IIgly-treated C6 cells (left panel). The bar graphs indicate the relative amounts of AIF and Endo G normalized to the respective laminin B level (right panel). Each bar represents the mean of three independent experiments.
Treatment with Cas IIgly Induces ROS Formation and Lipoperoxidation
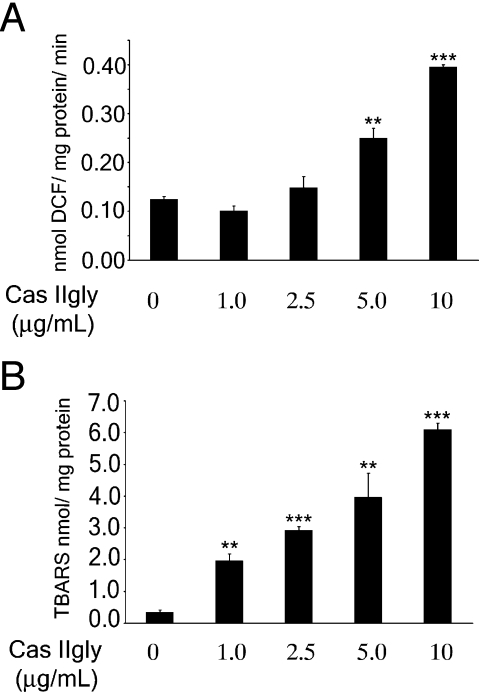
Some antineoplastic agents eliminate tumor cells through ROS formation [38]. We investigated if Cas IIgly is able to induce ROS formation and lipid peroxidation. Generation of ROS was determined in cellular lysates of glioma C6 cells treated with Cas IIgly using DCFH-DA as a specific fluorescent dye probe. A significant increase in ROS levels was observed at 5 and 10 g/ml (Figure 4A). Lipid peroxidation in membranes is a direct measure of the effect of ROS formation in biologic systems, and was measured using the thiobarbituric acid reaction. A dose-dependent increase of lipid peroxidation as the result of incubation of cells in the presence of Cas IIgly was also observed (Figure 4B). These data show that Cas IIgly induces lipid peroxidation by ROS formation.
Figure 4.
Treatment with Cas IIgly induces ROS and lipid peroxidation. (A) ROS generation was determined in lysed cells obtained from control and 24-hour Cas IIgly-treated C6 cells as described in Materials and Methods section. Each bar represents the mean ± SD (**P ≤ .001 and ***P ≤ .0001) of three independent experiments. (B) Levels of lipid peroxidation were determined in lysed cells obtained from control and 24-hour Cas IIgly-treated C6 cells, as described in Materials and Methods section. Each bar represents the mean ± SD (**P ≤ .001 and ***P ≤ .0001) of three independent experiments.
Increase in Cellular ROS Formation Is Associated with Apoptosis Induced by Cas IIgly
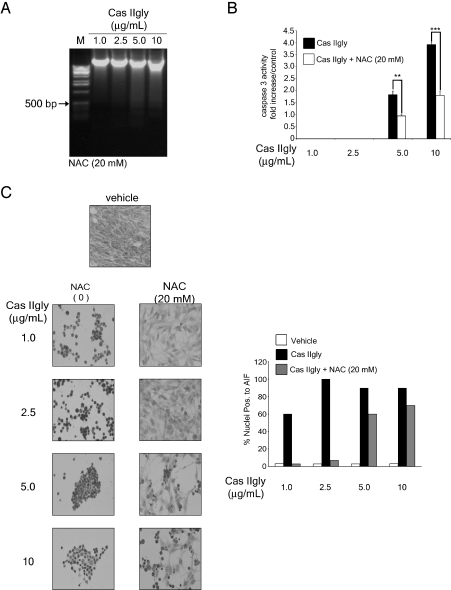
Glioma C6 cells were treated with Cas IIgly in the presence of the antioxidant NAC, a potent ROS scavenger (Table 1). The addition of this compound to cells treated with increasing doses of Cas IIgly produced a reduction of cell death and increased the fraction of cells in the G0/G1 phase analyzed through flow cytometry, whereas Cas IIgly increased the amount of apoptotic cells independently of the cell cycle phase. Table 1 presents data showing that at 20 mM, NAC completely inhibited the Cas IIgly-induced cell death at 1 and 2.5 µg/ml doses, whereas at 5 and 10 µg/ml doses plus NAC, the inhibition of cell death mediated by Cas IIgly was approximately 50%. NAC protected cells from death induced by Cas IIgly by arresting them in G0/G1 phase, and this effect was observed by DNA analysis from Cas IIgly-treated glioma C6 cells in the presence of NAC. Oligonucleosomal cleavage of DNA was shown only in cells treated with Cas IIgly at 5 and 10 g/ml plus NAC (Figure 5A), and DNA cleavage was less than that observed in cells treated with 5 and 10 g/ml Cas IIgly alone (Figure 2C). These results suggest that ROS play an important role in the regulation of cell death induced by Cas IIgly in glioma C6 cells. To investigate if the ROS formation induced by Cas IIgly is a triggering event of Cas IIgly-induced apoptosis, we examined caspase-3 activity by a colorimetric method, as well as the AIF translocation from the mitochondria to the nucleus by immunocytochemistry. When glioma C6 cells were treated with Cas IIgly in the presence of NAC (Figure 5B), the activation of caspase-3 was inhibited by approximately 50% at 5 and 10 g/ml concentrations, concentrations, whereas the translocation of AIF was significantly inhibited at 1 and 2.5 g/ml concentrations; however, doses of 5 and 10 g/ml Cas IIgly inhibited the AIF translocation only by 50% (Figure 5C). These results suggest that Cas IIgly at lower doses induces apoptosis through the formation of ROS, involving AIF translocation to the nucleus; whereas at higher doses, apoptosis is mediated through caspase activation and ROS-dependent and ROS-independent mechanisms, as evidenced by the fact that, in the presence of 20 mM NAC, AIF translocation, caspase-3 activation, and apoptosis were only partially inhibited.
Table 1.
Effect of Cas IIgly on the Cell Cycle in Glioma C6 Cells.
| Cas IIgly (g/ml) | % Sub-G0 | % G0/G1 | % S/M |
| 0 | 4.15 ± 2.65 | 53.10 ± 1.62 | 43.20 ± 0.74 |
| 1.0 | 27.78 ± 7.35* | 43.95 ± 4.06* | 27.78 ± 11.15 |
| 1.0 + NAC | 1.49 ± 0.74 | 73.50 ± 3.42† | 25.15 ± 3.64* |
| 2.5 | 50.97 ± 6.93† | 26.52 ± 0.91‡ | 22.54 ± 4.35* |
| 2.5 + NAC | 1.27 ± 0.07 | 85.30 ± 2.23‡ | 12.49 ± 2.77‡ |
| 5.0 | 73.17 ± 18.67* | 11.07 ± 2.75‡ | 16.82 ± 10.73* |
| 5.0 + NAC | 38.05 ± 5.6† | 47.15 ± 6.84 | 14.29 ± 1.61‡ |
| 10 | 80.37 ± 9.90† | 8.65 ± 0.84‡ | 10.45 ± 6.11† |
| 10 + NAC | 47.77 ± 1.24‡ | 37.73 ± 3.35* | 13.24 ± 2.75‡ |
Results are expressed as mean ± SD. Statistical significance was obtained by comparing untreated cells versus Cas IIgly treatment (1.0, 2.5, 5.0, and 10 g/ml) or Cas IIgly + NAC (20 mM) treatment.
P ≤ .05.
P ≤ .001.
P ≤ .0001.
Figure 5.
Increase in cellular ROS is associated with apoptosis induced by Cas IIgly. (A) Effects of NAC on DNA of 24-hour Cas IIgly-treated cells in the presence of 20 mM NAC. DNA extraction was performed as described in Materials and Methods section. The marker is a 1-kb extension ladder. (B) Immunocytochemistry of AIF in control C6 cells, treated with Cas IIgly and Cas IIgly +20 mM NAC for 24 hours (left panel). The figures shown are representative of at least three different experiments for each experimental condition. Original magnification, x20. The bar graphs indicate the mean of three independent determinations (left panel). Quantitative estimation of AIF-positive nuclei at different doses was determined by counting five fields of x20 for each determination. (C) Caspase-3 activity in control C6 cells, treated with Cas IIgly and Cas IIgly +20 mM NAC for 24 hours. Caspase-3 activity was determined as described in Materials and Methods section. Caspase-3 activities were presented as fold increases over control. Each bar represents the mean ± SD (**P ≤ .001 and ***P ≤ .0001) of three independent experiments.
In Vivo Antitumoral Effect of Cas IIgly
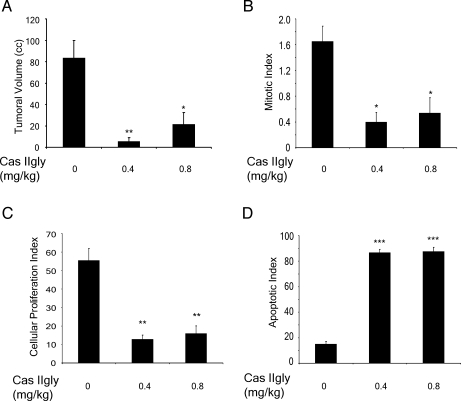
To determine whether Cas IIgly exerts an antitumoral effect in glioma C6, we performed individual microscopic analyses of tumors. Necrosis, mitosis, pleomorphic multinucleated cells, and vascular proliferation were less evident in tumors from animals treated with Cas IIgly at doses of 0.4 and 0.8 mg/kg per day. Reductions in the mean volume of tumors of about 94% and 75% were found in rats treated with 0.4 and 0.8 mg/kg per day, respectively (Figure 6A). In controls, the mean volume was 84 ± 16 ml; whereas in animals treated with 0.4 mg/kg per day of Cas IIgly, the volume was 5.3 ± 2 ml (P ≤ .001), and in animals treated with 0.8 mg/kg per day, the mean volume was 21.4 ± 10 ml (P ≤ .05). When the mitotic index from treated animals was compared with that of controls, reductions of 76% and 67% were observed (Figure 6B). For controls, the mitotic index in viable sections of the tumor was 1.65 ± 0.24; whereas in animals treated with Cas IIgly at doses of 0.4 and 0.8 mg/kg per day, the indexes were 0.4 ± 0.1 (P ≤ .05) and 0.54 ± 0.24 (P ≤ .05), respectively. Reductions of 77% and 71% in the index of cell proliferation were observed in animals treated with Cas IIgly (Figure 6C). The index in animals treated with Cas IIgly at 0.4 mg/kg per day was 12.9 ± 2.2 (P ≤ .001), and in those treated with 0.8 mg/kg per day, it was 16.02 ± 4.06 (P ≤ .001); whereas for controls, it was 55.5 ± 6.4. The apoptotic index for the treated groups was significantly higher than in controls (P ≤ .0001) (Figure 6D). For all variables analyzed, we found significant differences between treated animals and controls.
Figure 6.
In vivo antitumoral effect of Cas IIgly. (A) Comparison of tumoral volume determined through water displacement. (B) Mitotic index determined by microscopic analysis. (C) Cellular proliferation index determined by inmunohistochemistry for PCNA. (D) Apoptotic index determined by TUNEL assay for rats with glioma C6 treated with Cas IIgly at doses of 0.4 and 0.8 mg/kg per day for 21 days.
Side Effects
In the case of metal complexes, toxicity can be exacerbated by a retention or accumulation of the metal in tissues. To determine the extent of hepatic and renal damage induced by Cas IIgly in vivo, both hepatic function and blood chemistry tests were performed in control and treated groups. The mean values of laboratory blood tests were similar among the groups and no evidence of drug-induced toxicity was found (Table 2). No mortality was registered during the experiment.
Table 2.
Laboratory Parameters in Control and Cas IIgly-Treated Rats.
| Parameter | Control (n = 8) | Tumor (n = 5) | Tumor + Cas IIgly, 0.4 mg/kg per day (n = 10) | Tumor + Cas IIgly, 0.8 mg/kg per day (n = 9) |
| Glucose (mg/dl) | 147.96 ± 22.35 | 186.44 ± 61.08 | 116 ± 25.35* | 111.22 ± 28.98* |
| BUN | 24.40 ± 2.31 | 26.6 ± 5.24 | 25.4 ± 3.06 | 20.44 ± 2.92 |
| Urea (mg/dl) | 52.22 ± 4.93 | 56.92 ± 11.2 | 54.35 ± 6.55 | 43.74 ± 6.24 |
| Creatinine (mg/dl) | 0.63 ± 0.15 | 0.72 ± 0.044 | 0.63 ± 0.08 | 0.53 ± 0.07 |
| Uric acid (mg/dl) | 1.65 ± 0.43 | 4.16 ± 0.78† | 2.43 ± 1.45 | 3.64 ± 0.77† |
| Bilirubin (mg/dl) | 0.56 ± 0.20 | 1.1 ± 0.87 | 0.58 ± 0.21 | 0.53 ± 0.19 |
| SGOT (U/l) | 206.36 ± 102.56 | 344.22 ± 148.05 | 191.67 ± 73.02 | 211.44 ± 74.49 |
| SGPT (U/l) | 75.2 ± 17.2 | 67.12 ± 17.77 | 48.4 ± 13.7* | 43.77 ± 10.73‡ |
| Alkaline phosphatase (U/l) | 431.22 ± 119.71 | 277.36 ± 61.81* | 318.8 ± 153.46 | 238.22 ± 96.15* |
| GGT | 1.25 ± 1.03 | 5.96 ± 4.11* | 3.75 ± 2.96* | 2.5 ± 1.37 |
| Total protein (g/dl) | 7.26 ± 0.17 | 6.84 ± 0.75 | 6.32 ± 0.84* | 5.13 ± 1.0† |
| Albumin (g/dl) | 1.57 ± 0.20 | 2.08 ± 1.18 | 1.4 ± 0.36 | 1.5 ± 1.25 |
| Globulins (mg/dl) | 5.66 ± 0.15 | 5.36 ± 0.55 | ND | 4.0 ± 0.58 |
| A/G ratio | 0.25 ± 0.03 | 0.278 ± 0.075 | ND | 0.27 ± 0.044 |
Results are expressed as mean ± SEM. Statistical significance was obtained by comparing untreated rats without tumor (Control) versus untreated rats with tumor or Cas IIgly-treated rats (0.4 and 0.8 mg/kg per day) with tumor.
P ≤ .05.
P ≤ .001.
P ≤ .0001.
Discussion
Recent developments in cancer research suggest that a number of apoptotic stimuli share common mechanistic pathways characterized by the generation of ROS and the loss of mitochondrial membrane potential with subsequent changes in the outer mitochondrial membrane permeability and the release of apoptogenic factor [38,39]. Mitochondrio-nuclear translocation of AIF appears to be a constant feature of apoptosis, independently of triggering conditions (protein kinase inhibition, c-myc overexpression, ceramide, etc.) [40]. Redistribution of AIF to the nucleus, which correlates with large-scale DNA fragmentation, is a caspase-independent event [8]. The mitochondrio-nuclear translocation of AIF seems to be associated with UVB-induced apoptosis through a caspase-independent pathway; loss of mitochondrial membrane potential and ROS generated by UVB might also trigger AIF translocation [41]. In vitro, there is a close relation between AIF and the caspase-mediated cascade, as AIF stimulates the release of cyt c from isolated mitochondria [8]. In several models of cell death, AIF is released from mitochondria before cyt c [8,42], and its neutralization (e.g., by microinjection of an antibody or in knockout models) [42–44] prevents apoptosis. This phenomenon suggests that, at least in some cases, AIF might be necessary for cyt c-dependent caspase activation [36]. The kinetics for differential release of AIF might be explained by the preferential localization of cyt c within the inner mitochondrial membrane, and sustained by electrostatic interactions that delay its release [45,46]. It has been proposed that PARP-1 activation elicited by DNA damage leads to a decrease in NAD+ that is sensed by the mitochondria [42]. Cytosolic AIF is translocated into the nucleus and initiates nuclear condensation and large-scale chromatin fragmentation [8]. Additionally, cytosolic AIF acts on the mitochondrial membrane potential and initiates the release of cyt c, thus activating caspases [9]. The late activation of caspases, after the executioner step of AIF release, may facilitate its dissolution inside the cell.
In our studies, Cas IIgly, a novel copper-based chemotherapeutic agent, increased cell death by apoptosis in glioma C6 cells, and various mechanisms seem to be related to this effect. Cas IIgly may induce apoptosis in CH1 cells without showing DNA oligonucleosomal fragments, revealing a relatively low level of caspase activation [23]. Reduced inhibition of apoptosis by ZVAD-FMK, a wide broad caspase inhibitor, suggests that Cas IIgly may trigger the caspase-independent pathway of apoptosis [23]. At low concentrations (≤10 µM), Cas IIgly inhibits the rates of state 3 and uncoupled respiration of mitochondria isolated from the rat liver, kidney, heart, and AS-30D hepatoma. At concentrations higher than 10 µM, Cas IIgly stimulates basal respiration, which is followed by its inhibition, a K+-dependent swelling, collapse of membrane potential, and late cyt c release [47]. A single intravenous administration of Cas IIgly (1, 3, and 5 mg/kg) to rats produces hemolytic damage in a dose-dependent manner [48]; however, a lack of hemolytic damage at the lowest dose 12 hours after its administration was observed, suggesting that this dose could represent the limit for adverse reactions. In this study, the major toxic effect observed in hemolytic anemia was attributed to the dose of 5 mg/kg Cas IIgly; it was observed 12 hours and 5 days after Cas IIgly administration, and was accompanied by a marked neutrophilic leukocytosis. Recovery occurred 15 days after its administration.
Our findings also indicate that Cas IIgly, at doses between 1 and 2.5 g/ml, induces ROS generation, loss of mitochondrial membrane potential, AIF and Endo G mitochondrio-nuclear translocation and apoptosis without cytosolic release of cyt c, caspase-3 activation, or DNA laddering. An additional interesting finding is that NAC, a ROS scavenger, blocked the mitochondrio-nuclear translocation of AIF and apoptosis, suggesting that ROS generated by Cas IIgly acts as a trigger factor for mitochondrio-nuclear translocation of AIF only. In contrast, at higher doses of casiopeina (5 and 10 µg/ml), ROS production and loss of mitochondrial membrane potential are larger, and apoptosis occurs with DNA laddering associated with release of cyt c and caspase-3 activation, as well as mitochondrio-nuclear translocation of AIF and Endo G. In the latter case, NAC blocks only 50% of these processes. These results also suggest that ROS generated by Cas IIgly is one of the triggering events for apoptosis. The results mentioned above show that low doses of Cas IIgly induce apoptosis in glioma C6 cells through a caspase-independent pathway. Under these conditions, the mitochondrio-nuclear translocation of AIF is associated with this pathway, and ROS generated may be involved in triggering its translocation, whereas at higher doses, Cas IIgly induces apoptosis through both caspase-dependent and caspase-independent pathways (caspase-3 activation and mitochondrio-nuclear translocation of AIF).
The administration of Cas IIgly to murine tumors increased the lifespan similarly to other antineoplastic drugs [48]. According to our results, Cas IIgly exerts an intense antitumoral effect in vivo, evidenced by the decrease in all variables studied: tumoral volume, mitotic and cell proliferation, and increased apoptotic index. Unexpectedly, the degree of antitumoral activity of the casiopeina against glioma C6 cells was intense, and even low doses were sufficient to produce a substantial antineoplastic effect. All these features occurred in the absence of toxicity for the hapato-biliary or renal system. Hematic biometry performed on Wistar:Hsd rats 21 days after treatment with Cas IIgly (0.4 and 0.8 mg/kg per day, i.p.) showed a dose-dependent effect. The higher dose produced only a moderate decrease of hemoglobin (accompanied by light neutrophilic leukocytosis but no hemolytic anemia), platelet count was normal, and no histologic abnormalities were seen in the spleen (results not shown).
Most currently used antineoplastic drugs have limited efficacy and high toxicity, as they also affect normal cells. Lipophilic cation drugs are concentrated by cells into mitochondria because of the large negative-inside electric membrane potential [49,50]. The higher plasma and mitochondrial membrane potentials of tumors cells [51,52] may enhance the selective targeting of Cas IIgly into tumor cells and mitochondria. AS-30D hepatoma mitochondria also exhibited higher mitochondrial membrane potential values than did mitochondria from the liver, the organ from which tumor mitochondria were derived. Indeed, AS-30D and HeLa cells in culture died within 48 hours of exposure to CAS IIgly [47]. The effect of Cas IIgly (1, 2.5, 5, and 10 µg/ml) after 24 hours of incubation on the survival of normal fibroblasts was determined by the MTT assay. At 1 and 2.5 µg/ml doses of Cas IIgly, the cell viability of normal fibroblasts in culture was 100%; when the dose was increased to 5µg/ml, viability was 85%; and at 10 µg/ml, it was 50%, suggesting that metabolic effects of Cas IIgly at 1, 2.5, or 5 µg/ml doses are fairly specific against glioma cells (results not shown).
Our results indicate that Cas IIgly is a promising chemotherapeutic option against glial malignant tumors.
Acknowledgements
We gratefully acknowledge the assistance of Martha Arroyo and Carmen Escalante for performing the blood chemistry tests. Isabel Pérez Montfort corrected the English version of the manuscript.
Abbreviations
- Cas IIgly
Casiopeina IIgly
- PCD
programmed cell death
- AIF
apoptosis-inducing factor
- ROS
reactive oxygen species
- NAC
N-acetyl-l-cystein
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- MTT
3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- FACS
fluorescence-activated cell sorter
- NBT
4-nitro blue tetrazolium chloride
- BCIP
5-bromide-4-chloride-3-indolyl-phosphate 4-toluidine salt
- TUNEL
terminal deoxynucleotidyl transferase-mediated nick end labeling
- R123
rhodamine 123
- cyt c
cytochrome c
- Endo G
endonuclease G
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- DCF
2′, 7′-dichlorofluorescein
- TBARS
thiobarbituric acid reactive substances
- PMSF
phenymethylsulfonyl fluoride
- PCNA
proliferating cell nuclear antigen
- PAGE
polyacrylamide gel electrophoresis
- ECL
enhanced chemiluminescence
- SGOT
aspartate transaminase
- SGPT
alanine transaminase
- GGT
gamma glutamyl transferase
Footnotes
This work was supported by CONACyT grants U41997-MA1 and C01-7677-Salud, 2002.
References
- 1.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Huschtscha LI, Bartier WA, Ross CE, Tattersall MH. Characteristics of cancer cell death after exposure to cytotoxic drugs in vitro. Br J Cancer. 1996;73:54–60. doi: 10.1038/bjc.1996.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;28:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondria cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 7.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 8.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosisinducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 9.Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler N, et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugas E, Nochy D, Ravagnan L, Loeffler N, Susin SA, Zamzami N, et al. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82/83:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez M, Vélez PC. Transition metal-induced apoptosis in lymphocytes via hydroxyl radical generation, mitochondria dysfunction, and caspase-3 activation: an in vitro model for neurodegeneration. Arch Med Res. 2004;35:185–193. doi: 10.1016/j.arcmed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 14.France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson's disease. J Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- 15.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, et al. Bcl-2 inhibition of neural death decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 16.Greenlund LJ, Deckwerth TL, Johnson EM., Jr Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 17.Majima HJ, Oberley TD, Furukawa K, Matsson MP, Yen HC, Szweda LI, et al. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998;273:8217–8224. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- 18.Kiningham KK, Oberley TD, Lin S, Mattingly CA, St Clair DK. Overexpression of manganese superoxide dismutase protects against mitochondrial-initiated poly(ADP-ribose) polymerase-mediated cell death. FASEB J. 1999;13:1601–1610. doi: 10.1096/fasebj.13.12.1601. [DOI] [PubMed] [Google Scholar]
- 19.Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem. 1999;274:29294–29302. doi: 10.1074/jbc.274.41.29294. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Ramírez L, De La Rosa ME, Gracia-Mora I, Méndoza A, Pérez G, Ferrer-Sueta G, et al. Casiopeinas, metal-based drugs a new class of antineoplastic and genotoxic compounds. J Inorg Biochem. 1995;207:2–3. [Google Scholar]
- 22.Ruiz-Ramírez L, Gracia-Mora I, De La Rosa ME, Sumano H, Gómez C, Pimentel E, et al. Cytostatic, mutagenic, antineoplastic activities and preliminary toxicity of copper (II) drugs: casiopeinas I, II, III. J Inorg Biochem. 1993;406:1–2. [Google Scholar]
- 23.De Vizcaya-Ruiz A, Rivero-Muller A, Ruiz-Ramirez L, Kass GE, Kelland LR, Orr RM, et al. Induction of apoptosis by a novel copper-based anticancer compound, casiopeina II, in L1210 murine leukaemia and CH1 human ovarian carcinoma cells. Toxicol In Vitro. 2000;14:1–5. doi: 10.1016/s0887-2333(99)00082-x. [DOI] [PubMed] [Google Scholar]
- 24.Gracia-Mora I, Ruiz-Ramírez L, Gómez-Ruiz C, Tinoco-Méndez M, Márquez-Quiñones A, Romero de Lira L, et al. Knight's move in the periodic table, from copper to platinum, novel antitumor mixed chelate copper compounds, casiopeinas, evaluated by an in vitro human and murine cancer cell line panel. Met-Based Drug. 2001;8:19–29. doi: 10.1155/MBD.2001.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruíz-Azuara L. US Patent, Ap 21 (1992), no. 5,107,005; US Patent, Re 35,458, February 18 (1997) 1993 US, Patent November 19 (1996), no. 5,576,326, 407543 SECOFI. [Google Scholar]
- 26.Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179–193. doi: 10.1016/s0037-1963(01)90051-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen XC, Zhu YG, Chen LM, Fang F, Zhou YC, Zhao CH. Nitric oxide induced PC12 cells apoptosis and the protective effect of gingenoside Rg1. Chin Pharmacol Bull. 1998;18:516–519. [Google Scholar]
- 28.Scott JA, Homcy CJ, Khaw BA, Rabito CA. Quantitation of intracellular oxidation in a renal epithelial cell line. Free Radic Biol Med. 1988;4:79–83. doi: 10.1016/0891-5849(88)90067-6. [DOI] [PubMed] [Google Scholar]
- 29.LeBel CA, Ischiropoulous H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescein as an indicator of reactive species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 30.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Muñoz R, Glender W, Díaz-Muñoz M, García-Sainz JA, Chagoya de Sánchez V. Effects of adenosine on liver cell damage induced by carbon tetrachloride. Biochem Pharmacol. 1984;33:2599–2604. doi: 10.1016/0006-2952(84)90631-2. [DOI] [PubMed] [Google Scholar]
- 32.Smirnova IV, Douglas C, Bittel Rudravajhala R, Huimin J, Glen KA. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem. 2000;275:9377–9384. doi: 10.1074/jbc.275.13.9377. [DOI] [PubMed] [Google Scholar]
- 33.Guevara P, Sotelo J. C6 rat glioma grown into the peritoneal cavity, a large source of tumoral cells for subcutaneous transplant of glioma. J Neuro-Oncol. 1999;44:91–92. doi: 10.1023/a:1006112422132. [DOI] [PubMed] [Google Scholar]
- 34.Tamayko M, Reynolds P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 35.Van GM, Festjens N, Van LG, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 36.Cande C, Cecconi F, Dessen F, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 37.Damien A, Brigitte G, Mariusz K, Juanita CS, Francesco C, Richard JY. Mitochondrial release of AIF and Endo G requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22:4385–4399. doi: 10.1093/emboj/cdg423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siro S, Minoru T, Kazuo U, Masaya I. Requirement of caspase-3 (-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900–26907. doi: 10.1074/jbc.273.41.26900. [DOI] [PubMed] [Google Scholar]
- 39.Decaudin D, Marzo I, Brenner C, Kroemer G. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy. Int J Oncol. 1998;12:141–152. [PubMed] [Google Scholar]
- 40.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- 41.Murahashi H, Azuma H, Zamzami N, Furuya KJ, Ikebuchi K, Yamaguchi M, et al. Possible contribution of apoptosis-inducing factor (AIF) and reactive oxygen species (ROS) to UVB-induced caspase-independent cell death in the T cell line Jurkat. J Leukoc Biol. 2003;73:399–406. doi: 10.1189/jlb.0702335. [DOI] [PubMed] [Google Scholar]
- 42.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 43.Ferri KF, Jacotot E, Blanco J, Este JA, Zamzami N, Susin SA, et al. Apoptosis control in syncytia induced by the HIV-envelope glycoprotein complex. Role of mitochondria and caspases. J Exp Med. 2000;192:1081–1092. doi: 10.1084/jem.192.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, et al. Essential role of the mitochondrial apoptosis inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 45.Salamon Z, Tolli G. Interaction of horse heart cytochrome c with lipid bilayer membranes: effects on redox potentials. J Bioenerg Biomembr. 1997;29:211–221. doi: 10.1023/a:1022401825287. [DOI] [PubMed] [Google Scholar]
- 46.Jutila A, Rytomaa M, Kinnunen PK. Detachment of cytochrome c by cationic drugs from membranes containing acidic phospholipids: comparison of lidocaine, propranolol, and gentamycin. Mol Pharmacol. 1998;54:722–732. [PubMed] [Google Scholar]
- 47.Marin-Hernandez A, Gracia-Mora I, Ruiz-Ramirez L, Moreno-Sanchez R. Toxic effects of copper-based antineoplastic drugs (Casiopeinas) on mitochondrial functions. Biochem Pharmacol. 2003;65:1979–1989. doi: 10.1016/s0006-2952(03)00212-0. [DOI] [PubMed] [Google Scholar]
- 48.Gracia-Mora I, Bravo-Gómez ME, Ruiz-Ramírez L, Tinoco-Méndez M, Huerta L. New antineoplastic in vitro and in vivo screening of mixed chelate copper(II) coordination compounds (casiopeinas) in several tumoral models. J Bioorg Med Chem. 2004 (in press) [Google Scholar]
- 49.Vizcaya-Ruíz A, Rivero-Muller A, Ruíz-Ramirez L, Howarth JA, Dobrota M. Hematotoxicity response in rats by the Koper-based anticancer agent: casiopeina II. Toxicology. 2003;194:103–113. doi: 10.1016/j.tox.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Nadakavukaren KK, Nadakavukaren JJ, Chen LB. Increased rhodamine 123 uptake by carcinoma cells. Cancer Res. 1985;45:6093–6099. [PubMed] [Google Scholar]
- 51.Koya K, Li Y, Wang H, Ukai T, Tatsuta N, Kawakami M, et al. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996;56:538–543. [PubMed] [Google Scholar]
- 52.Davis S, Weiss MJ, Wong JR, Lampidis TJ, Chen LB. Mitochondria and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Biol Chem. 1985;260:13844–13850. [PubMed] [Google Scholar]