Abstract
The force required to rupture bonds between individual Staphylococcus aureus MSCRAMMs and surfaces coated with extracellular matrix molecules has been quantified by using optical tweezers. The observed binding forces between fibrinogen or fibronectin and S. aureus MSCRAMMs occurred as an approximate integer multiple of 20 or 25 pN, respectively.
Bacterial adhesion to host tissue is an early step in the process of infection (5). In some gram-positive cocci, such as Staphylococcus aureus, this adhesion is mediated by covalently anchored transmembrane molecules called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (13). Developing a greater understanding of the interactions between MSCRAMMs and their ligands can lead to improved methods of reducing bacterial adhesion, thus preventing the formation of a biofilm. As a first step toward this goal, we have used optical tweezers to measure the force required to detach individual S. aureus cells from surfaces coated with extracellular matrix (ECM) proteins.
The optical tweezers system in our experiments utilized a tunable titanium-sapphire laser pumped by a solid-state, frequency-doubled neodymium yttrium orthovanadate (Nd-YVO4) laser emitting light at 532 nm. The titanium-sapphire laser was tuned to 830 nm, a wavelength that induces negligible damage to cells (7, 8, 10). The laser beam was expanded five times to fill the back aperture of a high numerical aperture (numerical aperture, = 1.3) oil-immersion microscope objective lens of an inverted microscope and focused into the chamber containing the cells. A light attenuator allowed for laser power adjustment. A halogen lamp illuminated the cell chamber from above. The emitted visible light was reflected toward a charge-coupled device camera; coupled with a video monitor, the camera allowed for visualization of the cell chamber. The microscope stage was mounted on a plate that could be moved manually with micromanipulators in the plane normal to the beam propagation axis. A piezoelectric stage was mounted on top of the manually controlled plate to control the movement of the sample chamber during calibration experiments. By varying the amplitude of a ramp waveform supplied by a function generator, it was possible to control the velocity of the piezoelectric stage. For calibration of the trapping force, the piezoelectric stage was moved with increasing velocities until a cell escaped the trap. The escape velocities for various laser powers after the microscope objective lens were converted into forces by using Stokes' law for drag on a sphere (3, 22, 24).
We coated 10-μm-diameter polystyrene microspheres (Polysciences, Warrington, Pa.) with an ECM protein solution by adsorption. Following coating, the microspheres were incubated with albumin fraction V to block nonspecific adhesion. The microspheres were then centrifuged and resuspended in phosphate-buffered saline. Uncoated microspheres and those coated with only albumin fraction V served as controls. S. aureus strains were inoculated into tryptic soy broth and were cultured overnight in a 37°C shaking water bath. At the time of an experiment, a small amount of the liquid culture was removed and resuspended in phosphate-buffered saline. Coated microspheres were allowed to adhere to a coverslip for at least 10 min prior to an experiment so that they were firmly fixed, as demonstrated by the fact that we were unable to move them with the optical tweezing at maximum power.
To determine detachment forces we moved the stage to bring a trapped cell in contact with the surface of a coated microsphere immobilized on the coverslip and held it there for 10 s. The trapping power was subsequently increased in a stepwise fashion until it was possible to detach the cell from the microsphere by simultaneously moving the manually controlled stage. The stage movement and increased trapping force opposed the adhesion between the cell and the coverslip-bound microsphere until, at some point, the laser power was sufficient to overcome the binding force between cell and microsphere. We determined the minimum laser power necessary to detach the cell from the microsphere and converted this power into a force. Each trial was performed with a different cell and microsphere.
First we quantified detachment forces of S. aureus 8325-4 (11) from microspheres incubated in 5- and 25-μg/ml solutions of fibronectin (Fn). S. aureus 8325-4 expresses two Fn-binding MSCRAMMs, FnbpA and FnbpB, which have structurally similar binding domains (6).
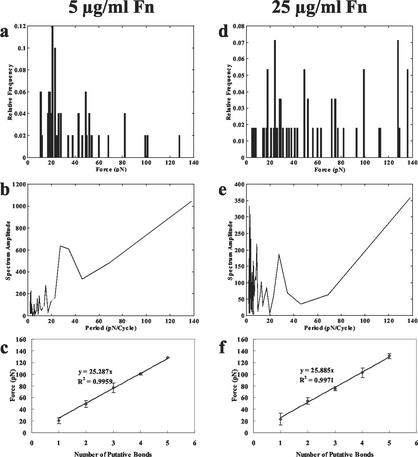
Upon examining a histogram of the force data (Fig. 1a and d), it was noted that the detachment forces occurred in a series of separate clusters instead of an evenly distributed group of force values, and the means within each of these clusters were similar for both Fn coating concentrations. Higher force values were measured more frequently as Fn coating concentration increased. The existence of force quanta was confirmed through analysis of the spectrum amplitude associated with the Fourier transforms of the histograms depicted in Fig. 1a and d. This frequency analysis is presented via the periodograms in Fig. 1b and e. For each Fn coating concentration the periodograms display noticeable peaks at 27 pN, which indicates that this is approximately the separation between successive peaks in the histograms. We used the Fourier analysis to assist in grouping the force values. It is thought that the clustering of force data is related to the number of bonds formed (Fig. 1c and f). Each point on the graph represents the mean force value from all trials that fit into one force quantum. The slope of the regression line signifies the estimated force per bond of approximately 25 pN for Fn concentrations of both 5 and 25 μg/ml.
FIG. 1.
(a) Histogram of detachment forces between S. aureus 8325-4 MSCRAMMs and a 5-μg/ml concentration of Fn. Data are presented as the percentages of data points falling within each interval. The initial contact time between the cell and microsphere before the detachment process was 10 s. (b) Periodogram for frequency analysis of S. aureus 8325-4 MSCRAMM detachment forces from a 5-μg/ml concentration of Fn. The periodogram exhibits a noticeable peak at 27 pN. (c) Data clusters were separated and placed into bond number groups (1 to 5) on the basis of periodogram analysis. Descriptions for panels d to f are similar to those for panels a to c, respectively, except that the Fn concentration was 25 μg/ml.
The substantial ramp on the right half of the periodograms is due to the fact that the majority of the force values in the histograms occur below approximately 40 pN on the force scale shown in Fig. 1a and d. This concentration of small forces results from the short 10-s contact time between cell and microsphere during each trial; if the contact time were increased, the likelihood of achieving higher detachment forces would increase. As a result, the frequency analysis identifies a component with a very low frequency. The slope of the ramp was reduced when the peaks below 40 pN were lowered to be the same magnitude as those on the higher end of the force scale.
There is also a series of very close peaks on the left end of each periodogram. In the histograms of Fig. 1a and d, the peaks on the lower end of the force scale are much less defined than those at the higher end of the scale; that is, there are many force values that are very close together. This is an inherent problem with the light attenuator used during our experiments in that the trapping force increases exponentially as the attenuation level is decreased. There is some judgment involved in determining the attenuator setting at which detachment occurs, and since there is very little difference between two adjacent attenuator settings for lower trapping forces, this judgment becomes even more significant. Therefore, these very close force values are interpreted as very short period components in the frequency analysis.
We also performed several control experiments. Since albumin fraction V was used as a blocking agent to prevent nonspecific adhesion to coated microspheres, we examined the adhesion of S. aureus 8325-4 to microspheres coated only with albumin fraction V and found that little or no adhesion was present. Furthermore, the strength of adhesion between cells and uncoated polystyrene microspheres exceeded the maximum optical trapping force the laser could produce. Therefore, albumin fraction V served as an effective blocking agent in these experiments, so the measured forces are not due to nonspecific adhesion. It is also highly unlikely that the measured forces were related to the removal of adsorbed Fn from the polystyrene microspheres. Other researchers have studied the process of adsorption and found that, within milliseconds, the force required to rupture the nonspecific adhesion between Fn and hydrophobic silica was approximately 200 pN (4). Furthermore, after adsorption for minutes to hours the adhesive forces between proteins and polystyrene are on the order of nanonewtons, several orders of magnitude higher than the forces we are reporting (18, 19).
When an excess of soluble Fn was added to the solution containing the cells, no adhesion was observed. This result, which confirms the specificity of the obtained force values, indicates that the soluble Fn molecules saturated the MSCRAMMs on the cells and blocked any adhesion between cells and microspheres coated with Fn.
In addition to the aforementioned control experiments, we sought to ensure that the quantified detachment forces were measures of specific binding and not measures of the forces required to extract MSCRAMMs from the bacterial cell wall. Since the MSCRAMMs are covalently anchored in the bacterial cell wall, extraction would be unlikely; however, the possibility warranted investigation. FnbpA and FnbpB, Fn-binding MSCRAMMs in S. aureus, bind fibrinogen (Fg) in addition to binding Fn (23). Therefore, we tested the binding of these two different ligands to the same MSCRAMM and then compared the detachment forces, the assumption being that if MSCRAMMs were extracted from the membrane, then the same extraction force would be required regardless of which ligand the MSCRAMM was bound to. In these experiments we used a mutant type of S. aureus P1 (generously donated by T. Foster, University of Dublin, Ireland) which did not express the primary Fg-binding MSCRAMMs, ClfA and ClfB; therefore, this strain was only able to adhere to Fg via FnbpA and FnbpB. The S. aureus P1 mutant cells adhered to microspheres coated with 0.1-μg/ml concentrations of Fn and Fg with forces of approximately 24 and 20 pN per bond, respectively. Similar to the aforementioned Fn-binding experiments, the frequency analysis of Fg binding demonstrated the existence of a peak close to the estimated single-bond value of 20 pN (Fig. 2). A two-tailed paired t test was used to compare the mean of each Fn force quantum to the corresponding Fg quantum. The results of the t test indicated that there is a significant difference between binding to Fn and Fg (P < 0.05). If the force measurements were related to MSCRAMM extraction from the membrane, then we would expect the same base force regardless of which ligand the MSCRAMM was bound to. However, we achieved different forces for Fn and Fg binding to the same MSCRAMM, which indicates that MSCRAMMs were not being extracted.
FIG. 2.
(a) Histogram of detachment forces between S. aureus P1 MSCRAMMs and 0.1 μg of Fg/ml. The initial contact time between the cell and microsphere before the detachment process was 10 s. (b) Periodogram for frequency analysis of S. aureus P1 MSCRAMM detachment forces from 0.1 μg of Fg/ml. The periodogram exhibits a noticeable peak at 17 pN. (c) Data clusters were separated and placed into bond number groups (1 to 5) on the basis of periodogram analysis.
This study also demonstrated that, though the number of MSCRAMMs expressed may vary from one strain to another, the single-bond force does not change. A paired t test comparing the S. aureus P1 wild type (20) and S. aureus 8325-4 binding to Fn showed that there is no statistically significant difference in the binding force of these two strains (P > 0.05). We hypothesize that S. aureus P1 has a higher density of Fn-binding MSCRAMMs than S. aureus 8325-4, since a lower concentration of Fn had to be used with S. aureus P1 in order to successfully detach cells with the available trapping force.
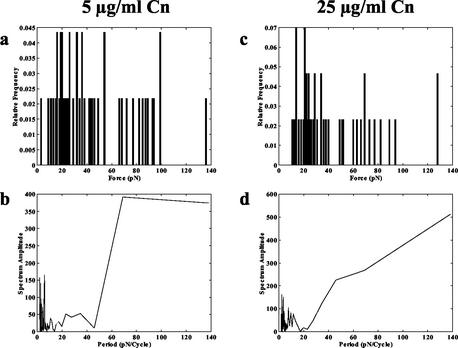
Finally, we measured S. aureus binding to microspheres coated with 5 and 25 μg of type I collagen (Cn)/ml. Here, we used S. aureus Phillips, since this strain expresses the Cn-binding MSCRAMM Cna while 8325-4 does not. In contrast to the results of the Fn adhesion experiments, the force values obtained in the Cn experiments did not exhibit any apparent quantization. No clear correlation emerged between the forces for 5 and 25 μg/ml in that they did not appear to share any common clusters (Fig. 3a and c). Furthermore, the periodogram analysis did not elucidate any obvious peaks in the data (Fig. 3b and d). The results of this experiment are consistent with the knowledge that Cna binds to various sites on the Cn molecule with different affinities (17). Our model of Cna interaction with Cn depicts multiple sites along the Cn triple helix that can fit into the binding trench of the MSCRAMM, and these different sites exhibit different affinities for the binding protein dependent on the surface complementarity of the Cn site and the binding trench. It is likely that, depending on which of these sites is involved in a binding event, the detachment force could differ; this could explain the lack of discernible clusters like those that were observed in the Fn-binding experiments.
FIG. 3.
(a) Histogram of detachment forces between S. aureus Phillips MSCRAMMs and 5 μg of Cn/ml. The initial contact time between the cell and microsphere before detachment process was 10 s. (b) Periodogram for frequency analysis of S. aureus Phillips detachment forces from 5 μg of Cn/ml. The periodogram does not have any distinct peaks. Descriptions for panels c and d are similar to those for panels a and b, respectively, except that the Cn coating concentration was 25 μg/ml.
In summary, we have estimated the forces required to rupture single MSCRAMM-ECM bonds. These results are not dependent on the number of MSCRAMMs expressed per cell, since our data are presented as estimates of the force required to break a single bond. In addition, the measurements do not depend on cell concentration, since each trial involves only a single cell.
Other studies have quantified the forces involved with bacterial adhesion to surfaces; however, none have considered the forces involved with MSCRAMM-mediated adhesion. The initial nonspecific interaction forces between Escherichia coli and biomaterials have been quantified by using atomic force microscopy (2, 12, 14-16). However, these studies did not quantify the force of specific interactions. The forces measured in these nonspecific binding experiments are on the order of nanonewtons, several orders of magnitude greater than the forces we can generate with optical tweezers; therefore, optical tweezers are best applied to studies of smaller, specific binding forces. For instance, by using optical tweezers Liang and coworkers quantified the adhesion of E. coli pili to carbohydrate-coated surfaces and found that the minimum force required to detach a pilus from a carbohydrate molecule is about 1.7 pN (9).
Our estimates of single-bond detachment forces are close to other specific protein-protein interactions that have been investigated with optical tweezers. For example, the unbinding force of plasma von Willebrand factor from the platelet glycoprotein Ib-IX has been estimated to be in the range of 6 to 9 pN (1), and the detachment force of staphylococcal protein A from goat immunoglobulin G is approximately 25 pN (21).
A potential limitation of these experiments is that we have not yet investigated the effect of pulling rate on the detachment forces; this will be studied in future experiments. The optical tweezers technique may also be utilized to explore the effects of mutations, MSCRAMM truncations, and antibodies on detachment forces. We are presently investigating the adhesion of mutants lacking various domains of FnbpA.
Acknowledgments
This work was supported in part by a Special Opportunity grant from The Whitaker Foundation, a student (K.H.S.) research grant from the American Society for Laser Medicine and Surgery, and NIH grants AI20624 and AI10629-02.
REFERENCES
- 1.Arya, M., B. Anvari, G. M. Romo, M. A. Cruz, J. F. Dong, L. V. McIntire, J. L. Moake, and J. A. Lopez. 2002. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood 99:3971-3977. [DOI] [PubMed] [Google Scholar]
- 2.Camesano, T. A., and N. I. Abu-Lail. 2002. Heterogeneity in bacterial surface polysaccharides, probed on a single-molecule basis. Biomacromolecules 3:661-667. [DOI] [PubMed] [Google Scholar]
- 3.Feigner, H., O. Muller, and M. Schliwa. 1995. Calibration of light forces in optical tweezers. Appl. Optics 34:977-982. [DOI] [PubMed] [Google Scholar]
- 4.Gergely, C., J. Voegel, P. Schaaf, B. Senger, M. Maaloum, J. K. Horber, and J. Hemmerle. 2000. Unbinding process of adsorbed proteins under external stress studied by atomic force microscopy spectroscopy. Proc. Natl. Acad. Sci. USA 97:10802-10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gristina, A. G. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588-1595. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson, K., C. Signas, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 7.Leitz, G., E. Fallman, S. Tuck, and O. Axner. 2002. Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence. Biophys. J. 82:2224-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang, H., K. T. Vu, P. Krishnan, T. C. Trang, D. Shin, S. Kimel, and M. W. Berns. 1996. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 70:1529-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang, M. N., S. P. Smith, S. J. Metallo, I. S. Choi, M. Prentiss, and G. M. Whitesides. 2000. Measuring the forces involved in polyvalent adhesion of uropathogenic Escherichia coli to mannose-presenting surfaces. Proc. Natl. Acad. Sci. USA 97:13092-13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman, K. C., E. H. Chadd, G. F. Liou, K. Bergman, and S. M. Block. 1999. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 77:2856-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novick, R. P. 1967. Properties of a cryptic high frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 12.Ong, Y. L., A. Razatos, G. Georgiou, and M. M. Sharma. 1999. Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir 15:2719-2725. [Google Scholar]
- 13.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 14.Razatos, A., Y. L. Ong, F. Boulay, D. L. Elbert, J. A. Hubbell, M. M. Sharma, and G. Georgiou. 2000. Force measurements between bacteria and poly(ethylene glycol)-coated surfaces. Langmuir 16:9155-9158. [Google Scholar]
- 15.Razatos, A., Y. L. Ong, M. M. Sharma, and G. Georgiou. 1998. Evaluating the interaction of bacteria with biomaterials using atomic force microscopy. J. Biomater. Sci. Polym. Ed. 9:1361-1373. [DOI] [PubMed] [Google Scholar]
- 16.Razatos, A., Y. L. Ong, M. M. Sharma, and G. Georgiou. 1998. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA 95:11059-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich, R. L., C. C. Deivanayagam, R. T. Owens, M. Carson, A. Hook, D. Moore, J. Symersky, V. W. Yang, S. V. Narayana, and M. Hook. 1999. Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors, alpha(1)beta(1) integrin and Staphylococcus aureus Cna MSCRAMM. J. Biol. Chem. 274:24906-24913. [DOI] [PubMed] [Google Scholar]
- 18.Sagvolden, G. 1999. Protein adhesion force dynamics and single adhesion events. Biophys. J. 77:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagvolden, G., I. Giaever, and J. Feder. 1998. Characteristic protein adhesion forces on glass and polystyrene substrates by atomic force microscopy. Langmuir 14:5984-5987. [Google Scholar]
- 20.Sherertz, R. J., W. A. Carruth, A. A. Hampton, M. P. Byron, and D. D. Solomon. 1993. Efficacy of antibiotic-coated catheters in preventing subcutaneous Staphylococcus aureus infection in rabbits. J. Infect. Dis. 167:98-106. [DOI] [PubMed] [Google Scholar]
- 21.Stout, A. L. 2001. Detection and characterization of individual intermolecular bonds using optical tweezers. Biophys. J. 80:2976-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svoboda, K., and S. M. Block. 1994. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 23:247-285. [DOI] [PubMed] [Google Scholar]
- 23.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]
- 24.Wright, W. H., G. J. Sonek, and M. W. Berns. 1994. Parameteric study of the forces on microspheres held by optical tweezers. Appl. Optics 33:1735-1748. [DOI] [PubMed] [Google Scholar]